Abstract
Threat of global warming due to carbon dioxide (CO2) emissions has stimulated research into carbon sequestration and emissions reduction technologies. Alkaline scrubbing allows CO2 to be captured as bicarbonate, which can be photochemically fixed by microalgae. The carbon concentrating mechanism (CCM), of which external carbonic anhydrase is a key component, allows organisms to utilise this bicarbonate. In order to select a suitable strain for this application, a screening tool is required. The current method for determining carbonic anhydrase activity, the Wilbur and Anderson assay, was found to be unsuitable as a screening tool as the associated error was unacceptably large and tests on whole cells were inconclusive. This paper presents the development of a new, whole cell assay to measure inorganic carbon uptake and external carbonic anhydrase activity, based on classical pH drift experiments. Spirulina platensis was successfully used to develop a correlation between the specific carbon uptake (C) and the specific pH change (dpH). The relationship is described by the following: C[mmol C (g dry algae)−1 h−1] = 0.064 × (dpH). Inhibitor and salt dissociation tests validated the activity and presence of external carbonic anhydrase and allowed correlation between the Wilbur and Anderson assay and the new whole cell assay. Screening tests were conducted on S. platensis, Scenedesmus sp., Chlorella vulgaris and Dunaliella salina that were found to have carbon uptake rates of 5.76, 5.86, 3.86 and 2.15 mmol C (g dry algae)−1 h−1, respectively. These results corresponded to the species' known bicarbonate utilisation abilities and validated the use of the assay as a screening tool.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global impact and consequences of climate change, due to greenhouse gas emissions, are increasingly recognised as a concern for policy makers. Reductions in carbon dioxide (CO2) emissions, in particular, would require significant lifestyle adjustments, which are difficult to achieve. An attractive addition to emissions reductions and other sequestration methods is the utilisation of CO2 through biological means. The use of microalgae (including cyanobacteria) to photosynthetically fix CO2 is a potentially favourable method, given their high productivities. In addition, microalgal systems may be more environmentally benign than other alternatives (geological storage and mineral carbonation) that are energy intensive (Binaghi et al. 2003; Maeda et al. 1995). Traditionally, CO2 is fixed from the atmosphere or directly injected into the algal solution as a gas. However, CO2 uptake efficiencies are low, particularly due to limitations in mass transfer and photosynthetic efficiency (Stewart and Hessami 2005; Ugwu et al. 2008; Langley et al. 2012).
Carbon dioxide emissions from industrial sources are effectively scrubbed using monoethanolamine (MEA) (Wong and Bioletti 2002). Alternatively, an alkaline scrubber can be used, where the dissolved CO2 reacts with the base to form bicarbonate ions. This solution can then be used as a dissolved carbon source for alkaline-tolerant microalgae (Stewart and Hessami 2005).
The speciation of dissolved inorganic carbon (Ci) is pH dependent. Above pH 4.5, the proportion occurring as CO2(aq) decreases and bicarbonate (HCO3 −) increases, to the bicarbonate equivalence point at pH 8.3, above which the equilibrium begins to shift towards carbonate (CO3 2−). In neutral and alkaline systems, the dissolved CO2 concentrations are typically low (<10 μM) (Moroney and Somanchi 1999). The K m (CO2) value of RuBisCO, the enzyme responsible for carbon fixation, is significantly higher than this, having been reported as being as high as 200 μM in some cyanobacteria (Moroney and Somanchi 1999). Therefore, the provision of a sufficiently high CO2 concentration around the RuBisCO enzyme is a challenge to these organisms and has been found to represent the rate-limiting step in the conversion of inorganic carbon to biomass (Moroney and Tolbert 1985). Thus, in order to provide the required Ci concentration, most microalgal species have developed a carbon concentrating mechanism (CCM), enabling them to thrive in CO2-deficient environments (Colman et al. 2002; Funke et al. 1997).
The CCM is composed of three transport mechanisms, which each move Ci into the cell. They are the following: the active uptake of bicarbonate (HCO3 −), a diffusion driven CO2 transport mechanism, and an external carbonic anhydrase (CA) typically found in the periplasmic space (Giordano et al. 2005; Badger and Price 2003; Colman et al. 2002). Carbonic anhydrase is a metalloenzyme that catalyses the reversible decomposition of HCO3 − to CO2 and OH− (Moroney et al. 2001). The metal ion is typically zinc, but both cobalt (Yee and Morel 1996) and cadmium (Park et al. 2007) have been shown to substitute for zinc, where the environment is zinc deficient, although the activity is typically lower than the native zinc form. Transport of CO2 is the preferred mechanism of Ci transport in many green algal species growing under pH-neutral conditions (Moroney and Somanchi 1999). Under alkaline conditions, the external CA maintains the CO2 supply to the Ci transporters by decomposing HCO3 − in the media surrounding the cell (Williams and Colman 1995). This ensures that all Ci transport mechanisms are saturated and the largest possible flux of Ci is achieved.
