Abstract
Bio-based succinic acid production has attracted global attention since its consideration as a potential replacement to petroleum-based platform chemicals. This study used three different CO2 sources, namely NaHCO3, K2CO3 and MgCO3 for fermentation of succinic acid (SA) by Actinobacillus succinogenes under three distinct substrate conditions i.e. lactose, whey and whey devoid of any supplements. Batch experiments were performed in both anaerobic flasks and 5L benchtop fermenter. SA fermentation in anaerobic flasks was unfettered by supplementary nutrients. However, fermentation in the benchtop fermenter devoid of supplementary nutrients resulted into 42% reduction in SA yield as well as lower SA productivities. Furthermore, a significant reduction of cell growth occurred in anerobic flasks at pH < 6.0, and complete termination of bacterial activity was noted at pH < 5.3. The highest SA titer, yield and productivity of 15.67 g/L, 0.54 g/g and 0.33 g/L/h, respectively, was recorded from whey fermentation with MgCO3. The present study further highlights significant inhibitory effect of K2CO3 buffered medium on Actinobacillus succinogenes. Thus, we can claim that environmental pollution as well as costs of SA production from whey can be reduced by leveraging on whey residual nutrients to support the activity of Actinobacillus succinogenes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Succinic acid (butanedioic acid, CH2)2(CO2H)2) has been identified as one of the top ten chemicals with potential to replace petroleum-based platform chemical by the U.S. Department of Energy (Bozell and Petersen 2010; Jansen and van Gulik 2014). Succinic acid (SA) is a crucial compound in the manufacture of degradable biopolymer and could also be used for synthesis of 1, 4-butanediol, N-methyl pyrrolidone and polybutylene succinate (Wang et al. 2011; Huang et al. 2019). Consequently, many investigations have focused on economic viability of bio-based commercial succinic acid production. Different microorganisms such as Anaerobiospirillum succiniciproducens, Actinobacillus succinogenes, Mannheimia succiniciproducens, Basfia succiniciproducens and Escherichia coli have been assessed for succinic acid production (Olajuyin et al. 2016; Shen et al. 2018). Therein, Actinobacillus succinogenes, a Gram-negative, rod-shaped bacterium isolated from the bovine rumen (Guettler et al. 1999), has been identified as one of the most favorable strains for commercial production of succinic acid due to its tolerance to high acid concentration and ability to produce succinic acid at high yields from wide range of carbon sources.
Succinic acid production from renewable feedstocks such as whey, sugarcane molasses, straw hydrolysate, textile waste hydrolysate, duckweed hydrolysate and glycerol have been examined by many researchers with the objective of reducing the feedstock costs involved in SA production (Lee et al. 2003; Wan et al. 2008; Zheng et al. 2009; Shen et al. 2015, 2018). In this study, whey was used for fermentation of succinic acid by Actinobacillus succinogenes. Whey is a liquid waste generated in dairy industries during cheese-making or coagulation of the milk casein (Saidi et al. 2020). In the fermentation process, nutrients including amino acids and vitamins are required by microorganisms for their growth and development. Actinobacillus succinogenes is auxotrophic, which makes it unable to synthesize amino acids and vitamins necessary for growth and replication. To cater for this dysfunction, nitrogen sources such yeast extract or corn steep liquor are added as supplements during fermentation (McKinlay et al. 2005; Rajendra et al. 2016). Undoubtedly, these nutrient supplements are very expensive and highly affect the cost of commercial succinic acid production, rendering the process economically unviable. Whey is composed of approximately 7% solids, of which 75% is lactose and about 10–15% soluble lactate, proteins, lipids, vitamins, complex nitrogen sources other mineral salts (Samuelov et al. 1999; Yadav et al. 2015). Therefore, leveraging on the existing whey nutrients can enhance profitability of SA production. Moreover, biochemical oxygen demand (BOD) for whey ranges from about 25–60 g/L (Carvalho et al. 2013; Yadav et al. 2015). This possesses a significant pollution risk if directly discharged into the environment without treatment.
