Abstract
Propionic acid (PA) is a short-chain fatty acid with wide industrial application including uses in pharmaceuticals, herbicides, cosmetics, and food preservatives. As a three-carbon building block, PA also has potential as a precursor for high-volume commodity chemicals such as propylene. Currently, most PA is manufactured through petrochemical routes, which can be tied to increasing prices and volatility due to difficulty in demand forecasting and feedstock availability. Herein described are research advancements to develop an industrially feasible, renewable route to PA. Seventeen Propionibacterium strains were screened using glucose and sucrose as the carbon source to identify the best platform strain. Propionibacterium acidipropionici ATCC 4875 was selected as the platform strain and subsequent fermentation optimization studies were performed to maximize productivity and yield. Fermentation productivity was improved three-fold to exceed 2 g/l/h by densifying the inoculum source. Byproduct levels, particularly lactic and succinic acid, were reduced by optimizing fermentor headspace pressure and shear. Following achievement of commercially viable productivities, the lab-grade medium components were replaced with industrial counterparts to further reduce fermentation costs. A pure enzymatically treated corn mash (ECM) medium improved the apparent PA yield to 0.6 g/g (PA produced/glucose consumed), but it came at the cost of reduced productivity. Supplementation of ECM with cyanocobalamin restored productivity to near lab-grade media levels. The optimized ECM recipe achieved a productivity of 0.5 g/l/h with an apparent PA yield of 0.60 g/g corresponding to a media cost <1 USD/kg of PA. These improvements significantly narrow the gap between the fermentation and incumbent petrochemical processes, which is estimated to have a manufacturing cost of 0.82 USD/kg in 2017.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Propionic acid (PA) is a short-chain fatty acid that is commonly used as a food preservative and is also used as a chemical intermediate for the manufacture of pharmaceuticals, herbicides, and cosmetics. As a three-carbon building block, PA also has potential for being a precursor for high-volume commodity chemicals such as propylene. Currently, the vast majority of propionic acid is manufactured through petrochemical processes using the hydroformylation or “oxo” process where ethylene is oxidized in the presence of a transition catalyst and syngas to produce propionaldehyde, then propanol, and ultimately PA. Unfortunately, the economic viability and sustainability of this process is dependent on the availability and price of petroleum feedstock, which has proven to be geographically dependent and volatile. Market demand for propionic acid is currently ~300 kta in both Europe and the US [2]. Latin America currently has a small (9 kta) market, but has shown a significant annual growth rate, exceeding 5 % [2]. This growth, in combination with the availability of sugar feedstock, represents an opportunity to switch from the petrochemical route to a bio-based alternative. Some of these opportunities, such as the production of polyethylene from fermentation-derived ethanol, have already come to fruition [13].
A PA fermentation process provides not only a more sustainable approach, but it also opens the door for PA manufacturing capacity in geographies with limited petroleum supply. PA fermentation processes require an abundant and economical feedstock such as sucrose (sugar cane derivatives), glucose (corn derivatives), glycerol or cellulosics [7, 8, 15, 17–19, 24, 27, 31]. While cellulosic-based feedstocks have several advantages including cost, reduced competition with food sources, and more widespread availability, their application remains very challenging at an industrial scale [21]. On the other hand, glucose- and sucrose-based PA fermentations are well studied and have achieved PA titers exceeding 100 g/l with yields near theoretical maximum [1, 11, 14, 19, 25]. Flexibility between glucose and sucrose as fermentation substrates is desired as availability is geographically dependent. United States (US) sugar production is primarily glucose from corn while in countries like Brazil production is primarily sucrose from sugar cane [6].
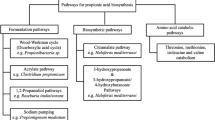
Propionic acid is naturally produced by a group of bacteria classified as Propionibacteria when fermented under anaerobic conditions [23, 24, 28, 29]. At least 60 different Propionibacterium strains have been characterized, but unfortunately much of this data is limited and was collected under a variety of fermentation conditions, making it difficult to compare strain performance. Most, if not all, Propionibacteria produce propionic acid from pyruvate through a dicarboxylic acid cycle known as the Wood-Werkman cycle (see Figure S1 of Supplementary Materials). Unfortunately, this pathway is redox-constrained when either glucose or sucrose is catabolized via glycolysis, limiting substrate yield to ~0.55 g/g [5, 15, 18]. Propionibacteria also produce significant amounts of byproducts such as succinic, lactic, and acetic acids, which detract from PA yield and complicate product recovery. Previous work has shown that some of these byproducts can be reduced, but not eliminated, by controlling the fermentation process conditions [23].
PA itself causes significant growth and product inhibition in Propionibacterium making strain selection and adaptation necessary [7, 11, 25, 29, 30]. In fact, just 1 % (w/v) of PA was shown to reduce Propionibacterium growth rate by 50 % [3]. Inhibition occurs because at high PA concentrations, undissociated PA can diffuse into the cell where it dissociates in the presence of the alkaline cytoplasm forming an abundance of protons within the cell. This ultimately disturbs the pH gradient across the plasma membrane, affecting metabolism and ultimately cell growth [10]. Attempts have been made to engineer an extractive fermentation system to prevent the PA concentration from achieving inhibitory levels; however, these systems require large amounts of solvent and increased capital expenditure [9, 16]. Thus, in order to achieve the most industrializable and economical bioprocess, a strain must be selected that is sufficiently tolerant to high titers of PA such that the concentration is amenable to efficient recovery operations. Furthermore, byproducts must be minimized as it is very difficult to selectively remove PA from similar organic acids. Unfortunately, the formation of some byproducts could be required to maintain redox potential, while the formation of other byproducts are likely caused by over-flow metabolism as a result of rapid sugar catabolism.
Relatively little progress has been made in improving the yield of PA from sugars, which despite over 50 years of research has remained in the range of 0.55–0.65 g/g, depending on the substrate. Fermenting a more reduced substrate such as glycerol generally produces higher PA yields, but the economics and centralized availability of glycerol as a large-scale feedstock remains questionable [27, 28, 30]. For this reason, a high-yield glucose or sucrose process is likely the most economically viable route to high-volume PA.