In situations where microalgae are grown on bicarbonate ions, a decrease in acidity and a subsequent increase in pH occurs, typically forcing the Ci speciation to carbonate. This phenomenon has been employed for the treatment of hard and metal-contaminated waters (van Hille et al. 1999). This has a particular application in the final polishing stage of waste water treatment, particularly for the removal of calcium, magnesium and manganese, which precipitate more favourably as carbonates (Costa and Leite 1990; Hoffmann 1998; van Hille 2001; Inthorn et al. 2002).
The amount of CA expressed is dependent on the growth conditions, species and strain, allowing some species to make better use of bicarbonate than others (Matsuda and Colman 1995). As a result of this divergence, there will be specific strains of algae and cyanobacteria that are better suited to applications requiring growth on bicarbonate-rich media, such as those supplemented with CO2 scrubbed from industrial sources. To identify these strains, there is a need for a whole cell assay of external CA activity and bicarbonate utilisation potential.
The current CA activity assay was developed by Wilbur and Anderson (1948) and uses an electrometric technique to determine the activity of CA by the change in the pH of a buffer solution, typically veronal buffer. While some authors have used the Wilbur and Anderson assay on whole cells (Moroney et al. 1985; Williams and Colman 1995), the method has been reported to have several flaws that negatively affect its application as a screening tool (van Hille 2001). The enzyme has a very large turnover number which, coupled with the short reaction time, makes the measurement of activity by typical experimental methods difficult. Hence, the assay typically produces results with low confidence levels, which are then inconclusive (Husic et al. 1989; van Hille 2001; Williams and Colman 1993). In addition, the method requires the extraction of the CA from the cells, which may be difficult and can result in loss of enzyme activity. Furthermore, the assay does not differentiate between internal and external CA, which perform different functions in the cell (Funke et al. 1997; Colman et al. 2002). Other assay methods, such as mass spectroscopy, exist although most require enzyme isolation (van Hille 2001). This study, therefore, aimed to develop and test a new whole cell assay based on solution chemistry for inferring CA activity as an indicator of the suitability of an algal strain for carbon fixation from alkaline media and water treatment applications.
The pH drift technique has been used for many years to assess the ability of aquatic plants and algae to utilise bicarbonate for photosynthesis and calculate the CO2 compensation point (Allen and Spence 1981; Maberly and Spence 1983). The method involves measuring the pH change, in a sealed unit, as a consequence of photosynthetic carbon accumulation. A defined amount of inorganic carbon is provided and the rate of pH change measured. The inorganic carbon accumulation rate could be calculated using the pH change data and aqueous chemistry equations. Maberly and Spence (1983) used the quotient of the final inorganic carbon concentration to alkalinity (CT/alk) to assess whether inorganic carbon was accumulated as dissolved CO2 or bicarbonate. The early studies did not assess different bicarbonate uptake mechanisms, but Spijkerman et al. (2005) used specific CA inhibitors to differentiate between active uptake of bicarbonate and CA-mediated accumulation. Their study focused on desmids and showed that bicarbonate uptake was primarily through active transport, with very little CA activity. The bicarbonate concentrations used in the study were low (maximum 2 mM) and the uptake rates were significantly lower, with a maximum of 130 μmol CT (mg Chl a)−1 h−1 for Staurastrum planctonicum Teiling at the highest bicarbonate concentration. Values for Spirulina platensis (Nordst.) Gomont obtained in the current study were approximately 30 times higher, necessitating the use of a different methodology. This work presents a modified pH drift assay that was developed to rapidly screen algal species for their ability to utilise bicarbonate.
Materials and methods
Algal cultures and media
The alkaliphilic cyanobacterium, Spirulina platensis (UTEX LB 2340), was cultivated in stirred flasks on Zarrouk medium (Zarrouk 1966). Dunaliella salina (Dunal) Teodoresco (WCSA, Upington) was grown in natural seawater collected from Seapoint, Cape Town, which had been supplemented with trace metals and vitamins as specified in f/2 medium (Anderson 2005) as well as (gram per litre): NaNO3 (0.25), NaH2PO4 (0.0004), FeC6H5O7 (0.0029) and Na2EDTA·2H2O (0.0087). Chlorella vulgaris Beij. (UTEX 395) and Scenedesmus sp. (Cape Carotene, Upington) were grown on Bold 1NV medium (UTEX) without the biotin vitamin addition. The C. vulgaris, Scenedesmus sp. and D. salina flask cultures were bubbled with air in order to supply the algae with CO2 as well as to keep the algae in suspension. All cultures were grown under constant illumination. Dry cell mass (data not shown) was related to optical density (OD) at 680 nm for each species as a measure of cell concentration. A parallel study in our laboratory (Griffiths et al. 2011) confirmed that measuring OD at 750 nm yielded a more accurate correlation to dry mass, with an average error of 6 % (750 nm) compared to 10 % (680 nm).
