Abstract
Introduction
Regenerative therapy has shown promising results in the treatment of osteoarthritis (OA) knee with Kellgren–Lawrence (KL) Grades I–III. We compared the safety, efficacy, functional, and clinical outcomes of intra-articular implantation of autologous adipose tissue-derived stromal vascular fraction (SVF) isolated using direct ultrasonic cavitation (Sahaj therapy–Cell Innovation Patented Technology) and saline injection in knee osteoarthritis.
Materials and Methods
The present prospective observational study was conducted over 3 years. We enrolled 120 patients in our study, where four patients got excluded as they did not meet the inclusion criteria. The remaining 116 patients were randomized into two groups, one with autologous adipose tissue-derived SVF and the other group with saline injection. A comparison of mean KOOS and VAS scores at different follow-ups was done using Paired ‘t’ test. A p value of < 0.05 was considered significant.
Results
The results show that the SVF group had significantly higher KOOS scores (78.49 ± 6.54 in the SVF group vs 59.19 ± 5.14 in the saline group), respectively (p < 0.001). Similarly, the SVF group had significantly lesser VAS scores (3.17 ± 0.94 in the SVF group vs 3.89 ± 1.04 in the saline group), respectively (p < 0.001).
Conclusions
Autologous adipose tissue-derived SVF is a better choice for treating knee osteoarthritis. For individuals with degenerative osteoarthritis, autologous SVF grafting in the same surgical procedure is an innovative and promising treatment modality. Even after 3 years of follow-up, the study participants with OA knee have shown a good clinical and functional outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) of the knee is a chronic, degenerative disease in which there is a gradual loss of articular cartilage [1, 2]. According to the 2013–2015 National Health Interview Survey (NHIS), the overall doctor-diagnosed arthritis in adults was 22.7% and the age-adjusted prevalence was 23.5% in women and 18.1% in men [3]. They reported an increase in the prevalence of arthritis with an increase in age [3]. By 2040, CDC reported that there will be 78.4 million patients with doctor-diagnosed arthritis in the age group of 18 years or more [4, 5]. The situation in India is much worse that more than 180 million patients with OA knee in India with an increasing trend [6, 7]. OA knee is the 10th leading cause of non-fatal burden with female preponderance with clinical evidence in 45% of women more than 65 years and radiological evidence in 70% of women more than 65 years [6, 8]. The challenges of treating early OA in the’40 s,’50 s, and early’60 s prevail, where effective and proven treatments are absent. The presentation of OA knee varies with individuals, but these symptoms gradually become more severe with time progression or overuse of the knees [6, 9]. The symptoms seen in OA knee include gradual onset of knee pain, knee stiffness, swelling, and pain from sitting for long periods [9]. OA knee is commonly seen among females around the fourth or fifth decade of life [10, 11].
Radiological evaluation [X-rays and MRI] helps in assessing the grade of OA knee, which guides an orthopaedic surgeon to decide on the management which ranges from lifestyle modifications, pharmacological [analgesics (oral or injectables), nutraceuticals (glucosamine, diancerin, chondroitin sulphate, collagen, ω3 and ω6 fatty acids), intra-articular steroids, and intra-articular viscosupplementation], physical [active quadriceps exercises, ankle pump movements, and wax bath], minimally invasive day-care procedures [intra-articular platelet rich plasma injection, intra-articular autologous conditioned plasma, intra-articular autologous protein solution, intra-articular gold induced cytokines, intra-articular injectable platelet rich fibrin, intra-articular bone marrow aspirate concentrate, intra-articular stromal vascular fraction, intra-articular nanofat, intra-articular mircofragmented adipose tissue, intra-articular culture-expanded mesenchymal stromal cells (MSCs), intra-articular MSC derived exosomes, allogenic MSCs, and prolotherapy] to surgical modalities [high tibial osteotomy, proximal fibular osteotomy, unicondylar knee replacement, and total knee replacement] [12,13,14]. For end-stage OA knee, total knee replacement is the mainstay of management which has its advantages and disadvantages.
With the robust development in biomedical technologies, regenerative medicine gained momentum in regenerating tissues. Mesenchymal stromal cells (MSCs) play a vital role in regenerating diseased or degenerated tissues. Among all the sources of MSCs, bone marrow is the most versatile source and is most commonly preferred by orthopaedic surgeons followed by adipose tissue [15]. Adipose tissue gives rise to various regenerative products such as adipose tissue-derived MSCs (AD-MSCs), microvascular fragments (MVF), microfat, nanofat, the stromal vascular fraction (SVF), and exosomes [16, 17]. Due to the robust regenerative potential rendered by adipose tissue products, researchers are interested to utilize in regenerating bone, cartilage, tendons, and ligaments. With the global acceptance of SVF biology, various groups explored the plausible role in terms of safety and efficacy in regenerating the cartilage in OA knee [18]. Currently, in India, there is very limited research using an autologous adipose tissue-derived SVF in the treatment of knee OA. The isolation of SVF using Sahaj therapy falls under “minimal manipulation of cells” category under government regulations. Therefore, considering these lacunae, the present study was undertaken to compare the safety, efficacy, clinical, and functional outcome of implantation of autologous adipose tissue-derived SVF and saline injection done in a single surgical sitting in patients with knee OA.
Materials and Methods
The present prospective observational study was conducted on 231 knee joints of 116 patients suffering from OA knee with Kellgren–Lawrence (KL) grades I–III. The study has been registered with the Clinical Trial Registry of India (CTRI/2018/02/011844). Voluntary written informed consent was obtained from the patient and/or his/her legally acceptable representative for participation in the study.
