Abstract
Background
The response to warfarin, as an oral anticoagulant agent, varies widely among patients from different ethnic groups. In this study, we tried to ascertain and determine the relationship between non-genetic factors and genetic polymorphisms with warfarin therapy; we then proposed a new warfarin dosing prediction algorithm for the estimation of drug sensitivity and resistance in the Iranian population.
Methods
Overall, 200 warfarin-treated patients with stable doses were recruited, the demographic and clinical characteristics were documented, and genotyping was done using a sequencing assay.
Results
The outcomes of our investigation showed that the genetic polymorphisms of VKORC1(-1639 G > A), CYP2C9*3, CYP2C9*2, amiodarone use, and increasing age were found to be related to a significantly lower mean daily warfarin dose. In contrast, the CYP4F2*3 variant and increased body surface area were linked with an increased dose of warfarin in the Iranians. Our descriptive model could describe 56.5% of the variability in response to warfarin. This population-specific dosing model performed slightly better than other previously published warfarin algorithms for our patient’s series. Furthermore, our findings provided the suggestion that incorporating the CYP4F2*3 variant into the dosing algorithm could result in a more precise calculation of warfarin dose requirements in the Iranian population.
Conclusions
We proposed and validated a population-specific dosing algorithm based on genetic and non-genetic determinants for Iranian patients and evaluated its performance. Accordingly, by using this newly developed algorithm, prescribers could make more informed decisions regarding the treatment of Iranian patients with warfarin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Notwithstanding the more recent endorsement of oral anticoagulants not requiring routine laboratory monitoring, the most widely prescribed anticoagulant is warfarin [1]. Due to the narrow therapeutic index, the erratic monitoring of laboratory results, and weak patient conformity in warfarin therapy, inadequate or excessive anticoagulation may lead to acute complications such as thromboembolism or bleeding [2].
It is generally accepted that the diversity observed in responding to warfarin therapy is recognized in various patients; it can be mainly described by clinical, demographic, environmental, and genetic determinants. Genetic variations are associated with both pharmacodynamics as well as pharmacokinetics of warfarin, thus serving an essential function in inter-patient variability in the warfarin response [3].
Key genetic single nucleotide polymorphisms (SNPs) that determine warfarin therapeutic response have occurred in cytochrome P450-2C9 (CYP2C9) and vitamin K epoxide reductase complex subunit 1 (VKORC1) genes, which can contribute to from 35 to 50% in the warfarin doses variability in diverse populations [4].
The CYP2C9 enzyme is known to be involved in the clearance of S-warfarin from the body, the more active enantiomer of warfarin. The polymorphic alleles of the CYP2C9, CYP2C9*2 (rs1799853), and CYP2C9*3 (rs1057910) have an association with impaired enzyme activity and S-warfarin metabolism, thus reducing warfarin dose requirements and leading to the variability observed in the warfarin response [5].
The most common noncoding VKORC1 gene polymorphism, known as VKORC1 (-1639G > A, rs9923231), is one single nucleotide transition in the VKORC1 promoter region; it is significantly related to the reduced synthesis of functional VKORC1 protein. The VKORC1 enzyme is essential for the transformation of vitamin K into a form of activating coagulation proteins [5].
Moreover, the CYP4F2*3 (rs2108622) polymorphism, as a functional missense variant in cytochrome P450 4F2 (CYP4F2), a metabolizing enzyme vitamin K, has also been discovered to have moderate involvement in warfarin dose variability. Prior studies in many ethnic groups ranging from Caucasian to Asian populations have described that the carriers of the CYP4F2*3 variant needed a higher warfarin dose to achieve the same impact of anticoagulant response compared to the wild-type genotype [6, 7] However, a limited number of studies have explored the influence of the CYP4F2*3 genetic variant on the variance in the warfarin maintenance dose among Iranians, although Iran is a country in the Middle East region with the largest ethnic groups [8, 9].
To calculate the suitable maintenance warfarin dose by the consideration of demographic, genetic as well as clinical data, warfarin dosing prediction algorithms have been proposed in various populations around the world [10,11,12,13,14]. Further studies have revealed that the involvement of factors affecting the sensitivity as well warfarin resistance may vary significantly between subjects from diverse ethnic groups and the requirements for the dosing algorithm are more populated and customized [15,16,17]. However, the dosing algorithm models that were employed to estimate the required warfarin dose in Iranian patients were restricted; furthermore, the vast majority of the studies performed in Iran for warfarin genotyping are limited to showing the SNP alleles frequency.