Wilbur and Anderson assay
Carbonic anhydrase activity was measured using a modified version of the electrometric technique developed by Wilbur and Anderson (1948). The enzyme was suspended in 3 mL ice-cold 20 mM veronal buffer (pH 8.3) in a vessel that contained a pH electrode and temperature probe. The time taken for the pH to drop from 8.3 to 7.3 on the addition of 1.5 mL of ice-cold CO2-saturated water was then measured. The activity was calculated in Wilbur-Anderson Units (WAU) using the following formula:
where t 0 and t refer to the time taken in the absence and the presence of the enzyme, respectively. Assays on bovine CA (Sigma C3934), with a known activity of 3,160 WAU mg-1, were completed to determine the accuracy of the method. A mass of 0.316 μg (1 WAU) bovine CA was added to each of these assays. A minimum of four repeats were performed for each experiment, except for the determination of t 0 where 10 repeats were conducted due to the large degree of variance in the assay.
Whole cell CA assay development
The whole cell assay was conducted in a sealed, stirred 25-mL glass reactor (diameter of 36 mm, height 70 mm) that had been fitted with a pH electrode. Assays were performed under a nitrogen atmosphere to prevent the absorption of atmospheric CO2. Thermodynamic modelling using Visual MINTEQ (version 2.53, developed by Gustafsson, 2007) indicated that the activity of dissolved CO2 in a 10 mM NaHCO3 solution was 1.18 × 10−4 M (1.18 % of Ci). The solubility of CO2 in pure water at 25 °C is 3.40 × 10−2 M and, while this is reduced in increasingly saline water (Al-Anezi et al. 2008), it remains more than an order of magnitude greater than the equilibrium concentration in the assay system, justifying the use of a nitrogen atmosphere in the headspace. The reactor was illuminated using a daylight bulb (approximately 100 μmol photons m−2 s−1) at 24–26 °C to ensure that the algal suspension was not light limited and to reduce temperature effects on enzyme activity. An appropriate volume of 1 M NaHCO3 was added to bicarbonate and nitrate-free Zarrouk media, which had been bubbled with nitrogen to remove any dissolved CO2, to a final volume of 20 mL. The pH of the solution was corrected to pH 8.3 using 0.1 N H2SO4 in order to ensure that bicarbonate was the dominant Ci species. S. platensis was filtered through a cheese cloth and washed with NaCl (1 g L−1 solution) to remove any residual alkalinity. To start the run, the appropriate amount of biomass was added to the reaction solution to make up a suspension of the chosen cell density. The pH was recorded every minute for the first 30 min and every 3 min thereafter for the duration of the run (typically 1 h) to monitor the pH trend. The doubling time of S. platensis was found to be approximately 2 days, so no significant growth would be observed over the assay time.
The cell density and bicarbonate concentrations were varied between 0.26–0.98 g L−1 and 1–35 mM, respectively, in order to find a suitable combination where there was a significant change in pH, without extensive buffering and acceptable light limitation due to mutual shading or substrate (bicarbonate) limitation. At the end of each run, the OD of the suspension was measured and the algal concentration determined using an empirical correlation between OD and dry mass. Multiple assays at the chosen conditions were performed to confirm reproducibility of the assay. Control experiments were performed where no algae was added to the reaction solution.
Chemical speciation of reaction solution
Whole cell assays were allowed to run for 0, 10, 20, 30, 40, 50, 60 and 90 min. At the end of each assay, the suspension was vacuum filtered using 0.45-μm Whatman membrane filter to halt the reaction. A measured volume (10–15 mL) of the filtrate was titrated against 0.01 N H2SO4 down to pH 8.3 and then pH 4.5 in order to quantify the alkalinity of the solution. From this, the carbonate and bicarbonate concentrations at the end of the run were determined according to the method presented by Clesceri et al. (1989). Titrations were also conducted with bicarbonate-free Zarrouk medium under a nitrogen atmosphere in order to decouple the alkalinity due to the presence of carbonate and bicarbonate ions from the other species, particularly phosphate, in the Zarrouk solution. This contribution to the alkalinity was then excluded from the assay titrations. The carbon uptake was calculated as the difference between the initial number of moles of bicarbonate and the final number of moles of carbonate and bicarbonate in the solution. The results of these calculations were used to develop an empirical model.
Thermodynamic modelling and validation of assay results
The changes in pH and dissolved Ci speciation resulting from the carbon uptake were modelled using a thermodynamic package (Visual MINTEQ, version 2.53). The experimental setup was simulated by varying the bicarbonate, carbonate and hydroxide concentrations of the defined media, under a nitrogen atmosphere, as measured during the experimental runs. The resulting solution pH was predicted using Davies correlations. This was used to validate the experimental data.
