Abstract
Peripheral nerve injury (PNI) is associated with considerable functional impairment. Photobiomodulation (PBM) has demonstrated positive effects regarding neuromuscular repair after PNI when applied locally to the nerve or injured muscle. However, the effects of systemic PBM with transcutaneous application over an important artery, which is also denominated vascular PBM (VPBM), remain unclear. The aim of the study was to compare the effects of VPBM with low-level laser (LLL) and light-emitting diode (LED) on gait, sensitivity and muscle morphology following a PNI. PNI was induced on Wistar rats using the sciatic nerve crushing technique. VPBM was performed over the rat’s artery tail region with LED (850 nm, 40 mW, 3.2 J) and LLL (780 nm, 40 mW, 3.2 J). Gait functionality, mechanical (nociceptive) sensitivity, and morphology of the tibialis anterior muscle were evaluated at 7, 14, and 21 days after injury. An improvement in functional gait was shown in the VPBM-LLL group in all periods. Motor sensitivity was found after 14 days in the VPBM-LLL group. The left/right (L/R) muscle mass ratio revealed a reduction in muscle atrophy in the VPBM-LLL group at 7 days. Muscle fiber diameter increased in the VPBM-LED group at 14 days and increases in the cross-section area were found in the VPBM-LED and VPBM-LLL groups at 7 days. VPBM with both light sources (LED and LLL) positively modulated functioning and neuromuscular recovery following sciatic nerve injury in rats, with more pronounced results when using LLL.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Statements and declarations
1.1 Introduction
Peripheral nerve injury (PNI) constitutes an interruption of nerve impulses, causing the loss of functioning and sensitivity as well as muscle weakness and a consequent loss of muscle mass. PNI affects 13–23 people per 100,000 individuals per year and results in a reduction in quality of life [1].
The repair process is initiated after the occurrence of a PNI, which involves neuronal growth, reinnervation and functional recovery. However, when the injury is accompanied by Wallerian degeneration, as occurs in crush injuries, the muscles innervated by the affected fibers undergo progressive deterioration, with insufficient replacement of the degenerated tissue [2]. This type of PNI leads to muscle atrophy, a reduction in the cross-sectional area (CSA) of muscle fibers, and a reduction in nicotinic acetylcholine receptors in the endplate due to the impairment of motor fibers, resulting in a functional disorder [3, 4].
Denervation directly affects the active mechanical characteristics of muscles. In the early stage, the greatest difficulty in performing muscle contraction is due to the decrease in the CSA of the muscle fibers. As a consequence, the maximum tension generated by the contraction of the denervated muscle decreases proportionally. With the prolongation of denervation, the progressive disorganization and replacement of muscle fibers with collagen reduces the capacity for strength [5].
The use of light irradiation to modulate biological processes is denominated photobiomodulation (PBM), which has demonstrated positive results with regards to neuromuscular repair following a PNI [6]. Low-level laser (LLL) has demonstrated positive effects in such cases by modulating neuromuscular repair. Most LLL studies have used a power of up to 50 mW and total energy of up to 15 J administered at various points over the affected muscle and/or nerve. Moreover, determining the best dosimetric parameters is the key to establishing a PBM protocol for improving the regeneration process following a PNI [3].
Light-emitting diode (LED) has been increasingly explored as an alternative light source for PBM. It was believed that the coherence of LLL was essential to obtaining therapeutic results, but studies involving LED have recently challenged this theory, as this modality emits non-coherent light over a wide wavelength range yet has achieved important therapeutic results. The therapeutic differences between LED and LLL have not yet been precisely determined, but a very common assertion is that laser penetrates deeper than LEDs. The difference between the PBM sources are the coherence and monochromatic beam of LLL light and enable the irradiation of a larger area [7].
Systemic PBM—also known as vascular PBM (VPBM) [8]—is an alternative to local and direct irradiation in which the focus is on the irradiation of blood, which can be performed in two ways: intravascular or non-invasive transcutaneous irradiation. VPBM enables irradiating areas close to large blood vessels with no need for the previous determination of irradiation points [9].
The indirect effects of VPBM, considering the non-static structures, is based on blood modification through the influence of the light, which promotes effects in the enhancement of the regeneration muscle process [10], and along with an improvement in microcirculation, especially in structures of the central nervous system, such as the hypothalamus, which has a highly developed microvascular system [11]. Intravenous irradiation stimulates functional activity of the hypothalamus and limbic system, which leads to the activation of hormonal, immunological, metabolic, and vegetative processes, with the mobilization of adaptive reserves [12]. Brill et al. (1993) [12] showed a regulation of cytochemical parameters of neutrophils, lymphocytes and monocytes in an in vitro irradiation of blood and later Kilik et al. (2009) [11] correlated the increase of protein levels in the blood plasma of animals treated with VPBM in the heart area with a protective effect of the irradiation from oxidative stress and the damage.
