Abstract
Pressurized liquid extraction with water and ethanol was employed to obtain high antioxidant extracts from olive leaves (Olea europaea L.). The influence of solvent ratio (100–50% v/v water/ethanol), temperature (100–200 °C), and solvent flow rate (1–5 mL min−1) on the extraction process was investigated using a Box-Behnken experimental design. ANOVA was applied to determine the overall goodness of fit of the dependent variables (extraction yield, antioxidant capacity by DPPH, total flavonoids, total iridoid-glycoside, and total phenolic content) in second-order models. For the optimization, these models were used to find the optimal conditions using the Sequential Simplex optimization procedure combined with Derringer and Suich’s desirability function to maximize antioxidant capacity, total iridoid-glycoside recovery, and total phenolic content. The optimal region conditions for extraction were determined at a solvent ratio of 53–65% v/v water/ethanol, the extraction temperature of 100–120 °C, and a 3–4 mL min−1 solvent flow rate. Fifteen phenolic compounds were identified in the samples using HPLC–DAD-ESI/MSn, including two phenolic acids (hydroxytyrosol and hydroxytyrosol glucoside), four flavonols (apigenin-6,8-C-dihexoside, quercetin-3-O-rutinoside, luteolin-O-hexoside, and derivatives), and nine iridoid-glycosides (verbascoside, oleuropein, and derivatives). Oleuropein and derivates were identified as the major phenolic compounds in the extract obtained at optimal conditions as a percentage of 89% of the extract composition.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyphenols are important plant-derived secondary metabolites, presenting in their molecular structure a hydroxyl radical attached to an aromatic ring, which makes them easily oxidizable. Thereby, the phenolic compounds have natural and attractive antioxidant properties (Carabias-Martínez et al. 2005; Kumari et al. 2018). In addition to the antioxidant characteristic, polyphenols show other remarkable properties, such as radical-scavenging, antibacterial, antiviral, negative enzymatic modulation, and antimutagenic (Quideau et al. 2011). These properties make phenolic compounds a target of application in several areas, such as in functional food products (Bouaziz et al. 2010; Neto et al. 2022), also as natural antioxidants in various food formulations (Souilem et al. 2017; More et al. 2021), cosmetics production (Kishikawa et al. 2015) and the treatment and prevention of diseases influenced by oxidative stress (Bulotta et al. 2014; Boss et al. 2016; Mahmoudi et al. 2018; Omar et al. 2018).
Due to the recent increase in polyphenols demand, many works are focused on polyphenols extraction from plants, showing the importance of natural antioxidants for the scientific community and productive sectors (Vergara-Salinas et al. 2015; Aissa et al. 2017; Bodoira et al. 2017; Veličković et al. 2017; Gullón et al. 2018; Favareto et al. 2019; Jaski et al. 2019; Monroy et al. 2020). Moreover, the use of biowastes as initial substrates for the recovery of polyphenolic compounds may be an economical and viable solution (Avila-Sosa et al. 2010; Tsakona et al. 2012; Garmus et al. 2019; Sorita et al. 2020; More et al. 2021; Moreno-González and Ottens 2021).
In this context, the olive tree (Olea europaea L.) leaves are considered not only a bio residue (or biowaste) from olive oil production (Squillaci et al. 2021) but also a viable source for the extraction of phenolics (Lee and Lee 2010; Ranieri et al. 2018; Otero et al. 2021). In the olive oil production process, large amounts of waste are generated, including leaves and wood from the olive trees (Roselló-Soto et al. 2015), and for this reason olive leaves may be considered as a cheap and easily available natural source of phenolic compounds (Quirantes-Piné et al. 2012; Souilem et al. 2017).
The main differential from the phenolic compounds found in the olive oil biowastes, as well as in olive fruits (Brahmi et al. 2012), concerning other phenolic sources is the presence of a class of compounds named secoiridoids, an exclusive characteristic of the Oleaceae family (Talhaoui et al. 2015). This characteristic distinguishes the olive oil biowastes as a special source of bioactives since the extracts’ biological effects are strongly attributed to this class (Pereira et al. 2007; Lozano-Sánchez et al. 2011). Further to secoiridoids (most representative compounds: oleuropein, ligstroside, dimethyloleuropein, and oleoside), olive leaves phenolic content also presents flavonoids (typical representative compounds: apigenin, kaempferol, luteolin) and phenolic acids (mainly: tyrosol and hydroxytyrosol) (Rahmanian et al. 2015; Şahin and Bilgin 2017; Paulo and Santos 2020).
Obtaining polyphenols from natural sources for their application in the food and pharmaceutical industries involves an adequate extraction process and different extraction techniques are commonly used to achieve this goal (Pasrija and Anandharamakrishnan 2015). The most traditionally processes used are based on the solid–liquid extraction system, in which the matrix remains in contact with an organic solvent. Examples of traditional techniques are mixing, maceration, sonication, and Soxhlet extraction (Mustafa and Turner 2011; Sorita et al. 2020).
In the last two decades, a massive effort has been made by the scientific community to replace the traditional extraction techniques with several emerging techniques motivated by the need to reduce costs, replace the high consumption of organic solvents, speed up the extractive process and increase the selectivity of recovered compounds (Cvjetko Bubalo et al. 2018). More specifically, for the extraction of phenolic compounds from olive leaves, several methods have been proposed, including the use of advanced technologies, such as microwave, ultrasonic extraction, and pressurized liquid extraction (Taamalli et al. 2012; Xynos et al. 2014; Şahin and Bilgin 2017).
Pressurized Liquid Extraction (PLE) is characterized by using high temperature and pressure to facilitate solute extraction of a solid matrix (Abaza et al. 2015). In this technique, temperature and pressure conditions are always below the predicted critical point for the solvent (Mendiola et al. 2007; Herrero et al. 2013), as a result, the solvent remains in the liquid phase, even above the boiling point, due to pressure action.
In PLE, the combination of high temperature and pressure is responsible for increasing the solubility and mass transfer properties of the solid matrix analytes, which explains the high efficiency attributed to the process when compared to extraction techniques performed at temperatures and pressures close to atmospheric values (Mustafa and Turner 2011). Also, the high temperature and pressure are responsible for reducing the viscosity and surface tension of the solvent, which facilitates its penetration into the matrix and, at the same time, increases the solid–liquid contact surface (Herrero et al. 2013).
Temperature is pointed as a critically important variable in PLE as it reflects its efficiency and selectivity. In theory, the higher the temperature, the greater the results in terms of extraction yield (Plaza et al. 2013). The thermal energy of the solvent is also responsible for overcoming the molecular interactions of cohesion and adhesion since it decreases the activation energy required for the desorption of analytes. High solvent temperatures help the breakdown of analyte-matrix interactions, caused for example by Van der Waals forces or hydrogen bonds, facilitating solubilization (Aliakbarian et al. 2011). Also, the temperature causes a decrease in the solvent viscosity, providing greater penetration into the matrix and its surface tension, making the solvent more capable in terms of sample wettability (Mustafa and Turner 2011).
When PLE occurs under static conditions, equilibria between the matrix components are still bounded and that solubilized in the solvent might be achieved. If this happens, the efficiency of the extraction procedure will not increase, even with the increase in extraction time. Under the dynamic mode, the fresh solvent is continuously introduced into the extraction vessel. Consequently, the equilibria might be displaced and the efficiency of the extraction increases, providing higher extraction yields than static extractions (Herrero et al. 2013).
Water and ethanol are largely applied as extraction solvents for PLE since are considered GRAS substances (Generally Recognized As Safe) (Chemat et al. 2012; Talhaoui et al. 2014; Abaza et al. 2015; Castro-Puyana et al. 2017; Panja 2018; Jaski et al. 2019), as well as present stable physicochemical characteristics, low volatility, are safe and reusable (Cvjetko Bubalo et al. 2018). In the case of olive leaves as a natural source, hydro-alcoholic mixtures have been investigated due to their ability to extract both lipophilic and hydrophilic phenols (Tsakona et al. 2012). Solvent mixtures can increase extraction efficiency because analytes with different affinities may be extracted by interacting with two solvents simultaneously. Most typical polyphenols present relatively low solubility in water at ambient conditions (Song et al. 2018). This aspect changes significantly when water at a high temperature is used. In this condition, water is an able solvent for extracting phenolic compounds from a plant matrix (Mustafa and Turner 2011).