Final whole cell CA assay
Following the analysis of the results of the whole cell assay development, standard conditions of 10 mM NaHCO3 and 0.45 g dry cell mass L−1 were chosen. The assays were conducted for 1 h with the pH recorded every minute for the first 30 min and every 5 min for the remainder of the time, although only the final pH value is required.
Inhibitor studies
Carbonic anhydrase activity was inhibited using acetazolamide (AZA). The AZA was dissolved in 1 M NaOH to a final concentration of 0.135 M. For the Wilbur and Anderson assay, the appropriate volume of the AZA stock solution was added before the CO2-saturated water so that the AZA concentrations in the reaction volume were 1, 10 and 100 μM for the experiments using bovine CA. For the Wilbur and Anderson assays using whole cells, the chosen AZA concentrations were 100, 500, 1,000 and 2,000 μM. In the case of the new whole cell assay, the AZA stock solution was added before the algae. For the S. platensis experiments, AZA concentrations of 200, 315, 500, 1,000 and 2,000 μM were tested, while lower concentrations (1, 10 and 100 μM) were used for C. vulgaris. The lower concentrations for C. vulgaris were selected based on preliminary assays that showed significantly lower external CA activity.
Salt-induced dissociation of CA
Carbonic anhydrase was dissociated from intact algal cells using a modified version of the procedure described by Husic (1991). A volume of 5 mL of algal slurry was used (which corresponded to approximately 0.2–0.5 g dry biomass). For S. platensis, the biomass was concentrated by filtering through cheese cloth, while for the non-filamentous C. vulgaris, centrifugation was required. The biomass was washed twice with 200 mL of distilled water and centrifuged at 2,500 ×g for 5 min. This was repeated once with a 20 mM phosphate buffer solution at pH 7.4. The algae were then suspended in 25 mL of 0.5 M KCl and 20 mM phosphate buffer (pH 7.4) solution and left at 4 °C for 10 min. The suspension was centrifuged again at 2,500 ×g for 5 min after which the supernatant was retained and kept at 4 °C, while the pellet was resuspended in another 25 mL of 0.5 M KCL and 20 mM phosphate buffer and left for 10 min at 4 °C. This procedure was repeated for a third time and the supernatants pooled for protein analysis. The recovered biomass was retained for use in the whole cell CA assay.
Protein concentration
The protein washed off with salt-induced dissociation was concentrated by ammonium sulphate precipitation. The protein suspension was saturated with ammonium sulphate at 4 °C (107 g ammonium sulphate/150 mL suspension). The solution was centrifuged at 17,696 ×g for 20 min and the supernatant was removed. The protein pellet was resuspended in TE buffer (10 mM tris–HCl, 1 mM EDTA, pH 7.5) and dialysed against a total of 10 L of TE buffer at 4 °C over 20 h. The protein solution was mixed with acetone (pre-chilled to −20 °C) and 100 % v/v trichloroacetone–water solution in the ratio of 1:8:1 and maintained at −20 °C for 1 h. The precipitate was recovered by centrifugation (12,500 ×g for 15 min). The pellet was washed with 1 mL cold acetone and re-centrifuged at 12,500 ×g for 15 min. The final pellet was dissolved in rehydration buffer (tris–HCl pH 8.0). This protein solution was loaded onto a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel to confirm the molecular weight of the proteins.
Results
Wilbur and Anderson assay
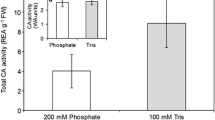
The Wilbur and Anderson assay was performed using a solution of bovine CA of known concentration in order to evaluate the method statistically. The solution was prepared to have an activity of 1 WAU. The experiments gave a result of 1.2 ± 0.51 WAU (Fig. 1), so the expected activity fell within the observed activity range.
The assay was then performed using the same bovine CA solution with added AZA. The activity decreased with increasing AZA concentration. From these results, the concentration of inhibitor required to reduce the activity by 50 % (I50) was calculated to be 2.85 μM AZA. The relative error associated with t 0 (121 s) was calculated to be 12.4 %. This error compounded with the error associated with t from each run that resulted in the large errors in activity shown in Fig. 1.
Wilbur and Anderson assays were conducted on three masses of S. platensis, namely 1.35, 3.08 and 1.51 mg dry mass (Fig. 2). The average activity determined using 1.51 mg was higher than that of the 3.08 mg run, indicating that the activities observed did not correspond to the mass of algae and, thus, of the mass of CA present. Furthermore, the large errors associated with these experiments render the results inconclusive with respect to CA activity. The inhibited runs exhibited similarly inconclusive results, with the assayed CA activity not decreasing with increasing AZA concentrations.
