Abstract
This investigation had as objective to study the effective recovery of olive leaf (OLL) polyphenols using a blend of an L-lactic acid/ammonium acetate deep eutectic solvent (DES) with β-cyclodextrin (β-CD). Following an initial DES screening, the extraction process was optimised with response surface methodology by implementing Box-Behnken design. The optimised extraction conditions thus determined were stirring speed 300 rpm, DES concentration 56.4% (w/w), liquid-to-solid ratio 100 mL g−1 and β-CD concentration 0.7% (w/v). Maximum extraction yield (113.66 mg caffeic acid equivalents g−1 dry mass) was achieved at 80 °C, without compromising antioxidant activity. Comparative assessment of the extraction medium DES/β-CD with other green solvents showed that it was a high-performing system providing polyphenol-enriched extract with improved antioxidant characteristics. Stability test of the extract obtained under optimal conditions performed over a period of 30 days at ambient conditions revealed that, while ferric-reducing power fluctuated within narrow limits, the antiradical activity was enormously enhanced by 100%. These changes in the antioxidant activity were not associated with the decline of major polyphenols in the OLL extract. However, the overall polyphenolic profile remained largely unchanged. It is suggested that the DES/β-CD is an efficient green solvent that may confer the OLL polyphenol extract satisfactory stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There is globally an outstanding amount of waste biomass generated from agricultural practices and industrial processing of plant material. These rejected residual streams are of particularly high environmental concern, owed to their polluting organic load, but on the other hand, they also represent an abundant, low-cost feed, rich in valuable substances. To date, there is an enormous effort being expended on the valorisation of food processing wastes, to produce an array of value-added products, in the framework of biorefinery concept [1]. Some of the most significant agri-food wastes in the Mediterranean basin are those deriving from olive (Olea europaea) cultivation and processing, including tree branches and bark, olive leaves, olive pomace and olive mill wastewater [2].
Olive leaves (OLL) is a residue produced from table olives and olive oil manufacturing, and they are a particularly rich source of polyphenolic phytochemicals, including oleuropein and flavone glycosides, with important bioactivities, such as antimicrobial, antihypertensive, hypoglycaemic and antioxidant properties [3]. By virtue of this peculiar composition, polyphenol extraction from OLL has been a subject of a wide number of studies. Recent research has been focused on green solid-liquid extraction processes, including supercritical fluids [4], ultrasound-assisted extraction [5], pressurised liquid extraction [6], instant controlled pressure drop [7], and microwave-assisted extraction [8].
However, a major concern in prospect industrial applications is the replacement of volatile conventional solvents with green ones. Over the past few years, there has been an ongoing search for low-price, sustainable and eco-friendly solvents, an issue of high importance for the development of green chemical and biochemical processes. Deep eutectic solvents (DES) are innovative liquids that share some attractive properties, including thermal and chemical stability, increased solubility for various substances and low volatility. Furthermore, they are characterised by the “designer solvent” flexibility, that is, the fine tuning of their physicochemical properties through modification of DES composition [9]. Because of the plethora of components used to synthesise DES, and the facile and straight-forward DES preparation, these materials are considered a potentially greener alternative to conventional solvents possessing additional advantages such as lower price, higher biodegradability, lower toxicity and wide-range designability (use of various cations, anions, hydrogen bond donors, etc.). Therefore, DES are currently attracting prominent industrial interest, with a broad range of applications in organic synthesis, extraction processes, biocatalysis, etc. [10].
In recent studies, there has been substantial evidence that combination of DES with cyclodextrins may boost polyphenol extraction efficiency [11, 12]. However, studies indicated that β-CD may have either positive or negative contribution in polyphenol extraction, depending on the DES [13]. On this basis, the present examination was carried out to model OLL polyphenol extraction, using a combination of an L-lactic acid/ammonium acetate DES with β-CD. The optimised composition of this solvent was then compared with other green solvents used for OLL polyphenol extraction, to demonstrate its high potential. Finally, extract stability was tested over a 30-day period, to obtain some evidence regarding the effect of the DES/β-CD combination on major polyphenols and antioxidant properties of OLL extracts.
2 Materials and methods
2.1 Chemicals
L-Lactic acid was from Fisher Scientific (Loughborough, UK). Ammonium acetate, sodium carbonate, caffeic acid, sodium acetate trihydrate, 2,2-diphenylpicrylhydrazyl (DPPH), oleuropein, aluminium chloride and rutin (quercetin 3-O-rutinoside) were from Sigma-Aldrich, GmbH (Darmstadt, Germany). β-Cyclodextrin was from Wacker Chemie AG (Burghausen, Germany). Folin-Ciocalteu was from Merck (Darmstadt, Germany). Ethanol and methanol were from Honeywell/Riedel-de Haen (Seelze, Germany). Iron chloride hexahydrate was from Honeywell/Fluka (Steinheim, Germany). Apigenin and luteolin were from Extrasynthese (Genay, France).
