Abstract
Objective: In our study, the effectiveness of avanafil, a second-generation phosphodiesterase-5 (PDE5) inhibitor, on testicular torsion (TT) related ischemia/reperfusion injury via NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3), which triggers inflammatory response, are studied molecularly, biochemically and histopathologically. Material and Method: This study was performed on 24 male Wistar albino rats randomized into four groups. Testicular ischemia/reperfusion (I/R) model was created for groups 2, 3 and 4. Groups 3 and 4, respectively, were administered a dose of 5 and 10 mg/kg avanafil before reperfusion by gavage. The testicles which were left in ischemia for two hours, were detorsioned for four hours. All animals were sacrificed after reperfusion. Testicular tissues were examined molecularly, biochemically and histopathologically. Results: The NLRP3, Interleukin-1β (IL-1β) and Tumor Necrosis alpha (TNF-α) mRNA expression levels were observed to be significantly increased in the I/R group compared to the healthy group (p < 0.001). After both doses of avanafil, NLRP3, IL-1β and TNF-α mRNA expression levels, which increased as a result of I/R, decreased in both avanafil groups. (p < 0.001). The greatest decrease was seen at the dose of 10 mg/kg (p < 0.001). Increased Malondialdehyde (MDA) levels due to I/R were statistically significantly decreased in both doses of avanafil (p < 0.001). Decreased Superoxide Dismutase (SOD) levels due to I/R damage increased statistically significantly in both doses of avanafil (p < 0.001). Conclusion: It was found that avanafil can reduce the damage caused by testicular I/R and that it will find new applications in the future with the support of advanced experimental and clinical studies.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Testicular torsion (TT) is a very serious surgical urgency resulting from the bending of the spermatic cord, which leads to the reduction of the blood perfusion to the testicle. Consequently, ischemia develops and correspondingly, testicular atrophy occurs. Urgent diagnosis and early surgical operation are mandatory to hinder germ cell loss that may occur with ischemic damage [1]. The condition can be observed at any age. However, it occurs more frequently among adolescents, and children [2]. It was considered to be the most common reason for referral to the pediatric urological emergency [3].
When TT occurs, two types of damage to the testicles that cause necrosis are present. The first type includes the ischemia-related injuries that occur during torsion. In this process, tissue perfusion is disturbed, and hypoxic by-products begin to accumulate in the tissue. The second type includes the damage that occurs after detorsion due to reperfusion [4]. After detorsion, blood flow returns to normal. However, increased amounts of oxygen flowing to the tissue after the resumption of blood flow cause an excessive accumulation of free oxygen radicals in the testicular tissue that has adapted to hypoxia. The resulting oxidative stress causes stimulation of inflammatory processes and apoptotic pathways. This subsequently leads to the inevitable consequences of DNA damage, endothelial damage, alteration in endothelial permeability, degradation of adhesion molecules, and apoptosis in germinal cells. Thus, ischemic damage escalates to reperfusion damage [5]. Long-term sperm count and motility decrease due to TT ischemia–reperfusion injury. The quality of semen decreases. The net result is infertility [6].
Among the pharmacological agents attracting the attention of researchers for the purpose of preventing I/R in recent years are phosphodiesterase (PDE) inhibitors. PDEs, which have 11 different isoenzymes, are found in many cells in the body. These enzymes catalyze the hydrolysis of the intracellular second messengers as cyclic Adenosine Monophosphate (cAMP) and cyclic Guanosine Monophosphate (cGMP) that play central role in signal transduction and regulating various physiological and pathophysiological processes of cells, including growth, differentiation, and proliferation. Through this process, they terminate cyclic nucleotide signaling [7]. Signal transduction begins with the activation of protein kinase A (PKA) and protein kinase G (PKG) by cAMP and cGMP, respectively. The corresponding proteins are then phosphorylated; thus, downstream pathways are activated. One of these targets is the inhibition of PDE5. Phosphorylation of PDE5 plays a regulatory feedback inhibition role in the signaling cascade of cGMP/PKG. PKG activation induced by cGMP phosphorylates a variety of intracellular proteins that regulate physiological functions such as cell differentiation and proliferation, modulation of vascular tone, and endothelial permeability [8]. Due to these vasoactive effects, many studies have been conducted on I/R using the first-generation PDE5 inhibitors sildenafil, tadalafil and vardenafil [9].
