Abstract
Shading, the predominant condition in most of the eucalyptus cultivation cycle, causes changes in the morphological and physiological weed's characteristics, which can alter their responses to glyphosate and carfentrazone-ethyl, important herbicides for the crop. The objective was to evaluate the influence of light on the efficiency of glyphosate and carfentrazone-ethyl used alone and in a mixture in Digitaria insularis control, a priority pest in the crop. The experiment was carried out in a 3 × 6 factorial scheme. The first factor corresponded to 3 cultivation environments (full sunlight, 45 and 63% shading) and the second factor to doses of glyphosate and carfentrazone-ethyl applied isolated (1920 and 40 g ai ha−1) and mixed (1536 + 8; 1152 + 16; 768 + 24 and 384 + 32 g ai ha−1), respectively. Shading increased D. insularis sensitivity to glyphosate alone and in a mixture with carfentrazone-ethyl. In shading, the glyphosate application alone at a dose of 1920 g ha−1 and in a mixture with carfentrazone-ethyl at doses of 1536 + 8 and 1152 + 16 g ha−1 were efficient in D. insularis control. In the environment of 63% shading, the dose of 768 + 24 g ha−1 was also efficient in this species control. None of the doses were effective in controlling D. insularis in full sunlight. Isolated carfentrazone-ethyl was inefficient in controlling D. insularis, regardless of the growth environment. Shading increases the quantum yield of photosystem II and reduces the electron transport rate, photosynthetic rate, stomatal conductance, and transpiration rate of D. insularis. In shady environments, it is possible to control D. insularis with lower glyphosate doses, used alone and mixed with carfentrazone-ethyl.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Eucalyptus sp. is considered the most planted forest species worldwide [1, 2]. In this scenario, Brazil stands out as one of the largest producers [3], obtaining high wood productivity per hectare/year in a shorter rotation period and growing crop expansion in the country [4, 5].
In areas of crop expansion or even the oldest stands, weed management is one of the most important practices in eucalyptus cultivation. Without control, weed interference can lead to losses of up to 40% in forest productivity [6, 7]. Of the five pests of economic importance and the greatest phytosanitary risk for eucalyptus, which are prioritized in the analysis of product registration processes and control technologies in Brazil, all are weeds [8]. Among them is Digitaria insularis [8], a perennial grass that is difficult to control, with biotypes resistant to glyphosate [9,10,11] and cross-resistance to acetyl-CoA carboxylase (ACCase) inhibiting herbicides [12]. Due to suitable climatic conditions and the constant use of the same mechanism of action, there is a risk of selecting glyphosate-resistant D. insularis biotypes in eucalyptus cultivation areas [13]. Digitaria insularis is also considered a weed in corn [14], soybean [15], cowpea [16], coffee [17], pastures [18], and in urban areas [10], which reinforces the species importance.
Glyphosate and carfentrazone-ethyl, herbicides that inhibit 5‐enolpyruvylshikimate 3‐phosphate synthase (EPSPS) and protoporphyrinogen oxidase (protox), respectively, are among the herbicides registered for eucalyptus in Brazil [19] and accepted by certifiers for use in the crop [20]. Both products have been used in eucalyptus plantations to control weeds between planting rows, even mixed in the tank as an alternative to increasing the control spectrum. In shaded environments, a condition commonly found under the canopies of eucalyptus planted forests [21], some weeds are more sensitive to glyphosate action, such as Euphorbia heterophylla [22] and Merremia cissoides [23] and protox-inhibiting herbicides, such as Commelina benghalensis [24], and can be controlled with lower doses of these products. The herbicides application in lower doses with high control efficiency, as may occur in shading conditions, helps to reduce the negative impacts of these pesticides on the environment, which is currently so questioned by society [25,26,27,28,29,30,31], in addition to reducing production costs.
Maintaining the viability of glyphosate and carfentrazone-ethyl is essential for successful weed management in eucalyptus crops. Mixing herbicides with different mechanisms of action increases the weed control spectrum, reduces selection pressure, delays the resistance emergence [32,33,34], and can also be used to control already resistant biotypes [35,36,37,38].
The mixture of glyphosate with protox-inhibiting herbicides has an additive and synergistic effect on the control of several species of broadleaf and grass weeds [39,40,41]. However, shading can interfere with the herbicide mixture's efficiency since the morphological and physiological plants characteristics [42,43,44,45,46] and herbicides efficiency when applied alone are altered under low light intensity [22,23,24].
The mixture of glyphosate and carfentrazone-ethyl can be a promising strategy to delay the emergence of resistant biotypes. However, the weed growth environment promoted by eucalyptus plantations must be considered when evaluating these herbicides' efficiency, which has been neglected. The objective of this study was to evaluate the effect of shading on the efficiency of glyphosate and carfentrazone-ethyl applied alone and in a mixture in the D. insularis control, a priority weed in the eucalyptus crop in Brazil.
2 Materials and methods
2.1 Site and plant material description
The experiment was carried out at the Instituto de Ciências Agrárias of the Universidade Federal de Minas Gerais, Brazil (16°40′58.1"S, 43°50′19.3"W). Köppen classifies the region’s climate as Aw—tropical with a dry season in winter [47].