2.2 Collection and pre-treatments of olive leaves
Olive leaves were manually collected from a plantation located within the premises of the Mediterranean Agronomic Institute of Chania. The trees belong to Olea europaea var. Koroneiki and they receive neither watering nor fertilising. Collection was accomplished from both shaded and sun-exposed parts of several trees, to minimise variations attributed to environmental factors. The gathered leaves were pooled, and a representative lot was spread over an aluminium tray and dried in a conventional laboratory oven for 24 h, at 50 °C. Following drying, the material was ground in a domestic blender to give a powder with average particle diameter of 1 mm. This feed was used for all examinations.
2.3 Synthesis of DES
For DES synthesis, the technique described elsewhere was used [14]. L-Lactic acid serving as HBD and ammonium acetate as HBA was accurately weighed, mixed and heated at 65–70 °C, approximately for 40–60 min, under magnetic stirring at 400 rpm, to obtain a transparent liquid. The HBD:HBA molar ratios (\( {\mathrm{R}}_{\mathrm{mol}}^{\mathrm{D}/\mathrm{A}} \)) used were 1:1, 3:1, 5:1, 7:1, 9:1 and 11:1. After their formation, all DES were stored in screw-cap glass flasks and inspected regularly over several days for crystal appearance.
2.4 Batch stirred-tank polyphenol extraction
Aliquot of 0.57 g of OLL powder was extracted with 20 mL solvent, giving a liquid-to-solid ratio (RL/S) of 35 mL g−1. Extraction was undertaken under stirring set at 500 rpm for 150 min and the temperature of the extraction was maintained at 50 °C, using a thermostated hotplate (YellowLine MST BasicC, Richmond, VA, USA). The extract was centrifuged at 10,000×g for 10 min and the supernatant was used for further analyses.
2.5 Design of experiment and response surface methodology
In the view of appraising the influence of critical parameters affecting extraction yield, an experimental design was set up including the stirring speed (SS, termed as X1), the concentration of aqueous solutions of DES (CDES, termed as X2), the liquid-to-solid ratio (RL/S, termed as X3) and the β-CD concentration (CCD, termed as X4) [11, 12]. The mode chosen was a Box-Behnken with three central points, composed in total of 27 design points. The yield in total polyphenols (YTP) was the screening response and the four independent variables (X1–X4) were coded from − 1 (lower limit) to 1 (upper limit) (Table 1). Codification was accomplished as follows [15]:
Δzi represents the distance between the real value at the central point of the design and the real value in the lower or upper limit of a variable. βd corresponds to the major coded limit value in the matrix for each variable, and z0 is the real value at the central point. The equation (mathematical model) found after fitting the function to the experimental data was assessed with analysis of variance (ANOVA). 3D surface response plots were built to visualise the predicted model equation.
2.6 Total polyphenol determination
Prior to analyses, samples were diluted 1:50 with 0.5% (w/v) aqueous trichloroacetic acid. Sample volume of 0.02 mL was mixed with 0.05 mL Folin-Ciocalteu reagent and 0.78 mL distilled water and left to react for 2 min. Then 0.15 mL sodium carbonate (20% w/v) was added, and the mixture was incubated at ambient temperature for 60 min. The absorbance at 760 nm was obtained and the total polyphenol concentration (CTP) was determined by a caffeic acid calibration curve (50–400 mg L−1). Yield in TP (YTP) was expressed as mg caffeic acid equivalents (CAE) g−1 dry mass (dm) [16].
2.7 Total flavonoid determination
For total flavonoid (TFn) determination, a previous protocol was used [17]. Aliquot of 0.1 mL sample was mixed with 0.86 mL aqueous ethanol (35% v/v) and 0.04 mL of a reagent containing 0.5 M sodium acetate and 5% (w/v) aluminium chloride. Following a 30-min reaction at room temperature, the absorbance was read at 415 nm. Total flavonoid concentration (CTFn) was determined with a rutin (quercetin 3-O-rutinoside) calibration curve (15–300 mg L−1). Total flavonoid yield (YTFn) was calculated as mg rutin equivalents (RtE) per g dry mass.
2.8 Antiradical activity determination
Samples were diluted with methanol prior to analysis. Then 0.025 mL of diluted extract was combined with 0.975 mL of DPPH solution (100 μM in methanol) and the absorbance at 515 nm was measured immediately after mixing (A515(i)) and after 30 min (A515(f)). Antiradical activity (AAR) was expressed as μmol DPPH per g dry mass as follows [18]:
CDPPH corresponds to the DPPH concentration (μM), CTP to the total polyphenol concentration (mg L−1) in the reaction mixture, A515(f) is the A515 at t = 30 min, A515(i) is the A515 at t = 0 and YTP is the total polyphenol extraction yield (mg g−1).