Avanafil is another PDE5 inhibitor that has more PDE isoform selectivity compared to first-generation PDE5 inhibitors and offers different physical and chemical properties [10]. Because of these properties, we used avanafil in the present study with the hope that it would have different effects on I/R caused by TT.
On the other hand, the modulation of the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome has a significant role in I/R. NLRP3 is a molecule that is considered a main regulator of inflammation. NLRP3 is thought to be effective in the pathophysiology of various diseases such as cancer, cardiac, metabolic and inflammatory diseases. Reactive oxygen species (ROS), increased by oxidative stress due to I/R, activate NLRP3 inflammasome. With activated NLRP3, procaspase-1 is activated to caspase 1, which in term causes the activation of the pro-inflammatory Interleukin-1β (IL-1β) and Interleukin-18 (IL-18). This process leads to the progression of inflammation and further aggravation of the damage. As a result, the interest in therapeutic approaches targeting the NLRP3 inflammasome in I/R is steadily increasing [11, 12]. NLRP3 has been found to be associated with the male reproductive system and with infertility. Activation of the NLRP3 inflammasome, leading to proinflammatory cytokine storms, oxidative stress and apoptosis, has been shown to be part of the multiple inflammatory mechanisms leading to male infertility [13]. In varicocele, a leading cause of male infertility, the NLRP3 inflammasome is activated and negatively affects spermatogenesis, resulting in sperm DNA fragmentation, mitochondrial dysfunction and motility [14]. Another study suggests that the NLRP3 inflammasome may be an important target for treatment as a new medical approach to reduce testicular damage associated with varicocele[15].
In the present study, the efficacy of avanafil, a second-generation PDE5 inhibitor, on TT-related I/R via NLRP3, which triggers the inflammatory response, were to be investigated molecularly, biochemically, and histopathologically.
Materials and Methods
Ethical Approval
Approval of the study was obtained from The Local Ethics Council of Animal Experiments of our University (approval number: 31.05.2022/2022–6/106). The study was conducted in accordance with ARRIVE guidelines and nationally accepted guidelines.
Animals
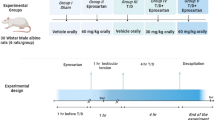
The rats used for the study were male Wistar albino rats of 8 to 10 weeks of age and 250 to 280 g in weight, and the determined number of rats was 24. The rats used in this study were provided by the Laboratory for Experimental Animals of the Medical and Experimental Application and Research Center of the University. Rats were fed ad libitum with standard pellet and tap water, hosted inside polypropylene cages with a 12-h dark/light photoperiod, under standard temperature (22 ± 1 °C) and humidity (50–60%).
Study Protocol
Rats were randomized into four groups for the study:
-
Group 1: Healthy (H)
-
Group 2: Torsion with ischemia and reperfusion (I/R)
-
Group 3: Torsion with ischemia and reperfusion + avanafil at 5 mg/kg (I/R + AVA5)
-
Group 4: Torsion with ischemia and reperfusion + avanafil at 10 mg/kg (I/R + AVA10)
To perform ischemia/reperfusion (I/R) of the testes, rats were given 25 mg/kg thiopental sodium intraperitoneally, and 2 cm vertical cuts were made 2–3 cm lateral to the abdominal midline along the scrotal midline. Group 1 underwent only surgical incision, and no torsion procedure was performed. A testicular I/R model was created for the second, third, and fourth groups.
Avanafil was suspended in distilled water and a single dose of avanafil 5 mg/kg and 10 mg/kg by oral gavage was given to groups 3 and 4, respectively, before reperfusion. To create the I/R model, the left testicle was subjected to torsion in the scrotal cavity together with the tunica vaginalis and the spermatic cord, by rotating it 720 degrees clockwise.
The testicles were subjected to ischemia for 2 h. At the end of 2nd hour, a detorsion procedure was performed and the testicles were exposed to reperfusion for 4 h. After the reperfusion, all rats were euthanized under general anesthesia by administering 50 mg/kg Na thiopental.
Testicular tissues taken for molecular and biochemical analyzes were kept at -80 °C until the analyses. For histopathological examination, some of the testicular tissues were placed in 10% formalin solution.
Avanafil (SML2799), ketamine (Ketalar 500 mg/10 ml) and xylazine (Bacilazine 2%) were obtained from Sigma-Aldrich Chemie GmbH (Munich, Germany), Pfizer (İstanbul, Turkey) and Biotek (İstanbul, Turkey), respectively.