In the current research study, we assessed the impact of key genetic polymorphisms of CYP4F2, VKORC1, and CYP2C9 genes, and non-genetic predictor variables on the warfarin therapy in our population. Moreover, we attempted to develop and then validate a population-specific warfarin dosing model for Iranian patients.
Methods
Study populations
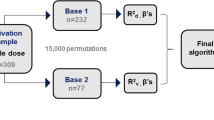
200 warfarin-treated patients maintaining a target international normalized ratio (INR) level of 2.0–3.0 for at least three consecutive intervals attended our anticoagulation clinic; they had been enrolled in Booali Sina Hospital, Qazvin, from, April 2019, to September 2021. All studied subjects needed to be aged ≥ 18 years. Participants with active cancer, severe renal or hepatic insufficiency, severe infection or respiratory failure, thyroid disease, pregnancy, and patients taking other anticoagulants or nonsteroidal anti-inflammatory medications were excluded. All obtained data were collected blindly from medical records as well as standardized interviews.
SNP genotyping
The genomic DNA related to all patients was drawn from peripheral blood by applying a GenEx™ DNA blood kit (GeneAll Biotechnology, Korea), in accordance with the manufacturer's instructions; then storing at – 20 °C was done for analysis. Designed regions of the SNPs CYP2C9*2 (rs1799853), CYP2C9*3 (rs1057910), VKORC1 (rs9923231, 1639G > A), and CYP4F2*3 (rs2108622) were amplified by PCR in a Veriti ABI system (Applied Biosystems, USA); and was visualized by electrophoresis on agarose gels with ethidium bromide staining, then nucleotide sequencing was done by the ABI Big Dye Terminator protocol by applying ABI 3500 Genetic Analyzer.
Statistical analysis
Statistical analysis was done by using SPSS, version 16 (SPSS Inc., Chicago, IL, USA). Numerical data were represented as mean ± standard deviation (SD), and the categorical data were expressed as frequency and percentages. Kolmogorov–Smirnov test was also applied to evaluate the numerical data normality. T-test or one-way ANOVA test followed by a Tukey corrected post hoc test were used f to compare two or more groups. Analysis of categorical variables was done by applying the Chi-square or Fisher’s exact test. The multiple regression analysis was used to predict warfarin dose from a combination of genetic as well as non-genetic predictor variables. The categorical characteristics were coded as dummy variables. The Stepwise selection method was used to include (p < = 0.05) and exclude (p > 0.10) variables into the model.
The dataset was randomly split into train and test datasets. The training dataset (75% of the data) was used to develop the regression model, and the testing dataset (25% of the data) was used for evaluation of the model's performance. Mean absolute error (MAE), mean absolute percentage error (MAPE), mean relative error (MRE), and Pearson’s correlation coefficient (r) were applied to evaluate and compare the developed model's performance with the previously warfarin pharmacogenetic algorithms. A p-value less than 0.05 was assumed to be significant.
Results
Patients' general characteristics
The demographic and clinical factors, mean daily warfarin maintenance dose, and diverse genotype groups of all patients on stable warfarin treatment (n = 200) are represented in Table 1. Of a total of 200 patients (mean age 59.77 ± 12.82 years), 117 (58.5%) were female, 19.5% (n = 39) used the warfarin concurrent medication amiodarone and 11.5% (n = 23) were smokers. The body surface area (BSA) average was 1.92 ± 0.19 m2. The main disease identified was valvular heart disease (n = 65, 32.5%) and the main comorbidity observed was hypertension (n = 50, 25.0%). The mean daily warfarin maintenance dose (mg/day) was 4.61 ± 1.51 and ranged from 1.3 to 9.2.
