Abstract
Despite the great potential for the industrial application of microalgae, production costs are still too high to make them a competitive raw material for commodities. Therefore, studying more efficient cultivation strategies in biomass production and economic viability is necessary. In this sense, this work aimed to reduce the production costs of biomass and biomolecules using phytohormone indole-3-acetic acid in different phases of Spirulina sp. LEB 18 cultivation. The experiments were conducted on bench scale indoor for 30 days. In each couple of experiments, the phytohormone was added on different days. The supplementation of indole-3-acetic acid on half of the growth deceleration phase of the microalga showed a cost reduction of 27%, 34%, and 75% for biomass, proteins, and carbohydrates, respectively. In addition, the strategy increased the final biomass concentration and carbohydrate content at 31.2 and 33.8%, respectively, compared to the condition without phytohormone. This study is the starting point for implementing phytohormone supplementation in industrial microalgal cultures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Interest in microalgae has increased mainly due to its potential for applications in different economic sectors, such as human food [1], animal feed [2], cosmetics, pharmaceuticals [3], building materials [4, 5], and biofuels [6]. In addition, these microorganisms can be a source of high value-added compounds, such as carotenoids [7], biopolymers [8], sulfated polysaccharides [9], and polyunsaturated fatty acids [10]. Spirulina is a microalgae genus that stands out in commercial cultivation and is the most studied prokaryotic microalgae globally [11]. This species can be successfully grown in open ponds since it has the ability to grow in highly selective environments, for example, at high pH and temperature [12]. Market opportunities for microalgae applications focus on high value-added products, as production costs are too expensive to make microalgae a competitive raw material for commodities [13]. As a result, it has been studied as an alternative to reduce the cultivation medium price [14, 15] or to improve biomass production systems, like supplementing microalgal cultures with phytohormones [16,17,18,19,20,21,22]. Phytohormones play control roles in plants, algae, and microalgae [23]. Studies also have shown that using these substances in microalgal cultivation can modify metabolic pathways, favoring the synthesis of compounds of interest, such as carbohydrates, proteins, and lipids [21, 22, 24].
The use of phytohormones in microalgal cultivation is related to increased production costs since it is an expensive input, despite being used in small quantities. Because of this, some studies have focused on evaluating the economic feasibility of this strategy. Park et al. [17] analyzed the economic viability of growing Chlamydomonas reinhardtii supplemented with phytohormones compared to a synthetic control medium. This study showed that the cost of biomass production using indole-3-acetic acid (IAA) was lower (approximately US$ 0.15 per gram) than without phytohormones. Salama et al. [19] also showed that the cost of biomass production of the microalga Scenedesmus obliquus supplemented with IAA (US$0.39 per gram) was lower than that of the standard culture medium (US$0.78 per gram). Silveira et al. [22] have added 0.1 mg L−1 IAA in the Spirulina sp. LEB 18 cultivations. In these conditions, the authors reported cost reductions of 41, 47, 55, and 19% in biomass, protein, carbohydrate, and lipid production, respectively.
Research on microalgae supplementation is based on cultivations conducted in batch mode, in which phytohormones are added to the culture medium at the beginning of the process [16, 21, 22]. However, it is known that the production of phytohormones by different microalgae occurs at various stages of microalgal growth [25] and that microalgae only produce phytohormones when needed for their growth. Otherwise, they are conjugated to other substances or degraded [26]. Thus, cultivation in fed-batch form can allow phytohormones to be supplemented just when they are needed.
As the growth phase of microalgae in which there is a greater need for phytohormones is not always the initial stage, by supplementing the substances in different cultivation periods, the microalgae can obtain a more significant benefit in the final concentration of biomass since supplemented IAA at the correct stages of cultivation prevents the phytohormone from being converted into other substances or degraded [27]. Furthermore, phytohormones in the proper microalgal growth phase can make the supplementation of microalgal cultivation an economically viable strategy. In this sense, this work aimed to define the growth phase of the microalga Spirulina sp. LEB 18 by adding the phytohormone indole-3-acetic acid (IAA), which stimulates an increase in biomass concentration and reduces the production costs of biomass and biomolecules.
