Abstract
The microbial production of valuable chemical feedstocks from renewable carbon sources has received increased attention by science and industry. The objective of this work was to study the biosynthesis of propionic acid (PA) by a mixed culture of Propionibacterium freundenreichii ATCC 6207 and Lactobacillus paracasei subsp. paracasei from whey cheese. The effects of L. paracasei inoculum, lactose and calcium carbonate (CaCO3) concentrations on propionic acid (PA) production by P. freundenreichii were investigated following a central composite rotational design with 18 treatments, using whey as source of carbon and nitrogen. Fermentation assays were carried out in Erlenmeyer flasks incubated at 30 °C without shaking during 120 h and for each treatment a kinetic study was done. A production of 23 g L−1 of propionic acid was obtained. Concentrations of lactose below 40 g L−1 and above 30 g L−1 of CaCO3 promoted higher PA production, productivity and yield and acetic acid production. This mixed culture can efficiently use whey for PA biosynthesis, reducing environmental damage from traditional synthesis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Propionic acid (PA) is a short chain organic acid with broad application in the food industry, mainly in food and in feed preservation (Vidra and Németh 2018; Ahmadi et al. 2017a, b) and it is recognized as a generally safe food additive (USFDA 2019). PA salts inhibit fungal growth, thereby improving food safety and prolonging shelf-life (Kagliwal et al. 2013). PA is also used in pharmaceuticals, herbicides, and cosmetics (Stowers et al. 2014; Liu et al. 2016) and is important chemical intermediate in the synthesis of cellulose fibers, perfumes, plastics and pesticides (Vidra and Németh 2018; Chen et al. 2012). Its presence in foods brings beneficial health effects as a satiety-inducing effect on human diet and PA can also be a by-product for microbial production of vitamin B12 (Ranaei et al. 2020; Khosravi-Darani et al. 2019).
Propionic acid is currently produced by chemical synthesis of non-renewable raw materials (petroleum) (Dishisha et al. 2012; Yang et al. 2018), as this is the most economical process. However, there is growing interest in sustainable production and in reducing the cost of synthesizing chemicals from renewable resources. In this context, obtaining PA through fermentation of agro-industrial waste is an excellent alternative, as these raw materials are rich in nutrients for microbial growth, are renewable resources and have low cost (Coral et al. 2008; Ahmadi et al. 2017a, b). Typical strains for PA production are Propionibacterium spp., especially P. freundenreichii and P. acidipropionici (Ranaei et al. 2020).
Fermentative processes offer the opportunity for countries with limited petroleum resources to manufacture organic acids such as propionic acid in a sustainable manner (Stowers et al. 2014). Reducing waste generated from technological processes is a significant concern for both manufacturing companies and environmentalists. Waste reduction has many benefits, such as reducing environmental pollution and treatment costs, and it can also enable the production of new products (Piwowarek et al. 2016). Therefore, alternative routes for producing PA from renewable products have been extensively investigated (Piwowarek et al. 2016; Wang et al. 2017; Sabra et al. 2013), but remains at the research scale (Dishisha et al. 2012).
Producing propionic acid via fermentation still has both low productivity and final concentration due to end-product inhibition of propionic bacteria growth (Dishisha et al. 2013). This inhibition is mainly due to the passage of PA (an undissociated weak acid) through the cell membrane into the cytoplasm where it releases H+ ions because of the intracellular alkaline environment. As a result, the pH gradient across the cell membrane is disturbed, which affects nutrient transfer and inhibits cell growth (Zhang and Yang 2009).
Several alternatives have been reported to significantly improve the propionic acid production and productivity, including extractive fermentation to reduce the effect of the acids generated during the production of propionate (Zhu et al. 2012); cellular immobilization, which increases the tolerance of bacterial cells to their metabolites (Zhu et al. 2012; Dishisha et al. 2015; Belgrano et al. 2018a, b); controlling the pH; strains to increase PA production (Ahmadi et al. 2017a, b) and propionic cells adapted by serial transfer in media containing increasing amounts of PA (Woskow and Glatz 1991; Zhu et al. 2010; Wang et al. 2015a). The pH also influences cell growth, substrate consumption, and by-product formation, with efficient production of 19.21 g L−1 of propionic acid obtained using a pH control strategy (Feng et al. 2010). The use of a mixed culture could be investigated to try to improve the yield of the microbial process or to reduce the co-generation of by-products.
Fermentation processes for PA production require abundant and economical raw materials, such as lactose (whey), sucrose (sugar cane derivatives), glucose (corn derivatives), glycerol or cellulosic materials (Feng et al. 2011; Coral et al. 2008), and control of the bacterial growth process by selecting ideal parameters (Coral et al. 2008; Farhadi et al. 2013; Ahmadi et al. 2017a, b; Ali et al. 2020). Thus, in this work the use of one of the most important by-products of the food industry, whey, and a mixed culture was studied for propionic acid biosynthesis, evaluating the effects of concentration of lactic acid bacteria, lactose, and calcium carbonate on the fermentative process with a mixed culture of + Propionibacterium freundenreichii ATCC 6207 and Lactobacillus paracasei subsp. paracasei.
Materials and methods
The whey was obtained from fresh Minas cheese production provided by the Milk Technology Pilot Plant in the Federal University of Lavras/Brazil. The microorganisms, P. freundenreichii ATCC 6207 and Lactobacillus paracasei subsp. paracasei were donated by the Tropical Cultures Collection belonging to the André Tosello Foundation, Campinas/SP. The reagents used were all analytical grade.