Molecular Studies
NLRP3, IL-1β and Tumor Necrosis alpha (TNF-α) mRNA expression levels in testis tissue were determined using reverse transcription polymerase chain reaction (RT-PCR) method.
RNA Extraction
After the tissue samples were treated with liquid nitrogen, homogenization was performed. RNA extraction and total RNA isolation were performed with a QIAcube device. The quantity of total mRNA was determined through NanoDrop spectrophotometer at 260 nm (Biotek, Shoreline, WA, USA, EPOCH Take 3 Plate). Obtained RNA was freezed at –80 °C.
Reverse Transcriptase Reaction and Synthesis of Complementary DNA (cDNA)
High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher, Waltham, MA, USA) was used. cDNA synthesis was achieved with VeritiTM 96-Well Thermal Cycler (Applied Biosystems, Waltham, MA, USA) according to the specified temperature values.
For cDNA synthesis, 10µL total RNA, 2µL 10X RT random primers, 0.8µL 25X dNTP mixes, 1µL MultiScribe reverse transcriptase, 2µL 10X RT buffer, and 4.2µL diethylpyrocarbonate-treated H2O were used. NanoDrop spectrophotometer (EPOCH Take3 Plate, BioTek) was used to determine the amount of cDNA. The obtained cDNA was then stored at -20 °C. [16].
Quantitative Detection of mRNA Expressions
The mRNA expressions of NLRP3, IL-1β, and TNF-α by RT-PCR were determined using the StepOnePlus RT-PCR System (Applied Biosystems) for amplification and quantification procedures. Examination of testicular mRNA expression was performed using TaqMan® Gene Expression Master Mix (Thermo Fisher). β-Actin was used as a reference gene.
For 200 ng cDNA, the subsequent TaqMan gene expression experiments were performed by pipetting 2 µL of 200 ng cDNA, 10 µL TaqMan Master Mix, and 1 µL assay, adding RNase-free H2O to make the total volume 20 µL. The assay was run for 40 cycles on the instrument. The number of cycles in which the fluorescence signal level exceeds the minimum or threshold value obtained in real-time PCR experiments is called the cycle threshold (CT). CT values were calculated by conversion to ΔΔCt [17].
Biochemical Measurements
Malondialdehyde (MDA) levels were determined using Ohkawa [18], Superoxide dismutase (SOD) enzyme activity measurements were determined using Sun and Oberley method [19], and total protein levels were determined using Lowry method [20]. MDA results were presented as nmol/mg protein, while SOD results were expressed as U/mg protein.
Histopathological Analyses
All histopathological analyses were performed in the Research Laboratory of the Histology and Embryology Department of the Faculty of Medicine, University of Kafkas.
For histopathologic analysis, the collected testicular tissue samples were placed in formalin solution and fixed for 48 h. The tissue processing procedure was as follows: Tissue samples were washed under running water for 2 h. Tissues were then dehydrated through a series of 50% (2 h), 70% (1 h), 80% (1 h), 96% (1 h), and 99% (1 h) alcohol solutions. The tissues were then soaked in three series of xylene solutions (3 × 15 min). Finally, the tissues were soaked in series of melted paraffin (2 × 1 h) and the tissue samples were embedded in paraffin to complete the processing procedure [21].
The resulting blocks were placed in a microtome, and 5-μm thick sections from each block were placed on poly-L-lysine slides. Routine hematoxylin and eosin staining was performed on the slides. Accordingly, Tissue samples placed on the slides were kept in a vacuum oven at 60 °C for 20 min, and then the samples were exposed to three series of xylene for 5 min. The slides were then dehydrated in a series of decreasing concentrations of alcohol (99%, 96%, 80%, 70%, and 50%) for 5 min, and nuclear staining was performed with Harris hematoxylin dye for 3 min. The samples were then stained for cytoplasm with Eosin Y solution for 2 min for contrast staining. Finally, the slides were passed through a series of 96% and 99% alcohol and xylene, and the surfaces of the slides were sealed with coverslips using a bonding agent. All slides were examined and photographed under a camera-attached microscope (Olimpus CX41) [22].
Statistical Analysis
For the evaluation of the data, SPSS Statistics 25.0 (IBM, NY, USA) was used for the statistical analyses and the results were presented as mean ± standard deviation (SD). The analyses of NLRP3, IL-1β, and TNF-α mRNA expression levels were carried out by using variance and Tukey tests (p < 0.001).