The patients with a history of idiopathic OA knee of age between 45 and 85 years, patients with more than 6 months of knee pain on the index side (left or right knee), patients with the severity of OA knee of KL grades I–III, patients with self-reported difficulty in at least one of the following activities such as lifting and carrying groceries, walking 400 m, getting in and out of a chair, or going up or down the stairs, patients with an adequate renal (serum creatinine < 1.5 mg/dl), cardiovascular, and respiratory functions, patients with PT/INR < 1.5 and normal APTT value, patients with an adequate immune system function and no known immunodeficiency diseases, and patients who have not received any intraarticular injection of steroid or hyaluronic acid within the last 3 months were included in the study.
The patients with ages less than 45 and more than 85 years, patients with an active neoplastic disease diagnosed in the last 3 years, patients with knee deformity of more than 10° varus or valgus, patients with a BMI more than 35, patients with the history of any surgery including arthroscopy or major trauma to the affected knee joint in the last 12 months, patients with the signs of active infection or inflammation over the joint, patients with congenital or acquired diseases leading to significant knee deformities, patients taking corticosteroid medicines or hyaluronic injections in the last 3 months, patients with inflammatory joint diseases or laxity in joint, patients who are positive HIV, HbsAg, HCV or VDRL were excluded from the study.
After obtaining consent for participation and other consents relevant to the treatment, the study procedures were initiated. Isolation and implantation of SVF into knee joints were performed as an in-patient procedure. Using n = Z1-α/2pq/d2, where p = 23.6% [3], d = 5% and adding 5% attrition rate, the estimated total sample size is 120. We enrolled 120 patients in our study. Four got excluded as they did not meet the inclusion criteria. The remaining 116 patients were double blinded and randomized into two groups concerning their joints. Among 116 patients, 231 joints were included. We assigned each participant a random number from 1 to 231. The first 115 participants were assigned numbers from 1 to 115 to the SVF group. The remaining 116 participants were assigned numbers from 116 to 231 to the saline group (Fig. 1).
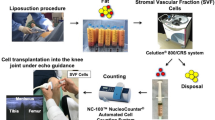
The whole procedure from harvesting the autologous adipose tissue to the isolation and implantation of SVF into the knee was done in the same surgical sitting which took around 60–90 min. The following steps were performed, as depicted in Fig. 2.
Preparation of Stromal Vascular Fraction (SVF) by Sahaj therapy. A Canister preparation, B marking of the abdomen for lipoaspiration, C lipoaspiration in the canister, D separation of the aqueous solution from adipose tissue, E adipose tissue disruption by ultrasonic cavitation process, F post-centrifugation cycles filtration process, and G intra-articular SVF grafting in same surgical sitting
Lipoaspiration
The patient was prepared for the procedure with all aseptic precautions. Pre-procedural antibiotics, anxiolytics, and/or opioid-based pain medications were administered if needed. Under sedation/short general anesthesia, a stab incision on the abdomen using a #11 blade was made for cannula entry after local infiltration with 1% lidocaine with epinephrine 1:100,000. 40 ml of 2% lidocaine plus 1 ml of 1% epinephrine was added to a 1000 ml bag of 0.9% normal saline and was infiltrated with the tumescent anesthesia on the infraumbilical area of the abdomen. Approximately 200–300 cc of adipose tissue was aspirated into the patented sterile Lipoaspiration Jar (Design No. 316580-001) containing 0.9% sterile normal saline and sodium bicarbonate.
Recovery of the Autologous Adipose Tissue (ACRU Unit)
Approximately 200–300 cc of autologous adipose tissue was harvested. In Class II Bio-Hood, the sample was divided into 50 ml tubes, and tissue fragmentation was done using direct ultrasonic cavitation (Australian Patented Technology) which was used to separate the SVF from fat. These 50 ml tubes were centrifuged, a pellet being formed at the bottom of the tube. The tube was turned upside down after screwing a tube filter to the 50 ml tube. The pellet separated was the SVF or cellular fraction, which is the heterogeneous population of cells. The cell count and viability were checked using the Muse Cell Flow cytometer.
Intra-articular Grafting of the SVF or Cellular Fraction
Chlorhexidine was used for knee preparation. Then, the prepared autologous SVF (5–8 cc with the cellular dosage of 5.0 × 107 cells with the viability of > 85% SVF cells) was implanted intra-articularly in each joints.
Intra-articular Saline Injection
Approximately 5–8 cc of saline injection was injected intra-articularly in each joints.
Follow-Up
Though SVF/saline implantation was an in-patient procedure, these patients were followed up on the following day or within the next 7 days. Patients were instructed to contact the surgeon in case of fever, pain, and any other adverse events. Patients were followed up regularly at 1, 6, 12, 24, and 36 months after the procedure by telephone, email, or in person for radiological documentation as depicted in Figs. 3 and 4. KOOS self-administered questionnaire and pain assessment using VAS were administered before the procedure and at all follow-ups.
Statistical Analysis
Statistical software such as GraphPad, Epi Info, etc. was used for statistical analysis. Descriptive statistics were presented as numbers and percentages. Paired ‘t’ test was applied to compare the preoperative KOOS and VAS scores at various follow-ups. A p value of < 0.05 was taken as statistically significant.