Purity of propionic and lactic bacteria cultures
The purity of the cultures Lactobacillus paracasei and Propionibacterium freundenreichii ATCC 6207 was verified using Matrix Assisted Laser Desorption/Ionisation—Time of Flight Mass Spectrometry (MALDI-TOF MS) (Microflex-Bruker Daltonics/BioTyper ™). The strain Escherichia coli K12 was used as the standard for the external calibration of MALDI-TOF MS following the methodology described by Lima-Neto et al. (2014), Patel (2013) and Clark et al. (2013).
Preparation of whey
The whey was filtered to remove the micelles and homogenize the raw material. This whey was then concentrated using a rotary evaporator to half the initial volume. In parallel, aliquots were taken for whey chemical characterization.
Activation, stock, and standardization of the inoculum
A lyophilized culture of L. paracasei was transferred to a test tube containing 10 mL of Man Rogosa and Sharpe broth (MRS), previously sterilized at 121 °C for 15 min and incubated at 37 °C for 48 h. For propagation, culture in the tube was transferred to an Erlenmeyer flask containing 100 mL of MRS broth and incubated at 37 °C for 24 h. For storage, 1 ml aliquots of the culture contained in the Erlenmeyer flask were transferred to several 1.5 ml eppendorfs. The eppendorfs were centrifuged at 27,000 g for 5 min in the Spinlab (SL-5AM) centrifuge spectrophotometer and the supernatant was removed. The culture of L. paracasei was stored added in pre-autoclaved freezing medium containing: 15 mL glycerol, 0.5 g bacteriological peptone, 0.3 g yeast extract, and 0.5 g NaCl in 100 mL of deionized water pH adjusted to 7.0.
The inoculum was standardized with the growth curve. After reactivation, aliquots of 100 μL of the inoculum were transferred to 300 mL of MRS broth, incubated at 37 °C. In parallel samples were taken at regular times of 1 h intervals for absorbance readings and viable cell counting. The OD (Optical Density) at 600 nm was determined in a Biospectro (SP-22) spectrophotometer. The culture was plated on MRS agar for incubation at 37 °C for 96 h in anaerobic conditions. The cultures were standardized for fermentation based on the ratio of absorbance to the number of viable cells, corresponding to 108 CFU mL−1.
The lyophilized culture of P. freundenreichii ATCC 6207 was activated, stored, and standardized following the same methodology as that for L. paracasei. However, the culture medium used was lactate broth (w v−1): 1% yeast extract, 1% meat peptone, 0.025% K2HPO4 and 1% sodium lactate, and the propionic bacteria were incubated at 30 ºC for 48 h and plated on lactate agar ((w v−1): 1% yeast extract, 1% meat peptone, 0.025% K2HPO4, 1% sodium lactate and 2% agar–agar). The plates were incubated at 30 °C for 96 h in anaerobic conditions.
Effect of bacterial cell, lactose, and carbonate concentration on propionic acid biosynthesis
A Central Composite Rotational Design (CCRD) was performed totaling 18 experiments (Rodrigues and Iemma 2014) with three independent variables: L. paracasei inoculum concentration in log CFU mL−1–LacInoc (× 1), lactose (× 2), and calcium carbonate (× 3) concentrations. The choice of levels of the factors was based on the results of a previous research (Ngome et al. 2017) and literature review (Coral et al. 2008; Wang et al. 2015b). The studied range of the variables is shown in Table 1. The dependent variables (responses) were the organic acid production (propionic, acetic, and lactic acid), propionic acid productivity and yield, lactose consumption, total acidity, and pH.
For fermentation, a mixed culture of P. freundenreichii ATCC 6207 and L. paracasei was used, with a fixed initial inoculum of 108 CFU mL−1 P. freundenreichii ATCC 6207 for all treatments. The fermentation medium was prepared with concentrated whey from fresh Minas cheese. The calcium carbonate and lactose (from the whey cheese) concentrations were determined by CCRD. All treatments were performed in duplicate; the Erlenmeyer flasks were sealed with cotton and incubated in a BOD oven without shaking at 30 °C. For each treatment, a kinetic study was carried out in which 10 mL aliquots were aseptically removed at time intervals of 0, 24, 48, 72, 96, and 120 h. A 0.1 mL aliquot of each treatment was also taken aseptically at the beginning (0 h) and at the end of the fermentation (120 h) to count cells by plating on lactate agar medium and incubating in anaerobic jars placed in an oven at 30 °C for 4 days. The collected samples (10 mL) were centrifuged at 1428 g for 25 min and the supernatant was collected in capped flasks and then stored frozen (-10 °C) for the analysis of organic acids, lactose, total acidity, and pH. The PA yield (YP/S), PA productivity (Pr), and growth factor (GF) were calculated according to Eqs. (1, 2 and 3).
where P–final concentration of PA (g L−1); P0–initial concentration of PA (g L−1); S–final lactose concentration (g L−1); S0–initial lactose concentration (g L−1); Pr –productivity of PA (g L−1 h−1); tf–fermentation time (h); Log CFU mL−1–logarithm of the total cell forming units per mL at the final and initial times.
Statistical analysis was performed using Statistic 8.0 software (Statsoft 2008), with 5% significance level. Models were adjusted (Eq. 4) and generated contour curves, according to methodology recommended by Rodrigues and Iemma (2014).