In this work, we describe a survey of 17 different Propionibacterium strains in serum bottles to identify the best platform strain for PA production. The top performing strains were then evaluated at the fermentor scale to gauge performance in terms of PA productivity, yield, and purity. Using the Propionibacterium strain with the highest baseline fermentation performance as a development platform, overall performance was then improved by modifying the media and process conditions. Lastly, fermentation costs were reduced by identifying industrially available media sources to replace the traditional lab-grade media components.
Materials and methods
Strain
Seventeen different Propionibacterium strains were acquired from the American Type Culture Collection (ATCC) strain bank as identified in Table 1. Each strain was initially cultured as described in “Medium” and “Culture conditions” in serum bottles. An optical density (OD) of 0.5 AU (600 nm) culture was then preserved in 15 % (v/v) glycerol stock stored at −80 °C.
Medium
Propionibacterium strains were cultured in a complex medium containing (per liter): 10 g yeast extract (Fisher Scientific BP14222, Indianapolis, IN, USA), 5 g trypticase soy broth (Fisher Scientific B11768), 0.25 g dibasic potassium phosphate (Fisher Scientific BP363), 0.056 g MnSO4 • H2O (Sigma Aldrich M7899, Indianapolis, IN) and varying amounts of glucose or sucrose. If mentioned in the experimental design, 50 g/l of calcium carbonate was used for pH buffering in serum bottles. The medium was made concentrated to accommodate the addition of 500 g/l carbon source and was sterilized (void of carbon source) by treatment in an autoclave or by steam in place technology for 30 min at 121 °C and 15 psig. The carbon source was sterilized separately and aseptically combined with the sterile medium to typically target a concentration of 100 g/l. If enzymatically treated corn mash (ECM) was used in the experiment, the material was supplied by Abengoa Biosciences as a milled and alpha amylase treated corn mash with a solid content of about 30 %. The ECM was subsequently prepared as follows. ECM was treated with glucoamylase to hydrolyze starch fragments into free glucose by adding 14 ppm of glucoamylase (1,425 glucose units per gram) to the corn mash and then heating the material to 60 °C in a 30-l fermentor with 200-rpm agitation for approximately 15 min to activate the enzymatic hydrolysis. After the saccharification, the corn mash was cooled, the glucose content was measured, and then the material was diluted appropriately with water to achieve the desired glucose concentration for the experiment. In ECM experiments utilizing vitamin and mineral supplementations, the ECM supplements were added post-sterilization under aseptic conditions as described. The minerals comprised of ZnCl2, FeCl2, MnCl2, and CaCl2 at a final fermentation concentration of 0.05 mM each. The vitamins comprised of riboflavin, pantothenic acid, biotin, thiamine, and cyanocobalamin at a final fermentation concentration of 2 mg/l each. In medium-optimization studies, the complex nitrogen components (i.e., yeast extract, trypticase) were directly replaced with an industrial counterpart by weight.
Anaerobiosis was achieved initially by sparging 99.9 % pure nitrogen through the medium in fermentors or by purging the headspace of serum bottles immediately following sterilization.
Culture conditions
Fermentations were conducted in Biostat C plus, 30-l fermentation vessels (Sartorius AG, Goettingen, Germany). The post-inoculation fermentation volumes were 15 l. During fermentation, overlay 99.9 % pure nitrogen was used to maintain anaerobic conditions in fermentors and supply headspace pressure. The temperature was controlled at 32 °C. Automatic addition of concentrated ammonium hydroxide (14.6 M) was used to control pH at 6.5 in fermentors. Pressure was maintained in fermentors at 0–1,500 mBar, typically 700 mBar. Fermentor agitation was provided by three 4.75″-diameter Rushton design impellers with tip speeds ranging from 0.31 to 6.79 m/s, typically 0.61 m/s.
Small-scale fermentations were completed in 125-ml stoppered and crimp-sealed serum bottles (Fisher Scientific 06-406J). No atmospheric control was implemented with the serum bottles. However, during sampling, some of the gas pressure that had built up in the bottles was released. Serum bottles were generally inoculated from a seed culture at 0.6 % (v/v) as described below. During strain comparative studies, a 0.6 % (v/v) inoculum amount was targeted but inoculum volumes were normalized by OD600. Sampling occurred typically every 12 h and all samples were immediately frozen at −20 °C until fermentation completion.
To prepare seed serum bottles, 300 μl of culture was thawed in an anaerobic vial and cryogenically sealed with a septum. The 300-μl culture was transferred via syringe to 50 ml of medium described above in a serum bottle supplemented with glucose or sucrose and incubated statically at 32 °C. After approximately 24 h, this culture typically achieved an OD600 of 0.5 and could be used to inoculate subsequent production serum bottles at 0.6 % (v/v). If additional biomass accumulation was required for bioreactor-scale experiments, 8 ml of the typically OD600 of 0.5 serum bottle seed was transferred into an anaerobically treated 2-l bottle with 1 l of identical media, grown under the same conditions to again reach an OD600 of 0.5. This culture was then used to inoculate a seed bioreactor at 5 % (v/v) and operated at the conditions described above. Once the seed bioreactor achieved an OD600 of 5–10, it was used to inoculate production fermentors at 5–10 % (v/v). A figure detailing the process steps involved in this cultivation from the inoculation of the seed serum bottle to the production fermentation is available in the Supplementary Information (see Figure S2).
For high cell-density experiments in serum bottles and bioreactors, the seed culture was aseptically concentrated by centrifugation. Sampling occurred typically every 12 h and all analytical samples were immediately frozen at −20 °C until fermentation completion.
Analytical methods
Optical density measurements were made using blank medium for background subtraction and dilution where applicable. Optical density was measured using 1-cm path cuvettes on a Thermo Genesis A10 UV spectrophotometer immediately after sampling.