Results
Autologous adipose tissue-derived SVF was implanted in 58 patients (115 knees). One patient had already undergone hemiarthroplasty, so implantation was done in 1 knee only. The harvesting of adipose tissue from the abdomen, isolation of SVF using ultrasonic cavitation (Sahaj therapy–Australian Patent technology), and implantation of SVF into the affected knee were done in the same surgical sitting. In our study, 5 (8.6%) patients were in the 45–50-year age group, 9 (15.5%) in 51–55 years, 20 (34.5%) in 56–60 years, 7 (12.1%) in 61–65 years, 7 (12.1%) in 66–70 years, 7 (12.1%) in 71–75 years and 3 (5.2%) in 76–80-year age group. There was a female preponderance in the study (62.1%). 1 (1.7%) patient was in the underweight group, 12 (20.7%) in normal, 29 (50.0%) in overweight, 9 (15.5%) in Class I obesity, 6 (10.3%) in Class II obesity, and 1 (1.7%) in Class III obesity group. 32 (55.2%) patients were having hypertension, 13 (22.4%) had diabetes mellitus type-2, 4 (6.9%) had hypothyroidism, and 1 (1.7%) each was having benign prostatic hyperplasia, bronchitis, bypass surgery, coronary artery disease, migraine, and previous hemiarthroplasty, respectively, as the comorbidities. 1 (1.7%) patient was in KL grade I, 8 (13.8%) in grade II, and 49 (84.5%) were in grade III OA knee.
Saline was injected in 58 patients (116 knees). In our study, 5 (10%) patients were in the 45–50-year age group, 11 (20%) in 51–55 years, 20 (35%) in 56–60 years, 5 (10%) in 61–65 years, 7 (12%) in 66–70 years and 8 (13%) in 71–75 years. There was a female preponderance in the study (70%). Three (5.2%) patients were in the underweight group, 16 (30.7%) in normal, 30 (52.0%) in overweight, 2 (4.5%) in Class I obesity, 3 (5.2%) in Class II obesity, and 2 (3.4%) in Class III obesity group. 26 (45.2%) patients were having hypertension, 10 (17%) had diabetes mellitus type-2, 3 (5.4%) had hypothyroidism, and 2 (2.4%) each was having benign prostatic hyperplasia, bronchitis, and coronary artery disease, respectively, as the comorbidities. Two (3.4%) patients were in KL grade I, 6 (10.34%) in grade II, and 50 (86.2%) were in grade III OA knee.
SVF Group
KOOS score: The mean overall preoperative KOOS score was 43.25 ± 12.13, 62.89 ± 11.82 at 1 month, 66.61 ± 9.90 at 3 months, 71.76 ± 9.38 at 6 months, 78.02 ± 10.67 at 12 months, 79.09 ± 7.74 at 24 months and 78.49 ± 6.54 at 36 months. The mean preoperative pain score was 49.63 ± 15.55, 73.34 ± 14.10 at 1 month, 77.10 ± 11.02 at 3 months, 80.50 ± 9.53 at 6 months, 84.23 ± 11.97 at 12 months, 85.01 ± 6.46 at 24 months and 85.58 ± 6.01 at 36 months. The mean preoperative symptom score was 46.45 ± 14.13, 64.21 ± 12.74 at 1 month, 71.16 ± 15.38 at 3 months, 76.90 ± 14.38 at 6 months, 85.14 ± 16.73 at 12 months, 89.65 ± 7.76 at 24 months and 87.75 ± 6.35 at 36 months. The mean preoperative Quality of Life score was 36.02 ± 13.41, 54.15 ± 13.75 at 1 month, 60.14 ± 14.23 at 3 months, 65.17 ± 14.41 at 6 months, 75.34 ± 12.76 at 12 months, 75.32 ± 13.01 at 24 months and 75.97 ± 13.69 at 36 months. The mean preoperative Activity of Daily Living score was 50.12 ± 14.83, 71.89 ± 16.47 at 1 month, 75.30 ± 11.87 at 3 months, 79.70 ± 11.85 at 6 months, 82.67 ± 12.74 at 12 months, 80.61 ± 9.10 at 24 months and 83.68 ± 7.94 at 36 months. There was a significant improvement in the overall KOOS score and KOOS subscales–pain score, symptom score, quality of life score, and average Activity of Daily Living score from preoperative level to 36 months (p = 0.001) (Table 1 and Fig. 5).
VAS score: The mean preoperative VAS score was 8.41 ± 0.56, 7.72 ± 0.52 at 1 month, 6.71 ± 0.68 at 3 months, 5.29 ± 0.46 at 6 months, 4.24 ± 1.06 at 12 months, 3.35 ± 0.95 at 24 months and 3.17 ± 0.94 at 36 months. There was a significant improvement in the mean VAS score from the preoperative level to 36 months (p = 0.001) (Table 2 and Fig. 6).