Despites the large amount of global whey production and the potential of being used as a renewable feedstock, very few studies have focused on whey fermentation for SA production. Lee et al. (2003) investigated the influence of adding yeast extract or corn steep liquor (CSL) as nitrogen source for whey and lactose fermentation by Mannheimia succiniciproducens MBEL55E, however, their report did not indicate the effect of eliminating all the other trace elements. At present, no study exists on succinic acid fermentation from whey devoid of any supplementary nitrogen sources and trace elements by Actinobacillus succinogenes. Furthermore, it is acknowledged that carbon dioxide (CO2) is a co-substrate in the production of succinic acid, and also serves as a pH buffer during SA fermentation (Zou et al. 2011; Pateraki et al. 2016; Herselman et al. 2017). The supply of dissolved CO2 to the bioreactor can be achieved through continuous sparging of gaseous CO2 or by addition of bicarbonate and carbonates salts. Nevertheless, previously published works have not provided any information on the inhibitory effect of K2CO3 as a source of CO2 or pH regulator on Actinobacillus succinogenes.
The present study used three different CO2 sources (NaHCO3, K2CO3 and MgCO3) to investigate the fermentation of succinic acid by A. succinogenes in three distinct substrate conditions i.e. lactose, whey and whey bereft of supplements. Experiments were carried out both in anaerobic flasks and 5L bioreactor. Part of our objectives was to test the hypothesis that succinic acid production can occur in whey devoid of nutrient supplements, hence establishing a basis for commercial succinic acid production from such a renewable feedstock. To the best of our knowledge, this is the first attempt in conducting succinic acid fermentation from whey devoid of nutrient supplements by A. succinogenes.
Materials and methods
Microorganism, growth media and culture conditions
Actinobacillus succinogenes130Z, was obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in 60% (v/v) glycerol solutions at − 40 °C. The inoculum was prepared by anaerobically growing 2 ml of the stock culture in 20 ml of sterilized tryptic soy broth (TSB) medium in 50 ml Erlenmeyer flasks. The incubation was performed in a rotary shaker (BIOSAN, incubator ES-20/60) at 37 °C and 150 rpm for 20 h.
Preparation of cheese whey
Cheese whey used in this study was acquired from Malkara Alliance Milk and Milk Products Inc., one of the largest producers of milk powder, whey powder, demineralized whey powder and lactose in the Balkans. Before use, the whey was filtered through 0.1 µm ceramic membrane to remove all suspended matter. Characteristics of the cheese whey used in this study are presented in Table1.
Succinic acid fermentation in anaerobic flasks
Flask experiments were carried in 500 mL shake flasks, containing 250 ml medium inoculated with 7 ml of inoculum and incubated at 37 °C. Based on previous studies (Corona-González et al. 2008, 2014; Corona-Gonzalez et al. 2010; Zhang et al. 2012), the fermentation medium contained per liter: 5.0 g yeast extract, 3.0 g KH2PO4, 1.5 g K2HPO4, 1.0 g NaCl, 0.3 g MgCl2, 0.3 g CaCl2, 0.07 g MnCl2. Three different CO2 sources namely, NaHCO3, K2CO3 and MgCO3 were investigated under three distinct substrate conditions i.e. lactose, cheese whey and cheese whey bereft of supplements. For each assay, 40 g/L of designated carbonate buffer (NaHCO3, K2CO3 or MgCO3) and 40 g/L of substrate (pure lactose or whey) was used. Assays without supplementary nutrients consisted of only substrate, carbonate buffer and inoculum. The fermentation medium was separately autoclaved (121 °C for 1 h) prior to inoculation with 10% (v/v) of the exponentially growing seed culture. The pH of fermentation medium was aseptically adjusted to 7.3 with H2SO4 (6 M) prior to culture seeding.
Fermentation in batch bioreactor
Batch fermentation was carried out in BIOSTAT® Bplus 5 L benchtop fermenter (Sartorius, Germany) equipped with temperature and pH control units. Fermentation medium was agitated at 200 rpm and temperature maintained at 37 °C, whilst pH was automatically kept at 6.8 by either 3 N NaOH or 3 N KOH or 3 N K2CO3 solution. The composition of the fermentation medium was identical to that used for anaerobic flasks in Sect. "Succinic acid fermentation in anaerobic flasks", except that 30 g/L of substrate was used in batch bioreactor.
Data analysis and experimental reproducibility
High-performance liquid chromatography (HPLC Shimadzu LC-20AD, Tokyo, Japan) equipped with a refractive index detector (RID-10A) was used for quantitative analysis of organic acids and sugars. Biorad Aminex HPX-87H column of 300 mm × 7.8 mm size was used. The mobile phase used for HPLC was 6.5 mM H2SO4 at a flow rate of 0.6 mL/min and oven temperature of 60 °C. All the samples were filtered through 0.45 µm prior to HPLC analyses. Retention times of the sample were identified through comparison with analytical standards. The cell growth was measured by optical density (OD600) using UV–visible spectrophotometer (Hach Lange, DR6000™). To ensure reproductivity of the results, experiments were performed in duplicate, and results presented as average values. SPSS version 21.0 was used for statistical analysis. One-way ANOVA (with Tukey post hoc test) was used for analysis of significance in comparison of multiple experimental results. The Student’s t test was used to evaluate statistical differences between experimental results, and tests were considered significant at p > 0.05.