2.9 Ferric-reducing power determination
Ferric-reducing power (PR) was measured following a published methodology [19]. Aliquot of 0.05 mL of extract was mixed with 0.05 mL FeCl3 (4 mM in 0.05 M HCl) and incubated for 30 min in a water bath, at 37 °C. After incubation, 0.9 mL of TPTZ solution (1 mM in 0.05 M HCl) was added and the absorbance at 620 nm was obtained after 10 min. PR was given as μM ascorbic acid equivalents (AAE) per g dry mass using an ascorbic acid calibration curve (50–300 μM).
2.10 Liquid chromatography-diode array-mass spectrometry
The apparatus consisted of a Finnigan (San Jose, CA, USA) MAT Spectra System P4000 pump, a UV6000LP diode array detector and a Finnigan AQA mass spectrometer. Separations were carried out on a Fortis RP-18 column, 150 mm × 2.1 mm, 3 μm, at 40 °C, with a 10-μL injection loop. Mass spectra were acquired with electrospray ionisation (ESI) in positive ion mode. Settings were the following: probe temperature 250 °C, acquisition 10 and 50 eV, source voltage 25 V, capillary voltage 4 kV and detector voltage 650 V. The eluents were (A) 2% acetic acid and (B) methanol. The flow rate was 0.3 mL min−1, and the elution programme used was 0–30 min, 0–100% methanol, 30–40 min, 100% methanol. Quantification was performed at 280 nm (oleuropein and derivatives) and 340 nm (flavones) using calibration curves constructed with oleuropein (1–50 μg mL−1, R2 = 0.9999), apigenin (1–50 μg mL−1, R2 = 0.9997) and luteolin (0.73–34 μg mL−1, R2 = 0.9996) [4].
2.11 Statistics
Extractions were performed at least twice and all measurements at least in triplicate. Values are means ± standard deviation. Statistics for the experimental design and response surface methodology, as well as distributions, were accomplished with JMP™ Pro 13 at a 95% significance level.
3 Results and discussion
3.1 Initial screening and the effect of \( {\boldsymbol{R}}_{\mathbf{mol}}^{\mathbf{D}/\mathbf{A}} \)
Previous studies highlighted the importance of molar analogies of HBD:HBA on the extraction efficiency of DES [20,21,22,23], and showed that tuning DES composition may desirably regulate polyphenol extraction yield. Thus, the first step in the process development was the synthesis of a series of DES, composed of various \( {R}_{\mathrm{mol}}^{\mathrm{D}/\mathrm{A}} \), ranging from 1:1 to 11:1. These DES were used as 70% (w/w) aqueous mixtures and screened to detect the highest-performing system in recovering polyphenols from OLL. Statistically significant difference (p < 0.05) was seen with the DES with \( {R}_{\mathrm{mol}}^{\mathrm{D}/\mathrm{A}} \) of 7, termed as LA-AmAc (7:1) (Fig. 1). The extraction with this solvent afforded 41.77 ± 1.04 mg CAE g−1 dm, which was by approximately 31% lower than that achieved with 60% (v/v) aqueous ethanol (60.90 ± 0.91 mg CAE g−1 dm).
In the case examined herein, there was no consistent pattern of extractability up to \( {R}_{\mathrm{mol}}^{\mathrm{D}/\mathrm{A}} \) of 7. Beyond this value, a gradual decline in YTP was recorded (Fig. 1). This is because the influence that \( {R}_{\mathrm{mol}}^{\mathrm{D}/\mathrm{A}} \) exerts on the extraction yield may lie on several parameters associated with the nature of the DES, including polarity and viscosity [24]. A decrease in \( {R}_{\mathrm{mol}}^{\mathrm{D}/\mathrm{A}} \) most probably contributed in reduced viscosity since the amount of solid (ammonium acetate) dropped. This might lead to higher diffusivity and increased mass transfer. On the other hand, low acetate proportion may disfavour interactions of polyphenols with acetate and/or ammonium ions, which might be critical in polyphenol extractability [25, 26]. Regarding basicity, the pH of the solvents used (70% w/w DES) varied from 5.10 (\( {R}_{\mathrm{mol}}^{\mathrm{D}/\mathrm{A}} \) = 1) to 2.52 (\( {R}_{\mathrm{mol}}^{\mathrm{D}/\mathrm{A}} \) = 11). However, no correlation between pH and YTP could be established. Therefore, it could be argued that \( {R}_{\mathrm{mol}}^{\mathrm{D}/\mathrm{A}} \) = 7 might represent the most appropriate combination, possessing low enough viscosity to allow for sufficient mass transfer, at the same time maintaining strong interactions between polyphenols and acetate/ammonium ions.