Since y is the response variable, β0 is the intercept term, β1, β2 e β3 are the coefficients of the model, β12, β13 e β23 are the interaction coefficients, β11, β22 e β33 are the quadratic coefficients, and x1, x2, and x3 are the independent variables in coded values. The interactions that did not have a significant effect (p > 0.05) were disregarded during the regression and eliminated from the final model.
Determination of organic acids (lactic, acetic, and propionic)
The organic acids were analysed by high performance liquid chromatography (HPLC), adapted from Ngome et al. (2017). A Schimadzu brand chromatograph was used with a diode array detection system (model SPD-M20A) and aSIL-20AHT automatic sampler. The column used was a Supelcogel C610H, 30 cm × 7.8 mm × 9.0 μm, equipped with a pre-column and operated at a temperature of 40 °C. The mobile phase was perchloric acid (HClO4) in H2O, with flow rate of 0.5 mL min−1 and a run time of 30 min. Acids were detected by UV absorbance at 210 nm. The acids were identified by comparison with sample retention times of the respective acid standards, and quantification was performed by preparing a standard curve. Samples were diluted and filtered with a 0.22 μm filter for further analysis. The results were processed using the LC-Solutions software.
Determination of physico-chemical parameters
Fermentation media pH values were determined according to the methodology of AOAC (2012). Lactose analysis was performed using the dinitrosalicylic acid (DNS) method according to Miller (1959).
Results and discussion
Using the MALDI-TOF MS technique the microorganisms were successfully confirmed and with a higher identification score of 2.167 and 2.112 for P. freundenreichii ATCC 6207 and Lactobacillus paracasei subsp. paracasei. This method corroborated all previous phenotypic identifications for these microorganisms in reference. Mass spectrometry using MALDI-TOF has been applied for the identification of a wide variety of bacterial species, where each profile can be automatically compared to a library of reference spectra, generating the list of the most closely related microorganisms. This ranking indicates the level of confidence in the identification and, depending on how high the value is, the organism is identified at the level of gender or species (Clark et al. 2013; Lima-Neto et al. 2014; Patel, 2013).
In fermentation, efforts have focused on the use of low-cost carbon and nitrogen sources (Yang et al. 2018). In the case of propionic fermentation, most of the carbon and nitrogen sources investigated have been agricultural and processing wastes (Vidra and Németh 2018). Whey is a by-product of cheese production, with valuable uses, and can reduce the cost in the fermentation process. In addition to lactose, whey proteins, amino acids and minerals are also found in the whey. Table 2 shows the composition of whey and whey concentrate from fresh Minas cheese used in this study.
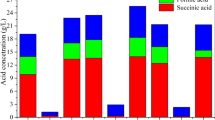
The results for lactate, acetate and propionate concentrations in g L−1, growth factor and propionic acid yield from the 18 treatments are shown in Table 3.
Analysis of the results of propionic acid production (PPA) revealed that the concentration ranged from 0.18 (treatment 13) to 23.27 g L−1 (treatment 14), indicating the large effect of calcium carbonate concentration on propionic fermentation. Similar results were obtained for propionic acid productivity, with a lowest value seen in treatment 13 and highest value in treatment 14 (0.19 g L−1 h−1). Finally, the highest yields of propionic acid were obtained in treatments 5 (0.74 g g −1) and 6, 11 and 14 (0.55—0.56 g g −1).
For lactic acid, highest concentrations were observed in treatments 7 (20.91 g L−1) and 12 (26.13 g L−1) and lowest concentrations were seen in treatments 5, 6, 11, and 14. Treatments 5 (5.08 g L−1) and 14 (5.88 g L−1) produced the highest concentrations of acetic acid. Since the purpose of this study was to produce propionic acid, the treatments with lowest levels of lactic and acetic acid are the most interesting. This can also be observed by analyzing the ratio of propionic acid/acetic acid (PA/AA), which was highest in treatments 10 (5.99) and 14 (3.96). There was little variation in the growth factor of lactic and propionic bacteria, ranging between 1.19 (treatment 2) and 1.82 (treatment 3).
The results of fermentation with the mixed culture of P. freundenreichii ATCC 6207 and L. paracasei were promising. Taking into account all factors, treatment 14, which contained 107 CFU mL−1 of L. paracasei, 45 and 40 g L−1 of lactose and CaCO3 respectively, had the best results with a maximum concentration of 23.27 g L−1 PA and yield of 0.55 g g−1 obtained after 120 h of the fermentation. In this treatment, the main by-product was 5.88 g L−1 of AA.
It was verified that in the treatments with highest concentrations of LA, the concentrations of propionic acid were low. The same result was observed in the production of PA from the fermentation of glucose and lactose by Lactobacillus zeae and Veillonella criteci. In this case, L. zeae metabolizes the glucose to lactate, causing the accumulation of lactic acid and the inhibition of V. criteci that should use lactate, resulting in the lowest propionic acid production (Sabra et al. 2013).
The results of this work were better than those obtained by Ngome et al. (2017), who fermented milk whey with a mixed culture of P. freudenreichii PS-1 and Lactobacillus helveticus and obtained a maximum propionic acid production of 3.78 g L−1, with 3.10 g L−1 of acetic acid, which competed with the main product. The highest productivity was 0.19 g L −1 h −1 over 120 h of fermentation and the yield was 0.74 g g −1. In the literature, there are many works with similar or better results, but the time required (productivity) for the fermentation or its complexity limit their eventual application on an industrial scale.