Digitaria insularis seedlings were produced from tillers collected from plants in areas with frequent glyphosate applications. The seedlings were transplanted into 10 dm3 pots containing a substrate of sandy soil and bovine manure in a 3:1 ratio (volume:volume). In each pot, two D. insularis plants were grown and taken to the cultivation environments after transplanting. The soil used had the following characteristics: pH (water) = 5.3; organic matter = 1.66%; sand = 72%; silt = 16%; and clay = 12%. The substrate was fertilized with NPK 4-30-10 fertilizer, as recommended for pots fertilization [48], and irrigated once a day to maintain humidity between 80 and 100% of field capacity.
2.2 Experimental design and treatments
The design adopted was randomized blocks with four replications. The treatments were established in a 3 × 6 factorial scheme, where the first factor consisted of plants in full sunlight and two shading levels (45 and 63%), and the second factor, by doses of glyphosate and carfentrazone-ethyl, applied isolated and in a mixture (Table 1).
Shading was obtained with a black polypropylene shade screen, installed on structures built with wooden posts and wire 2 m high, closing the sides. The shading levels adopted in the study are similar to those previously reported for eucalyptus planted forests [49, 50]. The incidence of photosynthetically active radiation (PAR) in the growth environments was determined in 20 days during the experiment execution, at 8:00, 12:00, and 16:00 h, with the fluorometer device Y (II) meter (OPTI- SCIENCES, Hudson, USA) (Fig. 1).
The plants remained in the cultivation environments for 52 days. A standardization cut was performed during this period at 37 cultivation days, at 5 cm height. At 15 days after plant cutting, the herbicides were applied. The application was carried out with a backpack sprayer pressurized with CO2 with a TTI 11002 nozzle model (Teejet, Wheaton, Illinois, USA) and a pressure regulating valve (Comam, Belo Horizonte, Brazil) constant at 300 kPa, calibrated to apply 116 L ha−1 of spray volume.
2.3 Assessments
At 3 and 6 days after application (DAA), photosynthetic rate (PN, μmol CO2 m−2 s−1), stomatal conductance (gs, mol H2O m−2 s−1), and transpiration rate (E, µmol H2O m−2 s−1) of the plants were analyzed using an infrared gas analyzer (IRGA, model LCpro-SD Portable, Hoddesdon, England) and the quantum yield of photosystem II (ΦPSII) and electron transport rate (ETR) with the fluorometer device Y (II) meter (OPTI-SCIENCES, Hudson, USA).
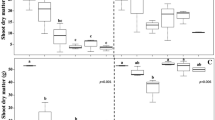
Visual control assessments were carried out at 28 and 60 DAA, adopting a scale from 0 to 100%, where 0 is the absence of herbicide injuries, and 100 is the plant death. Extra plants without herbicide application were maintained in each growth environment as a comparison parameter for treatment control scores. Three evaluators assigned control scores. The values per plot were determined by the arithmetic mean of the three scores. At 60 DAA, the plant biomass remaining in the pots was collected and weighed to determine the fresh biomass. For D. insularis, fresh biomass has the same behavior as dry biomass [51] and therefore was used as a parameter.
2.4 Statistical analysis
Data were submitted to analysis of variance (ANOVA), and when significant, means were grouped using the Scott-Knott test (p ≤ 0.05). ANOVA and the Scott-Knott mean clustering test were performed using the R Studio statistical program [52] and the ExpDes.pt package [53].
3 Results
3.1 Photosynthetic rate (PN), stomatal conductance (g s), and transpiration rate (E)
At 3 DAA, there was no difference between the growth environments in the plant's PN (Table 2). However, at 6 DAA, plants in shading showed lower PN. At 3 and 6 DAA, shading reduced the gs and E of D. insularis (Table 2).
At 3 DAA, the herbicide doses that most reduced PN, gs, and E were glyphosate applied alone at a dose of 1920 g ha−1 and mixed with carfentrazone-ethyl at doses 1536 + 8, 1152 + 16 and 768 + 24 g ha−1 (Table 3). At 6 DAA, the doses that most reduced these variables were glyphosate applied alone at a dose of 1920 g ha−1 and mixed with carfentrazone-ethyl at doses 1536 + 8 and 1152 + 16 g ha−1 (Table 3). Carfentrazone-ethyl applied alone was the treatment with the least impact on PN, gs, and E of D. insularis (Table 3).
3.2 Quantum yield of photosystem II (ΦPSII) and electron transport rate (ETR)
Shading increased ΦPSII and reduced the ETR of D. insularis at 3 and 6 DAA (Table 4). Between shading levels, there was no difference in ETR, however, the 63% shading environment showed higher ΦPSII.
The glyphosate application alone at a dose of 1920 g ha−1 and mixed with carfentrazone-ethyl at doses 1536 + 8 and 1152 + 16 g ha−1 caused the most significant reductions in ΦPSII and ETR of D. insularis at 3 and 6 DAA (Table 5). Carfentrazone-ethyl applied alone and in a mixture with glyphosate at doses of 768 + 24 and 384 + 32 g ha−1 caused the smallest impacts on these variables.