3.2 Process optimisation by response surface methodology
It has been previously demonstrated that major OLL polyphenolic constituents, such as oleuropein, may form stable inclusion complexes with β-CD [27]. A more recent study showed that β-CD may act as a very effective co-solvent when combined with glycerol to recover OLL polyphenols [28]. The same was also observed for the extraction of oak acorn phenolics using a blend of 2-hydroxypropyl β-cyclodextrin and glycerol [29]. Investigations involving polyphenol extraction using mixtures of DES and cyclodextrins were in concurrence, emphasising the role of cyclodextrins as polyphenol extraction boosters [11, 12]. However, a more illustrating study revealed that β-CD may be a high-performing co-solvent upon combination only with certain DES, including LA-AmAc (7:1), whereas for other DES, polyphenol yield suppression was encountered [13].
Thus, the process developed was destined to optimise the extraction of polyphenols from OLL, taking into consideration critical extraction parameters, including the stirring speed (SS), the liquid-to-solid ratio (RL/S), the concentration of DES (CDES) and the concentration of β-CD (CCD). On this ground, the experimental mode deployed was a Box-Behnken design considering the above-mentioned four independent (process) variables. The evaluation of model fitting was done with ANOVA (Fig. 2), taking into consideration the proximity of the measured and predicted values (Table 2). The mathematical model (second-degree polynomial equation) containing only the significant terms is given below:
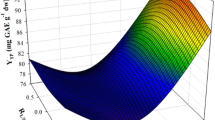
The square correlations coefficient (R2) of the model served as an indicator of the total variability around the mean given by the model. Because R2 was 0.94 and the p value (assuming a confidence interval of 95%) was highly significant, it can be supported that Eq. (3) showed very good adjustment to the experimental data. 3D plots crafted on the basis of the models (Fig. 3) portray at-a-glance the effect of the experimental variables on the response (YTP). The use of the desirability function (Fig. 2) permitted the computing of the optimal extraction conditions, which were SS = 300 rpm, CDES = 56.4% (w/w), RL/S = 100 mL g−1 and CCD = 0.70% (w/v). These settings enabled the estimation of the theoretically maximum YTP, which equalled 66.10 ± 8.01 mg CAE g−1 dm. To verify this value, three extractions were carried out under optimal conditions and the average YTP found was 68.98 ± 1.72 mg CAE g−1 dm. This outcome proved the validity of the model established.
3D plots depicting the effect of the simultaneous variation of the process variables on YTP. For codified variable assignment, see Table 1
Variable RL/S (X3) was shown to be highly significant (Fig. 2) and this finding pointed emphatically to the importance of the proportion between the solid feed and the liquid phase, in achieving high extraction yield. The optimal RL/S was rather high compared with previous values reported for polyphenol extractions with DES, which usually varied between 29 and 50 mL g−1 [30,31,32]. On the other hand, RL/S as high as 100–120 mL g−1 were demonstrated necessary to attain improved extraction yields with conventional solvents [18, 33]. Theoretically, higher RL/S would entail higher diffusivity, since a larger concentration gradient, which is the driving force of diffusivity, exists between the solid particles and the liquid phase. Furthermore, larger proportion of liquid phase would contribute in higher polyphenol solubilisation, leading in increased saturation concentration.
Contrary to that, the negative effect of the cross term X1X3, as revealed by the model, suggested that elevated SS might unfavourably interact with increased RL/S. Thus, practically, improved YTP may be achieved by maintaining low SS. Such a phenomenon might be manifested because switching SS to higher levels could shift the equilibrium towards adsorption of polyphenols onto the solid particles, instead of diffusion into the liquid phase, thus reducing the polyphenol saturation concentration. The positive effect of the cross term X2X4 indicated that YTP could be increased by increasing both CDES and CCD, most probably because CD is more soluble at higher CDES. Higher CD solubility could enable the formation of higher number of polyphenol/CD inclusion complexes, leading in improved YTP. However, CDES could be risen up to the optimal 56.4% (w/w) due to the limitation derived from the negative quadratic effect of CDES.
Regarding CCD, earlier investigations showed that maximum extraction performance of polyphenols from OLL could be achieved using 60% (w/w) glycerol and 2-hydroxypropyl β-cyclodextrin as co-solvent at 7% (w/v). In this study, the optimal CCD was 0.7% (w/v), stressing the effect of the DES used. On the other hand, only the cross term involving CCD and CDES was statistically significant, but not CCD. This finding underlined that it was the combination of the DES with the CD that affected extraction yield and not solely the presence of CD. CCD concentration higher than 0.7% (w/v) did not provide higher YTP, evidence that there might be a specific proportion of DES and CD that acted effectively towards YTP maximisation. The reason for such an observation was rather unclear, but it could be argued that since CD is a polyol, it could act antagonistically with polyphenols to form hydrogen bonds with the acetate ion, thereby lowering polyphenol extraction. This could happen because interactions of polyphenols with acetate ion would be the driving force for increased polyphenol solubilisation [25, 34].