The results of this paper showed that the mixed culture and whey can be used for PA production. Some authors show that conventional fermentation processes for PA production are mainly focused on monocultures (Gonzalez-Garcia et al. 2017; Sabra et al. 2013). However, Ngome et al. (2017) and Ahmadi et al. (2017a; b; ; 2015) investigated the use of mixed cultures to produce propionic acid with good results.
Whey is an inexpensive industrial by-product that has been investigated as a raw material for the production of propionic acid. Development of a continuous fermentation process with cell retention resulted in a productivity of 0.90 g L−1 h −1 and a yield of 0.40 g g −1 over a 192 h fermentation (Goswami and Srivastava 2001). Using a two-stage pH control strategy (pH 6.5 for 48 h and then 6.0), caused the PA concentration to increase from 14.58 g L−1 at a constant pH to a maximum of 19.21 g L −1 and the maximum propionic acid yield and glucose conversion efficiency reached 25.23 g L−1 and 47.76%, respectively (Feng et al. 2010). Chen et al. (2012) obtained high concentrations of PA, 136.0 ± 6.8 g L−1, with a productivity of 0.57 g L−1 h−1 after 240 ± 11 h, using glucose as a fermentation medium and P. freudenreichii CCTCC M207015 immobilized on sugarcane bagasse. Yang et al. (2018) fermented soybean residues at pH 6.5 with Propionibacterium acidipropionici and obtained a PA productivity of 0.8 g L−1 h −1 and a yield of 0.42 g g −1 sugar. Belgrano et al. (2018a) investigated the fermentation of propionic acid and obtained a concentration of 25.8 g L−1, a productivity of 0.46 g L−1 h−1 and a yield of 0.43 g g −1 using immobilization of microbial cells for bioprocess production of propionic acid. In all of these cases, the yields were lower than that achieved in this study.
AA is the main by-product of propionic acid fermentation. It is difficult to minimize its production during fermentation (Stowers et al. 2014) and makes recovery of the product difficult. In this study, the highest ratio of PA/AA was 5.99 in treatment 10, which unfortunately also showed low productivity and yield (0.02 g L −1 h −1 and 0.05 g g −1 respectively). Therefore, the most interesting PA/AA ratios were 3.72 and 3.95, which involved low levels of AA and yields of up to 0.74 and 0.55 g g −1 of PA, respectively. The low concentrations of acetic acid facilitate the purification of propionic acid from the fermented medium. Control of oxygen input during the fermentation process may result in a further reduction of the by-product. It has already been noted that aerobic conditions favor the production of acetic acid, which explains the increased production of this metabolite (Piwowarek et al. 2016).
Production of propionic, acetic, and lactic acid
The regression coefficients of the adjusted model for lactic acid, acetic acid, propionic acid production and propionic acid yield with only the significant parameters (p < 0.05) are presented in Table 4. According to the regression analysis it was verified that the concentration of the lactic acid bacteria inoculum did not significantly influence any of the response variables and that only the concentrations of lactose and calcium carbonate had statistically significant effects. It was found that an increase in the concentration of lactose decreased the production of propionic acid, while an increase in carbonate concentration increased the propionic acid content.
The Analysis of Variance (ANOVA) and the coefficients of determination (R2) above 88% indicated that the models which described lactic (LA), acetic (AA), and propionic acid (PA) production, and propionic acid yield (YP/S) as a function of the concentration of lactose and CaCO3 concentrations, can be considered adequate, since the regressions were statistically significant (p < 0.05) using the Fisher test (F regression > F tabulated).
For the growth factor (GF) and ratio (PA/AA) responses, the model adjustments were not statistically significant at a 5% threshold; for GF, no term was significant, whereas for PA/AA, only the linear effect of calcium carbonate concentration had a positive and statistically significant effect (p < 0.05) (Table 4).
The coded models for the production of PA, LA, and AA are represented by Eqs. (5, 6, and 7) and the model for the yield of propionic acid by Eq. (8).
Contour curves for the production and yield of propionic acid as a function of lactose and calcium carbonate concentrations in the fermentation medium are shown in Fig. 1A and B respectively.
Neither the LA, AA, PA, nor YP/S responses were affected by cell concentration (Log CFU mL−1) within the range studied (p < 0.05). For the PA, YP/S, LA, and AA responses, (Figs. 1A, B, 2A and B) respectively, only the concentrations of lactose and CaCO3 were statistically significant. For propionic acid yield (YP/S), it was verified that the region with the highest values is the area with lactose concentration below 40 g L−1 and carbonate calcium concentration above 30 g L−1 (Fig. 1B). This information is important because it is possible to minimize the quantities to be used, minimizing the cost of the fermentation process and consequently making it more competitive.
For lactic acid, the lowest concentrations were obtained using fermentation media containing more than 30 g L−1 of CaCO3 and less than 40 g L−1 lactose (Fig. 2A), equal to the optimal region for propionic acid. For acetic acid, the region with the lowest values corresponds to the area between 8 and 30 g L−1 of CaCO3 and above 60 g L−1 of lactose (Fig. 2B).
pH and lactose consumption
In Fig. 3A, the pH variation during the fermentation can be seen for all treatments. In the treatments 5, 6, 11, and 14, that showed higher levels of propionic acid production, pH variations during fermentation were the lowest and the final pH values reached around 6. In the other treatments higher pH variation was observed. In general, decreases in pH and increases in acidity were fastest during the first 48 h of fermentation. Treatment 13, in which the fermentation medium did not contain CaCO3, showed the lowest mean pH at the end of fermentation compared to the other treatments (3.47); it also started with the lowest pH (5.76). Coral et al. (2008) studied PA production by P. acidipropionici using fermentation media with sugarcane molasses, glycerol and lactate in batch at 30 °C. In media with glycerol the pH variation was very high and quick in the first 60 h and it reached a plateau phase around 4.4. In the media with 30 g L−1 sodium lactate the fermentation time was 130 h with small pH variation from 6.8 to 6.3, similar to the treatments with higher PA concentrations from this work which showed pH variation from around 7.2 to 6.0 after 120 h.