Results
Expressions of NLRP3, IL-1β, and TNF-α
The obtained mRNA expression levels of NLRP3, IL-1β and TNF-α of all study groups are given in Fig. 1.
NLRP3, IL-1β, and TNF-α mRNA expression levels obtained by RT-PCR. H: Healthy group; I/R: ischemia–reperfusion injury group; I/R + AVA5: group administered 5 mg/kg avanafil with ischemia–reperfusion injury; I/R + AVA10: group administered 10 mg/kg avanafil with ischemia–reperfusion injury. *: Comparison to the healthy group,δ: comparison to the I/R group
When tissue expression levels of NLRP3, IL-1β and TNF-α mRNA were examined in this rat model of TT with I/R, there was a meaningful statistical increase in these expression levels in the I/R groups regarding to the healthy group (p < 0.001).
When the efficacy of avanafil was investigated, NLRP3, IL-1β and TNF-α mRNA expression levels, which increased as a result of I/R, decreased in I/R + AVA5 and I/R + AVA10 groups. (p < 0.001). The greatest decrease in NLRP3, IL-1β and TNF-α levels was seen in the AVA10 group (p < 0.001).
Levels of SOD and MDA
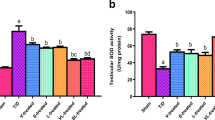
The obtained SOD enzyme activities and MDA tissue levels of all study groups are presented in Fig. 2. Accordingly, a significant decrease in SOD enzyme activities and a significant increase in MDA levels were found in the I/R group regarding to the healthy group (p < 0.001). A statistically significant increase in SOD enzyme levels and a statistically significant decrease in MDA levels were detected in the I/R + AVA5 and I/R + AVA10 groups compared to the I/R group (p < 0.001).
Comparisons of SOD enzyme activity and MDA levels in study groupsH: Healthy group; I/R: ischemia-reperfusion injury group; I/R + AVA5: group administered 5 mg/kg avanafil with ischemia-reperfusion injury; I/R + AVA10: group administered 10 mg/kg avanafil with ischemia-reperfusion injury. *: Comparison to the healthy group,δ:comparison to the I/R group
Histopathological Findings
When the testicular tissues were examined histopathologically, normal seminiferous tubule structures were detected in the healthy group. Healthy spermatogonia (Sg), primary spermatocytes (PS), late and early spermatids (LS-ES), spermatozoa (Sz), Sertoli cells (Srt), and Leydig cells (LC) were observed in the germinal epithelium (GM) (Figs. 3A and 3B).
Histopathological Findings of the Groups (A-B: Healthy, C-D: IR, E–F: IR + AVA5, and G-H: IR + AVA10)(ST: Seminiferous Tubule, IA: Interstitial Area, GE: Germinal Epithelium, Sz: Spermatozone, LS: Late Spermatitis, ES: Early Spermatitis: PS: Primary Spermatocyte, Sg: Spermatogonium, Str: Sertoli Cell, LC: Leydig Cell, Triangle: Edema, Star: Hemorrhage, Square: Basal Membrane Detachments, Round: Necrotic cells, X: Degenerated Spermatozones)
Areas of hemorrhage and edema in the interstitial spaces of rats in the I/R group were apparent. Detailed examination of the seminiferous tubule structures showed structural disorganization in the germinal epithelium. While some seminiferous tubules showed early spermatids and primary spermatocytes with necrotic appearances in the germinal epithelium, spermatozoa with damaged structures were observed in the centers of the tubules (Figs. 3C and 3D).
The areas of hemorrhage and edema in the interstitial spaces observed in the I/R group were not observed in the I/R + AVA5 group. However, the germinal epithelium was separated from the basal membrane of the seminiferous tubule in this group. In addition, the germinal epithelium of the seminiferous tubules in this group had a normal appearance, and healthy spermatozoa were observed in the lumen (Figs. 3E and 3F).
While hemorrhage in the interstitial spaces was not seen in the I/R + AVA10 group, areas of edema were also observed in this group. The appearances of the seminiferous tubules of this group were very similar to those of the healthy group. Irregular cellular structures were rarely observed in the germinal epithelium in this group (Figs. 3G and 3H).