Materials and Methods
Microalga and Phytohormone
The experiments were carried out using the microalga Spirulina sp. LEB 18. It was obtained from the strain bank of the Biochemical Engineering Laboratory (LEB) of the Federal University of Rio Grande (FURG). This strain was originally isolated from Mangueira Lagoon (latitude 33°31ʹ08″ S and longitude 53°22ʹ05″ W) [28] and cultivated in Zarrouk medium [29]. The commercial phytohormone IAA (Sigma-Aldrich, Brazil, 98%) was added to the experiments from 0.1 mg mL−1 stock solution with 0.1% (v v−1) sodium hydroxide (NaOH).
Cultivation Conditions
To evaluate which growth phase of the microalga is most suitable for adding the phytohormone, six experiments were carried out with 0.1 mg L−1 IAA [23] on different days. The experiments were called D0, D5, D10, D15, D20, and D25, which refer to the days when the phytohormone was added to each one: days 0, 5, 10, 15, 20, and 25, respectively. A control experiment (C) was also carried out in which phytohormones were not added.
The experiments were carried out for 30 days in duplicate in batch (C and D0) and fed-batch (D5, D10, D15, D20, and D25) forms in 0.5-L Erlenmeyer-type bioreactors, with a useful volume of 0.4 L, initial biomass concentration of 0.2 g L−1, and replacement of evaporation with sterile distilled water over time. The cultures were carried out in a culture chamber maintained at 30 °C with a photoperiod of 12 h light/dark [30], a luminous intensity of approximately 60 μmol m−2 s−1 provided by fluorescent lamps, and agitation by injecting sterile compressed air. At the end of the experiments, the biomass was centrifuged (Hitachi, Himac CR-GIII, Japan) at 2000 × g for 20 min, washed to remove the salts, frozen at ‒80 °C, lyophilized for 48 h, and stored at –20 °C until characterization.
Cultivation Monitoring
The biomass concentration was determined at the beginning and end of the cultivations by reading the optical density at 670 nm in a spectrophotometer (Shimadzu UV/VIS UVmin-1240 spectrophotometer, Japan), with a calibration curve that relates optical density to dry biomass mass [28, 31]. In addition, at time zero and the end of 30 days, the cell morphology was evaluated under an optical microscope (AxioCan ERc 5 s Microscope camera, Zeiss, Germany), and the pH of the cultures was determined using a digital pH meter (Mettler Toledo FiveGo, Switzerland).
Biomolecule Quantification
Lipids were extracted from the lyophilized biomass by the organic solvents chloroform and methanol (chloroform-to-methanol ratio, 1:2). Lipid quantification was performed colorimetrically using a tripalmitin standard curve [32].
Before analyzing carbohydrates and proteins, the lyophilized biomasses were treated to break the cell wall and release the intracellular material with the aid of an ultrasonic probe (Cole Parmer, CPX 130, USA). Extracts were prepared by adding 5 mg of lyophilized biomass in 10 mL of distilled water and sonicating with 10 operating cycles (59 s on/off).
The total carbohydrate content was determined using the phenol–sulfuric method [33] with a standard glucose curve. Protein content was quantified using the colorimetric method described by Lowry et al. [34] using bovine serum albumin as a standard and with a previous step of protein solubilization with sodium hydroxide (NaOH). Biomass moisture was determined using the official AOAC methodology [35].
Analysis of Biomass and Biomolecule Production Costs
Although there are many factors (energy, harvest, labor, among others) contributing to the cost of producing biomass and biomolecules, in this study, except for the addition of phytohormone, no other cultivation modification was performed. Therefore, only the costs of the culture medium with and without phytohormone were considered since all other factors were equal.
The prices of the IAA phytohormone (98%) and of all other reagents (ACS-grade reagents) that made up the culture medium used in this study (Table S1) were obtained from the Sigma-Aldrich website on December 23, 2021. The cost for producing the microalgal biomass, as well as for the biomolecules, was calculated using the equation described by Park et al. [17]: Cost (US$/g) = ((AB) + C)/D, where A is the amount of IAA added per liter of culture medium (mg L−1), B is the IAA price (US$ mg−1), C is the price of the culture medium (US$ L−1), and D is the final concentration of biomass or biomolecules produced at the end of the cultivation (g L−1).
Statistical Analysis
The results were analyzed in triplicate by analysis of variance (ANOVA) and Tukey’s test for comparison of means, with a confidence interval of 95% (p ≤ 0.05).