3.3 Control and fresh biomass of D. insularis
Shading increased the D. insularis sensitivity to glyphosate applied alone and in a mixture with carfentrazone-ethyl (Tables 6 and 7). At 28 and 60 DAA, in environments with 45 and 63% shading, the isolated glyphosate application at a dose of 1920 g ha−1 and in a mixture with carfentrazone-ethyl at doses 1536 + 8 and 1152 + 16 g ha−1 were efficient in D. insularis management. In the environment of 63% shading, the application of 768 + 24 g ha−1 was also efficient in controlling this species (Table 6). These treatments means were not statistically different, with control levels above 80%. These doses totally reduced the plant’s fresh biomass at 60 DAA (Table 7). For this variable, there was also no statistical difference between treatments. Although at 28 DAA, the doses 768 + 24 and 384 + 32 g ha−1 were efficient in environments with 45 and 63% shading, respectively, at 60 DAA, the control means for these treatments were statistically lower. The plants recovered from the herbicide injuries and the control was less than 80%, considered unsatisfactory (Table 6). In full sunlight, none of the applied doses effectively controlled D. insularis (Tables 6 and 7). In this environment, the plants recovered from the herbicide injuries, with drastic control reductions at 60 DAA compared to 28 DAA (Table 6). At 60 DAA in full sunlight, D. insularis control levels were less than 18% at all applied doses. Carfentrazone-ethyl applied alone is inefficient in controlling D. insularis, regardless of the growth environment (Tables 6 and 7).
4 Discussion
Shading increased D. insularis sensitivity to glyphosate applied alone and in a mixture with carfentrazone-ethyl. The increased D. insularis sensitivity to glyphosate applied alone and in a mixture with carfentrazone-ethyl in shading may be associated with lower ETR and PN of plants in these environments. These variables are related to carbon fixation and plant energy availability [54]. Plants with lower energy reserves are less likely to recover from the herbicides' injuries and may become more sensitive to these products' actions [55, 56]. The doses considered efficient in controlling D. insularis (1920 g ha−1 of glyphosate alone and mixed with carfentrazone-ethyl at doses 1536 + 8 and 1152 + 16 g ha−1) presented, for the physiological variables analyzed, means that were statistically lower than those treatments that did not control D. insularis. The shikimate metabolic pathway, inhibited by glyphosate, has an indirect role in plastoquinone production and ribulose-1,5-bisphosphate regeneration in the Calvin-Benson cycle [57], important proteins in electron transport rate and carbon fixation. The influence of glyphosate in plastoquinone production and ribulose-1,5-bisphosphate regeneration may explain the reductions in ETR and PN of plants. Similar results were observed in Euphorbia heterophylla [22] and Salix miyabeana [58] after glyphosate application.
In addition to the energy deficit, changes in D. insularis growth caused by shading may be associated with greater herbicide sensitivity. Digitaria insularis has rhizomes, reserve organs that, when present, make it difficult to control [59]. Digitaria insularis begin to produce rhizomes 45 days after emergence [60]. In the present study, the herbicides were applied at 52 cultivation days, after the formation beginning of these structures in full sunlight. However, shading alters the dry matter partition of some grasses, investing more resources in shoot development as a function of root growth [61, 62], which, combined with lower ETR and PN, may have delayed or compromised rhizome development and increased D. insularis sensitivity. Shading can also reduce wax deposition on the leaf surface [56]. The smaller wax amount in the shade can increase herbicide penetration and efficiency [56].
Digitaria insularis is a C4 metabolism grass. C4 metabolism plants have a high light and temperature saturation point [63]. The reductions in ETR, PN, gs, and E in D. insularis grown in shading are due to the lower light incidence in these environments. These results align with those found in other grasses grown in the shade [64,65,66,67].
Increased sensitivity in shading to glyphosate applied alone was also observed in Euphorbia heterophylla [22] and Merremia cissoides [23], and the mixture of glyphosate and carfentrazone-ethyl in Macroptilium atropurpureum [68]. Unlike what was observed in the present study, where no increase in the efficiency of carfentrazone-ethyl isolated under shading was found, Santos Júnior [24] found an increase in saflufenacil efficiency, another protox-inhibiting herbicide, in controlling Commelina benghalensis.
Although D. insularis sensitivity to herbicides increased under shade, control after the doses application of 40 g ha−1 of carfentrazone-ethyl and 384 + 32 g ha−1 of glyphosate + carfentrazone-ethyl were inefficient in this environment. Caron [50] did not identify the need for weed control in eucalyptus planted forests when the shading level was greater than 60% due to low plant growth. However, the study does not report the radiation level corresponding to the shading levels studied, a factor that depends on the time and region where the study was conducted and that directly impacts weed growth and the decision to manage them. In addition, the weed community in the study was mainly composed of the species Sida rhombifolia, Stellaria media, Sonchus oleraceus, and Echium plantagineum, different species from the present study, and which have an unknown ability to adapt to shading. Therefore, the decision to manage or not to manage weeds depends more on the incident radiation level and the species' ability to grow in the shade than just the shade level imposed by the forest canopy. Even after the application of isolated carfentrazone-ethyl, which promoted control levels equal in full sunlight and shade, D. insularis accumulated more fresh biomass in shade, which reinforces the need for attention to the species in forest areas.