Development of whole cell assay
As a first step in the assay development, standard conditions for NaHCO3 and algae concentrations needed to be determined. An algal concentration of 0.45 g dry mass L−1 was used to limit the time cells were not exposed to sufficient light. This was based on analyses of light penetration and mixing time (Langley 2010). Figure 3 shows trends observed at different initial NaHCO3 concentrations. The 1 mM NaHCO3 run was seen to reach a maximum pH, after which it remained constant indicating bicarbonate limitation. At the NaHCO3 concentration of 35 mM, pronounced buffering was observed that caused the pH to remain constant for the first 45 min. For the 10, 20 and 30 mM experiments, the rate of pH change decreased with increasing bicarbonate concentration. A compromise between these two phenomena was achieved at 10 mM initial NaHCO3, where the pH changes were measurable and significant without substrate limitation being observed.
Repeat runs were performed at the standard conditions over a number of time periods in order to assess the reproducibility of the assay. Figure 4 shows the results of these repeats by plotting the specific change in pH versus the specific carbon removed from the system by the microalgae and a significant relationship is evident (R 2 = 0.91). The data were used to develop an empirical model to describe the relationship between the change in pH and specific carbon uptake (per gram of biomass). The relationship is described by the following:
where dpH represents the measured increase in pH (final − initial) divided by the absolute mass of algae added (dry mass).
The solution pH increased from 8.30 to 9.31 in the longest run (90 min), when 7.27 mmol C g−1 dry mass was removed from the system. As the container was sealed, it can be inferred that this carbon was taken up by the microalgae, either by active bicarbonate transport or the action of its CA. The solution pH, which increased from pH 8.30 to 9.31 on the longest run, was modelled by inserting species concentrations into Visual MINTEQ. The predicted pH values did not differ significantly from the measured values, although the experimental pH was slightly higher than the thermodynamically predicted one toward the end of the assay.
Whole cell assays with inhibition and salt induced dissociation of external CA
Figure 5 shows the specific carbon uptake achieved at the different AZA concentrations. Increasing the amount of AZA added to the whole cell assay runs with S. platensis was found to decrease the rate of pH change. The data suggest complete inhibition of external CA occurred at an AZA concentration of between 1,000 and 2,000 μM, as the carbon uptake was only marginally lower at 2,000 μM. The uninhibited carbon uptake of 5.76 mmol C (g dry mass)−1 h−1, calculated using Eq. 1, dropped to 2.94 mmol C (g dry mass)−1 h−1 at complete inhibition, implying that 2.82 mmol C (g algae)−1 h−1 of the carbon uptake was a result of external CA activity. The 10 and 100 μM AZA inhibited runs done on C. vulgaris resulted in a decrease in carbon uptake, from 3.86 to 2.79 mmol C (g algae)−1 h−1, indicating a CA-dependent uptake of 1.07 mmol C (g algae)−1 h−1. The carbon assimilation not related to CA activity (active uptake or uncatalysed dissociation of HCO3 −) was similar in both C. vulgaris and S. platensis, at 2.79 and 2.57 mmol C (g dry mass)−1 h−1, respectively.
When S. platensis and C. vulgaris were washed using a buffer with a high KCl concentration (0.7 and 0.5 M, respectively), the washed cells assimilated carbon at a similar rate to cells for which external CA had been completely inhibited. Furthermore, when the protein from the wash solution was concentrated and run on an SDS-PAGE gel, a band at 35 kDa was observed, which is in the region of the weight of CA (31–45 kDa) (Yang et al. 1985) and corresponds to the 37 kDa molecular mass identified by Goyal et al. (1992). This indicated the wash off of the external CA. When the Wilbur and Anderson assay was performed on the protein extract, no activity was detected, possibly due to enzyme inactivation during purification.
Tests on different algal species
The whole cell assays performed on the different species yielded varying carbon uptake rates as illustrated in Fig. 6. Scenedesmus sp. and S. platensis accumulated Ci at a rate of 5.86 mmol C (g dry mass)−1 h−1 and 5.76 mmol C (g dry mass)−1 h−1, respectively, making them the most active bicarbonate consumers of the four species. C. vulgaris took up 3.86 mmol C (g dry mass)−1 h−1, while D. salina had a carbon uptake of 2.14 mmol C (g dry mass)−1 h−1.
Discussion
The Wilbur and Anderson (1948) assay is still accepted to estimate CA activity, although several studies reported problems with this approach (van Hille 2001; Williams and Colman 1993; Husic et al. 1989). Data obtained in this study was consistent with the studies cited above. Due to the high turnover number of CA, the Wilbur and Anderson assay time was short, generally less than 120 s; thus, any variation in the measured values gave a large relative error. The temperature dependence of the reaction, as well as errors in the pH measurements due to the response time of the pH electrode, could also have added to the error. These factors, combined with the fact that there was compound error in calculating WAU's, contributed to the lack of accuracy.