In addition to extraction solvent’s composition, the process parameters in PLE extraction that can interfere in compounds’ recovery are the temperature, time, solvent ratio, flow, as well as pressure. The influence of these process parameters on extraction selectivity is worth investigating in order to maximize PLE capacity of extracting compounds with different characteristics (Cvjetko Bubalo et al. 2018). Understanding how these extraction parameters affect the extraction from each natural source is essential for the phenolic compounds’ recovery. The effect of the extraction parameters on olive leaves extracts composition was already studied using a Plackett–Burman design followed by an optimization step using a central composite design (Xynos et al. 2014). The authors evaluated the effects of the following variables: ethanol content in the solvent mixture, the temperature of the extraction, static time, cycles of repeated extractions, the volume of solvent flush at the end of each extraction cycle, purge duration, pre-heat duration, and cell volume. Stamatopoulos et al. (2014) also investigated the polyphenol’s extraction from olive leaves by optimizing a multistage extraction scheme was performed in terms of particle size, pH, composition in the volume of aqueous ethanol solution, and solid-to-liquid ratio. Lama-Muñoz et al. (2019) have studied temperature, leaf moisture content, solvent/solid, and aqueous ethanol concentration, for the oleuropein and luteolin-7-O-glucoside recovery, optimized using the central composite and Box-Behnken experiment designs.
Although the optimization of bioactive compounds from olive leaves was studied, research in the area found only isolated optimal extraction points. In the scale up of extraction processes, working at a single point may turn the control of the process variables into a challenge, which may result in undesirable changes in the extract's composition. Also, most of the works studied extraction in static mode even with the recognition of the greater efficiency of extraction in dynamic mode. Thus, the search for an optimal extraction region using dynamic mode is a key point in the extraction of bioactive compounds from olive leaves not yet investigated. Therefore, the objective of the present study was to optimize the extraction of phenolic compounds from olive leaves using an environmentally friendly solvent mixture (water and ethanol) using a Box-Behnken experimental design and the desirability function approaches. The effect of three extraction variables temperature, flow rate, and ratio solvent, on the phenolic compounds recovery on the extraction yield, antioxidant capacity by DPPH (AOC), Total Iridoid-Glycoside (TIG), Total Flavonoids (TF), and Total Phenolic Content (TPC) was investigated. The multiobjective optimization took into account the antioxidant capacity and the polyphenol content recovery.
Materials and methods
Standards and reagents
Ethanol (Nuclear, ≥ 99.5% v/v, São Paulo, Brazil) and distilled water were used in the olive leaf extraction. For HPLC analysis were used HPLC grade acetonitrile (99.9% from Fisher Scientific, Lisbon, Portugal) and formic acid (Panreac Química S.L.U., Barcelona, Spain), the phenolic standards were from Extrasynthèse (Genay, France) and Milli-Q water (TGI Pure Water Systems, USA). DPPH (2,2-diphenyl-1-picrylhydrazyl, Sigma Aldrich, Germany) and DMSO (dimethyl sulfoxide, Dinâmica, Brazil) were used to the antioxidant capacity assay.
Extraction of phenolic compounds from the olive tree (Olea europeae L.) leaves
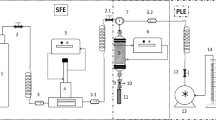
The raw material came from a commercial cultivation of olive trees located in Águas da Prata, Serra da Mantiqueira, in the state of São Paulo (22° 00′ 48.6ʺ S 46° 37′ 59.4ʺ W). The olive tree species used were the Arbequina, Koroneiki, and Arbosana varieties. Sample preparation and the extraction equipment were previously described in detail by Jaski et al. (2019). Briefly, the olive tree leaves were collected, dried in an oven (~ 36 °C, for 36 h, 400/4ND, Ethik Technology, Vargem Grande Paulista, SP, Brazil), then cut in a rotary knife cutter (SL-30, Solab, Piracicaba, SP, Brazil) and finally stored in sealed bags at ambient temperature. The extraction equipment consisted of a hollow cylindrical stainless-steel vessel (with approximate dimensions of 210 mm in length; 10 mm in internal diameter; 40 mm in external diameter and 33 mL in internal volume) surrounded by a metallic heat block with electrical resistances, according to the scheme described by (Jaski et al. 2019) and a syringe-type flow pump with a capacity of 500 mL (260D Syringe Pump, Tedelyne ISCO). The process was operated in dynamic mode for 15 min. In this regard 5 g of olive leaves were placed inside the extraction vessel (cotton wool was placed on the bottom and top of the leaves bed). The sample of olive leaves was added to the extractor vessel without any type of compression and filled the internal volume of the cylinder. The solvent was injected by the pump at a controlled flow rate (1, 3 and, 5 mL min−1 and residence time of 33, 11 and 6.6 min−1, respectively) and passed through a preheater (electrical resistance) and kept at 55 °C, before entering the extraction vessel. The upward flow of solvent, leaving the extraction vessel, entered a cooling system, which was maintained by a thermostatic bath at 20 °C. Before leaving the system, the solvent went through a “back pressure” type check valve, responsible for maintaining the pressurization of the process (100 kgf cm−2). After passing through the valve, the extract was then collected in a glass flask and oven-dried (NL-82, New Lab, Piracicaba, Brazil) at 60 °C until constant mass. Finally, the extract was stored in a freezer until the analyzes.
Phenolic compounds analysis
The phenolic profile was determined in the dried extracts, after being re-dissolved in a methanol:water (80:20, v/v) mixture, by HPLC-DAD-ESI/MSn (Dionex Ultimate 3000 UPLC, Thermo Scientific, San Jose, CA, USA), as previously described by Bessada et al. ( 2016). For the double online detection, were used 280 and 370 nm as preferred wavelengths for DAD and in a mass spectrometer (MS) connected to HPLC system via the DAD cell outlet. The MS detection was performed in negative mode, using a Linear Ion Trap LTQ XL mass spectrometer (ThermoFinnigan, San Jose, CA, USA) equipped with an ESI source. The identification of the phenolic compounds was performed using standard compounds, when available, by comparison their retention times, UV–Vis and mass spectra; and also, comparing the obtained information with available data reported in the literature giving a tentative identification. For quantitative analysis, a calibration curve for each available phenolic standard was constructed based on the UV signal (Hydroxityrosol (y = 124154x + 17,393, R2 = 0.9999, LOD = 0.22 µg/mL; LOQ = 0.68 µg/mL); apigenin-6-C-glucoside (y = 107,025x + 61,531, R2 = 0.9989, LOD = 0.19 µg/mL; LOQ = 0.63 µg/mL); oleuropein (y = 32226x + 12,416, R2 = 0.9999, LOD = 0.69 µg/mL and LOQ = 1.96 µg/mL); and quercetin-3-O-rutinoside (y = 13,343x + 76,751, R2 = 0.9998, LOD = 0.14 µg/mL; LOQ = 0.45 µg/mL). The quantification was performed through the calibration curve of the most similar available standard when a commercial standard for the identified phenolic compound was not available. The results were expressed as mg/g of extract.
Extraction yield
Extraction mass yield (%) in percentage was calculated by the relation between the dry extract mass and the initial mass of the milled olive leaf sample.
Antioxidant capacity by DPPH (%AOC)
The antioxidant capacity assay was based on the oxidation–reduction reaction of the DPPH·(2,2-diphenyl-1-picrylhydrazyl) radical, according to Brand-Williams, Cuvelier, and Berset (Brand-Williams et al. 1995), modified by others (Prior et al. 2005; Pires et al. 2017). For the test, 20 µL of each extract at an initial concentration of 0.3 mg/mL dissolved in DMSO 10% v/v. was added to 280 µL de DPPH (initial concentration 32 µg/mL). After remaining for 30 min in the dark, the color change was measured at 517 nm using a plate reader spectrophotometer (TP-Reader NM Thermo Plate). The antioxidant capacity by DPPH (AOC) was estimated by the difference between the absorbance of the control (20 µL DMSO 10% v. e 280 µl de DPPH 32 µg/mL) and the absorbance of the extracts, by the absorbance of the control. The results were expressed as %AOC.