3.3 Effect of temperature
Results from previous studies gave evidence regarding the unusual behaviour of YTP as a response to increasing temperature when DES was employed as solvents. In particular, extraction of red grape pomace polyphenols using various DES displayed fluctuations and YTP in some instances YTP was lower at 80 than at 60 °C [23]. In the same line, extraction of M. oleifera with DES exhibited a gradual decrease in YTP when temperature was shifted from 50 to 80 °C [21]. Therefore, to ascertain that temperature rising could indeed afford enriched extracts, a temperature assay was carried out within the range of 23 (room temperature) to 80 °C. Aside from YTP, YTFn, AAR and PR were also determined, to shape a more integrated picture of the effect of temperature. YTP showed a monotonous increase up to 80 °C (Fig. 4a) and the progress of PR was likewise (Fig. 4d). YTFn had a similar course up to 70 °C, but from 70 to 80 °C, a more abrupt increase was observed (Fig. 4b). On the contrary, AAR showed an increase up to 50 °C, then a sharp decline at 60 °C (Fig. 4c) and a recovery to the highest level at 80 °C. Considering all indices together, temperature rising up to 80 °C had a beneficial effect on the extraction yield, without compromising the antioxidant activity.
3.4 Comparison with other green solvents
To illustrate the efficiency of the combination of LA-AmAc (7:1) with β-CD to extract OLL polyphenols, a comparative appraisal was conducted using previously employed green solvents for OLL polyphenol recovery. Thus, extractions were carried out with:
-
60% (w/w) aqueous glycerol, containing 7% (w/v) 2-hydroxypropyl β-cyclodextrin, assigned as GL-CD [28].
-
60% (v/v) aqueous ethanol, containing 1 g L−1 citric acid, adjusted at pH 2, assigned as AqEt [35].
-
A 50% (w/w) aqueous solution of a DES composed of glycerol:sodium-potassium tartrate:water (7:1:2), assigned as GL-SPT [31].
The optimised solvent, composed of 54.6% (w/w) LA-AmAc with 0.7% (w/v) β-CD, was assigned as DES-CD. For a more credible assessment, not only YTP, but also YTFn, AAR and PR were considered. DES-CD and GL-CD performed equally giving almost 14 and 32% higher YTP than that found with AqEt and GL-SPT, respectively (Fig. 5a). However, AqEt was more effective in extracting flavonoids, giving 12% higher YTFn compared with DES-CD (Fig. 5b). Yet, the extracts obtained with DES-CD had by far superior antioxidant potency, possessing approximately 20% higher AAR and 37% higher PR, compared with AqEt (Fig. 5c, d). Such an outcome provided concrete evidence that the system LA-AmAc (7:1)/β-CD may be a high-performing solvent, producing OLL extracts enriched in polyphenols, with improved antioxidant characteristics.
3.5 Polyphenolic composition and stability
Polyphenol stability in DES is an issue of particular importance, largely unexamined. To obtain some information regarding any possible transformations of OLL polyphenols during storage under regular atmospheric conditions (T = 22 ± 2 °C), OLL extract prepared under optimised conditions was stored in a dark chamber of low moisture, for a period of 30 days. Since structural and quantitative changes may be well reflected on the antioxidant activity, both AAR and PR were monitored over the examination period and their evolution is depicted in Fig. 6. AAR increased progressively up to the 10th day, followed by a decline over the next 8 days, but at the end of the treatment (30th day), an overall rise up by 100% was recorded. By contrast, PR fluctuated within narrow limits (364–385 μmol AAE g−1 dm), to end up with a slight reduction by 3.4%. This finding suggested that PR remained virtually unaffected, as opposed to AAR, which displayed significant enhancement.
To clarify whether this behaviour was associated with specific polyphenol transformations, samples taken at day 0 and day 30 of the treatment were analysed with liquid chromatography-diode array-mass spectrometry (LC-DAD-MS). The analysis revealed the presence of 10 major compounds (Table 3), which were tentatively identified as oleuropein and an isomer thereof, 3 apigenin and 5 luteolin glycosides. All these metabolites represent typical OLL constituents and have been previously reported in other studies [35, 36], with the exception of compounds #2 and 7, for which no known structure could be proposed. Out of this profile, 5 major metabolites were selected (Fig. 7) to trace changes brought about during storage. As can be seen in Table 4, for all these substances, a decline was recorded, which varied from 4.4 (oleuropein isomer) to 42.2% (oleuropein). However, the overall profile was virtually unaffected, which suggested neither major interconversions of the native OLL polyphenols nor generation of novel derivatives. At this point, it should be stressed that earlier investigations on OLL extract storage in a glycerol/glycine DES containing methyl β-cyclodextrin demonstrated the formation of a yellow pigment [37].