The low control obtained by isolated carfentrazone-ethyl, regardless of the growth environment, is due to the advanced plant stage at the application time. Carfentrazone-ethyl is a contact herbicide that acts by inhibiting chlorophyll synthesis [69, 70] but has no action on already-formed chlorophylls. As it does not act on already-formed chlorophylls and does not translocate in the plant, it is only efficient in controlling plants in the early development stages [71]. The low control obtained by the mixture of glyphosate and carfentrazone-ethyl at the dose of 384 + 32 g ha−1, regardless of the growth environment, is associated with the low glyphosate dose used in the mixture. Carfentrazone-ethyl applied alone at a dose of 40 g ha−1 and mixed with glyphosate at a dose of 384 + 32 g ha−1 were the doses that had the least impact on D. insularis physiology.
Although isolated carfentrazone-ethyl has low control efficiency against D. insularis, it has good efficiency against other glyphosate-resistant weeds, such as Commelina ssp. [39]. Moreover, its use in a mixture with glyphosate can benefit D. insularis control in shading, as in the present study, and against other important weeds, like the glyphosate-tolerant species [39, 72]. Additionally, the constant glyphosate application, the most widely used herbicide in the world, led to the selection of resistant biotypes over time [73,74,75]. The use of two or more herbicides with different mechanisms of action has been an interesting agronomic practice because, in addition to improving the management efficiency of tolerant weeds [76,77,78], it decreases selection pressure for resistant weeds [32].
The application of glyphosate alone and in a mixture with carfentrazone-ethyl showed different behavior depending on the light intensity in the growth environments, indicating the need to consider this factor when defining weed control doses in eucalyptus areas. Considering the light availability in growing environments in the definition of herbicide doses for weed control creates a new possibility for efficient dose reduction and, consequently, gains in economy and sustainability. Weeds in shading conditions are widely found in crops of other forest species, orchards, and integration systems, adding up to millions of hectares where this new management approach could be applied. Studies on the absorption and translocation of glyphosate and carfentrazone-ethyl, starch accumulation and wax deposition in the leaves of D. insularis cultivated in environments with different light intensities are necessary to elucidate the mechanisms involved in the species susceptibility or tolerance when grown in shade or full sunlight, respectively.
5 Conclusions
The shading of 45 and 63% of the photosynthetically active radiation increases D. insularis sensitivity to glyphosate applied alone and mixed with carfentrazone-ethyl, requiring lower doses for its control in the understory of eucalyptus plantations. In full sunlight, D. insularis was not controlled. To manage this species in shade, glyphosate application alone at a dose of 1920 g ha−1 and in a mixture with carfentrazone-ethyl at doses of 1536 + 8 and 1152 + 16 g ha−1 were efficient. In the environment of 63% shading, the dose of 768 + 24 g ha−1 was also efficient. The light intensity in the growing environments must be considered when defining glyphosate doses, applied alone or mixed with carfentrazone-ethyl.
Data availability
The data used in this research is available upon request.
Code availability
Not applicable.
References
Myburg AA, Grattapaglia D, Tuskan GA, et al. The genome of Eucalyptus grandis. Nature. 2014. https://doi.org/10.1038/nature13308.
Costa SEL, Santos RC, Vidaurre GB, Castro RVO, Rocha SMG, Carneiro RL, Campoe OC, Santos CPS, Gomes IRF, Carvalho NFO, Trugilho PF. The effects of contrasting environments on the basic density and mean annual increment of wood from eucalyptus clones. For Ecol Manage. 2020. https://doi.org/10.1016/j.foreco.2019.117807.
Gonçalves JLM, Alvares CA, Higa AR, Silva LD, Alfenas AC, Stahl J, Ferraz SFB, Lima WP, Brancalion PHS, Hubner A, Bouillet JPD, Laclau JP, Nouvellon Y, Epron D. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For Ecol Manag. 2013. https://doi.org/10.1016/j.foreco.2012.12.030.
IBÁ. Indústria Brasileira de Árvores. In: Report 2019. 2019. https://www.iba.org/publicacoes. Accessed 22 August 2023.
Cunha TQG, Santos AC, Novaes E, Hansted ALS, Yamaji FM, Sette CR Jr. Eucalyptus expansion in Brazil: Energy yield in new forest frontiers. Biomass Bioenergy. 2021. https://doi.org/10.1016/j.biombioe.2020.105900.
Tarouco CP, Agostinetto D, Panozzo LE, Santos LS, Vignolo GK, Ramos LOO. Weed interference periods on in the initial growth of eucalyptus. Pesqui Agropecu Bras. 2009. https://doi.org/10.1590/S0100-204X2009000900010.