The Wilbur and Anderson assays performed on whole cells did not demonstrate a relationship between biomass concentration and activity, so did not give an accurate measure of the amount of CA present. Furthermore, Wilbur and Anderson activity was detected for S. platensis in the presence of 2,000 μM AZA, although subsequent inhibitor studies suggested that this concentration of AZA completely inhibits the external CA. This further suggests that the Wilbur and Anderson method is not appropriate for whole cell systems. The accumulation of carbon by whole cells renders the equilibrium-based Wilbur and Anderson assay inappropriate and confirms the need for a new assay. If the Wilbur and Anderson assay was to be used to compare species, the external CA would first need to be removed and isolated from each species, but quantitative extraction and purification are difficult and inaccurate.
Important factors in the development of the new assay were its applicability as a screening tool and the reproducibility of the method. A traditional Michaelis–Menten approach to determining external CA activity in whole cell systems was not appropriate due to the interaction of the carbon uptake by the cells and the subsequent buffering reaction of the substrate (HCO3 −) with the product (OH−) (Fogler 2006). The CA activity model was, therefore, developed empirically, in the form of a linear relationship. The pH values generated by Visual MINTEQ simulations, based on measured speciation data, confirmed that the experimentally determined carbon uptake and speciation shifts caused the observed changes in pH. Therefore, pH changes can be used to infer changes in Ci speciation from a specific initial condition with confidence. Furthermore, there was little variation in the pH changes recorded across repeat experiments at standard conditions, which indicated that the assay had good reproducibility and was suitable for continued development.
The inhibition curve (Fig. 5) follows the same backward ‘s’-shaped trend as has been observed in other studies, such as that by Moroney et al. (1985), where other CA assays have been used. The residual pH change measured and carbon uptake inferred on complete inhibition of the CA can be attributed to active transport of bicarbonate and the uncatalysed decomposition of the bicarbonate and subsequent CO2 uptake by the cells. The latter was confirmed in experiments, where the solution was actively sparged with nitrogen, in the absence of CA, and a significant increase in pH was observed as CO2 was stripped from the solution.
The inhibitor and salt wash studies confirmed that a substantial fraction of the carbon accumulation is dependent on external CA activity. The residual carbon uptake, in the absence of CA activity, is accounted for by uncatalysed decomposition and active uptake of bicarbonate. This shows that the assay gives a meaningful indication of total Ci uptake in whole cell systems and, when combined with inhibitor studies, can give a measure of external CA activity, thereby indicating the importance of external CA in the CCM. Experiments conducted where the system was sparged with nitrogen gas suggested that CA (bovine and whole cell) activity was higher than that determined using this assay, implying that photosynthetic carbon fixation is the limiting factor. However, the assay described here has been designed to assess Ci accumulation and effective CA activity in open pond systems, which are not actively aerated and productivity is typically limited by mass transfer of CO2. Therefore, this assay is applicable as a screening tool for species operating under these conditions, which are typical of most large-scale operations. Closed photobioreactors may be designed to optimise gas–liquid mass transfer, negating the requirement for a well-developed CCM.
Williams and Turpin (1987) showed that a 50 μM AZA solution completely inhibited external CA activity in Chlamydomonas reinhardtii. We found that this concentration was not sufficient for complete inhibition of external CA in S. platensis, with AZA concentrations between 1,000 and 2,000 μM being required. This suggests that the external CA expression in S. platensis is much higher than that in C. reinhardtii, which is expected as Spirulina is adapted to alkaline environments and may have evolved to express more external CA (Vonshak 1997). Conversely, only 100 μM AZA was required for complete inhibition of the external CA of C. vulgaris, indicating a lower expression of external CA in this neutraphilic species.
The whole cell assay was conducted on four algal species in order to evaluate the applicability of the assay as a screening tool to rank species on their ability to grow in bicarbonate-rich media (Fig. 6). S. platensis induced the fastest pH change initially, consistent with its status as a known alkaliphilic species.
Scenedesmus sp. is able to make use of either CO2 or bicarbonate as a Ci source, with various strains exhibiting different preferences for the two. They are, therefore, genetically adapted to tolerate alkaline systems and it was found by Cooper (1975) and Österlind (1950) that members of this taxon were able to tolerate pH values up to a maximum of pH 10.0–11.5. Our strain of Scenedesmus sp. showed the same high carbon uptake as S. platensis.
C. vulgaris grows well under a variety of conditions, although the normal growth medium is neutral to slightly acidic (Cooper 1975; Spoehr and Milner 1949). Moroney and Somanchi (1999) and Williams and Colman (1995) have found that while C. vulgaris will preferentially use CO2 as its Ci source, it is also able to make use of bicarbonate, although its growth rate has been found to drop off at higher pH where bicarbonate is the dominant Ci species (Mayo 1997; Österlind 1950). This implies that external CA expression by C. vulgaris is lower than the two alkaliphilic species and, hence, the lower carbon uptake was expected.