In general, concentrations of calcium carbonate above 30 g L−1 in the fermentation medium prevented drastic variations of acidity and pH, resulting in a positive effect on PA production (PPA) and yield—YP/S (Table 4 and Fig. 1A and B). It was also seen that, in the media with less CaCO3, pH levels were lower, such as the PA/AA ratios. In these treatments, the increase in acidity (drastic pH reduction after 24 h) may have led to inhibition of PA biosynthesis. The temperature was maintained constant at 30 °C, which is considered optimal for propionic bacteria to produce propionic acid. Concentrations of lactose greater than 45 g L−1 in the media had negative effects on the production of PA, AA, and PA YP/S (Table 4 and Figs. 1A, B and 2B).
The consumption of the substrate (lactose) throughout the fermentation is presented in Fig. 3B. It was verified that, after 120 h of fermentation, practically all lactose had been consumed. In the treatments with lactose concentrations below 30 g L−1 and calcium carbonate concentrations above 32 g L−1 (5, 6 and 14), consumption of lactose by the mixed culture was fast, with lactose depletion after 48 h of fermentation (Fig. 3B). These treatments had less acidity and lower pH variation. In contrast, in media with lactose concentrations above 60 g L−1 and less than 8 g L−1 of calcium carbonate, consumption of lactose was very slow, lasting about 120 h, as seen in treatments 3, 4, and 12. The axial points of carbonate (treatments 13 and 14) followed the trend of slow and fast lactose consumption, respectively. In the remaining treatments, lactose was consumed between 72 and 96 h.
Kinetics of organic acid production
A kinetic study of the formation of the organic acids over the course of fermentation was performed for treatment 14, which had the highest production of propionic acid. The complex interrelationship between the use of lactose and lactate and production of PA and AA in the fermentation by mixed culture is shown in Fig. 4. It was found that lactose was consumed almost entirely in just 48 h of fermentation, at which time lactate reached its maximum concentration of 38.31 g L−1, along with 7.64 and 3.92 g L−1 of PA and AA, respectively. After 48 h of fermentation, lactate consumption was observed until the end of fermentation at 120 h. By then, lactate had also been depleted, allowing the formation of 23.27 g L−1 of propionic acid. So, mixed culture was beneficial for this process of PA biosynthesis due to the availability of lactate in the fermentation medium.
In a previous study it had been observed that P. freundenreichii grows slower (32 h growth time) than L. paracasei (8 h) (Ngome et al. 2017). In Fig. 4, it was noted that at the beginning of the fermentation, L. paracasei metabolizes lactose, producing lactic acid, whose maximum concentration was observed in 48 h of fermentation when all lactose was metabolized. Then P. freundenreichii metabolized lactate, although lactose may also be used simultaneously, for PA production. The bacteria are able to produce propionic and acetic acids by utilizing lactic acid produced during the initial stage of fermentation (Chof and Mathews 1994). Ali et al. (2020) examined the influence of inoculum type, pH-value and thermal substrate pretreatment on PA production from dog food. Using a mixed bacterial culture isolated from goat cheese and in a fermentation medium adjusted to pH 6 it was observed that lactate is produced in the first days and propionic acid began to be produced after the maximum peak of lactate as a result of lactate convertion, similar to the behaviour found in this work. According to Seeliger et al. (2002) Zhang and Yang 2009, the propionic bacteria preferentially metabolize lactate to propionate and for each 3 mol of lactate consumed are formed 2 mol of propionate, 1 mol of acetic acid and 1 mol of carbon dioxide, plus 2 mol of ATP. At the end of the fermentation in this study, 0.35 g L−1 lactose and 1.01 g L−1 lactate were observed (Fig. 4). According to Ahmadi et al. (2017a, b), the use of mixed culture of P. freudenreichii and L. acidophilus at a ratio of 4:1 v v−1 increases the production of PA and biomass. In another work, Ahmadi et al. (2015) observed that the reason for the increase in acid production (PA and AA) after 48 h is the consumption of lactic acid by propionic bacteria as a carbon source corroborating the results of this work (Fig. 4).
This process of fermentation with a mixed culture showed good symbiosis between lactic and propionic bacteria, which resembles the production process of Swiss cheese, where enzymes produced by Lactobacillus spp. promote the release of peptides that stimulate the growth of P. freundenreichii. A high rate of pH decrease due to acid production causes a shock to P. freudenreichii ssp. shermanii (Ahmadi et al. 2015; Farhadi et al. 2013). This phenomenon leads to a decrease in the viability of these bacteria. However, L. acidophilus does not interfere with the growth of propionibacteria.
Several authors had previously observed that P. acidipropionici ACT-1 has a relatively high tolerance to propionate at pH 6.5. The highest propionic acid concentration of 42.7 g L−1 was found in the second batch (Wang et al. 2015a). Sequential batch fermentations in a fibrous-bed bioreactor (FBB) has much higher PA productivity (0.81 vs. 0.35 g L −1 h −1) and yield (0.42 vs. 0.39 g g− 1) at pH 6.5 (Yang et al. 2018) during subsequent batches. The production of propionic acid by propionic bacteria is inhibited by the extensive accumulation of by-products, mainly acetic acid, decreasing pH and thus inhibiting bacterial growth (Suwannakham and Yang 2005).