Experimental design and optimization
A three-level, three-factor Box–Behnken design was implemented with the following independent variables, water/ethanol ratio (v/v, X1), temperature (°C, X2), and solvent flow rate (mL min−1, X3) considered in the extraction of phenolic compounds from olive leaves. Three levels were attributed to each variable at low (−), central (0), and high (+), with an additional two replicated center points to measure the experimental error (pure error). This design required 15 experiments (Table 3), which were performed in a random order to avoid any systematic error. Maximum and minimum levels were selected by preliminary screening tests, instrumental aspects, and according to the literature reports (Plaza and Turner 2015).
For the dependent variables (extraction yield, antioxidant capacity by DPPH, total flavonoids, total iridoid-glycoside, and total phenolic content) a second-degree polynomial model was used to data fitting (Eq. 1), where Y represents the predicted response for dependent variables; X1, X2, and X3 are the independent variables; b0 is the intercept, bi is the linear coefficient for each component; bii is the quadratic coefficient, and bij is the binary interaction coefficient.
The statistical significance of the coefficients in the regression equations was evaluated by analysis of variance (ANOVA) (p < 0.05) for each response. The coefficient of determination (R2), adjusted coefficient of determination (R2adj), the p-value of the model, and the lack of fit test were performed to verify the predictive capacity of the model. Response surface plots were used to represent the results for each dependent variable, based on the models adjusted. In the optimization stage, these surfaces were used to find the desirable optimal conditions (maximize the % yield, total iridoid-glycoside recovery, and total phenolic content) using the desirability function of Derringer and Suich (1980). This method is a simultaneous optimization accomplished by obtaining the individual desirability for each response and then obtaining the combined desirability, which is the weighted geometric mean of the individual responses’ desirability (Sperb et al. 2018), and finally, employing a sequential simplex algorithm. Thus, the global desirability (D) is normally calculated by the geometric mean of the m specific desirability (d) as Eq. (2).
The individual desirability function (di) for an answer whose optimal target value is the value Ai, so y, that is a maximum value, will be located within an acceptance range with LLi as the lower limit and ULi as the upper limit. So, the individual desirability is then calculated by the equations (3), (4) and (5):
The rate of change of the individual desirability with the response estimated by the model is defined by the values of the exponents s and t. By varying them, different desirability can be attributed to the different levels of the response (Montgomery 2013). Once the target values or intervals of interest are defined, the method consists of feeding an optimization algorithm with several different choices for the exponents s and t. In this work, the chosen range of interest was defined by the minimum and maximum values of each studied response. The Sequential Simplex was used for the optimization of the global desirability (D). This algorithm forms an n-dimensional figure with n + 1 vertices, where n is the number of independent variables in the optimized function. The vertices are heuristically substituted until a satisfactory point is found (Bona et al. 2000; Gao and Han 2012; Tormena et al. 2017). The statistical analysis and the multiobjective optimization were performed by MATLAB R2021b software.
Results and discussion
Phenolic compounds of the aqueous and hydroethanolic extracts of the olive tree (Olea europeae L.) leaves
The characteristic peaks (retention time, λmax in the visible region, mass spectral data) and tentative identification of the phenolic compounds present in the aqueous and hydroethanolic extracts of fifteen samples of olive tree (Olea europeae L.) leaves are present in Table 1 and a representative phenolic profile recorded at 280 nm is shown in Fig. 1. The quantification of the phenolic compounds is shown in Table 2. Fifteen phenolic compounds were identified in the samples, two phenolic acids (hydroxytyrosol and hydroxytyrosol glucoside), four flavonols (apigenin-6,8-C-dihexoside, quercetin-3-O-rutinoside, luteolin-O-hexoside, and derivatives), and nine iridoid-glycosides (verbascoside, oleuropein, and derivatives). Numerous authors have already made an exhaustive description of the phenolic compounds present in olive fruits (Ryan et al. 1999; Vinha et al. 2005; Savarese et al. 2007; Jerman et al. 2010), and also in its leaves (Di Donna et al. 2007; Mylonaki et al. 2008; Quirantes-Piné et al. 2012) as in the present work, and even in olive mill wastewaters (D’Antuono et al. 2014). Nevertheless, all of the above-mentioned references were used as the basis for the tentative identification of the phenolic compounds described in Table 1.
For the two phenolic acids tentatively identification as hydroxytyrosol glucoside (peak 1, [M-H]− at m/z 315) and hydroxytyrosol (peak 2, [M-H]− at m/z 153), the chromatographic responses were compared by the previously described by Jerman et al. (2010) and Di Donna et al. (2007) in olive fruits. The elution order of both peaks is not consistent with the described by Di Donna et al. (2007), however, this may be explained by the different solvents used (water + formic acid 0.25% and methanol + formic acid 0.25%) and gradient solvent.
Regarding the flavonols group, peaks 3 ([M-H]− at m/z 593) and 7 ([M-H]− at m/z 609), apigenin-6,8-C-dihexoside and quercetin-3-O-rutinoside, respectively, were positively identified by comparing their retention time and UV spectra with the available standard compound. Peaks 8 and 9 were identified as luteolin derivatives. Peak 8 ([M-H]− at m/z 593) presented a unique MS2 fragment at m/z 285 that corresponded to the loss of 146 u + 162 u (deoxyhexosyl and hexosyl units, respectively), is tentatively identified as luteolin-O-deoxyhexoside-hexoside. Peak 9 ([M-H]− at m/z 447) also revealed a unique MS2 fragment at m/z 285 indicating the presence of a luteolin aglycone (characteristic UV spectra) and corresponding to the loss of a hexosyl moiety (162 u), being for that manner tentatively identified as luteolin-O-hexoside. This peak was previously identified in olive fruits (Savarese et al. 2007; Jerman et al. 2010), thus it was tentatively identified as luteolin-7-O-glucoside.
Finally, for the iridoid-glycosides group peaks, 6 and 11 presented the same pseudomolecular ion [M-H]− at m/z 623, and characteristics MS2 fragment at m/z 461 and 315, that lead to the tentative identification as verbascoside, as previously described in olive (Ryan et al. 1999; Savarese et al. 2007; Jerman et al. 2010). Peak 11 was assigned as verbascoside by comparing the retention time of the peak with the described by Živković et al. (2017) in Veronica teucrium L. and Veronica jacquinii Baumg., that performed the identification of these compounds for the first time in the same chromatographic conditions as in the present work. For that manner peak 6 was assigned as verbascoside isomer I. The tentative identification of peak 4 as β-hydroxyverbascoside diastereoisome ([M-H]− at m/z 639) followed the described by D’Antuono et al. (2014) in olive mill wastewaters in which the characteristics MS2 fragment at m/z 621, 529, 459, and 179, allowed the confirmation of the presence of the diastereoisomeric structures in the verbascoside compound.
The main compounds present in olive are oleuropein and derivatives. Oleuropein was tentatively identified in the samples (peak 13) and its corresponding isomer (peak 14), presenting a pseudomolecular ion [M-H]− at m/z 539, followed by characteristic MS2 fragments at m/z 377, 307, 197, and 153, as previously described by other authors (Ryan et al. 1999; Vinha et al. 2005; Savarese et al. 2007; Di Donna et al. 2007; Mylonaki et al. 2008; Jerman et al. 2010; Quirantes-Piné et al. 2012; D’Antuono et al. 2014) in olive extracts. Peaks 10 and 12 presented a pseudomolecular ion [M-H]− at m/z 701, followed by MS2 fragments corresponding to oleuropein structure (m/z at 539), which corresponds to the loss of an hexosyl moiety, being for that manner tentatively identified as oleuropein hexoside isomer I and II, respectively (Quirantes-Piné et al. 2012; D’Antuono et al. 2014). Peak 5 showed a main fragment at m/z 525 and MS2 fragments consistent with the description suggested, by other authors (Jerman et al. 2010), for demethyloleuropein. Lucidumoside C was also tentatively identified in the present samples (peak 15, [M-H]− at m/z 583), comparing the chromatographic responses obtained with the ones described by D’Antuono et al. (2014) in olive oil wastewaters.