D. salina is a highly salt-tolerant species (Goyal et al. 1992; Weldy and Huesemann 2007) and has been observed to tolerate pH of 6–9 (Baas-Becking 1931). However, Goyal et al. (1992) found that D. salina has very low levels of external CA in comparison to other species, including Chlorella. In the whole cell assay, it demonstrated the lowest carbon uptake of the four species, in agreement with the literature. This was likely compounded by the growth conditions, as Dunaliella was grown on bicarbonate-free media (pH c. 7), bubbled with CO2, and so had not been adapted to high bicarbonate and pH conditions.
Therefore, the whole cell assay was able to successfully differentiate between cultures known to have varying bicarbonate utilisation abilities. The studies presented here indicate the success of the whole cell assay as a screening tool for the ability to utilise bicarbonate. In combination with inhibitor studies, the assay provides a measure of the effective activity of external CA in whole cells.
References
Al-Anezi K, Somerfield C, Mee D, Hilal N (2008) Factors affecting the solubility of carbon dioxide in seawater at the conditions encountered in MSF desalination plants. Desalination 222:548–571
Allen ED, Spence DHN (1981) The differential ability of aquatic plants to utilize the inorganic carbon supply in fresh waters. New Phytol 87:269–283
Anderson RA (ed) (2005) Algal culturing techniques. Elsevier, China
Baas-Becking LGM (1931) Salt effects on swarmers of Dunaliella viridis teod. J Gen Physiol 14:765–779
Badger MR, Price GD (2003) CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. J Exp Bot 54:609–622
Binaghi L, Del Borghi A, Lodi A, Converti A, Del Borghi M (2003) Batch and fed-batch uptake of carbon dioxide by Spirulina platensis. Process Biochem 38:1341–1346
Clesceri LS, Greenberg AE, Trussell RR (eds) (1989) Standard methods for the examination of water and wastewater, 17th edn. American Public Health Association, Baltimore, Chapters 2 and 4
Colman B, Huertas IE, Bhatti S, Dason JS (2002) The diversity of inorganic carbon acquisition mechanisms in eukaryotic microalgae. Funct Plant Biol 29:261–270
Cooper JP (ed) (1975) Photosynthesis and productivity in different environments. International Biological Programme 3. Cambridge University Press, Cambridge
Costa ACA, Leite SGF (1990) Cadmium and zinc biosorption by Chlorella homosphaera. Biotechnol Lett 12:941–944
Fogler S (2006) Elements of chemical reaction engineering, 4th edn. Pearson Education International, Westford
Funke RP, Kovar JL, Weeks DP (1997) Intracellular carbonic anhydrase is essential to photosynthesis in Chlamydomonas reinhardtii at atmospheric levels of CO2. Plant Physiol 114:237–244
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation and evolution. Annu Rev Plant Biol 56:99–131
Goyal A, Shiraiwa Y, Husic HD, Tolbert NE (1992) External and internal carbonic anhydrases in Dunaliella species. Mar Biol 113:349–355
Griffiths MJ, Garcin C, van Hille RP, Harrison STL (2011) Interference by pigment in the estimation of microalgal biomass concentration by optical density. J Microbiol Meth 85:119–123
Hoffmann JP (1998) Wastewater treatment with suspended and nonsuspended algae. J Phycol 34:757–763
Husic HD, Kitayama M, Togasaki RK, Moroney JV, Morris KL, Tolbert NE (1989) Identification of intracellular carbonic anhydrase in Chlamydomonas reinhardtii which is distinct from the periplasmic form of the enzyme. Plant Physiol 89:904–909
Husic HD (1991) Extracellular carbonic anhydrase of Chlamydomonas reinhardtii: localization, structural properties, and catalytic properties. Can J Bot 69:1079–1087
Inthorn D, Sidtitoon N, Silapanuntakul S, Incharoensakdi A (2002) Sorption of mercury, cadmium and lead by microalgae. Sci Asia 28:253–261
Langley NM (2010) An investigation of the supply of carbon dioxide to microalgal systems, Ph.D. Dissertation, University of Cape Town
Langley NM, Harrison STL, van Hille RP (2012) A critical evaluation of CO2 supplementation to algal systems by direct injection. Biochem Eng J 68:70–75
Maberly SC, Spence DHN (1983) Photosynthetic inorganic carbon use by freshwater plants. J Ecol 71:705–724
Maeda K, Owada M, Kimura N, Omata K, Karube I (1995) CO2 fixation from the flue gas on coal-fired thermal power plant by microalgae. Energy Cons Manag 36:717–720
Matsuda Y, Colman B (1995) Induction of CO2 and bicarbonate transport in the green alga Chlorella ellipsoidea. Plant Physiol 108:247–252