However, no VPBM protocols have been performed following a sciatic nerve injury. It is necessary to fill this gap in knowledge to standardize a protocol that ensures the recovery of motricity, sensitivity and muscle trophism, as the insufficiency of these aspects can generate disabling sequelae in affected individuals. Therefore, the aim of this study was to compare the effects of VPBM using different light sources (LLL or LED) on neuromuscular recovery (motor function and sensitivity) as well as muscle atrophy and morphology following a compression-induced sciatic nerve injury in Wistar rats.
2 Materials and methods
This study was conducted at the research laboratory of Universidade Nove de Julho (UNINOVE, Sao Paulo, Brazil) after receiving approval from the Animal Experimentation Ethics Committee of the same university (certificate number: 3249161020). Animal care and procedures were performed by trained personnel and conducted in accordance with the “Guidelines for the care and use of laboratory animals” (8th edition, National Academy Press, Washington DC, 2011) and according to the ARRIVE Guidelines 2.0 checklist. Appropriate measures were taken to minimize pain, discomfort and stress in the animals.
2.1 Animals
Male Wistar rats, 12 weeks of age, weighing between 300 and 350 g were obtained from the UNINOVE animal lodging facility. The animals were housed in appropriate cages with temperature controlled between 22 and 25ºC, a 12-h light/dark cycle with free access to food and water.
2.2 Experimental groups
The animals were divided into four groups. The analysis periods were 7, 14, and 21 days following sciatic nerve injury or the beginning of the protocol (control group).
Control group: Animals not submitted to any procedure (n = 5);
Injury group: Animals submitted to sciatic nerve injury by compression, with no light treatment [n = 15, euthanized at 7 (n = 5), 14 (n = 5) and 21 (n = 5) days];
Vascular-PBM-LED group: Animals submitted to sciatic nerve injury by compression followed by VPBM with LED (850 nm, 40 mW, 3.2 J over a single point) [n = 15, euthanized at 7 (n = 5), 14 (n = 5) and 21 (n = 5) days];
Vascular PBM-LLL group: Animals submitted to sciatic nerve injury by compression followed by VPBM with LLL (780 nm, 40 mW, 3.2 J over a single point) [n = 15, euthanized at 7 (n = 5), 14 (n = 5) and 21 (n = 5) days].
2.3 Surgical procedure
The sciatic nerve crush procedure was performed as described by Belchior et al. (2009)[13] and Andreo et al. (2019) [4]. After acclimatization, the animals were weighed and submitted to anesthesia with an intraperitoneal injection of 10% ketamine (100 mg/kg, Vetbrands, São Paulo, Brazil) and 2% xylazine (10 mg/kg, Vetbrands, São Paulo, Brazil) using syringes with Ultra-Fine® needles (length: 12.7 mm; caliber: 0.33 mm; three-faceted bevel; BD, Brazil).
After the removal of fur from the surgical site, the animals were placed in the prone position and the skin on the posterior surface of the thigh was cleaned with a 2% chlorhexidine solution. An incision was made parallel to the ischium to enable isolation of the sciatic nerve from the other structures. The crushing injury was performed using hemostatic forceps (Rhosse Instruments and Surgical Equipment, Ribeirão Preto, SP, Brazil). Compression was performed 5 mm above the branch for 30 s with a pressure of 6.3 MPa [14, 15]. The surgical procedure was performed by the same operator on all animals. The surgical wound was sutured with polyamide thread and the animals were placed under observation. For the first four postoperative days, tramadol hydrochloride (5 mg/kg) and dipyrone (50 mg/kg) were administered subcutaneously every eight hours for analgesia and a single dose of tetracycline dihydrate was administered subcutaneously (0.1 mL/kg, Terramycin LA, Pfizer Inc, Guarulhos, SP, Brazil) for prophylaxis of complications secondary to possible infection [15].
2.4 VPBM procedure
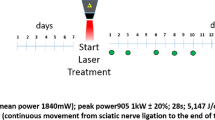
VPBM was performed using the parameters based on previous studies but applied in multipoint over the injured area [4, 16, 17]. The irradiation was applied following PNI using the Twin Laser® equipment (MM Optics, São Carlos, SP, Brazil) or using a prototype consisting of an infrared light-emitting diodes connected to a power source (MPS-3005B-MINIPA, São Paulo -SP, Brazil), transcutaneously over the vein/artery located on the dorsum of the base of the tail [10, 18]. The parameters are listed in Table 1.