Saline Group
The mean overall preoperative KOOS score was 35.15 ± 10.20, 37.13 ± 12.13 at 1 month, 39.31 ± 7.80 at 3 months, 47.14 ± 7.15 at 6 months, 49.31 ± 8.15 at 12 months, 54.13 ± 6.14 at 24 months and 59.19 ± 5.14 at 36 months. The mean preoperative pain score was 35.13 ± 11.25, 37.14 ± 12.15 at 1 month, 41.45 ± 9.12 at 3 months, 46.13 ± 10.13 at 6 months, 49.10 ± 10.17 at 12 months, 61.34 ± 2.64 at 24 months and 63.15 ± 3.29 at 36 months. The mean preoperative symptom score was 31.15 ± 12.30, 36.13 ± 10.45 at 1 month, 39.68 ± 10.80 at 3 months, 49.10 ± 13.80 at 6 months, 52.40 ± 14.30 at 12 months, 56.15 ± 6.60 at 24 months and 58.50 ± 5.15 at 36 months. The mean preoperative Quality of Life score was 32.20 ± 10.14, 34.50 ± 12.50 at 1 month, 40.46 ± 9.30 at 3 months, 45.72 ± 10.14 at 6 months, 49.40 ± 9.67 at 12 months, 55.20 ± 9.01 at 24 months and 59.70 ± 7.69 at 36 months. The mean preoperative Activity of Daily Living score was 35.20 ± 12.30, 32.90 ± 13.70 at 1 month, 39.29 ± 10.78 at 3 months, 45.78 ± 9.57 at 6 months, 51.76 ± 7.43 at 12 months, 58.13 ± 6.78 at 24 months and 60.18 ± 5.14 at 36 months. There was a significant improvement in the overall KOOS score and KOOS subscales–pain score, symptom score, quality of life score, and average Activity of Daily Living score from preoperative level to 36 months (p = 0.001) (Table 3 and Fig. 7).
VAS score: The mean preoperative VAS score was 7.31 ± 0.26, 7.62 ± 0.12 at 1 month, 7.10 ± 0.34 at 3 months, 6.16 ± 0.25 at 6 months, 5.42 ± 0.16 at 12 months, 4.21 ± 0.13 at 24 months and 3.89 ± 1.04 at 36 months. There was a significant improvement in the mean VAS score from the preoperative level to 36 months (p = 0.001) (Table 4 and Fig. 8).
Comparison Between SVF and Saline Groups
KOOS score: The mean KOOS score at the preoperative level and 1 month was not significant between the groups. At 3, 6, 12, 24, and 36 months, there was a significant improvement in the mean KOOS score between the groups (p = 0.001) (Table 5).
VAS score: The mean VAS score at 1 month was not significant between the groups. At 3, 6, 12, 24, and 36 months, there was a significant improvement in the mean KOOS score between the groups (p = 0.001) (Table 6).
There were no serious adverse effects of either autologous adipose tissue-derived SVF or saline injection. Some patients experienced pain, swelling, and ecchymosis at the lipoaspiration site, which resolved in a short period with the use of icing and common analgesics. All the patients were followed up till 36 months after SVF and saline injection. The study results demonstrated good safety and efficacy and significant improvement in the functional outcome and reduction in the pain score with SVF throughout the study period.
Discussion
OA knee is characterized by the loss of articular cartilage leading to degenerative joint disorder which affects the quality of life significantly [9]. World Health Organization estimated that 10% and 18% of men and women, respectively, aged over 60 years have OA knee [19, 20]. Cui et al. estimated the global prevalence of OA knee was 22.9% (95% CI 19.8% − 26.1%) in individuals aged > 40 years with female preponderance (21.7% [95% CI 19.0% − 24.5%]) [21]. Globally, the incidence of OA knee in individuals aged > 20 years was 203 per 10,000 (95% CI, 106–331) person-years. Management of OA knee has taken various dimensions with the recent technological developments in the healthcare industry. With a profound shift in industrialization towards industry 5.0, the novel biomedical and bioengineering technologies have revolutionized the dynamics of healthcare towards the improvisation of health indicators. Industry 5.0 deals with personalization and customization which form an integral part of Tissue Engineering and Regenerative Medicine (TERM) [22]. The debate on cartilage regeneration remains an unsolved technological development. In this connotation, MSCs paved a way for regenerating the cartilaginous tissue in a dose-dependent manner.
The regenerative potential of adipose tissue was well-accepted by various regenerative medicine experts and regenerative orthobiologists. Among the various translational products of adipose tissue, exosomes, and SVF possess an enhanced regenerative potential in terms of cartilage regeneration [16]. SVF is a collection of the non-expanded or uncultured cellular mixture obtained from lipoaspirate which is derived through either mechanical or enzymatic separation [23, 24]. SVF comprises a heterogeneous group of a cellular mixture such as MSCs (2–5%), AD-MSCs (1–15%), M2 macrophages/monocytes (10%), hematopoietic cells (0.004%), pericytes (10–70%), T regulatory cells (5–70%), dendritic cells, fibroblasts, vessel-forming cells, such as endothelial and smooth muscle cells, and their progenitors (7–30%), and extracellular matrix [25, 26]. SVF from adipose tissue contains 30% MSCs, 3% endothelial cells, and 14% endothelial precursor cells [27, 28]. The concentration of adipose-derived stromal cells in SVF varies from lesser than 1% to greater than 15% which depends on age, sex, the health status of the individual, and the harvesting method of adipose tissue [26]. The concentration of pericytes in the SVF cocktail aids in tissue regeneration in response to any injury or inflammation [29]. SVF follows the criteria for MSCs as laid out by the International Society for Cell and Gene Therapy (ISCT) which express 2% hematopoietic markers and 7% MSCs [30]. AD-MSCs in the SVF cocktail secrete various anti-inflammatory substances, such as IL-1RA, NO, TGF-β1, SDF-1, and LL37, which alleviate the inflammatory environment in the diseased joint [31]. Actual cartilaginous regeneration by SVF cells is yet to be fully elucidated. A few studies reported quantifiable hyaline cartilage regeneration observed [32, 33], whereas few studies reported no cartilage regeneration on application with SVF cells [34, 35].