The frequencies of our studied genetic variants in all patients on stable warfarin treatment (n = 200) are shown in Table 1. Most patients (56.5%, 113/200) carried the CYP2C9 *1/*1 wild-type genotype. Other patients were found to have mutant variants, with 1*/2* accounting for the greatest number of cases (27.0%, 54/200), followed by 1*/3* (10.5%, 21/200), 2*/2* (3.5%, 7/200), and 2*/3* (2.5%, 5/200). We did not observe the CYP2C9 *3/*3 genotype in this research study. The prevalence of VKORC1 wild-types (GG), heterozygous (GA), and homozygous (AA) mutation carriers were found to be 31.5% (63/200), 49% (98/200) and 19.5% (39/200), respectively, for all patients. The CC (wild-type), CT (heterozygous), and TT (homozygous) genotypes of the CYP4F2*3 variant were identified in 43.5% (87/200), 44.5% (89/200), and 12.0% (24/200) of all patients, respectively. All these genotyping results were revealed to be in Hardy–Weinberg equilibrium.
Dosage of warfarin based on non-genetic as well as genetic factors
In the training samples (n = 150), gender, current smoking status, as well as the use of amiodarone, were discovered to have a significant link to the mean daily warfarin dose (Table 2). The indicated treatment, hypertension, diabetes mellitus, and heart failure were found to have no significant impact on the mean daily warfarin dose (Table 2). Additionally, a Pearson correlation analysis of the data indicated that the warfarin maintenance dose had a significantly negative correlation with the patients' age (r = – 0.304; p < 0.001). In contrast, height (r = 0.282; p < 0.001) and weight (r = 0.279; p < 0.001), as well as BSA (r = 0.328; p < 0.001), were found to have a significant positive correlation with the warfarin's maintenance dose.
In the training samples (n = 150), observed differences in the daily maintenance warfarin dose in the considered genotype groups of CYP2C9, VKORC1, and CYP4F2 were calculated using one-way ANOVA (Table 3). Based on this analysis, there were significant correlations between all genotypes with warfarin's mean daily dose. As presented in Table 3, a comparison of the mean (± SD) warfarin's maintenance across CYP2C9 genotypes uncovered that patient with CYP2C9*1/*2, CYP2C9*1/*3, CYP2C9*2/*2, and CYP2C9*2/*3 variant genotypes presented significantly lower averages doses of warfarin (4.31 ± 1.11, 3.21 ± 0.83, 2.94 ± 1.21 and 2.67 ± 0.99 mg/day, respectively) than those with the wild-type CYP2C9*1/*1 genotype (5.08 ± 1.49 mg/day). Regarding the VKORC1 (-1639 G > A) polymorphism, it was revealed that the patients with the AA variant genotype needed significantly lower warfarin's average doses (3.27 ± 0.09 mg/day), as compared with AG or GG carriers (4.43 ± 1.2, or 5.57 ± 1.55 mg/day, respectively). The relative impact of CYP4F2*3 polymorphism on the warfarin maintenance dose indicated that patients with CT or TT genotype (4.75 ± 1.27, or 5.53 ± 1.77 mg/day, respectively) needed a remarkably higher daily warfarin dose when compared to those with the wild-type CC genotype (4.05 ± 1.5), (Table 3).
Warfarin dosing algorithm
We used multiple regression analysis to illustrate the effect of genetic as well as non-genetic parameters on the variability in warfarin doses. The results showed that this descriptive model, including the variant genotypes of VKORC1 (– 1639 G > A), CYP2C9*3, CYP2C9*2, and CYP4F2*3, and non-genetic factors such as age, BSA and amiodarone use, described 56.5% of individual differences in warfarin's maintenance dose in our Iranian patients (Table 4). Multiple regression analysis demonstrated that the presence of VKORC1 (− 1639G > A), CYP2C9*3, and CYP2C9*2 genetic polymorphisms, use of amiodarone, and increasing age, were negatively associated with the warfarin maintenance dose; meanwhile, increased BSA and CYP4F2*3 variant revealed a positive association (Table 4). Multicollinearity among factors was not observed.
Model evaluation
To validate the regression equation acquired from our training dataset, we estimated the predicted warfarin doses by employing the dosing algorithm and then the comparison of it to the actual doses in the considered test samples was done. We found a moderate correlation between the actual and predicted warfarin doses (Pearson’s correlation coefficient = 0.607, p < 0.001).