During the irradiation procedure, a 90-degree angle between the light emitter and skin was adopted to avoid refraction and reduce the energy lost during the light transmission. Laser light emission power was measured at the same angle at the beginning and end of the experimental procedure using the LaserCheck power meter (MM Optics, São Carlos, SP, Brazil).
In the VPBM groups, treatment was initiated two hours after the induction of injury with a single daily application five consecutive days per week until the completion of the different periods of analysis [15, 19]. The groups receive VPBM with LED or with LLL, the irradiation was administered transcutaneously over a single point at the base of the tail [10, 18, 20].
Functional evaluations were performed on days 7, 14, and 21 following the induction of sciatic nerve injury. After the evaluations in each period, five animals from each group were submitted to euthanasia with an overdose of 10% ketamine (300 mg/kg) and 2% xylazine (30 mg/kg) administered in an intraperitoneal injection. The tibialis anterior (TA) muscles were removed, weighed, and submitted to histological analysis for the determination of morphological aspects.
2.5 Functional gait analysis
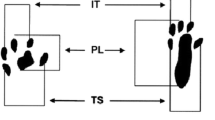
Functional gait analysis was performed using the Sciatic Functional Index (SFI) based on Bain et al. (1989) [4, 21]. For this evaluation, the paws were dyed in ink to obtain footprints as the animals walked along a track covered with sulfite strips, constructed as described by Medinaceli et al. (1982) [22], with closed sides and a small shelter at the end. The variables of interest were print length (PL), total spread of toes (TS—1st–5th toes), intermediate toes (IT—2nd–4th toes) and distance to other foot (TOF) [21].
The SFI was calculated as follows:
in which
E: experimental.
N: normal.
TOF: distance between paws.
PL: paw length.
TS: distance between 1st and 5th toes.
IT: distance between 2nd and 4th toes.
SFI: Sciatic Functional Index.
The SFI begins at zero (normal function), with progressively negative results indicating greater dysfunction (maximum dysfunction: -100) [21].
2.6 Analysis of mechanical (nociceptive) sensitivity
The von Frey test was used to assess mechanical (nociceptive) sensitivity, as described by Takasaki et al. (2000) [23]. The animals were placed in a transparent box on a wire mesh to enable access to the plantar surface of the paw and were habituated for 20 min. Additionally, before the execution of each test and in the three days prior to the first collection, the animals were habituated for one hour in an environment with no sound interruption that could interfere with the performance of the test (startling the animals).
A digital analgesiometer (Insight Ltda., Ribeirão Preto, SP, Brazil) with a 0.1–1000 g capacity transducer and reaction time of 1 ms was used for this evaluation. The test consisted of increasing pressure over the plantar region of the left hind paw (corresponding to the affected area). The pressure transducer was connected to a digital force counter and expressed pressure in grams. The transducer was applied linearly increasing pressure on the center of the left plantar surface until the animal response by withdrawing the paw from the stimulus. The stimulus was repeated three times and the arithmetic mean of the withdrawal response was calculated [23].
2.7 Muscle mass analysis
After euthanizing the animals, the right and left TA muscles were carefully removed, cleaned and weighed on a semi-analytical scale to obtain the muscle mass ratio calculated as described by Shen et al. (2013) [14].
in which
r: muscle mass ratio
L: muscle mass of injured limb
R: muscle mass of uninjured limb
2.8 Qualitative and quantitative assessment of morphological aspects
Muscle samples were also used for morphological analysis. For such, the samples were fixed in 10% formalin buffer (pH 8.0) and kept at room temperature. The material was then dehydrated in solutions with an increasing concentration of ethyl alcohol (70%, 80%, and 90%) for a period of one hour each, passed three times in absolute alcohol and three times in xylol (Reagen). The samples were embedded in paraffin (Paraplast, Sigma, USA) for 12 h at 60ºC with the central portions positioned more externally in the block. Three slices measuring 10 μm in thickness were obtained using a microtome (Leica RM2125, Nussloch, Germany), transferred to microscope slides and placed in an oven at 37 °C for 12 h.
The specimens were submitted to histological staining with hematoxylin and eosin (HE) for the evaluation of morphological aspects [muscle fiber diameter, fiber cross-sectional area (CSA) and location of the nucleus] by light microscopy (Axioplan 2 microscope, Zeiss, Germany). The quantitative analysis was performed by counting these aspects in five photographed areas per slide from three animals per group with a final magnification of 400X. The analysis was performed with the aid of the Image J software (National Institutes of Health, USA) and the data were submitted to statistical analysis.