Results and Discussion
Effect of IAA Supplementation in Different Phases of Spirulina Growth on the Final Biomass Concentration
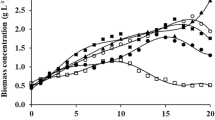
The microalga cultivation phase in which IAA was supplemented affected the final biomass concentration (Fig. 1). Phytohormone supplementation on days 5 and 15 of cultivation generated higher biomass concentrations than the other conditions. These results showed an increase of 30.4 and 31.2%, respectively, compared to the experiment without the addition of phytohormone. In addition, these conditions were also superior to the state with IAA supplementation on day 0 (D5 at 7.8% and D15 at 8.5%), the condition most commonly used for microalgal cultivation with phytohormone supplementation [16, 21, 22].
It can be reasonably assumed that the fifth day of cultivation corresponded to the exponential growth phase of the microalga and the 15th day to half of the growth deceleration phase (Fig. 2). Thus, it can be suggested that the addition of IAA in these growth phases of the microalga Spirulina sp. LEB 18 provided higher final biomass concentrations than phytohormone supplementation in the other 30-day cultivation periods of this microalga. If IAA is supplemented in cultivation phases where it is not needed for microalga growth, the phytohormone is converted into other substances or degraded. According to Sztein et al. [27], excess IAA is converted into storage conjugates, including indole-3-butyric acid, sugar-linked IAA esters, and amino acid– and peptide-linked amides of IAA. On the other hand, degradation can occur through two pathways: a decarboxylative pathway to indole-3-carboxylic acid or a nondecarboxylative pathway involving the oxidation of the indolic ring.
Similarly, Renuka et al. [18] obtained different maximum biomass productivity when phytohormones were supplemented in different phases of microalgal growth. The authors cultivated Acutodesmus obliquus in BG 11 medium for 14 days, with nitrogen limitation and the addition of cytokinin-type phytohormones (1 mg L−1 kinetin and 0.1 mg L−1 zeatin) in the lag growth phases (day 0), initial log (day 3), and intermediate log (day 7). The experiments with kinetin had higher biomass productivity when supplemented in the intermediate log phase (165 mg L−1 day−1). In comparison, the cultures with zeatin showed maximum productivity with the addition of the phytohormone in the initial log phase (176.79 mg L−1 day−1). This increase in productivity can be explained by phytohormones stimulating photosynthetic activity. When kinetin was added to the cultivation in the intermediate log phase and zeatin in the initial log phase, the cultures showed a higher relative electron transport rate (rETR), 52.4 and 63.1, respectively, compared to experiments with supplementation of phytohormones in other stages of growth.
In addition, Park et al. [17] found different maximum cell concentrations according to the microalga growth phase in which phytohormones were supplemented. The researchers cultivated Chlamydomonas reinhardtii CC124 in TAP medium supplemented with the phytohormones IAA (3 mg L−1), gibberellic acid (1 mg L−1), kinetin (5 mg L−1), 1-triacontanol (10 mg L−1), and abscisic acid (10 mg L−1). When abscisic acid was added to the culture in the lag phase, the maximum cell concentration was reduced (approximately 0.7 g L−1) compared to the culture without the addition of phytohormones (approximately 1.0 g L−1). The supplementation of other substances in this same phase had no significant effect. On the other hand, the authors observed a significantly increased maximum cell concentration (approximately 1.2 g L−1) when gibberellic acid and kinetin were added to the experiments at the beginning of the stationary phase.
The performance of phytohormones, depending on the microalgal strain and supplementation concentration, is consistent. The results presented in the studies mentioned above for Acutodesmus obliquus [18] and Chlamydomonas reinhardtii CC124 [17], in addition to the evidence in this study for Spirulina sp. LEB 18, demonstrate the importance of supplementing phytohormones in an adequate growth phase of microalga to increase the final biomass concentration.