In the literature, various methods and modifications of SVF isolation have been mentioned [24, 28, 36, 37]. In our study, we followed the mechanical method of SVF isolation using Australian Patent technology. Koh et al. suggested the need for the SVF cellular dosage in cartilage regeneration [38]. Cytori Therapeutics reported the cellular mixture in SVF pellet as 37% with CD34 + /CD31 − cells, 23% with tissue macrophages, 22% with WBCs, 9% with smooth muscle cells, 7% with endothelial cells, and 2% with MSCs [39]. Aronowitz et al. reported measurable amounts of the cellular mixture in SVF pellets generated by various commercial systems and hence a significant variability exists in the recovered viability of the cells [40].
Various studies demonstrated the use of SVF in OA knee as mentioned in Table 7. Yokota et al. reported a significant functional outcome in VAS, JKOM, and WOMAC scores with intra-articular injection of SVF in KL grade III and IV OA knee [41]. Tsubosaka et al. reported no significant difference in radiological evaluation of either lower (2.5 × 107 SVF cells) or higher (5.0 × 107 SVF cells) cellular dosage of SVF in OA knee but clinically the patients who received higher cellular dosage exhibited a superior pain relief than lower cellular dosage group [42]. Zhang et al. reported significant improvement in VAS and WOMAC scores in patients treated with SVF than the hyaluronic acid group at the end of 5 years. They concluded that bone marrow lesions and body mass index were independent predictors of the prognosis [43]. Mehling et al. reported better outcomes in grade III OA knee and hip when treated with intra-articular SVF. At the end of the 12-month follow-up, 84.9% of patients demonstrated better pain relief and improved mobility with enhanced quality of life [44]. Desando et al. demonstrated the delayed progression of OA knee in surgically induced OA knee models in rabbits [45]. Boada-Pladellorens et al. in their systematic review explained the need for standardization of SVF isolation protocols to obtain a standard cellular count in SVF pellets. They concluded that SVF is a promising regenerative agent in terms of pain reduction, functionality, and anatomical structural improvement in OA knee [46]. Garza et al. reported no MRI changes in the cartilage thickness were observed after 12 months of follow-up with SVF treatment of varied dosages [47]. A quantitative T2 cartilage mapping sequence provides early detection of cartilage degeneration in OA knee [48]. T2 cartilage mapping detects the change in water and collagen content in the cartilage and reflects the degree of cartilage degeneration [49]. Hong et al. demonstrated increased cartilage thickness quantitatively and qualitatively by magnetic resonance observation of cartilage repair tissue (MOCART) score at the end of 1 year with intra-articular injection of autologous adipose tissue-derived SVF in OA knee [50].
This comparative study of the VAS Score and KOOS Score between the autologous adipose tissue-derived SVF group and saline group indicates that the SVF group has better outcomes. The results show that the SVF group had significantly higher KOOS scores (78.49 ± 6.54 in the SVF group vs 59.19 ± 5.14 in the saline group), respectively (p < 0.001). Similarly, the SVF group had significantly lesser VAS scores (3.17 ± 0.94 in the SVF group vs 3.89 ± 1.04 in the saline group), respectively (p < 0.001). This suggests that autologous adipose tissue-derived SVF may be a better treatment option for knee osteoarthritis. Furthermore, the SVF group had a significantly lower rate of adverse events compared to the saline group, which further demonstrates the superiority of SVF. Therefore, it can be concluded that autologous adipose tissue-derived SVF is the better choice for treating knee osteoarthritis.
In our study, we observed a statistically significant difference (p < 0.001) in clinical and functional follow-up with autologous adipose tissue-derived SVF implantation in KL grade I–III OA knee. We performed radiological documentation in the form of a plain radiograph at every follow-up to demonstrate the maintenance of cartilage thickness. No adverse side effects were observed among the study population who underwent autologous adipose tissue-derived SVF for OA knee. The limitations of the study are a) no MR cartigram documentation of increased cartilage thickness and b) No pre- and post-op arthroscopy done after autologous adipose tissue-derived SVF implantation.
Conclusions
The results of our study support the long-term safety and efficacy of autologous grafting of adipose tissue-derived SVF implantation in the same surgical sitting in patients with OA knee with KL grades I–III. There are level I and II evidences are present that support use of adipose tissue-derived SVF for OA knee.
Data Availability
Not applicable.
References
Di Nicola, V. (2020). Degenerative osteoarthritis a reversible chronic disease. Regenerative Therapy, 15, 149–160. https://doi.org/10.1016/j.reth.2020.07.007
Haq, I., Murphy, E., & Dacre, J. (2003). Osteoarthritis. Postgraduate Medical Journal, 79(933), 377–383. https://doi.org/10.1136/pmj.79.933.377
Barbour, K. E., Helmick, C. G., Theis, K. A., Murphy, L. B., Hootman, J. M., Brady, T. J., et al. (2013). Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation United states, 2010–2012. Morbidity and Mortality Weekly Report, 62(44), 869–873.