Model fitting
Experimental results of extraction yield, antioxidant activity capacity by DPPH, total iridoid-glycoside, total flavonoids, and total phenolic content obtained under different conditions of extraction are presented in Table 3. The regression coefficients for each model and the statistical parameters determined by ANOVA are shown in Table 4. As can be seen (Table 4), the non-significant effects (p > 0.10) were eliminated or kept to improve the fit of the model. The regression models were statistically significant (p < 0.05) with satisfactory coefficients of determination (R2), ranging between 0.8450 and 0.9762 (Alexandre et al. 2017). The adjusted coefficient of determination (R2adj) was acceptable for all responses (0.7109—0.9445), indicating a good adjustment between the experimental and predicted values. No lack of fit was detected (p > 0.05). For the total phenolic acids, it was not possible to obtain a statistically significant polynomial model to describe the observed data.
Extraction yield
The extraction yield values ranged between 9.34 and 57.90% (run 6 and 3, respectively, Table 3). The results in Table 4 showed that the linear terms of water/ethanol ratio (b1), extraction temperature (b2) and flow rate (b3), and the quadratic terms of temperature (b22) and flow rate (b33) were statistically significant (p ≤ 0.05). The interactions between temperature and flow rate (b23) were significant (p ≤ 0.05). The regression model was highly significant with an adjusted coefficient of determination of 0.9445.
The regression coefficients of the adjusted model (Table 4) allowed the determination of the influence of each term independent variable on the responses. It is worth noting that a higher absolute value of the coefficient implies a greater effect of the independent variable, and the sign indicates if this contribution increase (+) or decrease (−) the response. The extraction temperature was the term that most affected yield, followed by the flow rate and water/ethanol ratio. The water/ethanol ratio had a negative effect on the response (− 6.3446), meaning that the higher the water percentage, the lower the yield, while the temperature and flow rate had a positive effect (10.6809 and 9.9791, respectively). Therefore, the increase in temperature and flow contribute to the extraction yield. These effects can be confirmed by the contour plot shown in Fig. 2. For the interactions, it was possible to note, by the positive coefficients in the equations, that the temperature acted in synergy with the flow rate.
According to Ahmadian-Kouchaksaraie et al. (2016), temperature is one of the factors that most influences efficiency during subcritical water extraction. In the present study, it was observed that increasing the temperature from 100 to 200 °C improved the extraction yield, and it was the most influential factor. Similar results in phenolic extraction were observed by Martín-García et al. (2020). According to the authors, the highest extraction yield was obtained at 198 °C and 100% of ethanol. Other studies have also reported higher yields in the extraction of compounds from olive leaves with increasing temperature (Herrero et al. 2011; Lama-Muñoz et al. 2019; Xynos et al. 2014). In the subcritical condition, higher temperatures reduce the dielectric constant of water and its polarity, thus increasing its ability to solvate less polar compounds (Cheng et al. 2021). Furthermore, high temperatures improve the solubility and diffusivity of compounds, thus increasing the mass transfer between the plant matrix and solvent (Herrero et al. 2011). As the flow rate increases, the amount of solvent injected per unit of time increases, allowing a faster separation of the analytes from the extraction matrix.
As it can be seen in Fig. 2, increasing the flow rate from 1 to 5 mL min−1 improved the yield, which may be related to enhanced mass transfer at a higher flow rate. The results also showed that the yield decreased as the percentage of water increased from 50 to 100%, which is in concordance with previous studies (Herrero et al. 2011; Martín-García et al. 2020; Xynos et al. 2014).
Antioxidant capacity by DPPH
The antioxidant capacity values determined by the DPPH method varied between 27.85 and 57.12% AOC (run 2 and 1, respectively, Table 3). In their work, Mkaouar et al. (2018) extracted the olive leaves with 95% ethanol at 55 °C for 3 h. The obtained extracts presented a 50% DPPH radical scavenging ability at relatively low concentrations (86.88 μg/mL). In the present work, the final concentration of the extract in the assay was equal to 20 μg/mL, and with this concentration, higher percentage inhibition of 50–57% were obtained for various conditions in the experimental design (runs 1, 5, 7, 9, 11 and 14, Table 3) compared to the result found by Mkaouar et al. (2018). This result must be associated with the ethanol concentration used in the extraction process (Tsakona et al. 2012).
The significant coefficients in the model equation were the linear coefficients of the water/ethanol ratio (p ≤ 0.05) and extraction temperature (p ≤ 0.10) and the quadratic term of the temperature (p ≤ 0.05). Although the linear and quadratic coefficient of the flow rate were not significant (p ≥ 0.05), they were kept improving the fit of the model. According to the coefficients in Table 4, the ethanol concentration (b1 = − 5.5638) and the extraction temperature (b2 = − 3.1638) showed a negative effect on the antioxidant capacity values. The interactions between water/ethanol ratio and temperature were significant (p ≤ 0.05) and showed synergism (b12 = 11.2709).
Figure 2 shows the contour plot from antioxidant capacity as a function of water/ethanol ratio and extraction temperature. As can be seen, when increasing the water ratio from 50 to 100%, there is a reduction in the extraction of antioxidant compounds. It is suggested that the extraction is favored at higher concentrations of ethanol. Lama-Muñoz et al. (2019) concluded that the concentration of ethanol can affect compounds solubility, by observing the higher antioxidant capacity results obtained with an aqueous ethanol concentration of 80%, Xynos et al. (2014) also found the same relation between ethanol concentration with improved antioxidant capacity, since samples obtained with 100% ethanol on the pressurized liquid extraction of olive leaves exhibited higher antioxidant activity. On the other hand, the reduction in the extraction of antioxidant compounds was observed with increasing the temperature from 100 to 200 °C, probably related to the degradation of thermosensitive antioxidant compounds at higher temperatures. In PLE with water as a solvent, an increase in temperature causes a change in the value of the dielectric constant of water (Carabias-Martínez et al. 2005; Brunner 2009; Herrero et al. 2011). With pressurized water at lower temperatures, the extraction of polar compounds is prioritized (Hassas-Roudsari et al. 2009). Thus, the extraction temperature influences the solvent properties, which shows the synergistic action of these two factors in the extraction of phenolic compounds from olive leaves.
Total iridoid-glycoside (TIG)
The maximum experimental value obtained for the TIG was equal to 91.00 mg g−1, reached in run 15 (25% v/v of ethanol concentration, 150 °C and flow rate of 3 mL min−1). The linear and quadratic coefficients (Table 4) of the water/ethanol ratio and the interaction between water/ethanol ratio and temperature (b12) were significant (p ≤ 0.05). The other coefficients were not significant (p ≥ 0.10) but were kept to improve the fit of the model, except for the coefficient of interaction b23 that was removed. The adjusted coefficient of determination of the regression was 0.8026 showing a good fit of the model to the experimental data.
A negative effect of water/ethanol ratio on the total iridoid-glycoside content was found (b1 = − 15.6463). This value implied that an increase in water concentration caused a decrease in the experimental value of TIG. The contour plot from TIG can confirm this effect as a function of water/ethanol ratio and extraction temperature (Fig. 2). The positive interaction coefficient between the water/ethanol ratio and temperature (b12 = 16.7500) indicated a synergistic effect between these variables.
According to Fig. 2, an increase in the ethanol ratio improved the extracted TIG content. This can be attributed to the increased solubility of the compounds in the solvent (Lama-Muñoz et al. 2019). In the same way, as for the AOC response, the joint action of temperature and solvent composition may be capable of extracting a greater amount of phenolic compounds in olive leaves. Herrero et al. (2011) reported greater recovery of oleuropein (the main component of the TIG class) in PLE with pure ethanol at 50 °C. As the temperature increased from 50 to 200 °C, the authors found that the amount of oleuropein in the extract dropped by 55%, which also illustrates the selectivity of this compound due to the action of temperature and concentration of the solvent used in the extraction.