Further insight into the comparison purpose, we chose four published dosing algorithms developed and validated with international multi-ethnic (Gage algorithm) [18], Caucasian (Zambon algorithm) [19], and Middle-East (Karaca-model [20] and Bader algorithm [10]) populations. Moreover, two Iranian algorithms (Namazi-algorithm [21] and Khaleqsefat-model [22]) were considered (Table 5). Comparisons of our algorithm to the previously published algorithms in the considered test samples are presented in Table 6. Based on the results, our algorithm's efficiency in Iranian patients was found to be slightly better than that attained when utilizing the six other algorithms in the case of our patient series.
Discussion
Numerous studies have previously revealed that the involving factors affecting the sensitivity as well as warfarin resistance may vary in a significant manner between subjects from different ethnic groups; also, the requirements for the dosing algorithm are more populated and customized. Nevertheless, the dosing algorithm models used to estimate the warfarin dose in Iranian patients were restricted, and most of the previous studies performed in Iran for warfarin genotyping were limited to showing the SNP alleles frequency [8, 9, 23,24,25]. Therefore, we conducted our research to develop and also, validate a novel dosing algorithm that was specifically designed for Iranian patients according to genetic polymorphisms and a series of non-genetic factors which were possibly involved in the prediction of the variance in warfarin response.
Results obtained from our research study indicated that the CYP2C9*2 allele prevalence was found at 18%, which was similar to other studies in the Iranian population, thus demonstrating the frequency of the CYP2C9*2 allele between 9 and 27% [23,24,25]. Likewise, the CYP2C9*3 prevalence had been previously demonstrated in 7–10% of the Iranians [23, 24], and the allele frequency was found at 7% in our study. The VKORC1 A allele's frequency was found to be 44% in our study. This distribution was consistent with those studied in the previous reports focusing on the Iranian population, which had been shown between 36 and 56% [25, 26]. The frequency of CYP2C9*2 and CYP2C9*3, and VKORC1 (−1639 G > A) genotypes, as described in this study, were consistent with other studies performed in the Middle Eastern region, like Qatari [10], Egyptian [27], Kuwaiti [28] and Turkish [29] populations; it was closer to that of Caucasians, as compared to Asians as well as African Americans [5, 14, 30]. The prevalence of the CYP4F2 T allele in our study was estimated to be 34%, which was almost similar to the previous studies on the Iranian population, which was between 24 and 41% [8, 9]. Furthermore, CYP4F2 T allele frequency was close to that found for Omani [31], Qatari [32], Egyptian [27], and Turkish [29] populations in the Middle Eastern region; it was also closer to that of Caucasians (30%), as compared to African Americans (7%) and Asians (22%) population. [7, 33,34,35].
The CYP2C9 enzyme metabolizes warfarin, and a reduction in the activity of the enzyme will increase the period of warfarin elimination and thereby raise the blood level of warfarin. CYP2C9*2 and CYP2C9*3 genetic polymorphisms cause an extremely damaging effect on the CYP2C9 enzyme activity that is related to sensitivity to warfarin’s effects as well as the heightened risk of bleeding [36]. As expected, individuals who had any decreased function CYP2C9 allele (*1/*2, *1/*3, *2/*2, and *2/*3) were related to a significantly lower warfarin dose, as compared to those with wild-type genotype. These results were greatly similar to those of other reports on the Iranian population and those observed in other countries in the Middle Eastern region and worldwide.
The VKORC1 enzyme is known to be involved in vitamin K regeneration and warfarin targets the VKORC1 enzyme, as an antagonist of vitamin K. The − 1639G > A genetic variant is a single nucleotide transition in the VKORC1 promoter region that creates a suppressor E-box binding site that can change the binding site related to the transcription factor of the VKORC1 gene. This contributes to the reduced protein expression and synthesis of functional VKORC1 enzyme, which is significantly related to warfarin sensitivity. Similar to other studies on the Iranian population, we revealed that carriers of the − 1639G > A variant (AG or AA carriers) required considerably lower doses for warfarin, as compared to subjects with the wild-type genotype.