After thawing and sufficient mixing of frozen samples, 1 ml was centrifuged and clarified by 0.2-μm filtration. Propionic (PA), acetic (AA), succinic (SA), and lactic acid (LA), as well as glucose or sucrose, concentrations were determined by high-performance liquid chromatography using an ion exclusion column Aminex HPX-87H, 300 × 7.8 mm (Bio-Rad, Hercules, CA, USA). The operation temperature was 45 °C with an isocratic elution of 0.005 M H2SO4 at a rate of 0.6 ml/min. The injection volume was 5 μl and the detector monitored refractive index for comparative quantitation to authentic standards.
Results and discussion
Organism selection
Approximately 60 different strains of Propionibacteria were identified through searches of the DSMZ, ATCC, NRRL, and CCC strain banks. While many of these strains had little or no PA fermentation data in the scientific literature, it was clear that the P. acidipropionici and P. freudenreichii species were consistently strong producers of PA. Based on this observation, 17 strains were selected for fermentation screening (see Table 1), of which ten were of P. acidipropionici and P. freudenreichii species. The remaining seven strains were selected in a manner to acquire a representative set from other species, but were somewhat contingent on strain availability from the strain banks.
Serum bottle screen
P. acidipropionici ATCC 4875 is one of the most well-studied Propionibacterium strains for PA production. Since there is an abundance of both internal and literature data for this strain, it was chosen as a reference to evaluate the relative performance of the remaining 16 strains. The goal of the screen was to identify a strain with high PA tolerance, high productivity, and PA purity comparable to or better than P. acidipropionici ATCC 4875. A two-tier, three-metrics system was devised to identify both PA tolerance and PA productivity mutants without compromising on PA purity. In the first tier, the two metrics for strain evaluation were PA titer at 120 h of fermentation and PA productivity between 24 and 72 h of fermentation. PA titer at 120 h was selected as a metric because the titer accretion curve of many strains began to flatten between 72 and 120 h, presumably due to PA or pH inhibition. Strains that had higher productivity and were tolerable to higher PA titers would produce the highest titers at 120 h of fermentation. The downside to this metric is that strains with longer lag phases or strains that were highly susceptible to PA inhibition would score poorly based on this metric. In order to not miss mutants that had an innate capacity to produce PA at higher rates, PA productivity between 24 and 72 h was also used as a metric. The 24–72 h time period was selected because many of the strains had a 24 h lag period for PA production. If productivities were calculated from the beginning of the fermentation, strains with longer lag phases would be penalized for a phenotype that could potentially be removed by strain adaptation or process optimization. Strains selected from both the 120-h PA titer and 24–72-h productivity were advanced to the second-tier screen where a PA/AA ratio was used as the sole metric. The PA/AA ratio was used as the second-tier metric since AA is a main byproduct that complicates product recovery and is very difficult to minimize during fermentation by modifying the fermentation conditions. Removal of AA production by metabolic engineering has also proven to be very challenging [25].
Table 1 and Fig. 1 summarize the glucose and sucrose screen data for the 17 Propionibacterium strains. Four strains (P. acidipropionici ATCC 55737, P. jensenii ATCC 4868, P. intermedium ATCC 14072, and P. acidipropionici ATCC 25562) out-performed P. acidipropionici ATCC 4875 in terms of 120-h titer on glucose. Two strains (P. acidipropionici ATCC 55737 and P. theonii ATCC 4874) out-performed P. acidipropionici ATCC 4875 in terms of 24–72 h productivity on glucose. Unfortunately, when these five strains were subjected to the PA/AA metric, only P. acidipropionici ATCC 55737 showed performance better than P. acidipropionici ATCC 4875 on glucose.
On sucrose, the strains (P. acidipropionici ATCC 4965, P. acidipropionici ATCC 25562, and P. theonii ATCC 4874) had 120-h PA titers higher than P. acidipropionici ATCC 4875. All three of these strains also had improved 24–72 h productivity when compared to P. acidipropionici ATCC 4875. In addition to these three strains, P. cyclohexanicum ATCC 700612 had higher 24–72-h productivity relative to P. acidipropionici ATCC 4875. When these four strains were subjected to the PA/AA metric, only P. acidipropionici ATCC 4965 and P. acidipropionici ATCC 25562 met the advancement criteria. Therefore, considering all three evaluation criteria for both the glucose and sucrose screens, only three strain-substrate combinations, P. acidipropionici ATCC 4965-sucrose, P. acidipropionici ATCC 25562-sucrose, and P. acidipropionici ATCC 55737-glucose, showed performance superior to P. acidipropionici ATCC 4875 in serum bottles.
Fermentor-scale strain evaluations
The serum bottle screen unfortunately cannot be perfectly predictive of fermentor-scale performance since serum bottles do not have pH control, continuous gas sparging, or internal agitation. Due to the limitation that serum bottles do not have pH control, the serum bottle screen will favor strains that are more tolerant to low pH or high free PA concentrations, but these phenotypes might not translate into improved PA productivities or byproduct ratios at the industrial fermentor scale. In the serum bottle screen, most fermentations reached a final pH of 4.0–5.0 with the higher-titer strains having a pH closer to 4.0. In the fermentor, pH was controlled at 6.5, so strains with lower free acid or pH tolerance would not have been disadvantaged.