In some cases (Fig. 4), and others treatments not shown, lactose and lactate depletion may have been another inhibiter of cell growth and PA biosynthesis. The highest production of metabolites occurred between 48 and 120 h of the fermentation process with a mean production of 5.21 g L−1 of propionic acid every 24 h. A study in which apple was used as a substrate for propionic/acetic acid fermentation showed a larger increase in the production of both metabolites between 96 and 120 h (Piwowarek et al. 2016). Zhuge et al. (2014) obtained a large production of propionic acid from glycerol with genetically modified Propionibacterium jensenii and a strategy of pH control, with a maximum yield of 37.26 g L−1 of PA and productivity of 0.163 g L−1 h −1. In this paper, the pH was not controlled. However, calcium carbonate was added to the fermentation medium at various concentrations according to CCRD in order to regulate abrupt pH variation during fermentation.
The PA biosynthesis in some treatments (Table 3) was not satisfactory. This can be explained by the low concentration of calcium carbonate in the medium, causing inhibition of propionic fermentation either by the low pH or the high concentration of lactate. Some authors have verified a metabolic change in P. acidipropionici, since P. acidipropionici ATCC 55,737 accumulated 25 g L−1 of LA in a fermenter compared to only 6 g L−1 of LA for P. acidipropionici ATCC 4875. Clearly, the metabolism of P. acidipropionici ATCC 55,737 was based towards the production of LA relative to P. acidipropionici ATCC 4875 (Stowers et al. 2014).
LA can then be converted into PA using propionic bacteria under glucose-limiting conditions, extending the fermentation time after glucose depletion (Wang et al. 2017). The production of PA by propionic bacteria from lactate is faster than from molasses because it does not need to be metabolized by the glycolytic pathway (Ahmadi et al. 2017a, b). Propionic acid is produced by propionic bacteria via carboxylic acids with acetic and lactic acid produced as by-products (Stowers et al. 2014).
According to the transcarboxylation reaction, they can convert pyruvate to oxaloacetate, which is then converted to succinate through the carboxylic acid cycle enzymes. Succinate is converted via intermediates of methylmalonyl coenzyme A (CoA) to propionate. The carboxylic group removed from methylmalonyl-CoA is then transferred to pyruvate to form oxaloacetate, making it a cyclic pathway (Huang et al. 2002). Although the occurrence of LA consumption and propionic acid production in Propionibacterium sp. is highly indicative of a Wood–Werkman cycle-based fermentation of lactate to propionate, it should not be forgotten that lactate is not the only substrate for propionibacteria (Ranaei et al. 2020). The production of AA from glucose by propionic bacteria is associated with redox balance, energy generation (ATP or NADH) and cell growth (Stowers et al. 2014; Wang et al. 2017) decreasing the PA yield (Vidra and Németh, 2018). Thus, the major challenge is to make P. freundenreichii more tolerant to higher substrate and final PA concentrations to obtain higher production and productivity.
Conclusions
A mixed culture of P. freundenreichii and L. paracasei can efficiently use lactose from whey for PA and AA biosynthesis and is therefore an alternative to exploit this by-product of the dairy industry. A symbiotic relationship between the microorganisms allowed the production of 23 g L−1 of propionic acid using 107 CFU mL−1 of L. paracasei, 40 g L−1 calcium carbonate and 45 g L−1 lactose from whey. It is important to develop sustainable processes such as fermentative processes to obtain commercial value products like propionic acid.
Abbreviations
- CCRD:
-
Central composite rotational design
- PA:
-
Propionic acid
- AA:
-
Acetic acid
- LA:
-
Lactic acid
- L.:
-
Lactobacillus
- P :
-
Propionibacterium
- V :
-
Veillonella
- CFU:
-
Colony forming units
- MRS:
-
Man Rogosa and Sharpe broth
- BOD:
-
Biochemical oxygen demand
- HPLC:
-
High performance liquid chromatography
- R2 :
-
Determination coefficient
- YP/S :
-
Yield of propionic acid
- Pr:
-
Productivity of propionic acid
- GF:
-
Growth factor
- PPA:
-
Production of propionic acid
- PLA:
-
Production of lactic acid
- PAA:
-
Production of acetic acid
- PA/AA:
-
Ratio between propionic and acetic acid production
References
Ahmadi N, Khosravi-Darani K, Zarean-Shahraki S, Mortazavian M, Mashayekh SM (2015) Fed-batch fermentation for propionic, acetic and lactic acid production. Orient J Chem. https://doi.org/10.13005/ojc/310174
Ahmadi N, Khosravi-Darani K, Mortazavian AM (2017a) An overview of biotechnological production of propionic acid: from upstream to downstream processes. Electron J Biotechnol. https://doi.org/10.1016/j.ejbt.2017.04.004
Ahmadi N, Khosravi-Darani K, Mortazavian AM, Mashayekh SM (2017b) Effects of process variables on fed-batch production of propionic acid. J Food Process Preserv. https://doi.org/10.1111/jfpp.12853