Total flavonoids (TF)
The total flavonoids (TF) present in the extracts ranged from 0.50 to 17.60 mg g−1 (run 2 and 15, respectively, Table 3). The significant coefficients in the model were the linear coefficients of the water/ethanol ratio (p ≤ 0.05) and extraction temperature (p ≤ 0.10) and the quadratic coefficient of the water/ethanol ratio (p ≤ 0.05). Similar to the DPPH’s model, the extraction of TF was negatively correlated with an increase in water/ethanol ratio and the temperature, being the effect of the water/ethanol ratio more important. Furthermore, the interaction between the water/ethanol ratio and extraction temperature was significant (p ≤ 0.10), and the positive value determined (b12 = 3.2200) evidence that the water/ethanol ratio acted in synergy with the temperature.
As can be observed in Fig. 2, when increasing both, water ratio and temperature, there is a reduction in TF extraction. Similar results were reported by Martín-García et al. (2020), which described the highest flavonoid content obtained using 125 °C and 100% ethanol, and that higher temperatures resulted in a reduction of the flavonoid content due to the thermo-labile nature of these compounds. Also, Lama-Muñoz et al. (2019) describe the operational conditions for maximizing the recovery of flavonoids were determined to be 190 °C and aqueous ethanol concentration of 80%. The value of the b12 interaction coefficient for the flavonoid response is smaller than the same interaction coefficient for the AOC, TIG, and TPC responses, which shows that the action between temperature and efficiency is less effective for this class of solvents. This may explain why different works found different conditions for the extraction of this class.
Total phenolic compounds (TPC)
The highest experimental value obtained for the total phenolic compounds was 123.0 mg g−1, reached in run 15 (25% v/v of ethanol concentration, 150 °C and flow rate of 3 mL min−1). According to the coefficients of the model (Table 4), only the linear and quadratic coefficients of the water/ethanol ratio (b1 and b11) and the interaction between water/ethanol ratio and temperature (b12) were statistically significant (p ≤ 0.05). The adjusted coefficient of determination of the regression was 0.7763. Similar to the trend observed for total iridoid-glycoside, the model presents a negative influence of the water concentration on TPC was found. The positive coefficients for the interaction (b12 = 23.2800) indicated that the water/ethanol ratio has a synergic effect on the extraction temperature. As the TPC class represents the totality of the phenolic content of the leaves, the synergistic behavior of temperature and solvent concentration presented by TF and TIG is also reflected in TPC.
Figure 2 shows, a decrease in the experimental value of TPC with the increase in water concentration from 50 to 100%. As previously mentioned, the ethanol concentration may affect the solubility of the phenolic compounds, improving their extraction (Lama-Muñoz et al. 2019). The authors found that using ethanolic aqueous solutions with a ethanol concentration above 80% resulted in higher TPC. Also, these results agree with those reported by Martín-García et al. (2020) who determined the highest TPC from olive leaves grown in Spain at 105 °C and using 100% ethanol.
Optimization and validation of the model
To evaluate how the desirability function interferes in the responses, around 50 optimization situations were performed, by varying the desirability levels between 1 and 10. Here, only the results of the five optimizations that were considered acceptable for the study will be described. The objective was to find a region for the extraction process that resulted in an extract with high antioxidant capacity and a phenolic composition rich in flavonoids and iridoid-glycosides, the major phenolic components of olive leaves.
The first situation considered the same desirability (value 1) for the five responses studied: yield, antioxidant activity by DPPH (AOC), total flavonoids (TF), total iridoid-glycosides (TIG), and total phenolic content (TPC). Then, a new optimization was performed considering higher desirability levels (value 10) only for the TIG and TPC responses. Subsequently, another optimization considered higher levels of desirability (value 10) only for the AOC and TF responses. Also, a new optimization, considering higher desirability only for AOC and TIG. Finally, the last optimization attempt was performed considering level 10 of importance for the responses antioxidant activity, total flavonoids, total iridoid-glycosides, and total phenolic content. The responses predicted values of the studied extraction process, as well as the optimal extraction points for each optimization, can be found in Table 5.
For the optimization considering equal importance for all responses, the optimal point found has values of concentration, temperature, and flow of 59% (v/v), 119 °C, and 3.5 mL/min, respectively. In the case of the optimization with high importance only for TIG and TPC, there was an increase in the concentration value of the optimal point, to 65% (v/v). At the same, the temperature rise by one degree, to 120 °C and the flow remained unchanged at 3.5 mL/min.
When higher importance was given to the AOC and TF responses, the values of the variables for the optimal point presented a decrease to the previous optimizations: the optimal concentration is now 53% (v/v), the temperature is 100 °C, and the flow rate around 3 mL/min. If the importance is greater for AOC and TIG, concentration and temperature remain at the previous values, and the flow increases to 4 mL/min.
Finally, considering high levels of desirability for AOC, TF, TIG, and TPC, there is a slight change in the optimal point. The values found are concentration 57% (v/v), temperature 100 °C, and flow rate 3.6 mL/min.
It is worth pointing out that the objective of the optimization procedure was to find an experimental point or region between the values of the variables that maximize one or more of the studied responses. As a multiobjective optimization tool, the desirability function simultaneously allows different importance attributed to different process responses (dependent variables). Thus, depending on the importance level or desirability level of each response, different optimal extraction points can be found for the same process (Montgomery 2013).
Analyzing the results in Table 5, it is possible to infer that the variation of the responses for the different desirability situations remains around a region that included all the found optimal points. Thus, to obtain a high antioxidant capacity and high phenolic content phenolic extract, any of the five optimal conditions is satisfactory.
For this reason, this region limited by the five optimal conditions found can be called an optimal extraction region. Thus, any point between 53 and 65% (v/v) of solvent concentration, between 100 and 120 °C of temperature, and 3–4 mL/min of flow rate, results in responses considered acceptable for the extraction process to obtain an extract with an antioxidant capacity of high phenolic content. Such a region can be easily intuited by imagining the overlapping contour surfaces of AOC, TF, TIG, and TPC in Fig. 2.
In an extraction process, controlling factors such as flow and temperature can present a certain challenge, as disturbances and fluctuations in these factors can inherently occur in the process, depending on the equipment, extraction mode used, and operator. The knowledge that small changes in the values of the factors do not significantly affect the collected extract content means that exists a robust extraction process that is not sensitive to small external and random variability. Thus, the extraction operation in the optimal region allows obtaining the desired extract, even with minor variations in the concentration, temperature, and flow values.
To test the optimization accuracy, an optimal point in to the optimal extraction region was chosen, a concentration of 53% (v/v) of water/ethanol, an extraction temperature of 115 °C, and a flow rate of 3 mL/min. Under these conditions, the observed experimental values for the responses were: 30.40% ± 1.36 of yield, 40.74% ± 0.0006 of antioxidant capacity, 8.17 ± 0.39 mg g−1 of total flavonoids, 109.11 ± 2.55 mg g−1 of total iridoid-glycosides, and 122.03 ± 2.38 mg g−1 total phenolic content. The tentative’compound identification and quantification of the phenolic compounds in the extraction’ optimal experimental condition are presented in Table 6.
The observed experimental values above were compared to the predicted values. For the yield response, the predicted value was 31%, so the experimental value showed good agreement with the model, staying within the estimated prediction interval (26–35%). For the antioxidant activity by DPPH, the experimental value was below the predicted value, which was 56%, with a prediction interval between 43–70%. In the total flavonoid response, the experimental value was also below the predicted value (13 mg g−1), and within the model's prediction interval (5–21 mg g−1). Furthermore, the total iridoid-glycosides response had an experimental value above that predicted by the model (71 mg g−1) and slightly above the predicted interval (37–104 mg g−1). For the total phenolic content, the experimental value was above the predicted value (91 mg g−1) and within the predicted interval (49–133 mg g−1).
The slight contradiction in the experimental and predicted values at the validation point may be related to the leaves composition of the different olive tree species under study. Despite the raw material homogenization, variations in the sample batches composition used to validate the model may have occurred. A comparison between the composition of the extracts Tables 1 and 6 shows differences in the phenolic compounds identified during the experimental design step and at the validation point, which proves the change in the phenolic composition of the samples.
In face of the previous, for the study in question with the goal to maximize the recovery of the olive leaves' phenolic content, the experimental values were considered satisfactory, and the observed experimental values are considered in agreement with the prediction intervals, showing a sufficient good predictive capacity of the models.