The CYP4F2 enzyme is known to be involved in the vitamin K main metabolization in the liver. The functional mechanism of warfarin involves diminishing the concentration of the vitamin K that is reduced form by blocking the vitamin K redox cycle. Therefore, variations in the bioavailability of vitamin K are assumed to interfere with the final response to the treatment done by applying warfarin. Regarding the CYP4F2*3 genotype, our results demonstrated that samples with the TT genotype needed about 1.48 mg/day more warfarin dose, as compared to patients with the CC allele (wild-type genotype); there was a significant relation between this polymorphism and the dose of warfarin requirements in Iranian patients. However, the significant impact of the CYP4F2*3 genetic variant on warfarin dose response in the Iranian-Azari population had not been addressed in Azarara et al. [8]. In contrast, another study done by Khosropanah et al. supported our data, showing that CYP4F2*3 polymorphism significantly impacted warfarin maintenance dose in Iranian patients [9].
Our warfarin dosing algorithm identified that genetic factors, including VKORC1(- 1639G > A), CYP2C9*3, CYP2C9*2, and CYP4F2*3 variants, and non-genetic characteristics such as age, BSA, and amiodarone use, as the significant determinants of warfarin dose, which could account for 56.5% of the variability in the warfarin dose. Our warfarin dosing model presented genetic factors as the most essential sources of the variability in warfarin's dose than clinical factors (44.7% versus 14.7%); this was in agreement with the prior studies done in other countries located in the Middle East region as well as worldwide [10,11,12,13].
In terms of non-genetic predictor variables studied, age was responsible for the greatest proportion, with almost 7% of the variance in warfarin dose. Furthermore, BSA and the use of amiodarone explained 5.5% and 2.3% of the observed variability in the dose of warfarin, respectively.
In regard to the contribution of genetic factors to determining the warfarin dose variance, the CYP2C9*2 and CYP2C9*3 genotypes were found to be effective predictors of warfarin dose, accounting for almost 12% of the variance. The predictive contribution of CYP2C9 alleles described 11.8% to 12.9% of the variability observed in warfarin's dose in Caucasians and between 1.7% and 5.4% in Asian populations [34,35,36]. In addition, we ascertained that VKORC1(– 1639G > A) variant was the most significant factor influencing the warfarin dose requirements and could describe almost 28% of the warfarin dose variance. Numerous studies focusing on different ethnic groups have reported the VKORC1 (– 1639 G > A) variant as a major genetic predictor of warfarin dose in different countries, from Middle Eastern region, Caucasians, and Asian populations, accounting for 11% to 34% of the variability in dose [5, 14]. It was also revealed that the CYP4F2*3 variant could account for almost 4% of the variability in the warfarin dose in our dosing algorithms, which is consistent with the findings reported in other countries from the Middle Eastern region, Asian and Caucasian populations, where the CYP4F2*3 variant contributed between 2 and 5% in the warfarin dose prediction [6, 7, 37].
To evaluate the performance of our population-specific algorithm, we compared the six warfarin dosing models with our warfarin dosing model in a sample of Iranian subjects. Finally, we found that our algorithm performed better than other models for our Iranian population. Iran is located in the Middle East region and the Iranian population's genetic background is an admixture of Middle Eastern, Caucasian, and Central Asian ancestries [38]. The Gage algorithm, as a diversified ethnic model, was developed on Caucasian, African American, and Asian (Chinese and Japanese) samples, as well as different Middle East subjects included in the European class in this cohort [18]. The Zambon- algorithm has been developed and then validated by applying data from Caucasian populations (Italian) [19]. Moreover, the Karaca model [20] and Bader algorithm [10] were conducted on the Middle East samples, Turkish and Qatari patients, respectively. However, in this study, we observed that our population-specific algorithm was more fruitful when compared to the multi-ethnic and Caucasian algorithms or even the algorithms that were designed for populations that were genetically and geographically close to our subjects. This discrepancy may occur owing to such evolutionary processes as gene drift, fluctuations in racial definitions, lifestyle or social contexts, clinical determinants, migration, and population boundaries expansion [39]. These findings, in agreement with the previous reports, propose the idea that warfarin dosing models could be micro-geographically described as population-specific [10, 13, 40]. Furthermore, in comparison with two Iranian algorithms (Namazi algorithm [21] and Khaleqsefat-model [22]), the warfarin dosing algorithm developed and validated in our study is different, mainly regarding the CYP4F2*3 polymorphism, which was included in our algorithm, in contrast to those which have been described by other Iranians authors. These results are in line with the previous studies, demonstrating the contribution of predictive variables considered and the proportion explained by these factors to the fluctuation of warfarin dosing even in the same ethnic population [6, 7, 12, 13].