The succinic acid yield for each fermentation experiment was calculated as the amount of succinic acid (g) produced per 1 g of sugars (lactose or glucose) consumed (Eq. (1). Whereas, productivity was determined from concentration of SA produced over fermentation time Eq. (2).
where YSA is SA yield and PSA is SA productivity.
Results and discussion
Succinic acid production in anaerobic flasks with different carbonate buffers
Dissolved CO2 in the chemostat serves as a pH controller, which is key for regulating intracellular enzymatic activities. Moreover, CO2 is used as a co-substrate in the production of succinic acid with a hypothetical ratio of 1 mol of succinic acid generated per mol of CO2 consumed (Pateraki et al. 2016; Longanesi et al. 2018) The results of succinic acid production in anaerobic flasks are presented in Figs. 1 and 2. One-way ANOVA and Levene's test of absolute deviations indicated SA titers from K2CO3 to be statistically different (p < 0.05) from that of NaHCO3 and MgCO3. The homogeneity of variance test (Levene's test of absolute deviations) showed non-significant variation between NaHCO3 and MgCO3 at (p < 0.05). As illustrated in Figs. 1 and 2, production of SA with K2CO3 was deplorable, less than 0.5 g/L of succinic acid was obtained for all trials with K2CO3 after 72 h of fermentation.
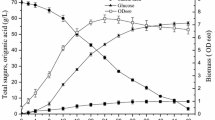
Fermentation results for variant carbonate buffers and substrate sources; a NaHCO3 + pure lactose + nutrient supplements; b K2CO3 + pure lactose + nutrient supplements; c MgCO3 + pure lactose + nutrient supplements; d NaHCO3 + whey + nutrient supplements; e K2CO3 + whey + nutrient supplements; f MgCO3 + whey + nutrient supplements; g NaHCO3 + whey only; H: K2CO3 + whey only; i MgCO3 + whey only
Contrastingly, fermentation with NaHCO3 and MgCO3 resulted into remarkable consumption of substrate. At initial substrate concentration of 40 g/L, consumption rate was calculated as 55.97%, 59.23% and 39.33% for lactose, whey with nutrient supplements and whey devoid of any supplements, respectively, after 72 h for NaHCO3 buffered medium. Under the same conditions, the respective substrate consumption rates for MgCO3 medium were 68.25%, 66.63% and 57.93%. Although statistically non-significant, the titer and yield of succinic acid produced using MgCO3 surpassed that of NaHCO3. This concurs with the findings of Liu et al. (2008a) and Yu et al. (2010) in which MgCO3 resulted in a higher succinic acid titer and lesser by-products compared to other pH buffers investigated. The effectiveness of MgCO3 in succinic acid production by A. succinogenes is attributed to the release of CO2 and Mg2+ ions, which are essential cofactors for phosphoenolpyruvate carboxykinase, the first enzyme in the reductive branch of the tricarboxylic acid (TCA) cycle (during succinate synthesis) (Pateraki et al. 2016).
Another commendable highlight of this study is the analogy of the results obtained from whey fermentation with nutrient supplements to that obtained without any nutrient supplements in anaerobic flasks. This supports the hypothesis that whey contains adequate complex nitrogen sources and nutrients for enzymatic and bacterial activity of A. succinogenes. From an economic point of view, leveraging on the existing whey nutrients and trace elements will reduce costs for industrial scale succinic acid production. Lee et al. (2003) examined the effect of supplementing yeast extract and corn steep liquor (CSL) as sources of nitrogen for whey fermentation by Mannheimia succiniciproducens MBEL55E and concluded that relatively inexpensive CSL can be used at low concentrations of 5gL−1 to boost succinic acid production from whey. However, their report was short of eliminating all the other trace elements indicated in Table 1.