2.9 Data analysis
Data were analyzed using the GraphPad Prism software, version 8.0.1 (GraphPad Software San Diego, California, USA). Normality was assessed using the Kolmogorov–Smirnov test. Data were expressed as mean and standard error of the mean and were submitted to analysis of variance one-way ANOVA followed by Tukey’s test for comparisons between groups. Confidence levels were adjusted to 95% (p ≤ 0.05).
3 Results
3.1 Functional gait analysis
The functional gait assessment using the SFI revealed results within the normal range (close to zero) in the control group, as expected. All injured groups (PNI, VPBM-LED and VPBM-LLL) exhibited greater impairment (more negative SFI scores), differing significantly from the control group (p < 0.001) on Days 7 and 14 (Fig. 1). A statistically significant (p < 0.05) improvement in gait was found on Day 7 in the VPBM-LLL group compared to the PNI and VPBM-LED groups (Fig. 1). At 14 days, gait was significantly better the VPBM-LLL group compared to the PNI group (p < 0.05) (Fig. 1). At 21 days, no difference in gait was found between the VPBM groups (LLL or LED) and the control group. In this same period, the injured group without irradiation had more negative scores compared to the control and VPBM groups (Fig. 1).
3.2 Analysis of mechanical (nociceptive) sensitivity
The nociceptive analysis (mechanical sensitivity) results using the von Frey test revealed no statistically significant difference among the experimental groups at 7 and 21 days (Fig. 2). At 14 days, the VPBM-LLL group exhibited a reduction in the pressure required to induce the paw withdraw (p < 0.05) in comparison to the injury group (PNI), that may induce an improvement in the sensitivity response in this irradiated group since the pressure values obtained were similar to the control (normal) group.
3.3 Muscle mass analysis
The results regarding L/R mass ratio of the TA muscle are shown in Fig. 3. The control group had values within the expected normality range (close to 1). At 7 days, only the VPBM-LLL group exhibited a statistically significant reduction in the L/R ratio compared to the control group (p < 0.05). After 14 and 21 days, statistically significant reductions in the L/R ratio were found in all injured groups (PNI, VPBM-LED and VPBM-LLL) compared to the control group (p < 0.05). In these latter two periods of analysis, no significant differences were found between the PNI group and groups that received VPBM with LLL or LED (Fig. 3).
3.4 Effects of PBM on muscle morphology
The histology images obtained after HE staining of the different experimental groups are shown in Fig. 4. As expected, the control group had peripheral nuclei with polygonal muscle fibers organized into an endomysium and perimysium. After 7 days, the PNI group exhibited an increase in the interstitial space, greater evidence of angled fibers as well as a smaller muscle fiber diameter and area compared to the VPBM-LED and VPBM- LLL groups. After 14 and 21 days, all experimental groups exhibited this same evolution (muscle atrophy, increase in the interstitial space between the fibers, greater deposition of collagen and angled fibers) similarly.
The analysis of muscle fiber diameter (Fig. 5a) at 7, 14, and 21 days revealed a statistically significant reduction (p < 0.05) in all injured groups (PNI, VPBM-LED, and VPBM-LLL) compared to the control group. At 14 days, the VPBM-LED group exhibited an increase in fiber diameter compared to the injury group without irradiation (PNI). At 21 days, no significant differences were found between the PNI group and VPBM groups.
The analysis of the cross-sectional area (CSA) of muscle fibers (Fig. 5b) revealed a significant increase in the irradiated groups (VPBM-LED and VPBM-LLL) at 7 days compared to PNI group. However, no differences were found between the PNI group and groups submitted to VPBM with LLL or LED at 14 and 21 days.
4 Discussion
This study investigated the effects of VPBM using different light sources (LED or LLL) on rats submitted to sciatic nerve injury. Previous studies have shown the beneficial effects of local PBM for the treatment of PNI, such as enhanced motor and sensory recovery, an increase in muscle trophism and modulated gene expression of myogenic regulatory factors and nicotinic acetylcholine receptors [4]. Using the same PNI model and local PBM, a previous study conducted by our research group showed positive effects of LLL with regards to neuromuscular repair, such as improvements in gait, sensitivity and muscle mass [4]. However, there is a lack of knowledge on the potential effects of vascular PBM on blood cells, such as peripheral leukocytes, which play an important role in neuromuscular recovery following a PNI. In this way, previous in vivo studies showed that VPBM irradiated in a preventive or therapeutic form (same LLL dosimetric parameters used in the present study), led to decrease inflammatory infiltrate, myonecrosis, biochemical markers, and increase immature muscle fibers in a model of acute muscle injury [10]. Silva et al. (2020) also showed that VPBM using LED in acute lung injury in rats reduced the recruitment of neutrophils into the bronchoalveolar lavage and reduce the production of the principals proinflammatory cytokines involved in this process [20]. Additionally, Martinelli et al. (2022) compared the vascular and local PBM and showed that both forms of irradiation were able to improve the muscle size and gait during the compensatory hypertrophy process with more pronounced effects with systemic irradiation (VPBM) [18]. Therefore, the purpose of this study was to determine how non-invasive VPBM with different light sources would influence these functional and morphological aspects.