The top three strain-substrate combinations from the serum bottle screen were evaluated in 30-l fermentors under identical conditions using pH control, headspace supplied nitrogen, and agitation. In a bioreactor fermentation on glucose, P. acidipropionici ATCC 55737 produced only roughly half the titer of P. acidipropionici ATCC 4875. As shown in Fig. 2, P. acidipropionici ATCC 55737 PA titer was considerably lower throughout the entire fermentation. This is a surprising result considering the serum bottle data indicated P. acidipropionici ATCC 55737 achieved a significantly higher 120-h PA titer and had a higher PA productivity between 24 and 72 h compared to P. acidipropionici ATCC 4875. Even more interesting is that the media composition used in the serum bottle and fermentor evaluations were identical, except for the 2.5-fold higher glucose concentration in fermentors. There are at least three potential explanations for the lower-than-expected P. acidipropionici ATCC 55737 performance in fermentors: differences in PA inhibition at higher titers or pH values, variable substrate inhibition due to higher glucose concentrations in the fermentor, or metabolic shifts induced by the headspace pressure of the fermentor. The metabolic shift hypothesis is supported by the fact that P. acidipropionici ATCC 55737 accumulated 25 g/l of LA in the fermentor compared to only 6 g/l of LA for P. acidipropionici ATCC 4875. Clearly, P. acidipropionici ATCC 55737 metabolism was shifted towards LA production relative to P. acidipropionici ATCC 4875. Further optimization of fermentor conditions could lead to improved P. acidipropionici ATCC 55737 performance. However, it is clear that under the experimental conditions employed in the fermentors, P. acidipropionici ATCC 4875 is the superior strain when grown on glucose.
Both P. acidipropionici ATCC 4965 and P. acidipropionici ATCC 25562 were evaluated in fermentors with sucrose relative to P. acidipropionici ATCC 4875. Similar to the P. acidipropionici ATCC 55737 results on glucose, P. acidipropionici ATCC 25562 produced less than half the titer of P. acidipropionici ATCC 4875 (see Fig. 3). The results with P. acidipropionici ATCC 4965 were however far more positive. After 72 h of fermentation, P. acidipropionici ATCC 4965 had produced ~40 g/l of PA, roughly 90 % of the P. acidipropionici ATCC 4875 PA titer. P. acidipropionici ATCC 4965’s LA levels were comparable to P. acidipropionici ATCC 4875, but it had produced ~4 g/l less AA and ~10 g/l less SA after 72 h of fermentation (data not shown). Thus, in this case, the P. acidipropionici ATCC 4965 PA/AA improvement identified in the serum bottle screen translated to the fermentor scale. Although P. acidipropionici ATCC 4965 has merit as a platform strain for PA production, it did produce a slightly lower final titer than P. acidipropionici ATCC 4875 during the fermentor evaluation. Furthermore, P. acidipropionici ATCC 4965 yield was lower at 0.37 g/g compared to 0.48 g/g for P. acidipropionici ATCC 4875. It is worth noting that despite all three strains outperforming P. acidipropionici ATCC 4875 in terms of 120 h PA titer and productivity between 24 and 72 h in serum bottles, none of the three selected strains reproduced this result at fermentor scale. Clearly the serum bottles, which are widely used as a strain evaluation and process development platform, were not very predictive of fermentor scale performance. Based on this observation, it is conceivable that one of the strains that underperformed in serum bottles, and therefore were not advanced to fermentor scale testing, could produce higher PA titers than P. acidipropionici ATCC 4875 in fermentors. However, fermentor-scale evaluations with all 16 strains would require a substantial amount of research capacity and would further delay process development work with P. acidipropionici ATCC 4875. So despite the risk, P. acidipropionici ATCC 4875 was chosen as the platform strain for process development research.
Fermentation process development with ATCC 4875
After selection of P. acidipropionici ATCC 4875 as the platform strain for fermentation process industrialization, a series of representative studies were conducted to optimize PA productivity, improve PA purity, and reduce media costs. The following three sections describe process and medium optimization studies which each resulted in a significant improvement in fermentation performance.
Optimization of fermentation process conditions to minimize byproducts
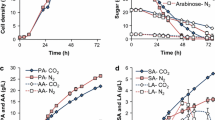
As shown in Figs. 2 and 3, ATCC 4875 fermentations accumulate significant amounts of organic acid byproducts. In order to maximize PA yield and minimize the cost of PA recovery, an industrializable PA fermentation process should minimize byproduct accumulation. As previously mentioned, we have found that AA accumulation is largely unaffected by the fermentation process conditions. This is likely related to the fact that AA accumulation is required for ATP or NADH generation (See Supplementary Data Figure S1). For this reason, we employed a strain selection criterion to identify strains with reduced AA levels and later focused fermentation process development activities around minimizing LA and SA. We have shown that LA is catabolized into PA under glucose-starvation conditions and therefore can be eliminated from the fermentation broth by simply extending the fermentation time beyond glucose exhaustion [22]. However, the approach of running PA fermentations under glucose limited conditions to minimize byproduct levels is not desirable from an industrial perspective because limiting glucose levels also limits productivity. Figure 4 below shows that fermentor headspace pressure can be used to significantly minimize LA production obviating the need to run the fermentation to glucose exhaustion. Neither SA nor AA titers were significantly affected by backpressure in the ranges tested. 1,500 mbar of pressure prevented the LA titer from exceeding 3 g/l, a 3–4 fold reduction compared to fermentations run at 0-350 mbar. The exact mechanism behind how headspace pressure reduces LA accumulation is unknown. However, it is known that the solubility of gases, such as carbon dioxide, increases at higher headspace pressures and can have an effect on cellular metabolism. As shown by Table 2, AA and SA titer were unaffected by headspace pressure so it is unclear where the LA metabolic flux is redirected to at higher pressures. However, considering that 1,100 and 1,500 mbar pressures produced marginally higher titers and propionic acid yields, one hypothesis is that the higher pressures drive the conversion of pyruvate to PA and away from LA by providing additional carbon dioxide substrate for pyruvate carboxylase. However, this hypothesis could explain only half of the carbon differential between the 0 and 1,500 mbar conditions considering that LA titer is reduced by ~8 g/l and PA titer increased by only ~4 g/l.