Formation of by-products such as acetic acid, formic acid and lactic acid is one of the major drawbacks of fermentative SA production. Besides reducing the SA yield, by-products also increase the downstream purification costs associated with SA production. Acetic and formic acid were the major by-products observed in the present study. The ratio of AC/SA and FA/SA varied from 0.41–0.53 g/g to 0.30–0.42 g/g, respectively, for fermentation with NaHCO3. Likewise, the respective ratios of AC/SA and FA/SA varied from 0.43–0.51 g/g to 0.11–0.27 g/g for MgCO3buffered medium. Lactic acid formation was not observed except when K2CO3 was used. It is manifested that succinic acid fermentation under anoxic condition produces phoshpoenolpyruvate (PEP), which is one of the central intermediates in mixed acid fermentation. PEP serves as a point of divergence between succinic acid, formic acid, acetic acid and ethanol producing pathways. The major route to succinic acid follows from PEP to oxaloacetate, malate, fumarate and lastly to succinate. These are catalyzed by PEP carboxykinase, malate dehydrogenase, fumarase and fumaratereductase enzymes, respectively (McKinlay et al. 2007; Wan et al. 2008; Pateraki et al. 2016). PEP carboxykinase is the key enzyme in succinic acid formation pathway and its activity is regulated by the amount of CO2 available to A. succinogenes (Van Der Werf et al. 1997). By-products such as acetic acid, formic acid, and ethanol are formed when pyruvate kinase converts PEP to pyruvate, which is thereafter transformed to by-products (Pateraki et al. 2016).
The pH of fermentation medium influences microbial growth and the conversion of substrate to product. Besides, pH affects the speciation of the carbonate buffers (CO2/HCO3/H2CO3 ratio) in solution and the availability of CO2. Thus, pH regulators are often used in SA fermentation. The optimal pH for A. succinogenes has been previously indicated as 6.7—7.2 (Van Der Werf et al. 1997; Wan et al. 2008). Regulation of fermentation pH was unfeasible for our anaerobic flask setup. Figure 3 illustrates the variation of pH during organic acid production by Actinobacillus succinogenes.
As shown in Fig. 3, the pH profile for K2CO3 medium differed strikingly from that of NaHCO3 and MgCO3. The K2CO3 buffered medium remained close to its initial pH value for 72 h of the experiment, indicating inactivity of the microorganisms. On the other hand, NaHCO3 and MgCO3 buffered medium showed minimal pH change during the initial hours of fermentation which is attributable to culture adaptation by A. Succinogenes (lag phase). Thereafter, pH decreased from about 7.2 at the start the fermentation to about 5.3 after 72 h. Also, cell growth significantly reduced at pH below 6.0 (data not shown), and bacterial activity completely ceased when pH reduced below 5.3. Similar observations have been reported by Van Der Werf et al. (1997) during fermentation of glucose, by A. succinogenes 130Z. The decrease in pH corresponds to the production of the organic acids and acidification of the medium. Literature reports for SA yields from whey generally ranged from 0.44 to 0.91 g/g (Samuelov et al. 1999; Lee et al. 2003; Wan et al. 2008). The lower succinic acid yields obtained from our anerobic flask experiments is certainly due to the lowering of pH in fermentation medium as the organic acids are produced, since our experimental setup couldn’t permit pH regulation during fermentation. Neutral pH enhances the exponential phase and production of organic acids (Van Der Werf et al. 1997; Wan et al. 2008). Corona-González et al. (2008) reported a reduction of the concentration in biomass after acids production exceeded 22 g/L. In another study by Lin et al. (2008), cell growth of A. succinogenes was decreased by 30% when only 5 g/L of formic acid was produced. Cimini et al. (2016) revealed that bioreactors attained higher succinic acid production compared to bottles, owing to possibility of pH control and CO2 sparging in the medium.
Succinic acid fermentation in batch benchtop bioreactor
Experimental studies in the batch benchtop bioreactor were conducted with working volume of 2.5 L. The initial substrate concentration was kept constant at about 30 g/L for all experimental runs. Pure substrates (glucose and lactose) were first fermented before attempting to ferment cheese whey. Results of SA fermentation by A. succinogenes are presented in Table 2 and discussed in following sections.