Ali R, Saravia F, Hille-Reichel A, Gescher J, Horn H (2020) Propionic acid production from food waste in batch reactors: effect of pH, types of inoculum, and thermal pre-treatment. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.124166
Belgrano FDS, Diegel O, Pereira N, Hatti-Kaul R (2018a) Cell immobilization on 3D-printed matrices: a model study on propionic acid fermentation. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.10.087
Belgrano FDS, Vercoza BRF, Rodrigues JCF, Hatti-Kaul R, Pereira NJ (2018b) EPS production by Propionibacterium freudenreichii facilitates its immobilization for propionic acid production. J Appl Microbiol. https://doi.org/10.1111/jam.13895
Chen F, Feng X, Xu H, Zhang D, Ouyang P (2012) Propionic acid production in a plant fibrous-bed bioreactor with immobilized Propionibacterium freudenreichii CCTCC M207015. J Biotechnol. https://doi.org/10.1016/j.jbiotec.2012.08.025
Chof CH, Mathews AP (1994) Fermentation Metabolism and Kinetics in the Production of Organic Acids by Propionibacterium acidipropionici. Appl Biochem Biotechnol. https://doi.org/10.1007/BF02779662
Clark AE, Kaleta EJ, Arora A, Wolk M (2013) Matrix-assisted laser desorption ionization–time of flight mass spectrometry : a fundamental shift in the routine practice of clinical. Clin Microbiol Rev. https://doi.org/10.1128/CMR.00072-12
Coral J, Karp SG, De Souza P, Vandenberghe L, Parada JL, Pandey A, Soccol CR (2008) Batch fermentation model of propionic acid production by Propionibacterium acidipropionici in different carbon sources. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-008-8196-1
Dishisha T, Alvarez MT, Hatti-Kaul R (2012) Batch- and continuous propionic acid production from glycerol using free and immobilized cells of Propionibacterium acidipropionici. Bioresour Technol. https://doi.org/10.1016/j.biortech.2012.05.079
Dishisha T, Ståhl Å, Lundmark S, Hatti-Kaul R (2013) An economical biorefinery process for propionic acid production from glycerol and potato juice using high cell density fermentation. Bioresour Technol. https://doi.org/10.1016/j.biortech.2012.08.098
Dishisha T, Ibrahim MHA, Cavero VH, Alvarez MT, Hatti-Kaul R (2015) Improved propionic acid production from glycerol: Combining cyclic batch- and sequential batch fermentations with optimal nutrient composition. Bioresour Technol. https://doi.org/10.1016/j.biortech.2014.11.013
Farhadi S, Khosravi-Darani K, Mashayekh M, Mortazavian AM, Mohammadi A, Shahraz F (2013) Production of propionic acid in a fermented dairy beverage. Int J Dairy Technol. https://doi.org/10.1111/1471-0307.12004
Feng X, Xu H, Yao J, Li S, Zhu H, Ouyang P (2010) Kinetic analysis and pH-shift control strategy for propionic acid production with Propionibacterium freudenreichii CCTCC M207015. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-008-8300-6
Feng X, Chen F, Xu H, Wu B, Li H, Li S, Ouyang P (2011) Green and economical production of propionic acid by Propionibacterium freudenreichii CCTCC M207015 in plant fibrous-bed bioreactor. Bioresour Technol. https://doi.org/10.1016/j.biortech.2011.02.087
Gonzalez-Garcia R, McCubbin T, Navone L, Stowers C, Nielsen L, Marcellin E (2017) Microbial propionic acid production. Fermentation 3(2):21. https://doi.org/10.3390/fermentation3020021
Goswami V, Srivastava AK (2001) Propionic acid production in an in situ cell retention bioreactor. Appl Microbiol Biotechnol. https://doi.org/10.1007/s002530000582
Huang YL, Wu Z, Zhang L, Cheung CM, Yang S (2002) Production of carboxylic acids from hydrolyzed corn meal by immobilized cell fermentation in a fbrous-bed bioreactor. Bioresour Technol. https://doi.org/10.1016/S0960-8524(01)00151-1
Kagliwal LD, Survase SA, Singhal RS, Granstrom T (2013) Wheat flour based propionic acid fermentation: an economic approach. Bioresour Technol. https://doi.org/10.1016/j.biortech.2012.12.154
Khosravi-Darani K, Zarean S, Ahmadi N, Hadian Z, Mortazavian AM (2019) Fed-Batch Production of a fermented beverage containing vitamin B12. Iran J Chem Chem Eng (IJCCE) 38(2):183–192
Lima-Neto R, Santos C, Lima N, Sampaio P, Pais C, Neves RP (2014) Application of MALDI-TOF MS for requalification of a Candida clinical isolates culture collection. Braz J Microbiol. https://doi.org/10.1590/S1517-83822014005000044
Liu L, Guan N, Zhu G, Li J, Shin H, Du G, Chen J (2016) Pathway engineering of Propionibacterium jensenii for improved production of propionic acid. Sci Rep. https://doi.org/10.1038/srep19963
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. https://doi.org/10.1021/ac60147a030
Ngome MT, Alves JGLF, Piccoli RH, Domingo EC, Pinto SA, Bernal OLM (2017) Inoculum concentration and inoculation time for propionic acid production from whey using mixed culture of Lactobacillus helveticus and Propionibacterium freudenreichii PS-1. Acta Sci Technol. https://doi.org/10.4025/actascitechnol.v39i0.29479
Patel R (2013) Matrix-assisted laser desorption ionization: time of flight mass spectrometry in clinical microbiology. Clin Infect Dis. https://doi.org/10.1093/cid/cit247