As shown in Fig. 5, fermentor agitation rate or shear also had an effect on byproduct accumulation levels, although in most cases, the effects were less significant than was seen when varying fermentor headspace pressure. Higher shear rates tended to increase LA production (Fig. 5d) and reduce SA production (Fig. 5c). PA yield was not significantly affected by agitation rate. The highest agitation rate, 1,100 rpm, produced marginally lower ~70 h titer, but it is difficult to say this difference is significant considering the earlier PA titer points were comparable to the fermentations with lower agitation rates. The increase in LA at higher shear rates is not of huge concern considering that LA accumulation can likely be avoided by using high pressures or running the fermentation to glucose starvation. It is, however, worth noting that high shear rates are often difficult to avoid at large scale due to the increased agitator tip speed and superficial gas velocities of large scale fermentors. Thus, pressures even higher than evaluated in this study may need to be employed to maintain LA titers at acceptable levels.
Fermentation productivity optimization
PA fermentation productivity with ATCC 4875 was substantially increased by densifying the biomass level of the inoculum. Triplicate serum bottles with complex medium including 50 g/l calcium carbonate to provide pH buffering and inoculated with variable biomass levels that were aseptically concentrated from identical stock culture. In each case, the volume of inoculum remained constant, but the biomass varied from 1× (control), 2×, 4×, 7×, and 10×, where 1× corresponds to 2.5 g DCW/l and 10× corresponds to 25 g DCW/l. Figure 6 shows that the productivity was significantly enhanced with higher cell density inoculum, but the effect was highly non-linear. Doubling the inoculum density had no measurable effect in productivity at 24 h, but increasing the inoculum by tenfold improved final productivity by ~45 %. Increasing inoculum level slightly improved PA/AA ratio, but no effect on the PA/SA ratio was observed. At an inoculum level of 2.5 g DCW/l, the final PA/AA ratio was 5.6 ± 0.7 compared to 8.6 ± 0.7 at an inoculum density of 25 g DCW/l. We suspect that the higher PA/AA ratio at higher inoculum densities is related to the fact that the higher inoculum cultures tended to grow less, as evident by a smaller increase in post-inoculation OD, and therefore required less ATP, which is produced through acetate generation.
A similar experiment was conducted in 30-l fermentors at 1× (control), 5× and 30× inoculum levels, where 1× corresponded to 0.14 g DCW/l biomass. Here, in order to demonstrate an integrated example, we also applied our learnings regarding fermentor backpressure and shear to minimize lactic acid accumulation. Thus, for all three inoculum levels, fermentor backpressure was held at 1,100 mbar and agitation was held at 50 rpm. As seen in the serum bottles, higher inoculum densities achieved substantially higher productivities. In fact, as shown in Fig. 7, the 30× inoculum achieved a sustained productivity of more than 2 g/l/h, one of the highest PA fermentation productivities reported to date. This is roughly a threefold improvement in productivity compared to the 1× inoculum condition, which had a productivity of 0.73 g/l/h. Furthermore, this was achieved while accumulating only ~2 g/l lactic acid by keeping backpressure high and agitation shear low. While further improvements in productivity are likely attainable, our previous work has shown that productivity improvements beyond 1 g/l/h have little return on investment for PA process economics [22]. For this reason, the remainder of our process development work focuses on variable fermentation cost reduction.
Thirty-liter fermentor organic acid titers as a function of inoculum density. Propionic acid is shown as the thick solid lines, lactic acid is shown as the long dashed lines, acetic acid is shown as the short dashed lines, and succinic acid is shown as the thin solid lines. The fermentor was operated at a backpressure of 1,100 mbar and agitation of 50 rpm to minimize lactic acid accumulation
Medium optimization to reduce manufacturing cost
The ATCC 4875 fermentation media was industrialized to reduce fermentation cash costs while preserving PA productivity and byproduct ratios. As detailed in the “Materials and methods” section of this paper, the base lab-grade medium consisted of tryptic soy broth (TSB), yeast extract (YE), dibasic potassium phosphate, manganese sulfate, and a carbon source such as glucose or sucrose. The most costly components of the lab-grade medium are the TSB and the YE. These two components alone account for 70 % of the media cost for the production of PA using lab-grade medium. In these studies, the yeast extract was directly replaced with an industrial equivalent and the TSB was replaced with an industrial-grade soy product. Although TSB in the lab-grade medium contains ~60 % casein and ~10 % soy, medium optimization was focused purely on soy products since these products are often less expensive than casein-based products.
Six different soy products from Marcor Corp. were evaluated in serum bottles relative to the standard lab media recipe. Three of the products were hydrolyzed through papaic digestion: soy peptone A, soy peptone H and soy peptone G, while the other three soy products were unhydrolyzed: Alpha DS, Alpha 5800, and Supro 595. The three peptones are fully soluble products whereas the unhydrolyzed soy products are not. The results of Fig. 8 show that the Soy Peptone A product improved the 168-h titer by more than 50 %, while Alpha DS, Alpha 5800, and Soy Peptone H had equivalent to slightly improved 168-h titer. Interestingly, all six of the products showed a statistically significant titer improvement 48 h into the fermentation (See Supplementary Information Figure S3). It is important to note that soy protein hydrolysis does not necessarily improve titer as two of the top three performers were unhydrolyzed. Furthermore, the lab-grade BBL TSB, which is hydrolyzed, produced the lowest 48- and 72-h titers (See Supplementary Information Figure S3). The relatively poor performance of the BBL TSB could be due to a difference in starting material or processing (hydrolysis, filtering, etc.). The titer improvement with the industrial soy products strongly correlates to a difference in biomass level as shown by the linear regression of OD and titer in Supplementary Information Figure S4. Thus, the improvement the soluble products afforded seems to be related to increased biomass and not an increase in specific cell productivity. Although all seven conditions were inoculated identically, the Soy Peptone A fermentations had produced up to twice the biomass by 72 h. OD could not be measured in the three unhydrolyzed soy products due to the presence of solids so it is unclear whether a correlation between cell growth and PA titer exists for these products.