Succinic acid production from pure substrate (glucose and lactose)
Pure glucose and lactose were fermented using NaHCO3 as CO2 source and 3 M NaOH as pH regulator. Glucose fermentation exhibited very rapid consumption of substrate, with almost no lag phase, the 30 g/L of glucose were consumed within 18 h resulting into 17.94 g/L of SA titer and corresponding yield of 0.59 g/g (Fig. 4). On the other hand, formation of succinic acid began after an initial 6 h lag phase and 48 h were required for complete consumption of the lactose resulting, into SA titer and yields of 15.86 g/L and 0.53 g/g, respectively. The SA productivity for glucose and lactose were 0.56 and 0.33 g/L/h, respectively. Similar results have been previously reported in literature (Kim et al. 2004; Yu et al. 2010). As expected, consumption rate for pure glucose was higher than that of pure lactose. This is because lactose is a disaccharide, which necessitates growth of β-galactosidase enzymes for hydrolysis by A. succinogenes prior to consumption. Despite its ability to metabolize a wide range of carbon sources, A. succinogenes exhibited picky characteristics when presented with more than one carbon source (data not shown). In scenarios where both lactose and glucose were initially present, it was observed that the bacteria first depleted the glucose before transitioning to the lactose. Furthermore, less than 55% of the generated galactose was consumed by A. succinogenes, which concurs with the observations of Salvachúa et al. (2016). In another study by Sorokina et al. (2020), A. succinogenes130Z first utilized glucose and arabinose, whereas metabolism of galactose started after the depletion of other monosaccharides. This phenomenon could be attributed to the blockage or silencing of nonpreferred nutrients by catabolite repression (molecular mechanisms).
Formation of lactic acid was not observed during fermentation of pure glucose, while very negligible amount of lactic acid was formed from pure lactose. Nevertheless, formic and acetic acids were the main by-products in both cases (Table 2).
Like the observations from anaerobic flask, use of K2CO3 yielded no SA and very poor growth of A. succinogenes, confirming the inhibition of the microorganism. It should be noted that 40 g/L of MgCO3 or NaHCO3 contains 0.5 mol/L of \({CO}_{3}^{2-}\), \({Na}^{+}\) or \({Mg}^{2+}\). On the other hand, the moles of \({K}^{+}\) and \({CO}_{3}^{2-}\) contained in 40 g of carbonate was relatively lower as 0.16 and 0.2, respectively. Apparently, no study has highlighted the inhibitory effect of K2CO3 on A. succinogenes. This inhibition could be attributed to annihilated transfer of substances from the growth medium into the cells owing to reduced biosynthesis. Liu et al. (2008b) compared the use of MgCO3, CaCO3, Na2CO3, NaOH and NH4OH in fermentation of glucose with A. succinogenes CGMCC1593. Their report revealed the inhibitory effect of NH4OH and indicated MgCO3 as the most efficient neutralizer.
Comparison of batch succinic acid fermentation from cheese whey with nutrient supplements and whey devoid of any supplements
Microbiological activities are highly affected by the composition of the medium, especially the nitrogen source. Despite being one of the most favorable strains for succinic acid production, A. succinogenes is auxotrophic. This implies its inability to synthesize the amino acids and vitamins necessary for growth and replication. For this reason, fermentation studies involve addition of yeast extract or corn steep liquor as source of the essential amino acids and vitamins (McKinlay et al. 2005; Rajendra et al. 2016). In commercial production of succinic acid, addition of yeast extract and other trace elements elevates the total production costs, rendering the process economically unrealistic. Whey contains high organic load and residual milk nutrients such as lactose, proteins, lipids and vitamins, which can be harnessed for microorganism growth. Herein, we attempt to investigate the possibility of whey fermentation without supplementary nutrients and trace elements listed in Table1, with the objective of reducing the costs involved in succinic acid production.
Figure 5 presents results of succinic acid fermentation from cheese whey by Actinobacillus succinogenes under both scenarios, with MgCO3 as CO2 source and 3 M NaOH as a pH regulator. Succinic acid yield decreased by 44% (from 0.54 to 0.31 g/g) for fermentation medium devoid of any nutrient supplements, and the amount of acetic acid generated rivaled that of succinic acid. Furthermore, lactose consumption was slower and poor growth of A. succinogenes was observed. For example, only 19.84 g/L lactose was consumed after 48 h, compared to the 29.16 g/L lactose consumption in medium with supplementary nutrients. Also, cell densities (OD600) were lower in fermentation experiments without supplementary nutrients. This phenomenon suggests that the available nutrients were insufficient for the activity of enzymes in the SA pathway. The eminent results attained in yeast extract supplemented medium could be because yeast extract contains traces of important nutrients like biotin, pantothenic acid, folic acid and vitamins B1, B2, B6, and B12, which are essential for the growth of A. succinogenes (Zhu et al. 2012).