Arthritis Related Statistics | CDC. (2021). https://www.cdc.gov/arthritis/data_statistics/arthritis-related-stats.htm. Published 12 Oct 2021. Accessed 12 Oct 2021. https://www.cdc.gov/arthritis/data_statistics/arthritis-related-stats.htm. Accessed 12 Oct 2021
Arthritis Prevalence to Jump from 54 to 78 Million by 2040. https://www.rheumatologynetwork.com/view/arthritis-prevalence-jump-54-78-million-2040. Accessed 12 Oct 2021. https://www.rheumatologynetwork.com/view/arthritis-prevalence-jump-54-78-million-2040. Accessed 12 Oct 2021
Pal, C. P., Singh, P., Chaturvedi, S., Pruthi, K. K., & Vij, A. (2016). Epidemiology of knee osteoarthritis in India and related factors. Ind J Orthop, 50(5), 518–522. https://doi.org/10.4103/0019-5413.189608
King, L. K., March, L., & Anandacoomarasamy, A. (2013). Obesity osteoarthritis. The Indian Journal of Medical Research, 138(2), 185–193.
Davis, M. A., Ettinger, W. H., Neuhaus, J. M., & Hauck, W. W. (1988). Sex differences in osteoarthritis of the knee The role of obesity. American Journal of Epidemiology., 127(5), 1019–1030. https://doi.org/10.1093/oxfordjournals.aje.a114878
Lespasio, M. J., Piuzzi, N. S., Husni, M. E., Muschler, G. F., Guarino, A., & Mont, M. A. (2017). Knee osteoarthritis: a primer. The Permanente Journal, 21, 16–183. https://doi.org/10.7812/TPP/16-183
Zhang, Y., & Jordan, J. M. (2010). Epidemiology of osteoarthritis. Clinics in Geriatric Medicine, 26(3), 355–369. https://doi.org/10.1016/j.cger.2010.03.001
Losina, E., Weinstein, A. M., Reichmann, W. M., Burbine, S. A., Solomon, D. H., Daigle, M. E., et al. (2013). Lifetime risk and age of diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care & Research. https://doi.org/10.1002/acr.21898
Gupta, K. B., Duryea, J., & Weissman, B. N. (2004). Radiographic evaluation of osteoarthritis. Radiologic Clinics of North America., 42(1), 11–41. https://doi.org/10.1016/S0033-8389(03)00169-6
Roemer, F. W., Demehri, S., Omoumi, P., Link, T. M., Kijowski, R., Saarakkala, S., et al. (2020). State of the Art: imaging of osteoarthritis revisited 2020. Radiology, 296(1), 5–21. https://doi.org/10.1148/radiol.2020192498
Braun, H. J., & Gold, G. E. (2012). Diagnosis of osteoarthritis: imaging. Bone, 51(2), 278–288. https://doi.org/10.1016/j.bone.2011.11.019
Muthu, S., Jeyaraman, M., Jain, R., Gulati, A., Jeyaraman, N., Prajwal, G. S., et al. (2021). Accentuating the sources of mesenchymal stem cells as cellular therapy for osteoarthritis knees—a panoramic review. Stem Cell Investigation, 8, 13. https://doi.org/10.21037/sci-2020-055
Sharma, S., Muthu, S., Jeyaraman, M., Ranjan, R., & Jha, S. K. (2021). Translational products of adipose tissue-derived mesenchymal stem cells: Bench to bedside applications. World Journal of Stem Cells, 13(10), 1360–1381. https://doi.org/10.4252/wjsc.v13.i10.1360
Jeyaraman, M., Muthu, S., Sharma, S., Ganta, C., Ranjan, R., & Jha, S. K. (2021). Nanofat: A therapeutic paradigm in regenerative medicine. World Journal of Stem Cells, 13(11), 1733–1746. https://doi.org/10.4252/wjsc.v13.i11.1733
Jeyaraman, M., Muthu, S., & Ganie, P. A. (2020). Does the Source of Mesenchymal Stem Cell Have an Effect in the Management of Osteoarthritis of the Knee? Meta-Analysis of Randomized Controlled Trials: Cartilage. https://doi.org/10.1177/1947603520951623
Jaiswal, A., Goswami, K., Haldar, P., Salve, H. R., & Singh, U. (2021). Prevalence of knee osteoarthritis, its determinants, and impact on the quality of life in elderly persons in rural ballabgarh, Haryana. Journal of Family Medicine and Primary Care, 10(1), 354–360. https://doi.org/10.4103/jfmpc.jfmpc_1477_20
Cross, M., Smith, E., Hoy, D., Nolte, S., Ackerman, I., Fransen, M., et al. (2014). The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Annals of the Rheumatic Diseases, 73(7), 1323–1330. https://doi.org/10.1136/annrheumdis-2013-204763
Cui, A., Li, H., Wang, D., Zhong, J., Chen, Y., & Lu, H. (2020). Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine, 29, 100587. https://doi.org/10.1016/j.eclinm.2020.100587
Jeyaraman, M., Nallakumarasamy, A., & Jeyaraman, N. (2022). Industry 50 in Orthopaedics. Indian Journal of Orthopaedics, 56(10), 1694–1702. https://doi.org/10.1007/s43465-022-00712-6
Cao, L., Xiaoming, F., Zhang, Q., Fang, J., Chu, C., Lv, J., et al. (2022). An Optimized method for adipose stromal vascular fraction isolation and its application in fat grafting. Aesthetic Plastic Surgery. https://doi.org/10.1007/s00266-021-02738-x
Senesi, L., De Francesco, F., Farinelli, L., Manzotti, S., Gagliardi, G., Papalia, G. F., et al. (2019). Mechanical and enzymatic procedures to isolate the stromal vascular fraction from adipose tissue: preliminary results. Frontiers in Cell and Developmental Biology, 7, 88. https://doi.org/10.3389/fcell.2019.00088