The screening of eight yeast extract products with the ATCC 4875 strain gave nearly a threefold difference in final titers. As shown in Fig. 8 and Supplementary Figure S5, five of the eight commercially available yeast extract products produced higher final titers than the lab-grade yeast extract. The three Bionis yeast extracts had the highest final titers with a range of 5.93–8.94 g/l, a 25–88 % improvement over the lab-grade yeast extract. The yeast extract screening titers had no statistically significant correlation with OD, but a weak correlation can be seen with the protein content of each material as shown in Supplementary Figure S6. The Bionis yeast extract products had a minimum protein content of 64.4–71.7 % (w/v), whereas the other products had 42.5–53.3 % (w/v) minimum protein content or lower. Since there was no correlation between titer and OD, it is clear that yeast extract protein content can affect PA specific productivity. The presence of additional protein could alleviate nitrogen, or more specifically an amino acid, limitation that allows the culture to produce PA at higher rates. More testing would be required to determine the specific molecule that is limiting PA productivity.
Based on the serum bottle yeast extract and soy product screening results, and considering the cost of each product, two yeast extract—soy product combinations were evaluated at the 30-l fermentor scale using pH control. Despite the superior performance of the soy peptone in the serum bottle screen, it was not selected for fermentor evaluation due to high relative cost. Rather independent Bionis YE NS—Alpha 5800 and Bionis YE NZ—Alpha DS combinations were evaluated. Figure 9 shows that the Bionis YE NZ—Alpha DS fermentation underperformed producing about 40 % lower PA titers, where the Bionis YE NS—Alpha 5800 fermentation closely matched the lab-grade medium performance. The Bionis YE NZ—Alpha DS fermentation accumulated ~15 g/l of LA, whereas the other media recipes had negligible amounts of LA at the end of the fermentation. There was very little difference in SA and only a modest difference in AA across the three media recipes (see Table 3). It is unclear whether the poor performance of Bionis YE NZ—Alpha DS is due to the Bionis YE NZ, Alpha DS, or a combination of both products. More experimentation could reveal that one of the products could be used without deterioration in performance.
In order to further reduce cost and simplify the fermentation process for industrialization, fermentation experiments were conducted using enzymatically treated corn mash (ECM) as both a replacement for sugar (glucose) and as a complete medium. ECM is an intermediate product to corn syrup and has been used for several years in ethanol fermentation [4]. ECM contains glucose as well as unrefined free sugars and other corn-based organic compounds such as proteins, amino acids, and trace minerals. ECM typically contains about 30 % dry solids, but only about 20 % glucose on a solids basis, meaning roughly 10 % of the material remains as a mixture of fermentable and non-fermentable substrates.
ECM was previously evaluated as a glucose replacement in lab-grade medium and shown to produce comparable results to lab-grade medium in fibrous bed bioreactors (FBB) [12]. We found a similar result in conventional free-cell fermentations using strain ATCC 4875 (data not shown). Far more surprising is that our experiments show that pure ECM itself is a suitable medium for PA production using ATCC 4875. With minimal supplementation, ECM performance can achieve 68 % of lab-grade medium performance in terms of productivity with no loss in fermentation yield and lower byproduct levels compared to lab-grade medium (see Fig. 10). In fact, Fig. 10 shows that the 25 g/l of PA was made from only 40 g/l of glucose indicating that in the ECM fermentation the apparent yield from glucose is enhanced, albeit likely artificially, from 0.48–0.55 g/g in lab-grade medium to 0.60 g/g in ECM. It seems likely that the apparent yield enhancement is due to the catabolism of non-glucose fermentable sugars within the ECM. In any case, since ECM is sold based on glucose content, this additional yield comes at practically no additional cost relative to using a more refined glucose supply. Supplementation with vitamins can significantly enhance productivity such that 0.5 g/l/h of propionic acid is produced during the first 40 h of fermentation as shown in Fig. 10. Further experimentation (data not shown) revealed that cyanocobalamin, which is a known Propionibacterium growth stimulant [26], was responsible for the productivity enhancement. Supplementation with inorganic minerals such as zinc, manganese, magnesium, or iron had no detectable effect on fermentation productivity or yield (data not shown). Overall, the supplemented ECM results represent the most attractive medium for an industrialized PA fermentation process since the medium is both low cost and simple. Based on the data shown with lab-grade medium, additional experimentation with higher-density inoculums could lead to even higher ECM productivities. The main disadvantage to this process is that ECM is available in large quantities only in certain geographies. ECM is widely available in the US, but not in countries such as Brazil where sugar production is heavily focused on sucrose from sugar cane. Sugar cane has been shown to be a suitable carbon source for PA fermentations [8]; however, analogous experiments using crude sucrose products such as direct sugar cane extracts as the main medium source have yet to be completed. It is certainly conceivable that sugar cane juice medium could be developed using minimal supplementation.
Process economics
The main purpose of industrializing a fermentation process is to deliver a process with lower overall cost such that the process is competitive with alternative, or in the case of PA, existing petrochemical routes. Two cost parameters are typically important from an industrial perspective: (1) capital cost or the investment required to initially build the industrial scale process, which typically includes equipment and installation costs, (2) cash costs which are the operating costs for the industrialized process, which typically include the cost of raw materials, utilities, labor, and permitting. Of most importance for our work is the fermentation operating or cash costs, which are largely dominated by the feedstock and fermentation media costs. Details on the capital cost estimates for an industrialized PA process can be found in a separate publication [22].
In this work, industrialization of the media composition using commercially available yeast extract and soy products, reduced the media cost nearly sevenfold. Still, due to limitations on yield and the cost of the other media components (trace minerals, potassium phosphate, etc.), the media cost per kilogram of PA was still ~ fourfold higher than the market cost for PA. Based on this observation, alternative media recipes were investigated with the intent of drastically reducing the fermentation media cost. ECM with or without vitamin B12 supplementation gave a final media cost of ~$1 per kilogram of PA, which is near the current market price for PA. A comparison of media costs and productivities is provided in Fig. 11.