Discussion
This study investigated the efficacy of avanafil, a second-generation PDE5 inhibitor, on TT-induced I/R. Molecular, biochemical, and histopathologic studies demonstrated that avanafil can prevent testicular I/R-induced damage. IL-1β, TNF-α, and NLRP3 levels were statistically higher in the I/R group compared to the groups treated with avanafil; in particular, high-dose administration of avanafil lowered those expression levels close to the levels of the healthy group.
Avanafil is effectively used as the first-line choice for erectile dysfunction, and for the treatment of pulmonary hypertension [23]. The morphological structures of PDE5 inhibitors are very similar to those of cGMP; thus, they bind competitively to PDE5 and block the hydrolysis of cGMP. They increase the amount of cGMP that does not undergo degradation. cGMP, in turn, activates PKG. Increased PKG levels play a role in the modulation of important cellular functions such as regulation of calcium concentration, inhibition of platelet aggregation, phosphorylation of proteins that regulate smooth muscle tone, and alterations of protein expressions [24]. In recent years, the use of PDE5 inhibitors for many different indications has been proposed due to these vasoactive effects and various studies have been conducted on these subjects [25]. These new indications led us to research the efficacy of the second-generation PDE5 inhibitor avanafil on TT-related I/R. It has been shown in the literature that there are potential beneficial effects of PDE5 inhibitors in different I/R models [26, 27]. However, most of these works were limited to the experimental level or had few applications in the field. Therefore, we used avanafil, which has higher PDE selectivity than first-generation PDE5 inhibitors, in our study. Studies have shown that this group of drugs modulates the GMP-PKG signaling pathway, causing vasodilation, increasing blood flow, and thus preventing the expansion of the ischemic area in I/R, and has antioxidant and anti-inflammatory effects [28, 29].
In the current study, we aimed to prove the positive effectiveness of avanafil on I/R by demonstrating a different mechanism of action. Hypoxic damage caused by TT is followed by severe reperfusion damage caused by detorsion. The production of acceptable quantities of ROS is allowed under normal physiological conditions, modulated by SOD, catalase (CAT), and glutathione peroxidase (GPx) enzymes. However, excessive amounts of ROS are produced in cases of I/R. Since this excessive production of ROS exceeds the neutralizing capacity of protective enzymes, an imbalance occurs between the ROS produced and the antioxidation system. This gives rise to a serious cellular injury [30]. Lipids are the chief biological targets of oxidative stress in cellular injury. Lipid peroxidation produces many secondary products, but primarily MDA. MDA is not only an indicator of lipid peroxidation, but also is extremely toxic molecule that interacts with proteins and DNA [31]. Thus, reduced level of MDA, a biomarker of oxidative stress, is an indicator of antioxidant activity [32]. The human body contains an antioxidant defense system that protects against oxidative injury from reactive oxidants. This defense system consists of enzymes such as SOD, CAT, and GPx and antioxidants such as glutathione, ascorbate, and thioredoxin. They resist oxidative stress and protect structures such as DNA, lipids, and proteins from damage [33]. Defense systems consisting of enzymes such as SOD, CAT, and GPx are also found in testicular tissue. Thus, they preserve testicular germinal and somatic cells from damage due to free radicals. In contrast to antioxidants, MDA is a noteworthy index of lipid peroxidation by ROS and give rise to damage to somatic and germinal cells [34].
It has been found that sildenafil, a first-generation PDE5 inhibitor, reduces oxidative damage arised by TT-related I/R in rats and has a cytoprotective effect on germ cells. The levels of MDA significantly increased in the group that underwent torsion-detorsion compared to the healthy group, and tissue CAT and GPx activities decreased. It was also observed that MDA levels decreased significantly, and CAT and SOD levels increased in the group that was given sildenafil before detorsion. When germ cells were examined histopathologically, it was found that apoptosis decreased with the administration of sildenafil [35].