Effect of IAA Supplementation in Different Phases of Spirulina Growth on the Biomolecule Content
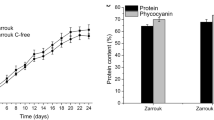
The protein portion of the biomass of Spirulina sp. LEB 18 showed no significant difference among most conditions tested (Table 1). Carbohydrates were the biomolecules that had the most significant change in content (Table 1). When comparing the control culture with the results of the experiments in which IAA was added on half of the growth deceleration phase (D15) and stationary phase (D25), an increase of 33.6 and 33.8% in carbohydrate content was obtained, respectively. Furthermore, when comparing these results to the experiment in which the phytohormone was added at the beginning of the cultivation (D0), as supplementation is usually carried out, an increase of 66.2% in the content of this biomolecule was observed (D25). Carbohydrate production was stimulated when IAA was made available to Spirulina sp. LEB 18 in the final phases of its growth.
Lipid production was not stimulated by supplementation with 0.1 mg L−1 IAA at different growth phases of Spirulina sp. LEB 18. However, the concentration of IAA used in this study is not adequate to stimulate the production of lipids by this strain. Silveira et al. [22] reported that adding 0.01 mg L−1 IAA to Spirulina sp. LEB 18 resulted in positive effects on lipid production (24%). The higher concentrations of IAA used in that study (0.1, 1, and 10 mg L−1) resulted in approximately 13% higher lipid content. Thus, it is believed that if IAA were used at a concentration of 0.01 mg L−1 in the current study, supplementation at different growth phases of Spirulina sp. LEB 18 could help define the form of IAA supplementation with better results for lipid production.
When IAA was added to the experiment on half of the growth deceleration phase (D15), the best composition of the biomass was reached. Under this condition, an increase in carbohydrate content was obtained, in addition to a high concentration of biomass (4.0 g L−1), as described in the previous section. These results, added to those described in the literature [17, 18, 20, 22], indicate that different supplementation strategies, such as the type of phytohormone, microalgal growth phase in which the phytohormone is added to the culture, supplementation concentration, and frequency of sampling from the cultivation, influence the composition of the microalgal biomass. Therefore, these cultivation conditions can be manipulated to obtain the microalgal biomass composition of interest. This manipulation possibility is enhanced by phytohormone supplementation, a versatile strategy that can be implemented in many microalgae-based products.
Analysis of Biomass and Biomolecule Production Costs of Spirulina Supplemented with IAA
Although IAA supplementation increases the price of a liter of Zarrouk medium by US$ 0.004 (from US$ 0.8677 to 0.8681), the production cost of 1 g of Spirulina sp. LEB 18 in any of the conditions with the addition of 0.1 mg L−1 phytohormone was inferior compared to the cultivation without supplementation since the biomass concentration was increased in the IAA cultures (Table 2). The biomass production costs with IAA supplementation on half of the growth deceleration phase (D15) were reduced by 31.5%. These conditions had a cost reduction of US$ 0.06 per gram of biomass produced when compared to the control condition. This is because IAA supplementation on days 5, 15, and 25 increased the final microalgal biomass concentration by approximately 30% compared to cultivation without supplementation.
This cultivation strategy presents significant cost savings industrially since it represents US$ 60 less in kilograms of produced biomass. For example, for a company producing Spirulina biomass for human consumption located in southern Brazil, which produces approximately 0.4 t of biomass per year [12], the use of phytohormones would save approximately US$27,000 annually.
It is also important to emphasize that this study used ACS-grade reagents to carry out the cultivation and cost analysis. Large-scale production uses bulk reagents, which reduces production costs. In the study by Park et al. [17], the production of 1 g of Chlamydomonas reinhardtii biomass with IAA supplementation was US$ 0.45 when using ACS-grade reagents. On the other hand, when the microalga was cultivated under the same conditions using bulk reagents, the production cost was US$ 0.024 per gram of biomass.
In addition to biomass production, biomolecules are also highlighted in the microalgae industry, as they are potential raw materials for other products. In this study, it was possible to reduce the production costs of proteins, carbohydrates, and lipids extracted from the biomass of the microalga Spirulina sp. LEB 18 from cultures supplemented with the IAA phytohormone (Table 2). Supplementing IAA on half of the growth deceleration phase of culture (15th day) reduced the cost of protein and carbohydrate production by 34.5% and 75.5%, respectively, compared to the control condition. Since carbohydrates were the biomolecules that increased their concentration the most with phytohormone use, they also showed the most significant reduction in production costs based on the strategy used. However, the biomolecules with the lowest production cost are proteins, representing the bulk of the microalgal biomass.