Fermentation media cost per kg of propionic acid produced and fermentation productivity using lab-grade and industrialized mediums. Cost per kilogram of propionic acid is shown as the grey bars and is plotted on the left axis. Productivity up to a titer of 30 g/l is shown as the black line with solid diamonds and is plotted on the right axis
Utilizing the fermentation cash cost factors developed in this study and assuming a fermentation yield of 0.50–0.60 g/g and product recovery costs detailed elsewhere, a comparison to the cash costs of the incumbent petrochemical “oxo” process can be done as shown in Fig. 12 [22]. The comparison reveals that in today’s dollars the PA fermentation process is still not competitive; however, at a yield of 0.55 g/g or greater, today’s process would be economically advantaged over the petrochemical route within the next 10–15 years. Further improvements in fermentation yield could drastically reduce the time for the bio-based PA process to become the more profitable route. As previously mentioned, the ECM process can produce apparent yields of 0.60 g/g PA, but productivity suffers with ECM causing a significant increase in capital expenditure and making it unreasonable for industrialization at this point [22]. Furthermore, biomass recovery becomes complicated due to the insolubles present in the ECM resulting in a yield penalty for losses in biomass between cycles. As a result, our current cash cost model assumes zero biomass recycle per fermentation batch in order to reflect an un-optimized process capable of significant refinement. Despite the fact that 0.60 g/g process is not yet industrially viable due to low productivity, a 0.60 g/g yield cash cost curve is shown in Fig. 12 for representative purposes. This curve shows that a 0.60 g/g PA fermentation process would be competitive as early as year 2020. The details of this economic analysis including an evaluation of cash cost sensitivity to productivity, yield, and raw material cost as well as estimations for capital costs based on fermentation productivity can be found in a separate publication [22].
Conclusions
Seventeen different Propionibacterium strains were screened in serum bottles and evaluated based on PA titer, productivity, and purity relative to organic acid byproducts. P. acidipropionici ATCC 4875 was selected as the platform strain when fermented on both glucose and sucrose in fermentors. To the best of our knowledge, this is the most extensive screen of Propionibacterium strains reported in a single study. Fermentation optimization was conducted using P. acidipropionici ATCC 4875 to reduce byproduct levels and increase productivity and yield. Optimization of agitation rate and fermentor head space pressure led to a sixfold reduction in LA and a 20 % reduction in SA. These optimization strategies provide a scalable method for controlling byproduct levels to potentially improve yield and product recovery efficiencies. Fermentation productivity was also increased more than threefold to achieve one of the highest sustained productivities reported to date of 2 g/l/h by densifying the inoculum source.
Medium optimization studies were conducted to reduce media costs and improve economics using industrial-grade media components. Fermentation with Bionis YE NS yeast extract and Alpha 5800 soy protein gave results comparable to the lab-grade medium components and reduced media costs more than sevenfold. Fermentation with pure and slightly supplemented ECM gave the most attractive results. Pure ECM media produced an apparent fermentation yield on glucose of 0.60 g/g compared to 0.48–0.55 for the previously evaluated media recipes, but unfortunately productivity was reduced significantly. An optimized ECM recipe with 2 mg/l cyanocobalamin supplementation partially restored productivity to 0.5 g/l/h. Further optimization, particularly looking at inoculum density, could lead to even higher ECM productivities. The optimized ECM recipe further reduced media costs fourfold to a final media cost of less than 1 USD per kilogram of PA, the lowest achieved in this study.
A cash costs comparison between an industrialized PA process and the current petroleum-based “oxo” process revealed than an economically competitive fermentation process is within reach, but fermentation yield must be further improved to be competitive in today’s dollars. Improving the actual fermentation yield from glucose or sucrose beyond 0.55 g/g will likely entail metabolic engineering to redistribute the carbon flow within the dicarboxylic acid pathway and alleviate redox constraints. While significant progress has been made in metabolic flux analysis and pathway engineering, metabolic engineering still remains as a major challenge in P. acidipropionici due to low transformation efficiencies and the lack of replicative plasmids. However, recently a transformation method was published for Propionibacterium that enabled the conversion of propionic acid into propanol [20].
Although significant progress was made in the development of an industrializable process, several challenges still remain. Utilization of the ECM as a medium supply will be limited to geographies with a large corn glucose supply such as the US, leaving countries such as Brazil without a feasible process. Furthermore, ECM PA productivity must be further improved to at least 1 g/l/h to reduce capital costs to economically viable levels [22]. Increasing biomass levels in the ECM media could improve productivity as seen with lab-grade media in this study, but at an industrial scale, recovery of biomass in a solid laden media could be challenging. Thus, the utilization of cell recovery and recycling methods must be thoroughly evaluated. Despite all of these remaining challenges, this work demonstrates that fermentation using P. acidipropionici ATCC 4875 to produce PA has significant potential as an economically competitive, industrializable process.