In Table 7 is presented optimal conditions for several researchs in olive leaf green extraction by PLE. The results for this research show an excellent recovery percentage of oleuropein and derivatives (iridoid-glycosides compounds) to PLE in dynamic mode with green solvents and moderate extraction temperatures: 85% of oleuropein and derivates in optimum extract.
Unfortunately the comparison with common methods to obtain olive leaves extracts may be difficult since the parameters in each technique usually do not correlate. However, using a conventional system of solid–liquid extraction by maceration of olive leaves in a solvent, studies show a phenolic content of 2.73% dry weight with ethanol for 3 h and 25 °C, and 2.48% of dry weight in the extraction with ethanol/water (1:1) for 3 h at 25 °C (Souilem et al. 2017). In the present research, the phenolic content achieved 12.2% of extract dry weight, in the optimal condition, and 15 min of extraction.
The use of pressurized liquids for the bioactives extraction is recognized to be a process that drastically reduces the solvent amount and the extraction time, consequently the extraction is efficient and faster with the use of high temperature and pressure. Dynamic mode extraction, the extraction mode chosen in this work, may be the best choice for an industrial scale extraction, considering that the permanent injection of the pure solvent increases extraction yield efficiency because the mass transfer equilibria in the process are displaced (Herrero et al. 2013) and also reduces the extraction time, which is a highly desirable characteristic in industrial-scale extraction (Souilem et al. 2017).
The solvents used in this research, water and ethanol, present stable physicochemical properties, low volatility, safe in use and reusable (Cvjetko Bubalo et al. 2018), advantageous characteristics in the recovery of bioactive compounds and that allow the use of extracts obtained without further processing. The ratio between water and ethanol in the solvent in the present work is also shown to be more economical, since a higher percentage of water in the extraction solvent reduces costs when compared to extraction with pure ethanol (Mustafa and Turner 2011). In the scale up of extraction processes, a higher percentage of water in the solvent and moderate temperatures are factors that can reduce energy and economic costs.
Therefore, future studies may be based on the optimal region found in this work for developing the PLE technique on an large-scale extraction process.
Conclusion
Extracts from olive leaves were obtained by pressurized liquid extraction (PLE) with water/ethanol. Fifteen phenolic compounds were identified in the samples and oleuropein derivates were identified as the major phenolic compounds in the extracts. The optimal experimental condition that simultaneously maximized the antioxidant activity and the phenolic content was found to be a region with a solvent composition of 53–65% (v/v) water/ethanol ratio, an extraction temperature between 100 and 120 °C and a flow rate from 3 to 4 mL/min. A satisfactory agreement between the experimental data and their predicted values was observed, demonstrating the good predictive capacity of the models. Also, the Box-Behnken design and Simplex optimization combined with the desirability functions proved to be an efficient tool for the optimization of subcritical water/ethanol extraction of phenolic compounds from of olive leaves.
Data availability
The data supporting the conclusions of this study are all in the text, there is no supplementary material.
References
Abaza L, Taamalli A, Nsir H, Zarrouk M (2015) Olive tree (Olea europeae L.) leaves: importance and advances in the analysis of phenolic compounds. Antioxidants 4:682–698. https://doi.org/10.3390/antiox4040682
Ahmadian-Kouchaksaraie Z, Niazmand R, Najafi MN (2016) Optimization of the subcritical water extraction of phenolic antioxidants from Crocus sativus petals of saffron industry residues: Box-Behnken design and principal component analysis. Innov Food Sci Emerg Technol 36:234–244. https://doi.org/10.1016/j.ifset.2016.07.005
Aissa I, Kharrat N, Aloui F et al (2017) Valorization of antioxidants extracted from olive mill wastewater. Biotechnol Appl Biochem 64:579–589. https://doi.org/10.1002/bab.1509
Alexandre EMC, Araújo P, Duarte MF et al (2017) Experimental design, modeling, and optimization of high-pressure-assisted extraction of bioactive compounds from pomegranate peel. Food Bioprocess Technol 10:886–900. https://doi.org/10.1007/s11947-017-1867-6
Aliakbarian B, Casazza AA, Perego P (2011) Valorization of olive oil solid waste using high pressure-high temperature reactor. Food Chem 128:704–710. https://doi.org/10.1016/j.foodchem.2011.03.092
Avila-Sosa R, Gastélum-Franco MG, Camacho-Dávila A et al (2010) Extracts of Mexican oregano (Lippia berlandieri Schauer) with antioxidant and antimicrobial activity. Food Bioprocess Technol 3:434–440. https://doi.org/10.1007/s11947-008-0085-7
Bessada SMF, Barreira JCM, Barros L et al (2016) Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: an underexploited and highly disseminated species. Ind Crops Prod 89:45–51. https://doi.org/10.1016/j.indcrop.2016.04.065
Bodoira R, Rossi Y, Montenegro M et al (2017) Extraction of antioxidant polyphenolic compounds from peanut skin using water-ethanol at high pressure and temperature conditions. J Supercrit Fluids 128:57–65. https://doi.org/10.1016/j.supflu.2017.05.011
Bona E, Borsato D, Sérgio R, Herrera P (2000) Aplicativo para otimização empregando o método simplex seqüencial. Acta Sci Technol 22:1201–1206
Boss A, Bishop K, Marlow G et al (2016) Evidence to support the anti-cancer effect of olive leaf extract and future directions. Nutrients 8:513. https://doi.org/10.3390/nu8080513
Bouaziz M, Feki I, Ayadi M et al (2010) Stability of refined olive oil and olive-pomace oil added by phenolic compounds from olive leaves. Eur J Lipid Sci Technol 112:894–905. https://doi.org/10.1002/ejlt.200900166
Brahmi F, Mechri B, Dabbou S et al (2012) The efficacy of phenolics compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Ind Crops Prod 38:146–152. https://doi.org/10.1016/j.indcrop.2012.01.023
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30
Brunner G (2009) Near and supercritical water. Part II: oxidative processes. J Supercrit Fluids 47:382–390. https://doi.org/10.1016/j.supflu.2008.09.001
Bulotta S, Celano M, Lepore SM et al (2014) Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: focus on protection against cardiovascular and metabolic diseases. J Transl Med 12:219. https://doi.org/10.1186/s12967-014-0219-9
Carabias-Martínez R, Rodríguez-Gonzalo E, Revilla-Ruiz P, Hernández-Méndez J (2005) Pressurized liquid extraction in the analysis of food and biological samples. J Chromatogr A 1089:1–17. https://doi.org/10.1016/j.chroma.2005.06.072
Castro-Puyana M, Marina ML, Plaza M (2017) Water as green extraction solvent: principles and reasons for its use. Curr Opin Green Sustain Chem 5:31–36. https://doi.org/10.1016/j.cogsc.2017.03.009
Chemat F, Vian MA, Cravotto G (2012) Green extraction of natural products: concept and principles. Int J Mol Sci 13:8615–8627. https://doi.org/10.3390/ijms13078615
Cheng Y, Xue F, Yu S et al (2021) Subcritical water extraction of natural products. Molecules 26:1–38. https://doi.org/10.3390/molecules26134004
Cvjetko Bubalo M, Vidović S, Radojčić Redovniković I, Jokić S (2018) New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod Process 109:52–73. https://doi.org/10.1016/j.fbp.2018.03.001
D’Antuono I, Kontogianni VG, Kotsiou K et al (2014) Polyphenolic characterization of olive mill wastewaters, coming from Italian and Greek olive cultivars, after membrane technology. Food Res Int 65:301–310. https://doi.org/10.1016/j.foodres.2014.09.033