Lipids contained in Spirulina sp. LEB 18, despite representing the lowest content of microalgal biomass biomolecules and not increasing their concentration due to supplementation, showed significant cost reduction with this strategy due to the increase in biomass production. The experiment with IAA supplementation on the 10th day of cultivation showed production values 24.8% lower than the control condition. However, two conditions in which phytohormone were used had higher lipid production costs than the control condition without supplementation: the condition in which IAA was added to the culture in the beginning (point 0) as microalgal culture is usually carried out with phytohormone supplementation and the condition with supplementation on day 20.
These results show that the use of phytohormone to increase the production of biomass and microalgal biomolecules can be advantageous in production costs, depending on how the strategy is used. This is an essential finding of this initial study before the use of phytohormones for large-scale cultivation since the economic impact of the use of any new strategy must be considered before its implementation in industrial production. Furthermore, it should be taken into account that the IAA used in this study is of high purity, which increases the costs of all products of this experiment. However, the large-scale production of microalga using bulk reagents and phytohormones must be further investigated. According to Park et al. [17], the use of phytohormones with different purities does not have other effects on the production of biomass and microalgae biomolecules. Nevertheless, it is an essential factor in cost reduction.
Conclusion
The cultivation of Spirulina sp. LEB 18 with IAA supplementation has been most effective when the IAA is added to half of the growth deceleration phase of cultivation. Under this condition, the costs of producing 1 g of biomass, proteins, and carbohydrates were reduced by 27, 34, and 75%, respectively. IAA supplementation in this phase of microalgal growth stimulated an increase in biomass and carbohydrates at 31.2 and 33.8%, respectively, compared to control cultivation. This study is the starting point to prove that the supplementation of phytohormones in microalgal cultures can be a viable strategy to increase production efficiency and reduce costs during industry implementation.
Data Availability
The data generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Lucas, B. F., Rosa, A. P. C., Carvalho, L. F., Morais, M. G., Santos, T. D., & Costa, J. A. V. (2020). Snack bars enriched with Spirulina for schoolchildren nutrition. Food Science and Technology, 40(suppl 1), 146–152. https://doi.org/10.1590/fst.06719
Rosa, A. P. C., Carvalho, L. F., Goldbeck, L., Enke, D. B. S., Rocha, C. B., Souza-Soares, L. A., Costa, J. A. V. (2020). Productive performance and fatty acid profile of Hungarian carp fingerlings fed with Spirulina enriched feed. Research, Society and Development, 9(3), 116932301. https://doi.org/10.33448/rsd-v9i3.2301
El-Baz, F. K., Aly, H. F., & Abd-Alla, H. I. (2020). The ameliorating effect of carotenoid rich fraction extracted from Dunaliella salina microalga against inflammation-associated cardiac dysfunction in obese rats. Toxicology Reports, 7, 118–124. https://doi.org/10.1016/j.toxrep.2019.12.008
Cruz, C. G., Silveira, J. T., Ferrari, F. M., Costa, J. A. V., & Rosa, A. P. C. (2019). The use of poly(3-hydroxybutyrate), C-phycocyanin, and phenolic compounds extracted from Spirulina sp. LEB 18 in latex paint formulations. Progress in Organic Coatings, 135, 100–104. https://doi.org/10.1016/j.porgcoat.2019.05.042
Ribeiro, E. S., Tavella, R. A., Santos, G. S., Figueira, F. S., & Costa, J. A. V. (2020). Thermal and acoustic insulation boards from microalgae biomass, poly-β-hydroxybutyrate and glass wool. Research, Society and Development, 9(4), e143942995. https://doi.org/10.33448/rsd-v9i4.2995
Cardias, B. B., Trevisol, T. C., Bertuol, G. G., Costa, J. A. V., & Santos, L. O. (2021). Hydrolyzed Spirulina biomass and molasses as substrate in alcoholic fermentation with application of magnetic fields. Waste and Biomass Valorization, 12(1), 175–183. https://doi.org/10.1007/s12649-020-00966-x
Schüler, L. M., Santos, T., Pereira, H., Duarte, P., Katkam, N. G., Florindo, C., & Varela, J. C. S. (2020). Improved production of lutein and β-carotene by thermal and light intensity upshifts in the marine microalga Tetraselmis sp. CTP4. Algal Research, 45, 101732. https://doi.org/10.1016/j.algal.2019.101732