Conclusions
The findings of the current research, thus, verified and heightened the function of genetic variants VKORC1(– 1639 G > A), CYP2C9*2, CYP2C9*3, and CYP4F2*3, in addition to age, BSA, and amiodarone use, on the variability in warfarin dose. By considering the significant ethnic variability in the response to warfarin therapy, the population-specific warfarin dosing algorithm can be useful to predict warfarin sensitivity and resistance in Iranian patients.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- BSA:
-
Body surface area
- CYP2C9 :
-
Cytochrome P450 2C9
- CYP4F2 :
-
Cytochrome P450 4F2
- INR:
-
International normalized ratio
- MAE:
-
Mean absolute error
- MAPE:
-
Mean absolute percentage error
- MRE:
-
Mean relative error
- SD:
-
Standard deviation
- VIF:
-
Variance inflation factor
- VKORC1:
-
Vitamin K epoxide reductase complex 1
References
Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the Vitamin K antagonists: the Seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:204S-233S.
Kocael A, Eronat AP, Tüzüner MB, Ekmekçi A, Orhan AL, İkizceli İ, et al. Interpretation of the effect of CYP2C9, VKORC1 and CYP4F2 variants on warfarin dosing adjustment in Turkey. Mol Biol Rep. 2019;46:1825–33.
Kim K-A, Song W-G, Lee H-M, Joo H-J, Park J-Y. Multiplex pyrosequencing method to determine CYP2C9* 3, VKORC1* 2, and CYP4F2* 3 polymorphisms simultaneously: its application to a Korean population and comparisons with other ethnic groups. Mol Biol Rep. 2014;41:7305–12.
Lee MTM, Klein TE. Pharmacogenetics of warfarin: challenges and opportunities. J Hum Genet. 2013;58:334–8.
Limdi NA, Wadelius M, Cavallari L, Eriksson N, Crawford DC, Lee M-TM, et al. Warfarin pharmacogenetics: a single VKORC1 polymorphism is predictive of dose across 3 racial groups. Blood J Am Soc Hematol. 2010;115:3827–34.
Carlquist JF, Horne BD, Mower C, Park J, Huntinghouse J, McKinney JT, et al. An evaluation of nine genetic variants related to metabolism and mechanism of action of warfarin as applied to stable dose prediction. J Thromb Thrombolysis. 2010;30:358–64.
Borgiani P, Ciccacci C, Forte V, Sirianni E, Novelli L, Bramanti P, et al. CYP4F2 genetic variant (rs2108622) significantly contributes to warfarin dosing variability in the Italian population. Pharmacogenomics. 2009;10:261–6.
Azarara M, Afrasibirad A, Farzamikia N, Alijani A, Sakhinia E. The effect of GGCX and CYP4F2 gene polymorphisms in genotype-guided dosing of warfarin in patients with a history of cardiac surgery. J Pharm Investig. 2017;47:349–55.
Khosropanah S, Faraji SN, Habibi H, Yavarian M, Mansoori R, Haghpanah S. Correlation between rs2108622 locus of CYP4F2 gene single nucleotide polymorphism and warfarin dosage in Iranian cardiovascular patients. Iran J Pharm Res IJPR. 2017;16:1238.
Bader L, Mahfouz A, Kasem M, Mohammed S, Alsaadi S, Abdelsamad O, et al. The effect of genetic and nongenetic factors on warfarin dose variability in Qatari population. Pharmacogenom J. 2020;20:277–84.
Wattanachai N, Kaewmoongkun S, Pussadhamma B, Makarawate P, Wongvipaporn C, Kiatchoosakun S, et al. The impact of non-genetic and genetic factors on a stable warfarin dose in Thai patients. Eur J Clin Pharmacol. 2017;73:973–80.
Krishna Kumar D, Shewade DG, Loriot M-A, Beaune P, Balachander J, Sai Chandran BV, et al. Effect of CYP2C9, VKORC1, CYP4F2 and GGCX genetic variants on warfarin maintenance dose and explicating a new pharmacogenetic algorithm in South Indian population. Eur J Clin Pharmacol. 2014;70:47–56.