Selected ion chromatograms of the OLL extract generated under optimal conditions, at 80 °C. a m/z = 271. The major peak is apigenin 7-O-rutinoside. b m/z = 287. The two major peaks are luteolin 7-O-rutinoside and luteolin 4′-O-glucoside. c m/z = 541. The two major peaks are oleuropein and a derivative thereof
The decline observed in major polyphenols apparently contrasted the drastic increase in AAR. On the other hand, other phenomena that might have taken place during storage could account for such a behaviour. One hypothesis could be that during storage, a greater number of inclusion complexes were formed, and this event contributed in enhanced antiradical activity of the extract. This theory could be based on previous results which demonstrated that inclusion complexes of cyclodextrins with polyphenols such as chlorogenic acid [38], quercetin [39], rosmarinic acid [40] and rutin [41] had significantly higher antioxidant activity compared with the non-encapsulated substances. Observations on cyclodextrin-encapsulated chlorogenate-rich coffee extracts were in the same line [42]. Therefore, the antioxidant behaviour seen with OLL extracts may not be simply correlated with polyphenol concentration, but interactions between OLL polyphenols and β-CD may also be critical in this regard.
4 Conclusions
In this study, a blend of a lactic acid/ammonium acetate DES with β-CD was used as an effective solvent for the recovery of OLL polyphenols. Following optimisation and temperature assay, this solvent was proven a highly efficient system, providing extracts enriched in polyphenols and improved antioxidant properties, compared with other green solvents. The stability test of the optimally obtained extract, performed for a period of 30 days at room temperature, showed that the polyphenolic profile of the extract suffered no major changes, yet some important quantitative differences were traced. These differences in the polyphenolic composition were not reflected on the antiradical activity, which exhibited significant enhancement. However, the ferric-reducing power was essentially unaffected. Overall, it was concluded that the DES/β-CD used may be a green, high-performing system with regard to OLL polyphenol extraction, conferring satisfactory stability to the extract. These findings may pave the way for future industrial applications in the food, cosmetics and pharmaceutical sector.
Abbreviations
- A AR :
-
Antiradical activity (μmol DPPH g−1)
- C DES :
-
Deep eutectic solvent concentration (% w/w)
- C TFn :
-
Total flavonoid concentration (mg RtE L−1)
- C TP :
-
Total polyphenol concentration (mg CAE L−1)
- dm:
-
Dry mass (g)
- P R :
-
Reducing power (μmol AAE g−1)
- \( {R}_{\mathrm{mol}}^{\mathrm{D}/\mathrm{A}} \) :
-
HBD:HBA molar ratio (dimensionless)
- R L/S :
-
Liquid-to-solid ratio (mL g−1)
- T :
-
Temperature (°C)
- Y TFn :
-
Yield in total flavonoids (mg RtE g−1)
- Y TP :
-
Yield in total polyphenols (mg GAE g−1)
- AAE:
-
Ascorbic acid equivalents
- CAE:
-
Caffeic acid equivalents
- β-CD:
-
β-Cyclodextrin
- DES:
-
Deep eutectic solvent
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl radical
- HBA:
-
Hydrogen bond acceptor
- HBD:
-
Hydrogen bond donor
- LA:
-
Lactic acid
- OLL:
-
Olive leaves
- RtE:
-
Rutin equivalents
- TPTZ:
-
2,4,6-Tripyridyl-s-triazine
References
Arancon RAD, Lin CSK, Chan KM, Kwan TH, Luque R (2013) Advances on waste valorization: new horizons for a more sustainable society. Energy Science Eng 1:53–71
Peralbo-Molina Á, de Castro MDL (2013) Potential of residues from the Mediterranean agriculture and agrifood industry. Trends Food Sci Technol 32:16–24
Şahin S, Bilgin M (2018) Olive tree (Olea europaea L.) leaf as a waste by product of table olive and olive oil industry: a review. J Sci Food Agric 98:1271–1279
Xynos N, Papaefstathiou G, Psychis M, Argyropoulou A, Aligiannis N, Skaltsounis A-L (2012) Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. J Supercrit Fluids 67:89–93
Şahin S, Şamlı R (2013) Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason Sonochem 20:595–602
Xynos N, Papaefstathiou G, Gikas E, Argyropoulou A, Aligiannis N, Skaltsounis A-L (2014) Design optimization study of the extraction of olive leaves performed with pressurized liquid extraction using response surface methodology. Separ Purif Technol 122:323–330
Mkaouar S, Bahloul N, Gelicus A, Allaf K, Kechaou N (2015) Instant controlled pressure drop texturing for intensifying ethanol solvent extraction of olive (Olea europaea) leaf polyphenols. Separ Purif Technol 145:139–146
Şahin S, Samli R, Tan ASB, Barba FJ, Chemat F, Cravotto G, Lorenzo JM (2017) Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: antioxidant and antimicrobial properties. Molecules 22:1056
Cunha SC, Fernandes J (2018) Extraction techniques with deep eutectic solvents. Trends Anal Chem 105:225–239
Yang Z (2018) Natural deep eutectic solvents and their applications in biotechnology. Adv Biochem Eng Biotechnol, Springer.