Piwowarek K, Lipińska E, Hać-Szymańczuk E (2016) Possibility of using apple pomaces in the process of propionic-acetic fermentation. Electron J Biotechnol. https://doi.org/10.1016/j.ejbt.2016.07.004
Ranaei V, Pilevar Z, Khaneghah AM, Hosseini H (2020) Propionic acid: method of production, current state and perspectives. Food Technol Biotechnol 58(2):115–127. https://doi.org/10.17113/ftb.58.02.20.6356
Rodrigues MI, Iemma AF (2014) Experimental design and process optimization. CRC Press, Boca Raton
Sabra W, Dietz D, Zeng AP (2013) Substrate-limited co-culture for efficient production of propionic acid from flour hydrolysate. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-013-4913-y
Seeliger S, Janssen PH, Schink B (2002) Energetics and kinetics of lactate fermentation to acetate and propionate via methylmalonyl-CoA or acrylyl-CoA. FEMS Microbiol Lett. https://doi.org/10.1111/j.1574-6968.2002.tb11204.x
StatSoft (2008) Inc., STATISTICA (data analysis software system), version 8.0. www.statsoft.com
Stowers CC, Cox BM, Rodriguez BA (2014) Development of an industrializable fermentation process for propionic acid production. J Ind Microbiol Biotechnol. https://doi.org/10.1007/s10295-014-1423-6
Suwannakham S, Yang S (2005) Enhanced propionic acid fermentation by Propionibacterium acidipropionici mutant obtained by adaptation in a fibrous-bed bioreactor. Biotechnol Bioeng. https://doi.org/10.1002/bit.20473
US Food and and Drug Administration (2019) Food Additive Status List. https://www.fda.gov/food/food-additives-petitions/food-additive-status-list#ftnP. Accessed 28 Nov 2020
Vidra A, Németh Á (2018) Bio-produced propionic acid: A review. Period Polytech Chem Eng. https://doi.org/10.3311/PPch.10805
Wang Z, Jin Y, Yang ST (2015a) High cell density propionic acid fermentation with an acid tolerant strain of Propionibacterium acidipropionici. Biotechnol Bioeng. https://doi.org/10.1002/bit.25466
Wang Z, Lin M, Wang L, Ammar EM, Yang S-T (2015b) Metabolic engineering of Propionibacterium freudenreichii subsp. shermanii for enhanced propionic acid fermentation: effects of overexpressing three biotin-dependent carboxylases. Process Biochem. https://doi.org/10.1016/j.procbio.2014.11.012
Wang X, Salvachúa D, Sànchez i Nogué V, Michener WE, Bratis AD, Dorgan JR, Beckham GT (2017) Propionic acid production from corn stover hydrolysate by Propionibacterium acidipropionici. Biotechnol Biofuels. https://doi.org/10.1186/s13068-017-0884-z
Woskow SA, Glatz BA (1991) Propionic acid production by a propionic acid-tolerant strain of Propionibacterium acidipropionici in batch and semicontinuous fermentation. Appl Environ Microbiol. https://doi.org/10.1128/aem.57.10.2821-2828.1991
Yang H, Wang Z, Lin M, Yang ST (2018) Propionic acid production from soy molasses by Propionibacterium acidipropionici: fermentation kinetics and economic analysis. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.11.016
Zhang A, Yang ST (2009) Propionic acid production from glycerol by metabolically engineered Propionibacterium acidipropionici. Process Biochem. https://doi.org/10.1016/j.procbio.2009.07.013
Zhu Y, Li J, Tan M, Liu L, Jiang L, Sun J, Lee P, Du G, Chen J (2010) Optimization and scale-up of propionic acid production by propionic acid-tolerant Propionibacterium acidipropionici with glycerol as the carbon source. Bioresour Technol. https://doi.org/10.1016/j.biortech.2010.06.070
Zhu L, Wei P, Cai J, Zhu X, Wang Z, Huang L, Xu Z (2012) Improving the productivity of propionic acid with FBB-immobilized cells of an adapted acid-tolerant Propionibacterium acidipropionici. Bioresour Technol. https://doi.org/10.1016/j.biortech.2012.01.055
Zhuge X, Liu L, Shin HD, Li J, Du G, Chen J (2014) Improved propionic acid production from glycerol with metabolically engineered Propionibacterium jensenii by integrating fed-batch culture with a pH-shift control strategy. Bioresour Technol. https://doi.org/10.1016/j.biortech.2013.11.063
Acknowledgements
The authors are grateful to Fapemig for financial support [grant number APQ 02822, 2015], to the Brazilian agency Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/CAPES [grant number 9246142, 2015], to the National Council for Scientific and Technological Development (CNPq) for scholarships, to Coleção de Culturas Tropical–FUNDAÇÃO ANDRÉ TOSELLO (CCT–FAT), Campinas / SP -Brazil for donations of microorganisms and to Laboratório Central de Biologia Molecular/Federal University of Lavras for chromatographic analysis.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MTN, ACFOM, DLHT, LRA, OLM-B, RHP and JGLFA. The first draft of the manuscript was written by MTN and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
About this article
Cite this article
Ngome, M.T., de Oliveira Meira, A.C.F., Torres, D.L.H. et al. Biosynthesis of propionic acid using whey and calcium carbonate by mixed culture of Propionibacterium freundenreichii ATCC 6207 and Lactobacillus paracasei. Braz. J. Chem. Eng. 38, 811–822 (2021). https://doi.org/10.1007/s43153-021-00143-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-021-00143-0