The results of our study seem to support previous studies. Increased MDA levels in the I/R group were detected as reduced after the administration of both dosages of avanafil. We believe that avanafil relieves lipid peroxidation by reducing oxidative stress. When we examined the SOD levels, another parameter measured in the current study, we found that the decreased SOD enzyme activities due to damage were increased after treatment with avanafil. Avanafil decreased the amount of oxidative stress markers and supported the antioxidant systems. Our histopathological results also provided clear evidence of the potential protective effects of avanafil. In contrast to studies conducted with other PDE5 inhibitors, in the present study, the interaction of avanafil with the NLRP3 inflammasome, which is activated by increased ROS due to reperfusion in I/R and creates tissue damage by causing severe inflammation, was also shown. Increased levels of ROS due to reperfusion in cases of I/R also cause NLRP3 inflammasome activation, which is a main modulator of inflammation [36]. NLRP3 is the most known inflammasome related to inflammation. The NLRP3 protein is found in a complex with the apoptosis-associated speck-like (ASC) adaptor protein and procaspase-1. Procaspase-1 is activated into caspase-1 by activated NLRP3; thus, the conversion of inactive proinflammatory cytokines IL-1β and IL-18 into their active forms is induced [37]. Caspase-1, IL-1β, and IL-18 have been found to increase in I/R in relation to activation of NLRP3. A review focusing on male reproductive disorders that impact fertility showed that activation of the innate immune system leads to increased levels of inflammatory cytokines, apoptosis, and pyroptosis, which is mediated by activation of the NLRP3 inflammasome [38]. A study to elucidate the mechanisms explaining inflammation in male infertility found that serum and semen NLRP3, IL1β, oxidant and antioxidant parameters were significantly higher in varicocele/azoospermia than in non-variocele/azoospermia [39]. They concluded that inflammasome mechanisms such as NLRP3 and IL1-β molecules may be of additional value in the assessment of the need for and benefit of surgical or medical treatment for infertility.
In this study, damage to testicular tissue and impaired spermatogenesis were associated with NLRP3 activation [40]. Oxidative damage to the testicular tissue initially leads to increased apoptosis, vacuolization of the seminiferous epithelium, and a decrease in sperm count [41]. As this process continues, testicular atrophy and impaired spermatogenesis are inevitable results [42]. Therefore, NLRP3 could be seen as a target in the group of innovative drugs to treat I/R caused by TT. In our study, the elevated expressions of NLRP3 and IL-1β due to I/R were decreased with the use of avanafil. Avanafil alleviated testicular inflammation by reducing the release of active proinflammatory cytokines via NLRP3. The histopathological findings of this study support this. In our study, we also examined variations of TNF-α expressions, one of the main cytokines of inflammation. We found that TNF-α, which increases in the event of I/R, was statistically significantly reduced in the avanafil applied groups. In addition to the oxidant/antioxidant balance deteriorating due to I/R, it is also seen that levels of various inflammatory cytokines such as TNF-α and IL-1β, which cause progression of testicular damage, are increased in I/R [37]. A decrease in these cytokine levels indicates a decrease in inflammation and is also a marker for demonstrating the effectiveness of the applied pharmacological agent [40]. In our study, it was shown that avanafil reduces TNF-α and IL-1β levels. Our histopathological findings also showed that avanafil alleviates tissue damage.
Limitations
The study has some limitations. First, because our study was an experimental study in rats, the results should be confirmed in clinical studies conducted in patients. Second, our study conducted histopathological and gene expression measurement, but not protein expression. In addition, long-term survival analysis should be performed in future studies to examine the effects of avanafil on both mortality and morbidity rates in testicular I/R.
Conclusion
The effects of avanafil, a second-generation PDE5 inhibitor, on NLRP3 and other cytokines and ROS have been explained in the context of the TT-based I/R model that we created. With the administration of avanafil, free oxygen radicals and NLRP3 inflammasome activation decreased, and, as a conclusion, the cellular injury produced by testicular I/R also decreased. We believe that avanafil can prevent the injury triggered by testicular I/R and that it will find new applications in the future with the support of advanced experimental and clinical studies.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Moslemi MK, Kamalimotlagh S. Evaluation of acute scrotum in our consecutive operated cases: A one-center study. Int J Gen Med. 2014;7:75–8. https://doi.org/10.2147/IJGM.S52413.
Dokmeci D. Testicular torsion, oxidative stress and the role of antioxidant therapy. Folia Med (Plovdiv). 2006;48:16–21.
Mäkelä EP, Roine RP, Taskinen S. Paternity, erectile function, and health-related quality of life in patients operated for pediatric testicular torsion. J Pediatr Urol. 2020;16:44.e1-44.e4. https://doi.org/10.1016/j.jpurol.2019.10.008.
Celik E, Oguzturk H, Sahin N, Turtay MG, Oguz F, Ciftci O. Protective effects of hesperidin in experimental testicular ischemia/reperfusion injury in rats. Arch Med Sci. 2016;5:928–34. https://doi.org/10.5114/aoms.2015.47697.