Athanasiadis V, Grigorakis S, Lalas S, Makris DP (2018) Methyl β-cyclodextrin as a booster for the extraction for Olea europaea leaf polyphenols with a bio-based deep eutectic solvent. Biomass Conver Bioref 8:345–355
Karageorgou I, Grigorakis S, Lalas S, Mourtzinos I, Makris DP (2018) Incorporation of 2-hydroxypropyl β-cyclodextrin in a biomolecule-based low-transition temperature mixture (LTTM) boosts efficiency of polyphenol extraction from Moringa oleifera Lam leaves. J Applied Res Med Arom Plants 9:62–69
Georgantzi C, Lioliou A-E, Paterakis N, Makris DP (2017) Combination of lactic acid-based deep eutectic solvents (DES) with β-cyclodextrin: performance screening using ultrasound-assisted extraction of polyphenols from selected native Greek medicinal plants. Agronomy 7:54
Bakirtzi C, Triantafyllidou K, Makris DP (2016) Novel lactic acid-based natural deep eutectic solvents: efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J Applied Res Med Arom Plants 3:120–127
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977
Karakashov B, Grigorakis S, Loupassaki S, Mourtzinos I, Makris DP (2015) Optimisation of organic solvent-free polyphenol extraction from Hypericum triquetrifolium Turra using Box–Behnken experimental design and kinetics. Inter J Ind Chem 6:85–92
Manousaki A, Jancheva M, Grigorakis S, Makris DP (2016) Extraction of antioxidant phenolics from agri-food waste biomass using a newly designed glycerol-based natural low-transition temperature mixture: a comparison with conventional eco-friendly solvents. Recycling 1:194–204
Philippi K, Tsamandouras N, Grigorakis S, Makris DP (2016) Ultrasound-assisted green extraction of eggplant peel (Solanum melongena) polyphenols using aqueous mixtures of glycerol and ethanol: optimisation and kinetics. Environ Proc 3:369–386
Makris DP, Passalidi V, Kallithraka S, Mourtzinos I (2016) Optimization of polyphenol extraction from red grape pomace using aqueous glycerol/tartaric acid mixtures and response surface methodology. Prep Biochem Biotech 46:176–182
Cui Q, Peng X, Yao X-H, Wei Z-F, Luo M, Wang W, Zhao C-J, Fu Y-J, Zu Y-G (2015) Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Separ Purif Technol 150:63–72
Karageorgou I, Grigorakis S, Lalas S, Makris DP (2017) Enhanced extraction of antioxidant polyphenols from Moringa oleifera Lam. leaves using a biomolecule-based low-transition temperature mixture. Eur Food Res Technol 243:1839–1848
Kottaras P, Koulianos M, Makris DP (2017) Low-transition temperature mixtures (LTTMs) made of bioorganic molecules: enhanced extraction of antioxidant phenolics from industrial cereal solid wastes. Recycling 2:3
Patsea M, Stefou I, Grigorakis S, Makris DP (2017) Screening of natural sodium acetate-based low-transition temperature mixtures (LTTMs) for enhanced extraction of antioxidants and pigments from red vinification solid wastes. Environ Proc 4:123–135
de los Ángeles Fernández M, Boiteux J, Espino M, Gomez FV, Silva MF (2018) Natural deep eutectic solvents-mediated extractions: the way forward for sustainable analytical developments. Anal Chim Acta 1038:1–10
Rakotondramasy-Rabesiaka L, Havet J-L, Porte C et al (2010) Estimation of effective diffusion and transfer rate during the protopine extraction process from Fumaria officinalis L. Separ Purif Technol 76:126–131
Mouratoglou E, Malliou V, Makris DP (2016) Novel glycerol-based natural eutectic mixtures and their efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from agri-food waste biomass. Waste Biomass Valor 7:1377–1387
Mourtzinos I, Salta F, Yannakopoulou K, Chiou A, Karathanos VT (2007) Encapsulation of olive leaf extract in β-cyclodextrin. J Agric Food Chem 55:8088–8094
Mourtzinos I, Anastasopoulou E, Petrou A, Grigorakis S, Makris D, Biliaderis CG (2016) Optimization of a green extraction method for the recovery of polyphenols from olive leaf using cyclodextrins and glycerin as co-solvents. J Food Sci Technol 53:3939–3947