Considering gait, the SFI is the gold standard for assessing sciatic nerve function [21]. However, some difficulties have been reported when performing the evaluation on rats. PNI can result in self-mutilation of the denervated part in rodents. This behavior is denominated autotomy and may be caused due to painful dysesthesia that projects to the three lateral toes of the foot [24]. Other problems have also been reported that could hinder the evaluation of the results, such as the development of flexion contractures, with the trailing of the tail and smudging during the recording of footprints [21]. In this study, autotomy occurred in two of the 21-day PNI group—the first performed self-mutilation at 16 days and the second at 18 days after the injury. These animals were excluded from the analysis.
Considering the SFI scores, a statistically significant improvement in movement recovery was found in the group irradiated with vascular LLL compared to the PNI group (without treatment) at all evaluation times as well as a statistically significant functional improvement at 7 days compared to the group irradiated with LED. However, VPBM with LED had a tendency toward positive results regarding this index at 21 days compared to the PNI group. Although the difference was not statistically significant, it arouses interest in seeking results in a period longer than 21 days. Thus, both light sources used systemically demonstrated positive effects on gait functioning after PNI, with more pronounced and earlier results when LLL was used, which is the most important finding of the present study.
These results are compatible with data described in the study carried out by Barbosa et al. (2010) [25] using the same PNI model (crushing of the sciatic nerve). The authors found that local irradiation with LLL (830 nm, 30 mW, 10/40/80 J/cm2) led to an improvement in SFI scores at 7 days in the groups irradiated with 40 J/cm2 (p < 0.05). At 14 days, the groups irradiated with both 40 J/cm2 and 80 J/cm2 exhibited better results compared to the untreated group. However, no difference between the irradiated and non-irradiated groups were found at 21 days (p > 0.05). This differs from the present findings, which revealed better functional results after sciatic nerve injury even at 21 days in the group irradiated with vascular LLL. Belchior et al. (2009)[13] used local PBM with LLL (660 nm, 26.3 mW, 4 J/cm2) after PNI (crushing injury) and found significant positive results regarding the functional recovery of the sciatic nerve after three weeks of treatment, which is in agreement with the present findings. Barbosa et al. (2010) [25] found that local PBM with LLL (830 nm, 30 mW, 4 J/cm2) accelerated the recovery of gait in the first two weeks after PNI (crushing of the sciatic nerve) and also found a significant improvement (p < 0.05) in functional gait recovery 14 days after sciatic nerve crush using local PBM with LLL (660/830 nm, 30 mW, 10 J/cm2) compared to the control group. Considering the SFI, the results of local PBM revealed a temporal variation between 7 and 14 days for the best results in the functional recovery scores.
Serafim et al. (2012) [26] conducted a study using PBM with LED (940 nm, 9.5 mW, 4 J/cm2) and a distance of 1 cm between the light-emitting source and irradiation site. The authors analyzed the SFI, edema, and mononuclear cell count after sciatic nerve injury and obtained statistically significant results for all variables, including reduced migration of mononuclear cells to the injured tissue, reductions in edema and degeneration of nerve fibers and an increase in functional recovery scores at 7, 14, and 21 days, suggesting that PBM at a wavelength of 940 nm improves morpho-functional recovery and nerve regeneration. These results are in agreement with those of the present study. However, the CSA of muscle fibers was not evaluated in the study cited. As demonstrated herein, VPBM induced an increase in muscle fiber diameter. This is an important finding that reflects a reduction in muscle atrophy, which can enhance neuromuscular recovery following a PNI.
Considering sensitivity assessed using the Von Frey test, the VPBM-LLL group exhibited the best result, as less pressure was required for the paw withdrawal response. Andreo et al. (2019)[4] report similar results using local PBM in the area corresponding to PNI (780 nm, 40 mW, 3.2 J of total energy at four points), as lower pressure was required for the withdrawal response after 7 days. LED is a non-collimated, non-coherent light and does not have waves in phase. Thus, the tissue response may be different, as the energy is more dispersed throughout the tissue, which may be explain the different results between the two groups. These photobiomodulatory effects on mechanical sensitivity are important, as sensory alterations can lead to disabling sequelae that directly affect the quality of life of individuals affected by a PNI [3, 4].