In comparison, the final SA yield (0.3 g/g) obtained by A. succinogenes from whey devoid of supplementary nutrients was higher than that produced by Escherichia coli strain YBS132 from glucose as wells as PGC01003 and PGC11505 engineered strains of Yarrowia lipolytica (Lin et al. 2005; Yu et al. 2018). The general, SA yields and substrate consumption rates were lower in whey compared to pure lactose and glucose. This can be explained by the osmolarity difference in the fermentation medium owing to high salt concentrations in whey, leading to water loss and cell shrinking (Shen et al. 2018). Moreover, the initial presence of lactic acid in whey also contributes to stress on microorganisms.
Overall, whey has proven to be a promising renewable feedstock for production of SA. Economic production of succinic acid from whey can be realized by leveraging on the existing whey nutrients for microbial growth. However, it should be noted that characteristics of whey vary depending on the sources of raw milk, the fraction of non-valorized cheese whey, the amount of water used for cleaning and the dairy products produced. Therefore, the decision to supplement whey with nitrogen sources and other trace elements depends on the specific properties of whey under consideration.
Table 3 presents a representative comparison of SA production from whey-based medium by various SA producing strains with the present study. The highest SA titer and yield was obtained by Samuelov et al. (1999) using A. succinogenes ATCC 29,305. From Table 3, the final yield in this study is comparable to that reported by Wan et al. (2008) and Longanesi et al. (2018). However, the relatively lower yields observed in this study could be due to high salinities in the fermentation medium, originating from the whey and carbonate buffer used.
Conclusion
This paper investigated three CO2 sources, namely NaHCO3, K2CO3 and MgCO3 for bio-based succinic acid production by A. succinogenes. In addition, three distinct substrate conditions i.e. lactose, whey and whey devoid of supplements were investigated. This is the first report to highlight inhibitory effect of K2CO3 as a pH buffer on succinic acid production by A. succinogenes. It was demonstrated that whey fermentation without nutrient supplements did not significantly affect SA production in small anaerobic flask. However, when the system was scaled up, additional nutrients were required to enhance SA production. Significant reduction in cell growth was observed at pH < 6.0, and bacterial activity completely ceased at pH < 5.3. The highest SA titer, yield and productivity of 15.67 g/L, 0.54 g/g and 0.33 g/L/h, respectively, was obtained from whey using MgCO3. To ensure more commercially appealing succinic acid production from cheese whey, future studies will focus on enhancing succinic acid yield and productivity without or with only very limited addition of trace elements and nutrients depending on the properties of whey under consideration. Finally, whey has demonstrated its potential as a unique substrate that if well harnessed, environmental pollution as well as industrial production costs of SA could be tremendously reduced.
References
Bozell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem 12:539–554. https://doi.org/10.1039/B922014C
Carvalho F, Prazeres AR, Rivas J (2013) Cheese whey wastewater: Characterization and treatment. Sci Total Environ 445–446:385–396. https://doi.org/10.1016/J.SCITOTENV.2012.12.038
Cimini D, Argenzio O, D’Ambrosio S et al (2016) Production of succinic acid from Basfia succiniciproducens up to the pilot scale from Arundo donax hydrolysate. Bioresour Technol 222:355–360. https://doi.org/10.1016/j.biortech.2016.10.004
Corona-González RI, Bories A, González-Álvarez V, Pelayo-Ortiz C (2008) Kinetic study of succinic acid production by Actinobacillus succinogenes ZT-130. Process Biochem 43:1047–1053. https://doi.org/10.1016/j.procbio.2008.05.011
Corona-González RI, Miramontes-Murillo R, Arriola-Guevara E et al (2014) Immobilization of Actinobacillus succinogenes by adhesion or entrapment for the production of succinic acid. Bioresour Technol 164:113–118. https://doi.org/10.1016/j.biortech.2014.04.081