Tiburcio RAS, Bacha AL, Alves PLCA, Salgado TP. Long-term growth response to weed-control strips in Eucalyptus urograndis plantations in Brazil. Aust For. 2023. https://doi.org/10.1080/00049158.2023.2167155.
MAPA. Ministério da Agricultura, Pecuária e Abastecimento. In: Portaria N° 112, de 8 de Outubro de 2018, Diário Oficial da União 198. 2018. https://pesquisa.in.gov.br/imprensa/jsp/visualiza/index.jsp?data=15/10/2018&jornal=515&pagina=4. Accessed 22 August 2023
Carvalho LB, Cruz-Hipolito H, González-Torralva F, Alves PLCA, Christoffoleti PJ, Prado R. Detection of sourgrass (Digitaria insularis) biotypes resistant to glyphosate in Brazil. Weed Sci. 2011. https://doi.org/10.1614/WS-D-10-00113.1.
Gazola T, Dias MF, Carbonari CA, Velini ED. Monitoring of resistance of sourgrass to glyphosate herbicide in urban areas of the state of São Paulo, Brazil. Planta Daninha. 2019. https://doi.org/10.1590/S0100-83582019370100121.
Takano HK, Oliveira RS, Constantin J, Mangolim CA, Machado MFPS, Bevilaqua MRR. Spread of glyphosate-resistant sourgrass (Digitaria insularis): Independent selections or merely propagule dissemination? Weed Biol Manag. 2018. https://doi.org/10.1111/wbm.12143.
Takano HK, Melo MSC, Ovejero RFL, Westra PH, Gaines TA, Dayan FE. Trp2027Cys mutation evolves in Digitaria insularis with cross-resistance to ACCase inhibitors. Pestic Biochem Physiol. 2020. https://doi.org/10.1016/j.pestbp.2019.12.011.
Barroso GM, Silva RS, Mucida DP, Borges CE, Ferreira SR, Santos JCB, Lins HA, Mendonça V, Silva DV, Santos JB. Spatio-temporal distribution of Digitaria insularis: risk analysis of areas with potential for selection of glyphosate-resistant biotypes in eucalyptus crops in Brazil. Sustainability. 2021. https://doi.org/10.3390/su131810405.
Gonçalves RM, Meirelles WF, Figueiredo J, Balbi-Peña MI, Paccola-Meirelles LD. Digitaria horizontalis and D. Insularis as alternative hosts for Pantoea ananatis in Brazilian maize fields. J Plant Pathol. 2015;97(1):177–81.
Gazziero DLP, Adegas FS, Silva AF, Concenço G. Estimating yield losses in soybean due to sourgrass interference. Planta Daninha. 2019. https://doi.org/10.1590/S0100-83582019370100047.
Neto FA, Oliveira Júnior JOL, Bezerra AAC, Silva Júnior GB, Albuquerque JAA, Zuffo AM, Aquino JPA, Borges AF. Floristic composition of weeds in a dystrophic red-yellow Argisol under the cultivation of cowpea, cv. BRS Novaera. Aust J Crop Sci. 2019. https://doi.org/10.2147/ajcs.19.13.05.p1553.
Carvalho LB, Alves PLCA, Bianco S. Sourgrass densities affecting the initial growth and macronutrient content of coffee plants. Planta Daninha. 2013. https://doi.org/10.1590/S0100-83582013000100012.
Chadhokar PA. Control of Digitaria insularis (l.) mez in tropical pastures. PANS. 1976. https://doi.org/10.1080/09670877609411460.
AGROFIT. Sistema de Agrotóxicos Fitossanitários do Ministério da Agricultura, Pecuária e Abastecimento (MAPA) do Brasil. 2023. http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Accessed 22 August 2023
FSC. FSC Lists of Highly hazardous pesticides. 2019. https://fsc.org/en/document-centre/documents/resource/315. Accessed 22 August 2023
Mattos EM, Binkley D, Campoe OC, Alvares CA, Stape JL. Variation in canopy structure, leaf area, light interception and light use efficiency among Eucalyptus clones. For Ecol Manag. 2020. https://doi.org/10.1016/j.foreco.2020.118038.
Ferreira GAP, Montes WG, Donato LMS, Faria RM, Santos LDT. Glyphosate doses should be lower for shaded environments: light and the sensitivity of Euphorbia heterophylla. Int J Pest Manag. 2022. https://doi.org/10.1080/09670874.2022.2056254.
Ferreira GAP, Donato LMS, Souza RF, Montes WG, Vaz V, Tuffi Santos LD. Adequacy of glyphosate doses in the Merremia cissoides (Lam.) Hallier f. control as a function of light intensity in the growth environments. J Environ Sci Health B. 2022. https://doi.org/10.1080/03601234.2022.2151790.
Santos Júnior A, Freitas FCL, Santos IT, Silva DC, Paixão GP, Sediyama CS. Management of Commelina benghalensis with saflufenacil in shaded environments. Planta Daninha. 2019. https://doi.org/10.1590/S0100-83582019370100051.
Gélinas P, Gagnon F, McKinnon C. Wheat preharvest herbicide application, whole-grain flour properties, yeast activity and the degradation of glyphosate in bread. Int J Food Sci Technol. 2018. https://doi.org/10.1111/ijfs.13741.