Liang R, Li L, Li C, Gao Y, Liu W, Hu D, et al. Impact of CYP2C9* 3, VKORC1-1639, CYP4F2rs2108622 genetic polymorphism and clinical factors on warfarin maintenance dose in Han-Chinese patients. J Thromb Thrombolysis. 2012;34:120–5.
Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J, Wadelius C, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum Genet. 2007;121:23–34.
Sridharan K, Al Banna R, Malalla Z, Husain A, Sater M, Jassim G, et al. Influence of CYP2C9, VKORC1, and CYP4F2 polymorphisms on the pharmacodynamic parameters of warfarin: a cross-sectional study. Pharmacol Rep. 2021;73:1405–17.
Naushad SM, Kutala VK, Hussain T, Alrokayan SA. Pharmacogenetic determinants of warfarin in the Indian population. Pharmacol Rep. 2021;73:1396–404.
Li J, Chen T, Jie F, Xiang H, Huang L, Jiang H, et al. Impact of VKORC1, CYP2C9, CYP1A2, UGT1A1, and GGCX polymorphisms on warfarin maintenance dose: Exploring a new algorithm in South Chinese patients accept mechanical heart valve replacement. Medicine (Baltimore). 2022;101(29):e29626. https://doi.org/10.1097/MD.0000000000029626.
Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther. 2008;84:326–31.
Zambon C-F, Pengo V, Padrini R, Basso D, Schiavon S, Fogar P, et al. VKORC1, CYP2C9 and CYP4F2 genetic-based algorithm for warfarin dosing: an Italian retrospective study. Pharmacogenomics. 2011;12:15–25.
Karaca S, Bozkurt NC, Cesuroglu T, Karaca M, Bozkurt M, Eskioglu E, et al. International warfarin genotype-guided dosing algorithms in the Turkish population and their preventive effects on major and life-threatening hemorrhagic events. Pharmacogenomics. 2015;16:1109–18.
Namazi S, Azarpira N, Hendijani F, Khorshid MB, Vessal G, Mehdipour AR. The impact of genetic polymorphisms and patient characteristics on warfarin dose requirements: a cross-sectional study in Iran. Clin Ther. 2010;32:1050–60.
Khaleqsefat E, Khalaj-Kondori M, Bonyadi MJ, Soraya H, Askari B. The Contribution of VKORC1 and CYP2C9 genetic polymorphisms and patients’ demographic characteristics with warfarin maintenance doses: a suggested warfarin dosing algorithm. Iran J Pharm Res IJPR. 2020;19:77.
Razavi FE, Zarban A, Hajipoor F, Naseri M. The allele frequency of CYP2C9 and VKORC1 in the Southern Khorasan population. Res Pharm Sci. 2017;12:211.
Marjani A, Gharanjik AM. Genetic polymorphism of CYP2C9 among Sistani ethnic group in Gorgan. Indian J Clin Biochem. 2018;33:208–13.
Azarpira N, Namazi S, Hendijani F, Banan M, Darai M. Investigation of allele and genotype frequencies of CYP2C9, CYP2C19 and VKORC1 in Iran. Pharmacol Rep. 2010;62:740–6.
SoltaniBanavandi MJ, Satarzadeh N. Association between VKORC1 gene polymorphism and warfarin dose requirement and frequency of VKORC1 gene polymorphism in patients from Kerman province. Pharmacogen J. 2020;20:574–8.
Shahin MHA, Khalifa SI, Gong Y, Hammad LN, Sallam MTH, El Shafey M, et al. Genetic and nongenetic factors associated with warfarin dose requirements in Egyptian patients. Pharmacogenet Genomics. 2011;21:130.
Alrashid MH, Al-Serri A, Alshemmari SH, Koshi P, Al-Bustan SA. Association of genetic polymorphisms in the VKORC1 and CYP2C9 genes with warfarin dosage in a group of Kuwaiti individuals. Mol Diagn Ther. 2016;20:183–90.
Özer M, Demirci Y, Hızel C, Sarıkaya S, Karaltı İ, Kaspar Ç, et al. Association of genetic polymorphisms in the VKORC1 and CYP2C9 genes with warfarin dosage in a group of Kuwaiti individuals. Basic Clin Pharmacol Toxicol. 2013;112:209–14.