Moradi, Z., Haghjou, M. M., Zarei, M., Colville, L., & Raza, A. (2021). Synergy of production of value-added bioplastic, astaxanthin and phycobilin co-products and Direct Green 6 textile dye remediation in Spirulina platensis. Chemosphere, 280, 130920. https://doi.org/10.1016/j.chemosphere.2021.130920
Liberman, G. N., Ochbaum, G., Mejubovsky-Mikhelis, M., Bitton, R., & Arad, S. (2020). Physico-chemical characteristics of the sulfated polysaccharides of the red microalgae Dixoniella grisea and Porphyridium aerugineum. International Journal of Biological Macromolecules, 145, 1171–1179. https://doi.org/10.1016/j.ijbiomac.2019.09.205
Chalima, A., Taxeidis, G., & Topakas, E. (2020). Optimization of the production of docosahexaenoic fatty acid by the heterotrophic microalga Crypthecodinium cohnii utilizing a dark fermentation effluent. Renewable Energy, 152, 102–109. https://doi.org/10.1016/j.renene.2020.01.041
Garrido-Cardenas, J. A., Manzano-Agugliaro, F., Acien-Fernandez, F. G., & Molina-Grima, E. (2018). Microalgae research worldwide. Algal Research, 35, 50–60. https://doi.org/10.1016/j.algal.2018.08.005
Costa, J. A. V., Freitas, B. C. B., Rosa, G. M., Moraes, L., Morais, M. G., & Mitchell, B. G. (2019). Operational and economic aspects of Spirulina-based biorefinery. Bioresource Technology, 292, 121946. https://doi.org/10.1016/j.biortech.2019.121946
Show, P. L. (2022). Global market and economic analysis of microalgae technology: Status and perspectives. Bioresource Technology, 357, 127329. https://doi.org/10.1016/j.biortech.2022.127329
Bezerra, P. Q. M., Moraes, L., Silva, T. N. M., Cardoso, L. G., Druzian, J. I., Morais, M. G., & Costa, J. A. V. (2021). Innovative application of brackish groundwater without the addition of nutrients in the cultivation of Spirulina and Chlorella for carbohydrate and lipid production. Bioresource Technology, 345, 126543. https://doi.org/10.1016/j.biortech.2021.126543
Costa, J. A. V., Cruz, C. G., & Rosa, A. P. C. (2021). Insights into the technology utilized to cultivate microalgae in dairy effluents. Biocatalysis and Agricultural Biotechnology, 35, 102106. https://doi.org/10.1016/j.bcab.2021.102106
Lv, H., Wang, Q., Wang, S., Qi, B., He, J., & Jia, S. (2019). Enhancing biomass production of Dunaliella salina via optimized combinational application of phytohormones. Aquaculture, 503(December 2018), 146–155. https://doi.org/10.1016/j.aquaculture.2018.12.077
Park, W., Yoo, G., Moon, M., Kim, C. W., Choi, Y.-E., & Yang, J.-W. (2013). Phytohormone supplementation significantly increases growth of Chlamydomonas reinhardtii cultivated for biodiesel production. Applied Biochemistry and Biotechnology, 171(5), 1128–1142. https://doi.org/10.1007/s12010-013-0386-9
Renuka, N., Guldhe, A., Singh, P., Ansari, F. A., Rawat, I., & Bux, F. (2017). Evaluating the potential of cytokinins for biomass and lipid enhancement in microalga Acutodesmus obliquus under nitrogen stress. Energy Conversion and Management, 140, 14–23. https://doi.org/10.1016/j.enconman.2017.02.065
Salama, E.-S., Kabra, A. N., Ji, M.-K., Kim, J. R., Min, B., & Jeon, B.-H. (2014). Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresource Technology, 172, 97–103. https://doi.org/10.1016/j.biortech.2014.09.002
Yu, Z., Pei, H., Jiang, L., Hou, Q., Nie, C., & Zhang, L. (2018). Phytohormone addition coupled with nitrogen depletion almost tripled the lipid productivities in two algae. Bioresource Technology, 247(17923), 904–914. https://doi.org/10.1016/j.biortech.2017.09.192
Yu, Z., Song, M., Pei, H., Jiang, L., Hou, Q., Nie, C., & Zhang, L. (2017). The effects of combined agricultural phytohormones on the growth, carbon partitioning and cell morphology of two screened algae. Bioresource Technology, 239, 87–96. https://doi.org/10.1016/j.biortech.2017.04.120
Silveira, J. T., Rosa, A. P. C., & Costa, J. A. V. (2022). Modulating phytohormone supplementation can efficiently increase biomass and lipid production in Spirulina (Arthrospira). BioEnergy Research, 15, 112–120. https://doi.org/10.1007/s12155-021-10310-3
Yamaguchi, I., Cohen, J. D., Culler, A. H., Quint, M., Slovin, J. P., Nakajima, M., Sakagami, Y. (2010). Plant Hormones. In H.-W. (Ben) Liu & L. Mander (Eds.), Comprehensive Natural Products II (pp. 9–125). Oxford: Elsevier. https://doi.org/10.1016/B978-008045382-8.00092-7
Saygideger, S. D., & Okkay, O. (2008). Effect of 2,4-dichlorophenoxyacetic acid on growth, protein and chlorophyll-a content of Chlorella vulgaris and Spirulina platensis cells. Journal of Environmental Biology, 29(2), 175–178.