Zoller O, Rhyn P, Rupp H, Zarn JA, Geiser C. Glyphosate residues in Swiss market foods: monitoring and risk evaluation. Food Addit Contam Part B Surveill. 2018. https://doi.org/10.1080/19393210.2017.1419509.
Fernandes G, Aparicio VC, Bastos MC, Gerónimo E, Labanowski J, Prestes OD, Zanella R, Santos DR. Indiscriminate use of glyphosate impregnates river epilithic biofilms in southern Brazil. Sci Total Environ. 2019. https://doi.org/10.1016/j.scitotenv.2018.09.292.
Leite GLD, Paulo PD, Tuffi-Santos LD, Alvarenga AC, Soares MA, Dourado LR, Bispo EPR. Efficacy of Trichogrammatidae species (Hymenoptera) submitted to the herbicide glyphosate. Planta Daninha. 2019. https://doi.org/10.1590/S0100-83582019370100147.
Lupi L, Bedmar F, Puricelli M, Marino D, Aparicio VC, Wunderlin D, Miglioranza KSB. Glyphosate runoff and its occurrence in rainwater and subsurface soil in the nearby area of agricultural fields in Argentina. Chemosphere. 2019. https://doi.org/10.1016/j.chemosphere.2019.03.090.
Agostini LP, Dettogni RS, Reis RS, Stur E, Santos EVW, Ventorim DP, Garcia FM, Cardoso RC, Graceli JB, Louro ID. Effects of glyphosate exposure on human health: insights from epidemiological and in vitro studies. Sci Total Environ. 2020. https://doi.org/10.1016/j.scitotenv.2019.135808.
Medalie L, Baker NT, Shoda ME, Stone WW, Meyer MT, Stets EG, Wilson M. Influence of land use and region on glyphosate and aminomethylphosphonic acid in streams in the USA. Sci Total Environ. 2020. https://doi.org/10.1016/j.scitotenv.2019.136008.
Busi R, Powles SB, Beckie HJ, Renton M. Rotations and mixtures of soil-applied herbicides delay resistance. Pest Manag Sci. 2020. https://doi.org/10.1002/ps.5534.
Liu C, Neve P, Glasgow L, Wuerffel RJ, Owen MDK, Kaundun SS. Modeling the sustainability and economics of stacked herbicide-tolerant traits and early weed management strategy for waterhemp (Amaranthus tuberculatus) control. Weed Sci. 2020. https://doi.org/10.1017/wsc.2020.6.
Sharpe SM, Boyd NS. Doveweed (Murdannia nudiflora) response to metsulfuron-methyl, trifloxysulfuron-sodium, and bentazon combinations. Weed Technol. 2020. https://doi.org/10.1017/wet.2019.104.
Miller MR, Norsworthy JK. Evaluation of herbicide programs for use in a 2,4-D–resistant soybean technology for control of glyphosate-resistant palmer amaranth (Amaranthus palmeri). Weed Technol. 2016. https://doi.org/10.1614/WT-D-15-00129.1.
Brunton DJ, Boutsalis P, Gill G, Preston C. Control of thiocarbamate-resistant rigid ryegrass (Lolium rigidum) in wheat in southern Australia. Weed Technol. 2020. https://doi.org/10.1017/wet.2019.72.
Pigatto CS, Tarouco CP, Nicoloso FT, Berghetti ÁLP, Leães GP, Werle IS, Ulguim AR. Barnyardgrass control using tank-mixed herbicides with saflufenacil and its influence in photosynthesis and chlorophyll fluorescence. Cienc Rural. 2020. https://doi.org/10.1590/0103-8478cr20190919.
Zargar M, Bayat M, Astarkhanova T. Study of postemergence-directed herbicides for redroot pigweed (Amaranthus retroflexus L.) control in winter wheat in southern Russia. J Plant Prot Res. 2020. https://doi.org/10.2442/jppr.2019.131272.
Werlang RC, Silva AA. Glyphosate—carfentrazone-ethyl interaction. Planta Daninha. 2002. https://doi.org/10.1590/S0100-83582002000100013.
Ramires AC, Constantin J, Oliveira RS, Guerra N, Alonso DG, Biffe DF. Control of Euphorbia heterophylla and Ipomoea grandifolia using glyphosate isolated or in association with broadleaf herbicides. Planta Daninha. 2010. https://doi.org/10.1590/S0100-83582010000300020.
Maciel CDG, Poletine JP, Amstalden SL, Gazziero DLP, Raimondi MA, Lima GRG, Oliveira Neto AM, Guerra N, Justiniano W. Glyphosate tank mixtures controlling Commelina benghalensis, Tridax procumbens and Cenchrus echinatus in soybean roundup ready®. Rev Ceres. 2011. https://doi.org/10.1590/S0034-737X2011000100006.
Hazrati S, Tahmasebi-Sarvestani Z, Modarres-Sanavy SAM, Mokhtassi-Bidgoli A, Nicola S. Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiol Biochem. 2016. https://doi.org/10.1016/j.plaphy.2016.04.046.