Gigo-Benato et al. (2010) [27] investigated the effects of local PBM (660 and 780 nm, 40 mW, 10/60/120 J/cm2) on the SFI, histology, morphometry and zymography of the sciatic nerve and tibialis anterior muscle after PNI (crushing) and found an improvement in muscle, myelin, and nerve fiber regeneration in the irradiated groups compared to the control group when using a wavelength of 660 nm with either 10 or 60 J/cm2. Moreover, the authors found that PBM with 780 nm did not prevent muscle fiber atrophy or promote functional recovery in the irradiated groups compared to the untreated group [27]. The present results differ somewhat, as both LLL or LED in the infrared wavelength were able to reduce atrophy (increase in CSA) compared to PNI without treatment. At 14 and 21 days, however, neither light source was able to delay the loss of CSA, as the values were close to that of the PNI group and remained low in comparison to the control group. Shen et al. (2013) [14] used local PBM with LLL (660 nm, 50 mW, 2 min, daily for 10 days, close to nerve injury area) in a PNI model by transection followed by tubing in Wistar rats and found a reduction in muscle atrophy in the denervated group treated with LLL compared to the non-irradiated group. The authors also found that PBM induced a greater neural regeneration, including increases in the nerve area, nerve fiber diameter and number of nerve fibers 12 days after injury [14]. Andreo et al. (2019) [4] used the same PNI model as that employed in the present study and found that the injured groups exhibited a progressive decrease in diameter at 7 days and CSA at 7, 14, and 21 days, followed by an increase in the diameter of muscle fibers in Week 4. The authors compared differences when local PBM was administered over the nerve and muscle regions, finding an increase in muscle fiber diameter and CSA at three and four weeks when the nerve was irradiated (780 nm, 40 mW, 3.2 J of total energy at four points around the nerve injury area) and an increase in CSA already at two weeks when the muscle region was irradiated (780 nm, 40 mW, 3.2 J of total energy at eight points) [4]. The present results showed that VPBM using LLL or LED was able to maintain the CSA close to the values found in the control group (without injury) up to seven days. After this period, however, all injured groups (with or without treatment) had similar values with no statistically significant difference.
VPBM with LED or LLL demonstrated promising results for the treatment of PNI in the first two weeks following the injury, offering an alternative to local application. VPBM-LLL achieved effective improvements in function at 7 and 14 days, while VPBM-LED demonstrated positive results in reducing sensitivity or the pain response and increasing muscle fiber diameter at 14 days. Moreover, both treatments (VPBM-LED or VPBM-LLL) were able to delay the loss of CSA in the first seven days, constituting an option for the acute treatment of PNI. However, the use LLL or LED in the present study were not directly compared due to the fact that some dosimetric parameters of irradiation were different, generated by the higher beam area of the LED device in comparison to LLL, consequently modifying the radiant exposure and irradiance.
However, further evaluations are needed to clarify the mechanisms and pathways involved in these responses to VPBM, including gene and protein expression of myogenic regulatory factors, nicotinic acetylcholine receptors, neurotrophic factors and cytokines as well as the phenotype, amount and distribution of inflammatory cells at the injury site. The investigation of these aspect could help to establish the possible events modulated by light and the consequences for nerve and muscle regeneration following a crush injury of the sciatic nerve.
5 Conclusion
This study showed that vascular photobiomodulation, using different light sources, was efficient in improving functional motor recovery, sensitivity, and muscle trophism following a peripheral nerve injury.
Data availability
All the data used to support the findings in this study are included in the article.