Derringer G, Suich R (1980) Simultaneous optimization of several response variables. J Qual Technol 12:214–219. https://doi.org/10.1080/00224065.1980.11980968
Di Donna L, Mazzotti F, Salerno R et al (2007) Characterization of new phenolic compounds from leaves of Olea europaea L. by high-resolution tandem mass spectrometry. Rapid Commun Mass Spectrom 21:3653–3657. https://doi.org/10.1002/rcm.3262
Favareto R, Teixeira MB, Soares FAL et al (2019) Extraction of bioactive compounds of leaves of duguetia furfuracea (annonaceae) using green and organic solvents. Braz J Chem Eng 36:549–556. https://doi.org/10.1590/0104-6632.20190361s20170451
Gao F, Han L (2012) Implementing the Nelder-Mead simplex algorithm with adaptive parameters. Comput Optim Appl 51:259–277. https://doi.org/10.1007/s10589-010-9329-3
Garmus TT, Kopf SFM, Paula JT et al (2019) Ethanolic and hydroalcoholic extracts of pitanga leaves (Eugenia uniflora L.) and their fractionation by supercritical technology. Braz J Chem Eng 36:1041–1051. https://doi.org/10.1590/0104-6632.20190362s20180159
Gullón B, Gullón P, Eibes G et al (2018) Valorisation of olive agro-industrial by-products as a source of bioactive compounds. Sci Total Environ 645:533–542. https://doi.org/10.1016/j.scitotenv.2018.07.155
Hassas-Roudsari M, Chang PR, Pegg RB, Tyler RT (2009) Antioxidant capacity of bioactives extracted from canola meal by subcritical water, ethanolic and hot water extraction. Food Chem 114:717–726. https://doi.org/10.1016/j.foodchem.2008.09.097
Herrero M, Temirzoda TN, Segura-Carretero A et al (2011) New possibilities for the valorization of olive oil by-products. J Chromatogr A 1218:7511–7520. https://doi.org/10.1016/j.chroma.2011.04.053
Herrero M, Castro-Puyana M, Mendiola JA, Ibañez E (2013) Compressed fluids for the extraction of bioactive compounds. TrAC Trends Anal Chem 43:67–83. https://doi.org/10.1016/j.trac.2012.12.008
Jaski JM, Barão CE, Morais Lião L et al (2019) β-Cyclodextrin complexation of extracts of olive leaves obtained by pressurized liquid extraction. Ind Crops Prod 129:662–672. https://doi.org/10.1016/j.indcrop.2018.12.045
Jerman T, Trebše P, Mozetič Vodopivec B (2010) Ultrasound-assisted solid liquid extraction (USLE) of olive fruit (Olea europaea) phenolic compounds. Food Chem 123:175–182. https://doi.org/10.1016/j.foodchem.2010.04.006
Kishikawa A, Ashour A, Zhu Q et al (2015) Multiple biological effects of olive oil by-products such as leaves, stems, flowers, olive milled waste, fruit pulp, and seeds of the olive plant on skin. Phyther Res 29:877–886. https://doi.org/10.1002/ptr.5326
Kumari B, Tiwari BK, Hossain MB et al (2018) Recent advances on application of ultrasound and pulsed electric field technologies in the extraction of bioactives from agro-industrial by-products. Food Bioprocess Technol 11:223–241. https://doi.org/10.1007/s11947-017-1961-9
Lama-Muñoz A, del Mar CM, Espínola F et al (2019) Extraction of oleuropein and luteolin-7-O-glucoside from olive leaves: optimization of technique and operating conditions. Food Chem 293:161–168. https://doi.org/10.1016/j.foodchem.2019.04.075
Lee O-H, Lee B-Y (2010) Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour Technol 101:3751–3754. https://doi.org/10.1016/j.biortech.2009.12.052
Lozano-Sánchez J, Giambanelli E, Quirantes-Piné R et al (2011) Wastes generated during the storage of extra virgin olive oil as a natural source of phenolic compounds. J Agric Food Chem 59:11491–11500. https://doi.org/10.1021/jf202596q
Mahmoudi A, Hadrich F, Feki I et al (2018) Oleuropein and hydroxytyrosol rich extracts from olive leaves attenuate liver injury and lipid metabolism disturbance in bisphenol A-treated rats. Food Funct 9:3220–3234. https://doi.org/10.1039/C8FO00248G
Martín-García B, Pimentel-Moral S, Gómez-Caravaca AM et al (2020) Box-Behnken experimental design for a green extraction method of phenolic compounds from olive leaves. Ind Crops Prod 154:112741. https://doi.org/10.1016/j.indcrop.2020.112741
Mendiola JA, Herrero M, Cifuentes A, Ibañez E (2007) Use of compressed fluids for sample preparation: Food applications. J Chromatogr A 1152:234–246. https://doi.org/10.1016/j.chroma.2007.02.046
Mkaouar S, Krichen F, Bahloul N et al (2018) Enhancement of bioactive compounds and antioxidant activities of olive (Olea europaea L.) leaf extract by instant controlled pressure drop. Food Bioprocess Technol 11:1222–1229. https://doi.org/10.1007/s11947-018-2098-1
Monroy YM, Rodrigues RAF, Sartoratto A, Cabral FA (2020) Purple corn (Zea mays L.) pericarp hydroalcoholic extracts obtained by conventional processes at atmospheric pressure and by processes at high pressure. Braz J Chem Eng 37:237–248. https://doi.org/10.1007/s43153-020-00009-x
Montgomery DC (2013) Design and analysis of experiments, 8th edn. Wiley, New York
More SB, Gogate PR, Waghmare JS (2021) Bioactives from pomegranate peel and moringa leaves as natural antioxidants for stability of edible oil blends. Braz J Chem Eng. https://doi.org/10.1007/s43153-021-00150-1
Moreno-González M, Ottens M (2021) A structured approach to recover valuable compounds from agri-food side streams. Food Bioprocess Technol 14:1387–1406. https://doi.org/10.1007/s11947-021-02647-6
Mustafa A, Turner C (2011) Pressurized liquid extraction as a green approach in food and herbal plants extraction: a review. Anal Chim Acta 703:8–18. https://doi.org/10.1016/j.aca.2011.07.018
Mylonaki S, Kiassos E, Makris DP, Kefalas P (2008) Optimisation of the extraction of olive (Olea europaea) leaf phenolics using water/ethanol-based solvent systems and response surface methodology. Anal Bioanal Chem 392:977–985. https://doi.org/10.1007/s00216-008-2353-9
Neto DCS, Ferreira VCS, Araújo ÍBS et al (2022) Solid–liquid extraction of bioactive compounds from Spondias mombin L. by-products : optimization and identification of phenolic profile. Braz J Chem Eng. https://doi.org/10.1007/s43153-021-00209-z
Omar SH, Scott CJ, Hamlin AS, Obied HK (2018) Biophenols: enzymes (β-secretase, Cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.). Fitoterapia 128:118–129. https://doi.org/10.1016/j.fitote.2018.05.011
Otero P, Garcia-Oliveira P, Carpena M et al (2021) Applications of by-products from the olive oil processing: revalorization strategies based on target molecules and green extraction technologies. Trends Food Sci Technol 116:1084–1104. https://doi.org/10.1016/J.TIFS.2021.09.007
Panja P (2018) Green extraction methods of food polyphenols from vegetable materials. Curr Opin Food Sci 23:173–182. https://doi.org/10.1016/j.cofs.2017.11.012
Pasrija D, Anandharamakrishnan C (2015) Techniques for extraction of green tea polyphenols: a review. Food Bioprocess Technol 8:935–950. https://doi.org/10.1007/s11947-015-1479-y
Paulo F, Santos L (2020) Encapsulation of the antioxidant tyrosol and characterization of loaded microparticles: an integrative approach on the study of the polymer-carriers and loading contents. Food Bioprocess Technol 13:764–785. https://doi.org/10.1007/s11947-020-02407-y
Pereira A, Ferreira I, Marcelino F et al (2007) Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 12:1153–1162. https://doi.org/10.3390/12051153
Pires J, Torres PB, Santos DYAC dos, Chow F (2017) Ensaio em microplaca do potencial antioxidante através do método de sequestro do radical livre DPPH para extratos de algas. Inst Biociências, Univ São Paulo 6
Plaza M, Turner C (2015) Pressurized hot water extraction of bioactives. Trends Anal Chem 71:39–54. https://doi.org/10.1016/j.trac.2015.02.022
Plaza M, Abrahamsson V, Turner C (2013) Extraction and neoformation of antioxidant compounds by pressurized hot water extraction from apple byproducts. J Agric Food Chem 61:5500–5510. https://doi.org/10.1021/jf400584f
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302. https://doi.org/10.1021/jf0502698