Ajmi A, Vázquez S, Morales F, Chaari A, El-Jendoubi H, Abadía A, Larbi A. Prolonged artificial shade affects morphological, anatomical, biochemical and ecophysiological behavior of young olive trees (cv. Arbosana). Sci Hortic. 2018. https://doi.org/10.1016/j.scienta.2018.06.089.
Artru S, Lassois L, Vancutsem F, Reubens B, Garré S. Sugar beet development under dynamic shade environments in temperate conditions. Eur J Agron. 2018. https://doi.org/10.1016/j.eja.2018.04.011.
Yasin M, Rosenqvist E, Andreasen C. The effect of reduced light intensity on grass weeds. Weed Sci. 2017. https://doi.org/10.1017/wsc.2017.17.
Yasin M, Rosenqvist E, Jensen SM, Andreasen C. The importance of reduced light intensity on the growth and development of six weed species. Weed Res. 2019. https://doi.org/10.1111/wre.12352.
Climate-Data. Dados climáticos para cidades mundiais. In: Montes Claros. 2022. https://pt.climate-data.org/america-do-sul/brasil/minas-gerais/montes-claros-2886/. Accessed 22 August 2023
Novais RF. Teores de nutrientes a serem adicionados ou atingidos em ensaios de vaso. In: Oliveira AJ, Garrido WE, Araújo JD, Lourenço S, editors. Métodos de pesquisa em fertilidade do solo. Brasília: Embrapa; 1991. p. 195–195.
Oliveira TK, Macedo RLG, Venturin N, Botelho SA, Higashikawa EM, Magalhães WM. Solar Radiation in Understory of Agrosylvopastoral System with Eucalypt on Different Spacings. Cerne. 2007; https://cerne.ufla.br/site/index.php/CERNE/article/view/288
Caron BO, Souza VQ, Costa EC, Eloy E, Behling A, Trevisan R. Interception of radiation by the canopy of forest species and its relationship with the weed control requirement. Cienc Rural. 2012. https://doi.org/10.1590/S0103-84782012000100013.
Carvalho SJP, Dias ACR, Shiomi GM, Christoffoleti PJ. Simultaneous addition of ammonium sulfate and urea to glyphosate spray solution. Planta daninha. 2010. https://doi.org/10.1590/S0100-83582010000300014.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2019.
Ferreira EB, Cavalcanti PP, Nogueira DA. ExpDes: an R package for ANOVA and experimental designs. Appl Math. 2014. https://doi.org/10.4236/am.2014.519280.
Saroussi S, Karns DAJ, Thomas DC, Bloszies C, Fiehn O, Posewitz MC, Grossman AR. Alternative outlets for sustaining photosynthetic electron transport during dark-to-light transitions. Proc Natl Acad Sci USA. 2019. https://doi.org/10.1073/pnas.190318511.
Tuffi Santos LD, Meira RMSA, Santos IC, Ferreira FA. Effect of glyphosate on the morpho-anatomy of leaves and stems of C. diffusa and C. benghalensis. Planta Daninha. 2004. https://doi.org/10.1590/S0100-83582004000100013.
Silva MSN, Santos LDT, Donato LMS, Ferreira GAP, Barbosa DMCR, Barros RE, Reis MM. Luminous restriction and chemical control with glyphosate in integrated management of Urochloa brizantha L. Res Soc Dev. 2022. https://doi.org/10.3344/rsd-v11i17.38758.
Maeda H, Dudareva N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu Rev Plant Biol. 2012. https://doi.org/10.1002/9780470015902.a0001315.pub2.
Gomes MP, Le Manac’h SG, Hénault-Ethier L, Labrecque M, Lucotte M, Juneau P. Glyphosate-dependent inhibition of photosynthesis in willow. Front Plant Sci. 2017. https://doi.org/10.3389/fpls.2017.00207.
Gemelli A, Oliveira Junior RS, Constantin J, Braz GBP, Jumes TMC, Oliveira Neto AM, Dan HDA, Biffe DF. Biology aspects of Digitaria insularis resistant to glyphosate and implications for its control. Rev Bras Herbic. 2012. https://doi.org/10.7824/rbh.v11i2.186.
Machado AFL, Ferreira LR, Ferreira FA, Fialho CMT, Tuffi Santos LD, Machado MS. Growth analysis of Digitaria insularis. Planta Daninha. 2006. https://doi.org/10.1590/S0100-83582006000400004.
Cruz P. Effect of shade on the carbon and nitrogen allocation in a perennial tropical grass, Dichanthium aristatum. J Exp Bot. 1997. https://doi.org/10.1093/jxb/48.1.15.
Fernández ME, Gyenge JE, Schlichter TM. Shade acclimation in the forage grass Festuca pallescens: Biomass allocation and foliage orientation. Agrofor Syst. 2004. https://doi.org/10.1023/B:AGFO.0000013276.68254.78.
Black CC. Ecological Implications of dividing plants into groups with distinct photosynthetic production capacities. Adv Ecol Res. 1971. https://doi.org/10.1016/S0065-2504(08)60203-2.