References
Taylor, C. A., Braza, D., Rice, J. B., & Dillingham, T. (2008). The incidence of peripheral nerve injury in extremity trauma. American Journal of Physical Medicine & Rehabilitation, 87(5), 381–385. https://doi.org/10.1097/PHM.0b013e31815e6370
Rochkind, S., Geuna, S., & Shainberg, A. (2013). Phototherapy and nerve injury: Focus on muscle response. International Review of Neurobiology (1st ed., Vol. 109). Elsevier Inc. doi:https://doi.org/10.1016/B978-0-12-420045-6.00004-3
Andreo, L., Soldera, C. B., Ribeiro, B. G., de Matos, P. R. V., Bussadori, S. K., Fernandes, K. P. S., & Mesquita-Ferrari, R. A. (2017). Effects of photobiomodulation on experimental models of peripheral nerve injury. Lasers in Medical Science. Springer, London. doi:https://doi.org/10.1007/s10103-017-2359-7
Andreo, L., Soldera, C. A., Ribeiro, B. G., de Matos, P. R. V., Sousa, P. B., Amorim, W. W., & Mesquita-Ferrari, R. A. (2019). Effects of Photobiomodulation on Functionality in Wistar Rats with Sciatic Nerve Injury. Photochemistry and Photobiology, 95(3), 879–885. https://doi.org/10.1111/php.13048
Lien, S. C., Cederna, P. S., & Kuzon, W. M. (2008). Optimizing Skeletal Muscle Reinnervation with Nerve Transfer. Hand Clinics, 24(4), 445–454. https://doi.org/10.1016/j.hcl.2008.08.001
Rochkind, S., Drory, V., Alon, M., Nissan, M., & Ouaknine, G. E. (2007). Laser Phototherapy (780 nm), a New Modality in Treatment of Long-Term Incomplete Peripheral Nerve Injury: A Randomized Double-Blind Placebo-Controlled Study. Photomedicine and Laser Surgery, 25(5), 436–442. https://doi.org/10.1089/pho.2007.2093
Heiskanen, V., & Hamblin, M. R. (2018). Photobiomodulation: Lasers: vs. light emitting diodes? Photochemical and Photobiological Sciences. Royal Society of Chemistry. doi:https://doi.org/10.1039/c8pp00176f
Fernandes, K. P. S., Ferrari, R. M., Bussadori, S. K., & Franca, C. M. (2021). Vascular Photobiomodulation. Photobiomodulation, Photomedicine, and Laser Surgery, 39(3), 143–144. https://doi.org/10.1089/photob.2020.4965
Tomé, R. F. F., Silva, D. F. B., dos Santos, C. A. O., de Vasconcelos Neves, G., Rolim, A. K. A., & de Castro Gomes, D. Q. (2020). ILIB (intravascular laser irradiation of blood) as an adjuvant therapy in the treatment of patients with chronic systemic diseases—an integrative literature review. Lasers in Medical Science, 35(9), 1899–1907. https://doi.org/10.1007/s10103-020-03100-4
Lopez, T. C. C., Malavazzi, T. C. S., Rodrigues, M. F. S. D., Bach, E. E., SilvaHi, D. T. E. M. B., & Fernandes, K. P. S. (2022). Histological and biochemical effects of preventive and therapeutic vascular photobiomodulation on rat muscle injury. Journal of Biophotonics. https://doi.org/10.1002/jbio.202100271
Kilik, R., Bober, P., Ropovik, I., Beňačka, R., Genči, J., Nečas, A., & Sabo, J. (2019). Proteomic analysis of plasma proteins after low-level laser therapy in rats. Phys Resear, 68, 399–404. https://doi.org/10.33549/PHYSIOLRES.934377
Brill, G. E., Grigoriev, S. N., & Romanova, T. P. (1992). Changes of leucocytes metabolism in He-Ne laser blood irradiation in vitro. SPIE, 1981, 204–209. Retrieved from http://proceedings.spiedigitallibrary.org/
Belchior, A. C. G., dos Reis, F. A., Nicolau, R. A., Silva, I. S., Perreira, D. M., & de Carvalho, P. D. T. C. (2009). Influence of laser (660 nm) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers in Medical Science, 24(6), 893–899. https://doi.org/10.1007/s10103-008-0642-3
Shen, C. C., Yang, Y. C., Huang, T., Chan, S. C., & Liu, B. S. (2013). Neural regeneration in a novel nerve conduit across a large gap of the transected sciatic nerve in rats with low-level laser phototherapy. Journal of Biomedical Materials Research Part A, 101(10), 2763–2777. https://doi.org/10.1002/jbm.a.34581
Silva-Couto, M. A., Gigo-Benato, D., Tim, C. R., Parizotto, N. A., Salvini, T. F., & Russo, T. L. (2012). Effects of low-level laser therapy after nerve reconstruction in rat denervated soleus muscle adaptation. Rev Bras Fisiot, 16(4), 320–327.