Žižková, E., Kubeš, M., Dobrev, P. I., Přibyl, P., Šimura, J., Zahajská, L., & Motyka, V. (2017). Control of cytokinin and auxin homeostasis in cyanobacteria and algae. Annals of Botany, 119(1), 151–166. https://doi.org/10.1093/aob/mcw194
Stirk, W. A., Ördög, V., Novák, O., Rolčík, J., Strnad, M., Bálint, P., & van Staden, J. (2013). Auxin and cytokinin relationships in 24 microalgal strains. Journal of Phycology, 49(3), 459–467. https://doi.org/10.1111/jpy.12061
Sztein, A. E., Cohen, J. D., Fuente, I. G., & Cooke, T. J. (1999). Auxin metabolism in mosses and liverworts. American Journal of Botany, 86(11), 1544–1555. https://doi.org/10.2307/2656792
Morais, M. G., Reichert, C. C., Dalcanton, F., Durante, A. J., Marins, L. F., & Costa, J. A. V. (2008). Isolation and characterization of a new Arthrospira strain. Zeitschrift für Naturforschung C, 63(1–2), 144–150. https://doi.org/10.1515/znc-2008-1-226
Zarrouk, C. (1966). Contribution à l’étude d’une cyanophycée Influence de divers facteurs physuques et chimiques sur la croissance et la photosynthèse de Spirulina maxima (Setch et Gardener) Geitler. PhD thesis, University of Paris, Paris, France.
Reichert, C. C., Reinehr, C. O., & Costa, J. A. V. (2006). Semicontinuous cultivation of the cyanobacterium Spirulina platensis in a closed photobioreactor. Brazilian Journal of Chemical Engineering, 23(1), 23–28. https://doi.org/10.1590/S0104-66322006000100003
Costa, J. A. V., Colla, L. M., Filho, P. D., Kabke, K., & Weber, A. (2002). Modelling of Spirulina platensis growth in fresh water using response surface methodology. World Journal of Microbiology & Biotechnology, 18, 603–607.
Marsh, J. B., & Weinstein, D. B. (1966). Simple charring method for determination of lipids. Journal of Lipid Research, 7, 574–576.
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356. https://doi.org/10.1021/ac60111a017
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
AOAC. (1995). Official methods of analysis of AOAC International. (P. Cunniff, Ed.) (16th ed.). Virginia: AOAC International.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. Financial support was also provided by the Ministry of Science, Technology, Innovations and Communications (MCTIC).
Author information
Authors and Affiliations
Contributions
JTS, APCR, MGM, and JAVC: conceptualization. JTS: data curation, formal analysis, validation, writing—original draft. APCR, MGM, and JAVC: writing—review and editing, supervision. MGM and JAVC: resources, funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with animals or human participants.
Consent to Participate
The authors agreed to participate in this work.
Consent for Publication
The authors agreed to publish this work.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silveira, J.T., Rosa, A.P.C., Morais, M.G. et al. Cost Reduction in the Production of Spirulina Biomass and Biomolecules from Indole-3-Acetic Acid Supplementation in Different Growth Phases. Appl Biochem Biotechnol 195, 2882–2892 (2023). https://doi.org/10.1007/s12010-022-04251-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04251-6