References
Barbirato F, Chedaille D, Bories A (1997) Propionic acid fermentation from glycerol: comparison with conventional substrates. Appl Microbiol Biotechnol 47:444–446
Bizzari SN, Blagoev M (2013) Propionic acid. In: Chemical economics handbook: IHS chemical. http://www.ihs.com/products/chemical/planning/ceh/propionic-acid.aspx
Blanc P, Goma G (1987) Kinetics of inhibition in propionic acid fermentation. Bioprocess Eng 4:175–179
Bothast RJ, Schlicher MA (2005) Biotechnological processes for conversion of corn into ethanol. Appl Microbiol Biotechnol 67:19–25
Boyaval P, Corre C (1995) Production of propionic acid. Lait 75:453–461
Process Chemical Program, IHS Chemical: Calculated from information obtained from Nexant, 2011
Coral J, Karp S, Porto de Souza Vandenberghe L, Parada J, Pandey A, Soccol C (2008) Batch fermentation model of propionic acid production by Propionibacterium acidipropionici in different carbon sources. Appl Biochem Biotechnol 151:333–341
Feng X, Chen F, Xu H, Wu B, Li H, Li S, Ouyang P (2011) Green and economical production of propionic acid by Propionibacterium freudenreichii CCTCC M207015 in plant fibrous-bed bioreactor. Bioresour Technol 102:6141–6146
Gu Z, Glatz BA, Glatz CE (1997) Propionic acid production by extractive fermentation. 1. Solvent considerations. Biotechnol Bioeng 57:454–461
Gu Z, Rickert DA, Glatz BA, Glatz CE (1999) Feasibility of propionic acid production by extractive fermentation. Lait 79:137–148
Himmi EH, Bories A, Boussaid A, Hassani L (2000) Propionic acid fermentation of glycerol and glucose by Propionibacterium acidipropionici and Propionibacterium freudenreichii ssp. shermanii. Appl Microbiol Biotechnol 53:435–440
Huang YL, Wu Z, Zhang L, Cheung CM, Yang ST (2002) Production of carboxylic acids from hydrolyzed corn meal by immobilized cell fermentation in a fibrous-bed bioreactor. Bioresour Technol 82:51–59
Process Chemical Program, IHS Chemical: Calculated from information obtained from Nexant, 2012, pp 1–31
Kagliwal LD, Survase SA, Singhal RS, Granström T (2013) Wheat flour-based propionic acid fermentation: an economic approach. Bioresour Technol 129:694–699
Leaver FW, Wood HG, Stjernholm R (1995) The fermentation of three carbon substrates by Clostridium propionicum and Propionibacterium. J Bacteriol 70:521–530
Lewis V, Yang ST (1992) A novel extractive fermentation process for propionic acid production from whey lactose. Biotechnol Prog 8:104–110
Liu Z, Ma C, Gao C, Xu P (2012) Efficient utilization of hemicellulose hydrolysate for propionic acid production using Propionibacterium acidipropionici. Bioresour Technol 114:711–714
Papoutsakis ET, Meyer CL (1985) Fermentation equations for propionic-acid bacteria and production of assorted oxychemicals from various sugars. Biotechnol Bioeng 27:67–80
Parizzi L, Grassi MC, Llerena L, Carazzolle M, Queiroz V, Lunardi I (2012) The genome sequence of Propionibacterium acidipropionici provides insights into its biotechnological and industrial potential. BMC Genom 13:562
Pereira GAG et al (2011) Microorganisms and process for producing n-propanol. WO2011029166 A1
Rawe B (2008) Breakthroughs and challenges in the production of cellulosic ethanol. MMG Basic Biotechnol EJ 4:10–15
Rodriguez B, Stowers CC, Cox BM (2014) The production of propionic acid, propanol, propylene via sugar fermentation: an industrial perspective on the progress, technical challenges and future outlook. Green Chem 16(3):1066–1076
Seshadri N, Mukhopadhyay SN (1993) Influence of environmental parameters on propionic acid upstream bioprocessing by Propionibacterium acidipropionici. J Biotechnol 29:321–328
Suwannakham S, Yang ST (2005) Enhanced propionic acid fermentation by Propionibacterium acidipropionici mutant obtained by adaptation in a fibrous-bed bioreactor. Biotechnol Bioeng 91:325–327
Suwannakham S, Huang Y, Yang ST (2006) Construction and characterization of ack knock-out mutants of Propionibacterium acidipropionici for enhanced propionic acid fermentation. Biotechnol Bioeng 94:383–395
Vorobjeva LI (1999) Propionibacteria. Kluwer, Dordrecht
Wang Z, Yang ST (2013) Propionic acid production in glycerol/glucose co-fermentation by Propionibacterium freudenreichii subsp. shermanii. Bioresour Technol 137:116–123
Zhang A, Yang ST (2009) Engineering Propionibacterium acidipropionici for enhanced propionic acid tolerance and fermentation. Biotechnol Bioeng 104:766–773
Zhang A, Yang ST (2009) Propionic acid production from glycerol by metabolically engineered Propionibacterium acidipropionici. Process Biochem 44:1346–1351
Zhu Y et al (2010) Optimization and scale-up of propionic acid production by propionic acid-tolerant Propionibacterium acidipropionici with glycerol as the carbon source. Bioresour Technol 101:8902–8906
Zhu L, Wei P, Cai J, Zhu X, Wang Z, Huang L, Xu Z (2012) Improving the productivity of propionic acid with FBB immobilized cells of an adapted acid-tolerant Propionibacterium acidipropionici. Bioresour Technol 112:248–253
Acknowledgments
The authors would like to recognize Dr. S.T. Yang’s lab at Ohio State University for helpful discussions. Barry Fish, Yomaira Pagan-Torres, Sanjib Biswas, Paul Larsen, and Vishesh Shah at The Dow Chemical Company are acknowledged for their contributions to the process economics study. We also gratefully acknowledge Babu Raman, Patrick Reifel, Matt Roach, Amudhan Venkateswaran, Nigel Mouncey, Paul Speakman, Amy Keeney, Kyle McFerran, Craig Finnegan, Paul Swanson, Paul Lewer, Scott Greenwalt, Bryan Ward, Karan Bansal, Prasanth Maddipati, Allison Lutocka, Samantha Hall, Paul Ketterer, Joe Brunson, Derek Jamrog, Kelly Hill and Mike Harris at Dow Agrosciences, LLC and Josh Watson, Kristie Means and Emma Patterson of Kelly Services on assignment at Dow AgroSciences for assisting with the fermentation studies. Sara Dorman from Marcor Corp. is appreciated for aiding raw material sourcing and product specifications while Mickey Boles, Emily Bredhold, and Joaquín Alarcón of Abengoa Bioenergy are thanked for supplying the corn mash. Lastly, Lars Nielsen, Esteban Marcellin, and Jens Kromer from the University of Queensland are acknowledged for contributing to the genomic analysis of Propionibacterium acidipropionici.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stowers, C.C., Cox, B.M. & Rodriguez, B.A. Development of an industrializable fermentation process for propionic acid production. J Ind Microbiol Biotechnol 41, 837–852 (2014). https://doi.org/10.1007/s10295-014-1423-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1423-6