Sun, Y., Chen, S., Zhang, X., & Pei, M. (2019). Significance of cellular cross-talk in stromal vascular fraction of adipose tissue in neovascularization. Arteriosclerosis, thrombosis, and vascular biology, 39(6), 1034–1044. https://doi.org/10.1161/ATVBAHA.119.312425
Ude, C. C., Shah, S., Ogueri, K. S., Nair, L. S., & Laurencin, C. T. (2022). Stromal vascular fraction for osteoarthritis of the knee regenerative engineering. Regenerative Engineering and Translational Medicine, 8(2), 210–224. https://doi.org/10.1007/s40883-021-00226-x
Ramakrishnan, V. M., & Boyd, N. L. (2018). The adipose stromal vascular fraction as a complex cellular source for tissue engineering applications. Tissue Engineering Part B, Reviews, 24(4), 289–299. https://doi.org/10.1089/ten.teb.2017.0061
Bora, P., & Majumdar, A. S. (2017). Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Research & Therapy, 8(1), 145. https://doi.org/10.1186/s13287-017-0598-y
Caplan, A. I. (2008). All MSCs are pericytes? Cell Stem Cell, 3(3), 229–230. https://doi.org/10.1016/j.stem.2008.08.008
Bourin, P., Bunnell, B. A., Casteilla, L., Dominici, M., Katz, A. J., March, K. L., et al. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics (IFATS) and Science and the International Society for Cellular Therapy (ISCT). Cytotherapy, 15(6), 641–648. https://doi.org/10.1016/j.jcyt.2013.02.006
Dimarino, A. M., Caplan, A. I., & Bonfield, T. L. (2013). Mesenchymal stem cells in tissue repair. Frontiers in Immunology, 4, 201. https://doi.org/10.3389/fimmu.2013.00201
Tran, T. D. X., Wu, C.-M., Dubey, N. K., Deng, Y.-H., Su, C.-W., Pham, T. T., et al. (2019). Time and kellgren-lawrence grade-dependent changes in intra-articularly transplanted stromal vascular fraction in osteoarthritic patients. Cells, 8(4), 308. https://doi.org/10.3390/cells8040308
Nguyen, P. D., Tran, T.D.-X., Nguyen, H.T.-N., Vu, H. T., Le, P.T.-B., Phan, N.L.-C., et al. (2017). Comparative clinical observation of arthroscopic microfracture in the presence and absence of a stromal vascular fraction injection for osteoarthritis. Stem cells translational medicine, 6(1), 187–195. https://doi.org/10.5966/sctm.2016-0023
Koh, Y.-G., Choi, Y.-J., Kwon, S.-K., Kim, Y.-S., & Yeo, J.-E. (2015). Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee surgery sports traumatology arthroscopy: Official journal of the ESSKA, 23(5), 1308–1316. https://doi.org/10.1007/s00167-013-2807-2
Fodor, P. B., & Paulseth, S. G. (2016). Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthetic Surgery Journal, 36(2), 229–236. https://doi.org/10.1093/asj/sjv135
Aronowitz, J. A., Lockhart, R. A., & Hakakian, C. S. (2018). A Method for isolation of stromal vascular fraction cells in a clinically relevant time frame. Methods in Molecular Biology., 1773, 11–19. https://doi.org/10.1007/978-1-4939-7799-4_2
van Dongen, J. A., Harmsen, M. C., & Stevens, H. P. (2019). Isolation of stromal vascular fraction by fractionation of adipose tissue. Methods in Molecular Biology., 1993, 91–103. https://doi.org/10.1007/978-1-4939-9473-1_8
Koh, Y.-G., Jo, S. B., Kwon, O.-R., Suh, D.-S., Lee, S.-W., Park, S.-H., et al. (2013). Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy The Journal of Arthroscopic & Related Surgery, 29(4), 748–755. https://doi.org/10.1016/j.arthro.2012.11.017
Lin, K., Matsubara, Y., Masuda, Y., Togashi, K., Ohno, T., Tamura, T., et al. (2008). Characterization of adipose tissue-derived cells isolated with the celution system. Cytotherapy, 10(4), 417–426. https://doi.org/10.1080/14653240801982979
Aronowitz, J. A., & Ellenhorn, J. D. I. (2013). Adipose stromal vascular fraction isolation: a head-to-head comparison of four commercial cell separation systems. Plastic and Reconstructive Surgery, 132(6), 932e–939e. https://doi.org/10.1097/PRS.0b013e3182a80652
Yokota, N., Yamakawa, M., Shirata, T., Kimura, T., & Kaneshima, H. (2017). Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regenerative Therapy, 6, 108–112. https://doi.org/10.1016/j.reth.2017.04.002
Tsubosaka, M., Matsumoto, T., Sobajima, S., Matsushita, T., Iwaguro, H., & Kuroda, R. (2021). Comparison of clinical and imaging outcomes of different doses of adipose-derived stromal vascular fraction cell treatment for knee osteoarthritis. Cell Transplantation, 30, 09636897211067454. https://doi.org/10.1177/09636897211067454
Zhang, S., Xu, H., He, B., Fan, M., Xiao, M., Zhang, J., et al. (2022). Mid-term prognosis of the stromal vascular fraction for knee osteoarthritis: A minimum 5-year follow-up study. Stem Cell Research & Therapy, 13(1), 105. https://doi.org/10.1186/s13287-022-02788-1
Mehling, B., Hric, M., Salatkova, A., Vetrak, R., Santora, D., Ovariova, M., et al. (2020). A Retrospective study of stromal vascular fraction cell therapy for osteoarthritis. Journal of Clinical Medicine Research, 12(11), 747–751. https://doi.org/10.14740/jocmr4354