Xie H-G, Prasad HC, Kim RB, Stein CM. CYP2C9 allelic variants: ethnic distribution and functional significance. Adv Drug Deliv Rev. 2002;54:1257–70.
Pathare AV, Al Zadjali S, Misquith R, Alkindi SS, Panjwani V, Lapoumeroulie C, et al. Warfarin pharmacogenetics: polymorphisms of the CYP2C9, CYP4F2, and VKORC1 loci in a genetically admixed Omani population. Hum Biol. 2012;84:67–77.
Sivadas A, Sharma P, Scaria V. Landscape of warfarin and clopidogrel pharmacogenetic variants in Qatari population from whole exome datasets. Pharmacogenomics. 2016;17:1891–901.
Pautas E, Moreau C, Gouin-Thibault I, Golmard J, Mahe I, Legendre C, et al. Genetic factors (VKORC1, CYP2C9, EPHX1, and CYP4F2) are predictor variables for warfarin response in very elderly, frail inpatients. Clin Pharmacol Ther. 2010;87:57–64.
Shendre A, Brown TM, Liu N, Hill CE, Beasley TM, Nickerson DA, et al. Race-specific influence of CYP 4F2 on dose and risk of hemorrhage among warfarin users. Pharmacother J Hum Pharmacol Drug Ther. 2016;36:263–72.
Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. https://doi.org/10.1038/nature15393.
Sano E, Li W, Yuki H, Liu X, Furihata T, Kobayashi K, et al. Mechanism of the decrease in catalytic activity of human cytochrome P450 2C9 polymorphic variants investigated by computational analysis. J Comput Chem. 2010;31:2746–58.
Singh O, Sandanaraj E, Subramanian K, Lee LH, Chowbay B. Influence of CYP4F2 rs2108622 (V433M) on warfarin dose requirement in Asian patients. Drug Metab Pharmacokinet. 2011;26:130–6.
Mehrjoo Z, Fattahi Z, Beheshtian M, Mohseni M, Poustchi H, Ardalani F, et al. Distinct genetic variation and heterogeneity of the Iranian population. PLoS Genet. 2019;15: e1008385.
Caulfield T, Fullerton SM, Ali-Khan SE, Arbour L, Burchard EG, Cooper RS, et al. Race and ancestry in biomedical research: exploring the challenges. Genome Med. 2009;1:1–8.
Pathare A, Al Khabori M, Alkindi S, Al Zadjali S, Misquith R, Khan H, et al. Warfarin pharmacogenetics: development of a dosing algorithm for Omani patients. J Hum Genet. 2012;57:665–9.
Acknowledgements
We appreciate each patient's continued involvement in this study. We also thank all the nurses and staff of Booali Sina Hospital for their assistance during the study. We would like to thank Dr. Haj Manouchehri, Bahar Laboratory, Qazvin, for contributing to method development. We highly thank Dr. Hamidreza Javadi, Qazvin University of medical science, for his valuable and constructive suggestions regarding the initial design of this study.
Funding
This research study was funded by the Cellular and Molecular Research Center, Institute for Prevention of Non-Communicable Diseases, Qazvin University of Medical Sciences, Qazvin, Iran (28/20/17123).
Author information
Authors and Affiliations
Contributions
MFD conceived the idea, carried out the experiments, gathered and evaluated the data, and wrote the main manuscript. FSR edited, guided, and supervised the manuscript. SSF examined and selected the appropriate patients to be included in the study. AJ analyzed and interpreted the data. SC and DHA conceived and designed the experimental methodologies. Finally, BR developed the concept of the presented idea, guided the experiments, supervised the study, and edited the whole manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no interest conflict.
Ethical approval
The local Ethical Committee of Qazvin University of Medical Sciences (QUMS), Qazvin, Iran, approved this study (IR.QUMS.REC.1397.367), and from all patients, before entering the study, written informed consent was acquired.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farajzadeh-Dehkordi, M., Samiee-Rad, F., Farzam, S.S. et al. Evaluation of a warfarin dosing algorithm including CYP2C9, VKORC1, and CYP4F2 polymorphisms and non-genetic determinants for the Iranian population. Pharmacol. Rep 75, 695–704 (2023). https://doi.org/10.1007/s43440-023-00476-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-023-00476-2