Ubierna N, Sun W, Kramer DM, Cousins AB. The efficiency of C4 photosynthesis under low light conditions in Zea mays, Miscanthus x giganteus and Flaveria bidentis. Plant Cell Environ. 2013. https://doi.org/10.1111/j.1365-3040.2012.02579.x.
Santos MV, Ferreira EA, Valadão D, Oliveira FLR, Machado VD, Silveira RR, Souza MF. Brachiaria physiological parameters in agroforestry systems. Cienc Rural. 2017. https://doi.org/10.1590/0103-8478cr20160150.
Sales CRG, Ribeiro RV, Hayashi AH, Marchiori PER, Silva KI, Martins MO, Silveira JAG, Silveira NM, Machado EC. Flexibility of C4 decarboxylation and photosynthetic plasticity in sugarcane plants under shading. Environ Exp Bot. 2018. https://doi.org/10.1016/j.envexpbot.2017.10.027.
Gomes FJ, Pedreira CGS, Bosi C, Cavalli J, Holschuch SG, Mourão GB, Pereira DH, Pedreira BC. Shading effects on marandu palisadegrass in a silvopastoral system: plant morphological and physiological responses. Agron J. 2019. https://doi.org/10.2134/agronj2019.01.0052.
Costa GA, Santos LDT, Ferreira GAP, Cruz LR, Machado VD, Rocha LM. Levels of shading and application of glyphosate and carfentrazone-ethyl in the control of Macroptilium atropurpureum. Rev Bras Eng Agric Ambient. 2018. https://doi.org/10.1590/1807-1929/agriambi.v22n12p819-824.
Matringe M, Camadro JM, Labbe P, Scalla R. Protoporphyrinogen oxidase as a molecular target for diphenyl ether herbicides. Biochem J. 1989. https://doi.org/10.1042/bj2600231.
Jacobs JM, Jacobs NJ, De Maggio AE. Protoporphyrinogen oxidation in chloroplasts and plant mitochondria, a step in heme and chlorophyll synthesis. Arch Biochem Biophys. 1982. https://doi.org/10.1016/0003-9861(82)90341-1.
Concenço G, Andres A, Galon L, Pontes CS, Correia VT. Macroptilium lathyroides control with pre and post-emergence herbicide applications. Rev Bras Herbic. 2012. https://doi.org/10.7824/rbh.v11i1.144.
Sharma SD, Singh M. Effect of timing and rates of application of glyphosate and carfentrazone herbicides and their mixtures on the control of some broadleaf weeds. Hortic Sci. 2007. https://doi.org/10.2127/HORTSCI.42.5.1221.
Goh SS, Yu Q, Han H, Vila-Aiub MM, Busi R, Powles SB. Non-target-site glyphosate resistance in Echinochloa colona from Western Australia. Crop Prot. 2018. https://doi.org/10.1016/j.cropro.2018.06.013.
Mora DA, Cheimona N, Palma-Bautista C, Rojano-Delgado AM, Osuna-Ruiz MD, de la Alcántara Cruz R, Prado R. Physiological, biochemical and molecular bases of resistance to tribenuron-methyl and glyphosate in Conyza canadensis from olive groves in southern Spain. Plant Physiol Biochem. 2019;144:14–21. https://doi.org/10.1016/j.plaphy.2019.09.023.
Chen J, Huang H, Wei S, Cui H, Li X, Zhang C. Glyphosate resistance in Eleusine indica: EPSPS overexpression and P106A mutation evolved in the same individuals. Pestic Biochem Physiol. 2020. https://doi.org/10.1016/j.pestbp.2020.01.014.
Kumar V, Jha P. Control of volunteer glyphosate-resistant canola in glyphosate-resistant sugar beet. Weed Technol. 2015. https://doi.org/10.1614/WT-D-14-00059.1.
McCullough PE, Johnston CR, Reed TV, Yu J. Indaziflam enhances buckhorn plantain (Plantago lanceolata) control from postemergence herbicides. Weed Technol. 2015. https://doi.org/10.1016/j.foreco.2020.118038.
Walsh KD, Soltani N, Shropshire C, Sikkema PH. Weed control in soybean with imazethapyr applied alone or in tank mix with Saflufenacil/Dimethenamid-P. Weed Sci. 2015. https://doi.org/10.1614/WS-D-14-00076.1.
Acknowledgements
The author’s thanks Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (Process PPM-00027-17) for funding the studies and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for providing scholarships to the first, second, and third authors (Finance Code 001).
Plant guidelines
The study followed the national/institutional guidelines during the study.
Funding
Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (Process PPM-00027-17).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by GAde PF, LMSD, WGM, LMR and LDTS. The first draft of the manuscript was written by GAdPF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferreira, G.A.P., Donato, L.M.S., Montes, W.G. et al. Control of Digitaria insularis (L.) Fedde in eucalyptus forests: shading increases sensitivity to glyphosate applied alone and in a mixture with carfentrazone-ethyl. Discov Agric 2, 3 (2024). https://doi.org/10.1007/s44279-024-00014-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44279-024-00014-5