Desando, G., Cavallo, C., Sartoni, F., Martini, L., Parrilli, A., Veronesi, F., et al. (2013). Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Research & Therapy, 15(1), R22. https://doi.org/10.1186/ar4156
Boada-Pladellorens, A., Avellanet, M., Pages-Bolibar, E., & Veiga, A. (2022). Stromal vascular fraction therapy for knee osteoarthritis: a systematic review. Therapeutic Advances in Musculoskeletal Disease, 14, 1117879. https://doi.org/10.1177/1759720X221117879
Garza, J. R., Campbell, R. E., Tjoumakaris, F. P., Freedman, K. B., Miller, L. S., Santa, M. D., et al. (2020). Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: a double-blinded prospective randomized controlled clinical trial. The American Journal of Sports Medicine, 48(3), 588–598. https://doi.org/10.1177/0363546519899923
Zhao, H., Li, H., Liang, S., Wang, X., & Yang, F. (2022). T2 mapping for knee cartilage degeneration in young patients with mild symptoms. BMC Medical Imaging, 22(1), 72. https://doi.org/10.1186/s12880-022-00799-1
Tsubosaka, M., Matsumoto, T., Sobajima, S., Matsushita, T., Iwaguro, H., & Kuroda, R. (2020). The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskeletal Disorders, 21(1), 207. https://doi.org/10.1186/s12891-020-03231-3
Hong, Z., Chen, J., Zhang, S., Zhao, C., Bi, M., Chen, X., et al. (2019). Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. International Orthopaedics, 43(5), 1123–1134. https://doi.org/10.1007/s00264-018-4099-0
Pak, J. (2011). Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. Journal of Medical Case Reports, 5, 296. https://doi.org/10.1186/1752-1947-5-296
Bui, K.H.-T., Duong, T. D., Nguyen, N. T., Nguyen, T. D., Le, V. T., Mai, V. T., et al. (2014). Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: A Clinical study. Biomedical Research And Therapy, 1(1), 2–8. https://doi.org/10.15419/bmrat.v1i01.11
Garza, J. R., Maria, D. S., Palomera, T., Dumanian, G. A., & Dos-Anjos, S. (2020). Use of autologous adipose- derived stromal vascular fraction to treat osteoarthritis of the knee: a feasibility and safety study. Journal of Regenerative Medicine. https://doi.org/10.4172/2325-9620.1000119
Gibbs, N., Diamond, R., Sekyere, E. O., & Thomas, W. D. (2015). Management of knee osteoarthritis by combined stromal vascular fraction cell therapy, platelet-rich plasma, and musculoskeletal exercises: a case series. Journal of Pain Research, 8, 799–806. https://doi.org/10.2147/JPR.S92090
Al-Salahat, N. (2016). Pre-SVF arthroscopy: A case report of new concept of meniscus and cartilage regeneration using arthroscopy followed by intra-articular injection of adipose-derived stromal vascular fraction. Stem Cell Biology and Research, 3(1), 2.
Pak, J., Lee, J. H., Park, K. S., Jeong, B. C., & Lee, S. H. (2016). Regeneration of cartilage in human knee osteoarthritis with autologous adipose tissue-derived stem cells and autologous extracellular matrix. BioResearch Open Access, 5(1), 192–200. https://doi.org/10.1089/biores.2016.0024
Bansal, H., Comella, K., Leon, J., Verma, P., Agrawal, D., Koka, P., et al. (2017). Retracted article: Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. Journal of Translational Medicine, 15(1), 141. https://doi.org/10.1186/s12967-017-1242-4
Schiavone, P. A., Vasso, M., Braile, A., Toro, G., De Cicco, A., Viggiano, D., et al. (2019). Preliminary results of autologous adipose-derived stem cells in early knee osteoarthritis: Identification of a subpopulation with greater response. International Orthopaedics, 43(1), 7–13. https://doi.org/10.1007/s00264-018-4182-6
Lapuente, J. P., Dos-Anjos, S., & Blázquez-Martínez, A. (2020). Intra-articular infiltration of adipose-derived stromal vascular fraction cells slows the clinical progression of moderate-severe knee osteoarthritis: Hypothesis on the regulatory role of intra-articular adipose tissue. Journal of Orthopaedic Surgery and Research, 15(1), 137. https://doi.org/10.1186/s13018-020-01664-z
Funding
Nil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Consent for Publication
Informed consent was obtained from all individual participants included in the study.
Ethical Standard Statement
Clinical Trial Registry of India (CTRI/2018/02/011844). This article does not contain any studies with human or animal subjects performed by the any of the authors
Informed Consent
For this type of study, informed consent is not required
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tantuway, V., Thomas, W., Parikh, M.B. et al. Clinical Outcome of Minimally Manipulated, Mechanically Isolated Autologous Adipose Tissue-Derived Stromal Vascular Fraction (Sahaj Therapy®) in Knee Osteoarthritis—Randomized Controlled Trial. JOIO 57, 1646–1658 (2023). https://doi.org/10.1007/s43465-023-00981-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43465-023-00981-9