Quideau S, Deffieux D, Douat-Casassus C, Pouységu L (2011) Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chemie Int Ed 50:586–621. https://doi.org/10.1002/anie.201000044
Quirantes-Piné R, Lozano-Sánchez J, Herrero M et al (2012) HPLC-ESI-QTOF-MS as a powerful analytical tool for characterising phenolic compounds in olive-leaf extracts. Phytochem Anal 24:213–223. https://doi.org/10.1002/pca.2401
Rahmanian N, Jafari SM, Wani TA (2015) Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci Technol 42:150–172. https://doi.org/10.1016/j.tifs.2014.12.009
Ranieri G, Mazzei R, Poerio T et al (2018) Biorefinery of olive leaves to produce dry oleuropein aglycone: use of homemade ceramic capillary biocatalytic membranes in a multiphase system. Chem Eng Sci 185:149–156. https://doi.org/10.1016/j.ces.2018.03.053
Roselló-Soto E, Koubaa M, Moubarik A et al (2015) Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: non-conventional methods for the recovery of high-added value compounds. Trends Food Sci Technol 45:296–310. https://doi.org/10.1016/j.tifs.2015.07.003
Ryan D, Robards K, Prenzler P et al (1999) Liquid chromatography with electrospray ionisation mass spectrometric detection of phenolic compounds from Olea europaea. J Chromatogr A 855:529–537. https://doi.org/10.1016/S0021-9673(99)00719-0
Şahin S, Bilgin M (2017) Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: a review. J Sci Food Agric 98:1271–1279. https://doi.org/10.1002/jsfa.8619
Savarese M, De Marco E, Sacchi R (2007) Characterization of phenolic extracts from olives (Olea europaea cv. Pisciottana) by electrospray ionization mass spectrometry. Food Chem 105:761–770. https://doi.org/10.1016/j.foodchem.2007.01.037
Song R, Ismail M, Baroutian S, Farid M (2018) Effect of subcritical water on the extraction of bioactive compounds from carrot leaves. Food Bioprocess Technol 11:1895–1903. https://doi.org/10.1007/s11947-018-2151-0
Sorita GD, Leimann FV, Ferreira SRS (2020) Biorefinery approach: is it an upgrade opportunity for peanut by-products? Trends Food Sci Technol 105:56–69. https://doi.org/10.1016/j.tifs.2020.08.011
Souilem S, Fki I, Kobayashi I et al (2017) Emerging technologies for recovery of value-added components from olive leaves and their applications in food/feed industries. Food Bioprocess Technol 10:229–248. https://doi.org/10.1007/s11947-016-1834-7
Sperb JGC, Costa TM, Bertoli SL, Tavares LBB (2018) Simultaneous production of biosurfactants and lipases from aspergillus niger and optimization by response surface methodology and desirability functions. Braz J Chem Eng 35:857–868. https://doi.org/10.1590/0104-6632.20180353s20160400
Squillaci G, Marchetti A, Petillo O et al (2021) Olive oil dregs as a novel source of natural antioxidants: extraction optimization towards a sustainable process. Processes 9:1064–1078. https://doi.org/10.3390/pr9061064
Stamatopoulos K, Chatzilazarou A, Katsoyannos E (2014) Optimization of multistage extraction of olive leaves for recovery of phenolic compounds at moderated temperatures and short extraction times. Foods 3:66–81. https://doi.org/10.3390/foods3010066
Taamalli A, Arráez-Román D, Barrajón-Catalán E et al (2012) Use of advanced techniques for the extraction of phenolic compounds from Tunisian olive leaves: phenolic composition and cytotoxicity against human breast cancer cells. Food Chem Toxicol 50:1817–1825. https://doi.org/10.1016/j.fct.2012.02.090
Talhaoui N, Gómez-Caravaca AM, León L et al (2014) Determination of phenolic compounds of “Sikitita” olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents “Arbequina” and “Picual” olive leaves. LWT Food Sci Technol 58:28–34. https://doi.org/10.1016/j.lwt.2014.03.014
Talhaoui N, Taamalli A, Gómez-Caravaca AM et al (2015) Phenolic compounds in olive leaves: analytical determination, biotic and abiotic influence, and health benefits. Food Res Int. https://doi.org/10.1016/j.foodres.2015.09.011
Tormena MML, de Medeiros LT, de Lima PC et al (2017) Application of multi-block analysis and mixture design with process variable for development of chocolate cake containing yacon (Smallanthus sonchifolius) and maca (Lepidium meyenii). J Sci Food Agric 97:3559–3567. https://doi.org/10.1002/jsfa.8211
Tsakona S, Galanakis CM, Gekas V (2012) Hydro-ethanolic mixtures for the recovery of phenols from mediterranean plant materials. Food Bioprocess Technol 5:1384–1393. https://doi.org/10.1007/s11947-010-0419-0
Veličković V, Đurović S, Radojković M et al (2017) Application of conventional and non-conventional extraction approaches for extraction of Erica carnea L.: chemical profile and biological activity of obtained extracts. J Supercrit Fluids 128:331–337. https://doi.org/10.1016/j.supflu.2017.03.023
Vergara-Salinas JR, Cuevas-Valenzuela J, Pérez-Correa JR (2015) Pressurized hot water extraction of polyphenols from plant material. Biotechnology of bioactive compounds. Wiley, Chichester, pp 63–101
Vinha AF, Ferreres F, Silva BM et al (2005) Phenolic profiles of Portuguese olive fruits (Olea europaea L.): Influences of cultivar and geographical origin. Food Chem 89:561–568. https://doi.org/10.1016/j.foodchem.2004.03.012
Xynos N, Papaefstathiou G, Psychis M et al (2012) Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. J Supercrit Fluids 67:89–93. https://doi.org/10.1016/j.supflu.2012.03.014
Xynos N, Papaefstathiou G, Gikas E et al (2014) Design optimization study of the extraction of olive leaves performed with pressurized liquid extraction using response surface methodology. Sep Purif Technol 122:323–330. https://doi.org/10.1016/j.seppur.2013.10.040
Živković J, Barreira JCM, Šavikin KP et al (2017) Chemical profiling and assessment of antineurodegenerative and antioxidant properties of Veronica teucrium L. and Veronica jacquinii Baumg. Chem Biodivers. https://doi.org/10.1002/cbdv.201700167
Acknowledgements
The authors thank the “Central Analítica Multiusuário da UTFPR Campo Mourão” (CAMulti-CM) for the analyses. The authors are also grateful to the Foundation for Science and Technology (FCT, Portugal).
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), process number 421541/2018-0, Chamada Universal MCTIC/CNPq n.º 28/2018. Also financial support through national funds FCT/MCTES to CIMO (UIDB/00690/2020); national funding by FCT, P.I., through the individual scientific employment program-contract for M.I. Dias and L. Barros contracts. This work was Conducted under the project “BIOMA—Bioeconomy integrated solutions for the mobilization of the Agro-food market” (POCI-01-0247-FEDER-046112), by “BIOMA” Consortium, and financed by European Regional Development Fund (ERDF), through the Incentive System to Research and Technological development, within the Portugal2020 Competitiveness and Internationalization Operational Program.
Author information
Authors and Affiliations
Contributions
PSS: Investigation, Formal analysis, Writing—Original Draft; FLGV: Formal analysis, Writing—Original Draft; RPI: Methodology, Formal Analysis, Writing—Review and Editing, Supervision; EB: Methodology, Formal Analysis, Writing—Review and Editing, Supervision; MID: Investigation, Formal analysis, Writing—Original Draft; ICFRF: Writing—Review and Editing, Funding acquisition, Project administration; LB: Formal analysis, Writing—Original Draft; Supervision; OHG: Formal Analysis, Writing—Review and Editing, Supervision; FVL: Methodology, Writing—Review and Editing, Supervision, Funding acquisition, Project administration; LCF: Methodology, Writing—Review and Editing, Supervision, Project administration.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva, P.S., Viell, F.L.G., Ineu, R.P. et al. Determination of an optimum extraction region for the recovery of bioactive compounds from olive leaves (Olea europaea L.) using green dynamic pressurized liquid extraction. Braz. J. Chem. Eng. 40, 843–861 (2023). https://doi.org/10.1007/s43153-022-00268-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-022-00268-w