Ribeiro, B. G., Alves, A. N., dos Santos, L. A. D., Cantero, T. M., Fernandes, K. P. S., da Dias, D., & Mesquita-Ferrari, R. A. (2016). Red and infrared low-level laser therapy prior to injury with or without administration after injury modulate oxidative stress during the muscle repair process. PLoS One, 11(4), e0153618. https://doi.org/10.1371/journal.pone.0153618
De Lima Rodrigues, D., Alves, A. N., Guimarães, B. R., de Alcântara Araujo Amorim, W. W., Bussadori, S. K., Fernandes, K. P. S., & Mesquita-Ferrari, R. A. (2018). Effect of prior application with and without post-injury treatment with low-level laser on the modulation of key proteins in the muscle repair process. Lasers in Medical Science, 33(6), 1207–1213. https://doi.org/10.1007/s10103-018-2456-2
Martinelli, A., Andreo, L., dos Malavazzi, T. C. S., Terena, S. M. L., da Cruz Tobelem, D., Bussadori, S. K., & Mesquita-Ferrari, R. A. (2022). Vascular photobiomodulation increases muscle fiber diameter and improves the gait during compensatory hypertrophy of plantar muscle in rats. Journal of Biophotonics. https://doi.org/10.1002/jbio.202200192
Alves, A. N., Fernandes, K. P. S., Melo, C. A. V., Yamaguchi, R. Y., França, C. M., Teixeira, D. F., & Mesquita-Ferrari, R. A. (2014). Modulating effect of low level-laser therapy on fibrosis in the repair process of the tibialis anterior muscle in rats. Lasers in Medical Science, 29(2), 813–821. https://doi.org/10.1007/s10103-013-1428-9
da Silva, J. G. F., dos Santos, S. S., de Almeida, P., Marcos, R. L., & Lino-dos-Santos-Franco, A. (2020). Effect of systemic photobiomodulation in the course of acute lung injury in rats. Lasers in Medical Science. https://doi.org/10.1007/s10103-020-03119-7
Bain, J. R., Mackinnon, S. E., & Hunter, D. A. (1989). Functional evaluation of complete sciatic peroneal and posterior tibial nerve lesions in the rat. Plastic and Reconstructive Surgery, 83(1), 129–136.
de Medinaceli, L., Freed, W. J., & Wyatt’, R. J. (1982). An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Experim Neurol, 77, 634–643.
Takasaki, I., Andoh, T., Shiraki, K., & Kuraishi, Y. (2000). Allodynia and hyperalgesia induced by herpes simplex virus type-1 infection in mice. Elsevier - Pain (Vol. 86, pp. 95–101). Retrieved from www.elsevier.nl/locate/pain
Varejão, A. S. P., Melo-Pinto, P., Meek, M. F., Filipe, V. M., & Bulas-Cruz, J. (2004). Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurological Research, 26(2), 186–194. https://doi.org/10.1179/016164104225013833
Barbosa, R. I., Marcolino, A. M., de Jesus Guirro, R. R., Mazzer, N., Barbieri, C. H., & de CássiaRegistro Fonseca, M. (2010). Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers in Medical Science, 25(3), 423–430. https://doi.org/10.1007/s10103-009-0750-8
Serafim, K. G. G., de Paula Ramos, S., de Lima, F. M., Carandina, M., Ferrari, O., Dias, I. F. L., & Siqueira, C. P. C. M. (2012). Effects of 940 nm light-emitting diode (led) on sciatic nerve regeneration in rats. Lasers in Medical Science, 27(1), 113–119. https://doi.org/10.1007/s10103-011-0923-0
Gigo-Benato, D., Russo, T. L., Tanaka, E. H., Assis, L., Salvini, T. F., & Parizotto, N. A. (2010). Effects of 660 and 780 nm low-level laser therapy on neuromuscular recovery after crush injury in rat sciatic nerve. Lasers in Surgery and Medicine, 42(9), 673–682. https://doi.org/10.1002/lsm.20978
Funding
This work was funded by São Paulo Research Foundation—FAPESP (2020/13976-0), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES (88887.481244/2020-00; 88887.475566/2020-00) and UNINOVE.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The planning, execution of experiments, and collection were performed by [T. A.], [L. A.], [D. C. T.], [T. S.] and [T. C. S. M.] and [A. M.]. The data analysis was performed by [T. A.], [L. A.], [T. C. S. M.] and [B. L.]. Original draft preparation was written by [T. A.], [L. A.] and [R. A. M.-F.]. The review and editing was performed by [K. P. S. F.] and [S. K. B.]. Supervision was made by [R. A. M.-F.], [K. P. S. F.] and [S. K. B.].
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study received approval from the Animal Research Ethics Committee of Universidade Nove de Julho (UNINOVE; process number: 3249161020). All experiments were performed in compliance with the guidelines of the Brazilian National Council for the Control of Animal Experimentation. This study does not include human participants.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Araujo, T., Andreo, L., Tobelem, D.C. et al. Effects of systemic vascular photobiomodulation using LED or laser on sensory–motor recovery following a peripheral nerve injury in Wistar rats. Photochem Photobiol Sci 22, 567–577 (2023). https://doi.org/10.1007/s43630-022-00335-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00335-8