Mayo AW (1997) Effects of temperature and pH on the kinetic growth of unialga Chlorella vulgaris cultures containing bacteria. Water Environ Res 69:64–72
Moroney JV, Somanchi A (1999) How do algae concentrate CO2 to increase the efficiency of photosynthetic carbon fixation? Plant Physiol 119:9–16
Moroney JV, Husic HD, Tolbert NE (1985) Effect of carbonic anhydrase inhibitors on inorganic carbon accumulation by Chlamydomonas reinhardtii. Plant Physiol 79:177–183
Moroney JV, Tolbert NE (1985) Inorganic carbon uptake by Chlamydomonas reinhardtii. Plant Physiol 77:253–258
Moroney JV, Bartlett SG, Samuelsson G (2001) Carbonic anhydrases in plants and algae. Plant Cell Environ 24:141–153
Österlind S (1950) Inorganic carbon sources of green algae. I. Growth experiments with Scenedesmus quadricauda and Chlorella pyrenoidosa. Physiol Plant 3:353–360
Park H, Song D, Morel FM (2007) Diversity of the cadmium-containing carbonic anhydrase in marine diatoms and natural waters. Environ Microbiol 9:403–413
Spijkerman E, Maberly SC, Coesel PFM (2005) Carbon acquisition mechanisms by planktonic desmids and their link to ecological distribution. Can J Bot 83:850–858
Spoehr HA, Milner HW (1949) The chemical composition of Chlorella; effect of environmental conditions. Plant Physiol 24:120–149
Stewart C, Hessami M-A (2005) A study of carbon dioxide capture and sequestration—the sustainability of a photosynthetic bioreactor approach. Energy Cons Manag 46:403–420
Ugwu CU, Aoyagi H, Uchiyama H (2008) Photobioreactors for mass cultivation of algae. Bioresour Technol 99:4021–4028
van Hille RP (2001) Biological generation of reactive alkaline species and their application in a sustainable bioprocess for the remediation of acid metal contaminated wastewaters. Ph.D. Dissertation, Rhodes University, South Africa
van Hille RP, Boshoff GA, Rose PD, Duncan JR (1999) A continuous process for the biological treatment of heavy metal contaminated acid mine water. Resour Conserv Recycling 27:157–167
Vonshak A (1997) Spirulina platensis (Arthrospira): physiology, cell-biology, and biotechnology. CRC, Boca Raton
Weldy CS, Huesemann M (2007) Lipid production by Dunaliella salina in batch culture: effects of nitrogen limitation and light intensity. J Undergrad Res US Dept Energy 7:115–122
Wilbur KM, Anderson NG (1948) Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 176:147–154
Williams TG, Colman B (1993) Identification of distinct internal and external isozymes of carbonic anhydrase in Chlorella saccharophila. Plant Physiol 103:943–948
Williams TG, Colman B (1995) Quantification of the contribution of CO2, HCO3 −, and external carbonic anhydrase to photosynthesis at low dissolved inorganic carbon in Chlorella saccharophila. Plant Physiol 107:245–251
Williams TG, Turpin DH (1987) The role of external carbonic anhydrase in inorganic carbon acquisition by Chlamydomonas reinhardii at alkaline pH. Plant Physiol 83:92–96
Wong S, Bioletti R (2002) Carbon dioxide separation technologies. Carbon and Energy Management, Alberta Research Council. Edmonton, Alberta, Canada. http://www.aidis.org.br/span/ftp/CARBON%20DIOXIDE%20SEPARATION%20TECHNOLOGIES.pdf. Accessed 14 December 2012
Yang S, Tsuzuki M, Miyachi S (1985) Carbonic anhydrase of Chlamydomonas: purification and studies on its induction using antiserum against Chlamydomonas carbonic anhydrase. Plant Cell Physiol 26:25–34
Yee D, Morel FMM (1996) In vivo substitution of zinc by cobalt in carbonic anhydrase of a marine diatom. Limnol Oceanogr 41:573–577
Zarrouk C (1966) Contribution a l'etude d'une cyanobacterie: influence de divers facteurs physiques et chimiques sur la croissance et la photosynthese de Spirulina maxima (Setchell et Gardner) Geitler. Dissertation, University of Paris, France
Acknowledgments
We thank Nathan van Wyk for his assistance with molecular techniques, Nicholas Langley for his assistance in algal cultivation, and Melinda Griffiths for providing us with the Scenedesmus sp. and C. vulgaris cultures. The authors acknowledge the financial contributions from the UCT Research Council and the National Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Hille, R., Fagan, M., Bromfield, L. et al. A modified pH drift assay for inorganic carbon accumulation and external carbonic anhydrase activity in microalgae. J Appl Phycol 26, 377–385 (2014). https://doi.org/10.1007/s10811-013-0076-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0076-6