Corona-Gonzalez RI, Bories A, González-Álvarez V et al (2010) Succinic acid production with Actinobacillus succinogenes ZT-130 in the presence of succinic acid. Curr Microbiol 60:71–77. https://doi.org/10.1007/s00284-009-9504-x
Guettler MV, Rumler D, Jain MK (1999) Actinobacillus succinogenes sp. nov., a novel succinic-acid-producing strain from the bovine 1umen. Int J Syst Bacteriol 49:207–216. https://doi.org/10.1099/00207713-49-1-207
Herselman J, Bradfield MFA, Vijayan U, Nicol W (2017) The effect of carbon dioxide availability on succinic acid production with biofilms of Actinobacillus succinogenes. Biochem Eng J 117:218–225. https://doi.org/10.1016/j.bej.2016.10.018
Huang M, Cheng J, Chen P et al (2019) Efficient production of succinic acid in engineered Escherichia coli strains controlled by anaerobically-induced nirB promoter using sweet potato waste hydrolysate. J Environ Manage 237:147–154. https://doi.org/10.1016/j.jenvman.2019.02.041
Jansen MLA, van Gulik WM (2014) Towards large scale fermentative production of succinic acid. Curr Opin Biotechnol 30:190–197. https://doi.org/10.1016/J.COPBIO.2014.07.003
Kim DY, Yim SC, Lee PC et al (2004) Batch and continuous fermentation of succinic acid from wood hydrolysate by Mannheimia succiniciproducens MBEL55E. Enzyme Microb Technol 35:648–653. https://doi.org/10.1016/J.ENZMICTEC.2004.08.018
Lee PC, Lee WG, Kwon S et al (2000) Batch and continuous cultivation of Anaerobiospirillum succiniciproducens for the production of succinic acid from whey. Appl Microbiol Biotechnol 54:23–27. https://doi.org/10.1007/s002530000331
Lee PC, Lee SY, Hong SH, Chang HN (2003) Batch and continuous cultures of Mannheimia succiniciproducens MBEL55E for the production of succinic acid from whey and corn steep liquor. Bioprocess Biosyst Eng 26:63–67. https://doi.org/10.1007/s00449-003-0341-1
Lin H, San KY, Bennett GN (2005) Effect of Sorghum vulgare phosphoenolpyruvate carboxylase and Lactococcus lactis pyruvate carboxylase coexpression on succinate production in mutant strains of Escherichia coli. Appl Microbiol Biotechnol 67:515–523. https://doi.org/10.1007/s00253-004-1789-x
Lin SKC, Du C, Koutinas A et al (2008) Substrate and product inhibition kinetics in succinic acid production by Actinobacillus succinogenes. Biochem Eng J 41:128–135. https://doi.org/10.1016/j.bej.2008.03.013
Liu YP, Zheng P, Sun ZH et al (2008a) Economical succinic acid production from cane molasses by Actinobacillus succinogenes. Bioresour Technol 99:1736–1742. https://doi.org/10.1016/j.biortech.2007.03.044
Liu YP, Zheng P, Sun ZH et al (2008b) Strategies of pH control and glucose-fed batch fermentation for production of succinic acid by Actinobacillus succinogenes CGMCC1593. J Chem Technol Biotechnol 83:722–729. https://doi.org/10.1002/jctb.1862
Longanesi L, Frascari D, Spagni C et al (2018) Succinic acid production from cheese whey by biofilms of Actinobacillus succinogenes: packed bed bioreactor tests. J Chem Technol Biotechnol 93:246–256. https://doi.org/10.1002/jctb.5347
Louasté B, Eloutassi N (2020) Succinic acid production from whey and lactose by Actinobacillus succinogenes 130Z in batch fermentation. Biotechnol Reports 27:e00481. https://doi.org/10.1016/j.btre.2020.e00481
McKinlay JB, Zeikus JG, Vieille C (2005) Insights into Actinobacillus succinogenes fermentative metabolism in a chemically defined growth medium. Appl Environ Microbiol 71:6651–6656. https://doi.org/10.1128/AEM.71.11.6651-6656.2005
McKinlay JB, Shachar-Hill Y, Zeikus JG, Vieille C (2007) Determining Actinobacillus succinogenes metabolic pathways and fluxes by NMR and GC-MS analyses of 13C-labeled metabolic product isotopomers. Metab Eng 9:177–192. https://doi.org/10.1016/j.ymben.2006.10.006
Olajuyin AM, Yang M, Liu Y et al (2016) Efficient production of succinic acid from Palmaria palmata hydrolysate by metabolically engineered Escherichia coli. Bioresour Technol 214:653–659. https://doi.org/10.1016/j.biortech.2016.04.117
Pateraki C, Patsalou M, Vlysidis A et al (2016) Actinobacillus succinogenes: Advances on succinic acid production and prospects for development of integrated biorefineries. Biochem Eng J 112:285–303. https://doi.org/10.1016/J.BEJ.2016.04.005