Kyriakidou K, Mourtzinos I, Biliaderis C, Makris DP (2016) Optimization of a green extraction/inclusion complex formation process to recover antioxidant polyphenols from oak acorn husks (Quercus robur) using aqueous 2-hydroxypropyl-β-cyclodextrin/glycerol mixtures. Environments 3:3
Jancheva M, Grigorakis S, Loupassaki S, Makris DP (2017) Optimised extraction of antioxidant polyphenols from Satureja thymbra using newly designed glycerol-based natural low-transition temperature mixtures (LTTMs). J Applied Res Med Arom Plants 6:31–40
Dedousi M, Mamoudaki V, Grigorakis S, Makris D (2017) Ultrasound-assisted extraction of polyphenolic antioxidants from olive (Olea europaea) leaves using a novel glycerol/sodium-potassium tartrate low-transition temperature mixture (LTTM). Environments 4:31
Slim Z, Jancheva M, Grigorakis S, Makris DP (2018) Polyphenol extraction from Origanum dictamnus using low-transition temperature mixtures composed of glycerol and organic salts: effect of organic anion carbon chain length. Chem Eng Com 205:1494–1506
Adjé F, Lozano YF, Lozano P, Adima A, Chemat F, Gaydou EM (2010) Optimization of anthocyanin, flavonol and phenolic acid extractions from Delonix regia tree flowers using ultrasound-assisted water extraction. Ind Crop Prod 32:439–444
Bi W, Tian M, Row KH (2013) Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J Chrom A 1285:22–30
Mylonaki S, Kiassos E, Makris DP, Kefalas P (2008) Optimisation of the extraction of olive (Olea europaea) leaf phenolics using water/ethanol-based solvent systems and response surface methodology. Anal Bioanal Chem 392:977
Taamalli A, Arráez-Román D, Ibañez E, Zarrouk M, Segura-Carretero A, Fernández-Gutiérrez A (2012) Optimization of microwave-assisted extraction for the characterization of olive leaf phenolic compounds by using HPLC-ESI-TOF-MS/IT-MS2. J Agric Food Chem 60:791–798
Athanasiadis V, Grigorakis S, Lalas S, Makris DP (2018) Highly efficient extraction of antioxidant polyphenols from Olea europaea leaves using an eco-friendly glycerol/glycine deep eutectic solvent. Waste Biomass Valor 9:1985–1992
Shao P, Zhang J, Fang Z, Sun P (2014) Complexing of chlorogenic acid with β-cyclodextrins: inclusion effects, antioxidative properties and potential application in grape juice. Food Hydrocol 41:132–139
Celik SE, Özyürek M, Güçlü K, Apak R (2015) Antioxidant capacity of quercetin and its glycosides in the presence of β-cyclodextrins: influence of glycosylation on inclusion complexation. J Inclus Phenom Macroc Chem 83:309–319
Medronho B, Valente AJ, Costa P, Romano A (2014) Inclusion complexes of rosmarinic acid and cyclodextrins: stoichiometry, association constants, and antioxidant potential. Colloid Polymer Sci 292:885–894
Alvarez-Parrilla E, Laura A, Torres-Rivas F, Rodrigo-Garcia J, González-Aguilar GA (2005) Complexation of apple antioxidants: chlorogenic acid, quercetin and rutin by β-cyclodextrin (β-CD). J Inclus Phenom Macroc Chem 53:121–129
Budryn G, Nebesny E, Pałecz B, Rachwał-Rosiak D, Hodurek P, Miśkiewicz K, Oracz J, Żyżelewicz D (2014) Inclusion complexes of β-cyclodextrin with chlorogenic acids (CHAs) from crude and purified aqueous extracts of green Robusta coffee beans (Coffea canephora L.). Food Res Inter 61:202–213
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Novelty statement
This study is on the extraction of polyphenols from olive leaves with deep eutectic solvent and cyclodextrin. This is the first report of such an extraction process, with obvious industrial prospect.
Rights and permissions
About this article
Cite this article
Chakroun, D., Grigorakis, S., Loupassaki, S. et al. Enhanced-performance extraction of olive (Olea europaea) leaf polyphenols using L-lactic acid/ammonium acetate deep eutectic solvent combined with β-cyclodextrin: screening, optimisation, temperature effects and stability. Biomass Conv. Bioref. 11, 1125–1136 (2021). https://doi.org/10.1007/s13399-019-00521-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00521-2