Rajendra U, Vijayan P, Nicol W (2016) Continuous production of succinic acid with Actinobacillus succinogenes biofilms: Effect of complex nitrogen source on yield and productivity
Saidi V, Sheikh-Zeinoddin M, Kobarfard F, SoleimanianZad S (2020) Bioactive characteristics of a semi-hard non-starter culture cheese made from raw or pasteurized sheep’s milk. 3 Biotech 10:85. https://doi.org/10.1007/s13205-020-2075-z
Salvachúa D, Mohagheghi A, Smith H et al (2016) Succinic acid production on xylose-enriched biorefinery streams by Actinobacillus succinogenes in batch fermentation. Biotechnol Biofuels 9:1–15. https://doi.org/10.1186/s13068-016-0425-1
Samuelov NS, Datta R, Jain MK, Zeikus JG (1999) Whey fermentation by Anaerobiospirillum succiniciproducens for production of a succinate-based animal feed additive. Appl Environ Microbiol 65:2260–2263. https://doi.org/10.1128/aem.65.5.2260-2263.1999
Shen N, Qin Y, Wang Q et al (2015) Production of succinic acid from sugarcane molasses supplemented with a mixture of corn steep liquor powder and peanut meal as nitrogen sources by Actinobacillus succinogenes. Lett Appl Microbiol 60:544–551. https://doi.org/10.1111/lam.12399
Shen N, Zhang H, Qin Y et al (2018) Efficient production of succinic acid from duckweed (Landoltia punctata) hydrolysate by Actinobacillus succinogenes GXAS137. Bioresour Technol 250:35–42. https://doi.org/10.1016/j.biortech.2017.09.208
Sorokina KN, Samoylova YV, Gromov NV et al (2020) Production of biodiesel and succinic acid from the biomass of the microalga Micractinium sp IC-44. Bioresour Technol. 317:124026. https://doi.org/10.1016/j.biortech.2020.124026
Van Der Werf MJ, Guettler MV, Jain MK, Zeikus JG (1997) Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentation by Actinobacillus sp. 130Z. Arch Microbiol 167:332–342. https://doi.org/10.1007/s002030050452
Wan C, Li Y, Shahbazi A, Xiu S (2008) Succinic acid production from cheese whey using Actinobacillus succinogenes 130 Z. Appl Biochem Biotechnol 145:111–119. https://doi.org/10.1007/s12010-007-8031-0
Wang D, Li Q, Song Z et al (2011) High cell density fermentation via a metabolically engineered Escherichia coli for the enhanced production of succinic acid. J Chem Technol Biotechnol 86:512–518. https://doi.org/10.1002/jctb.2543
Yadav JSS, Yan S, Pilli S et al (2015) Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol Adv 33:756–774
Yu J, Li Z, Ye Q et al (2010) Development of succinic acid production from corncob hydrolysate by Actinobacillus succinogenes. J Ind Microbiol Biotechnol. https://doi.org/10.1007/s10295-010-0750-5
Yu Q, Cui Z, Zheng Y et al (2018) Exploring succinic acid production by engineered Yarrowia lipolytica strains using glucose at low pH. Biochem Eng J 139:51–56. https://doi.org/10.1016/j.bej.2018.08.001
Zhang Y, Li Q, Zhang Y et al (2012) Optimization of succinic acid fermentation with Actinobacillus succinogenes by response surface methodology (RSM). J Zhejiang Univ Sci B 13:103–110. https://doi.org/10.1631/jzus.B1100134
Zheng P, Dong JJ, Sun ZH et al (2009) Fermentative production of succinic acid from straw hydrolysate by Actinobacillus succinogenes. Bioresour Technol 100:2425–2429. https://doi.org/10.1016/j.biortech.2008.11.043
Zhu LW, Wang CC, Liu RS et al (2012) Actinobacillus succinogenes ATCC 55618 fermentation medium optimization for the production of succinic acid by response surface methodology. J Biomed Biotechnol. https://doi.org/10.1155/2012/626137
Zou W, Zhu LW, Li HM, Tang YJ (2011) Significance of CO 2donor on the production of succinic acid by Actinobacillus succinogenes ATCC 55618. Microb Cell Fact 10:1–10. https://doi.org/10.1186/1475-2859-10-87
Acknowledgements
We wish to thank the Scientific and Technological Research Council of Turkey (TÜBITAK) for providing the financial support under project No. 115Y824
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest in the publication.
Rights and permissions
About this article
Cite this article
Omwene, P.I., Yağcıoğlu, M., Öcal-Sarihan, Z.B. et al. Batch fermentation of succinic acid from cheese whey by Actinobacillus succinogenes under variant medium composition. 3 Biotech 11, 389 (2021). https://doi.org/10.1007/s13205-021-02939-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02939-w