Zhong L, Yang M, Zou X, Du T, Xu H, Sun J. Human umbilical cord multipotent mesenchymal stromal cells alleviate acute ischemia-reperfusion injury of spermatogenic cells via reducing inflammatory response and oxidative stress. Stem Cell Res Ther. 2020;11:294. https://doi.org/10.1186/s13287-020-01813-5.
Jacobsen FM, Rudlang TM, Fode M, Østergren PB, Sønksen J, Ohl DA, et al. The Impact of Testicular Torsion on Testicular Function. World J Mens Health. 2020;38:298. https://doi.org/10.5534/wjmh.190037.
Bender AT, Beavo JA. Cyclic Nucleotide Phosphodiesterases: Molecular Regulation to Clinical Use. Pharmacol Rev. 2006;58:488–520. https://doi.org/10.1124/pr.58.3.5.
Hofmann F, Bernhard D, Lukowski R, Weinmeister P. cGMP Regulated Protein Kinases (cGK). cGMP Gener. Eff. Ther. Implic., Berlin, Heidelberg: Springer Berlin Heidelberg; 2009; p. 137–62. https://doi.org/10.1007/978-3-540-68964-5_8.
Das A, Durrant D, Salloum FN, Xi L, Kukreja RC. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol Ther. 2015;147:12–21. https://doi.org/10.1016/j.pharmthera.2014.10.003.
Evans J, Burke R. Avanafil for treatment of erectile dysfunction: review of its potential. Vasc Health Risk Manag 2012;517. https://doi.org/10.2147/VHRM.S26712.
Xu Q, Zhao B, Ye Y, Li Y, Zhang Y, Xiong X, et al. Relevant mediators involved in and therapies targeting the inflammatory response induced by activation of the NLRP3 inflammasome in ischemic stroke. J Neuroinflammation. 2021;18:123. https://doi.org/10.1186/s12974-021-02137-8.
Zahid A, Li B, Kombe AJK, Jin T, Tao J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front Immunol 2019;10. https://doi.org/10.3389/fimmu.2019.02538.
Tavalaee M, Rahmani M, Drevet JR, Nasr-Esfahani MH. The NLRP3 inflammasome: molecular activation and regulation in spermatogenesis and male infertility; a systematic review. Basic and Clinical Andrology. 2022;32(1):8. https://doi.org/10.1186/s12610-022-00157-9.
Poli G, Fabi C, Sugoni C, Bellet MM, Costantini C, Luca G, et al. The Role of NLRP3 Inflammasome Activation and Oxidative Stress in Varicocele-Mediated Male Hypofertility. Int J Mol Sci [Internet]. 2022;23(9):52333.
Antonuccio P, Micali AG, Romeo C, Freni J, Vermiglio G, Puzzolo D, et al. NLRP3 Inflammasome: A New Pharmacological Target for Reducing Testicular Damage Associated with Varicocele. Int J Mol Sci [Internet]. 2021;22(3):1319.
Kose D, Kose A, Halici Z, Cadirci E, Tavaci T, Gurbuz MA, et al. Bosentan, a drug used in the treatment of pulmonary hypertension, can prevent development of osteoporosis. Iran J Basic Med Sci. 2021;24:922–7.
Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25(4):402–8. https://doi.org/10.1006/meth.2001.1262.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8. https://doi.org/10.1016/0003-2697(79)90738-3.
Sun Y, Oberley LW, & Li Y. (1988). A simple method for clinical assay of superoxide dismutase. Clinical Chemistry 34(3), 497–500. https://www.ncbi.nlm.nih.gov/pubmed/3349599. Accessed 28 Feb 2024
Waterborg JH, & Matthews HR (n.d.). The Lowry Method for Protein Quantitation. In Basic Protein and Peptide Protocols (pp. 1–4). Humana Press. https://doi.org/10.1385/0-89603-268-X:1.
Aksak Karamese S, Toktay E, Unal D, Selli J, Karamese M, Malkoc I. The protective effects of beta-carotene against ischemia/reperfusion injury in rat ovarian tissue. Acta Histochem 2015; 117 (8):790–7. https://doi.org/10.1016/j.acthis.2015.07.006.
Çelikçi B, Uğan RA, & Toktay E. (2021). Effects of fisetin to letrozole-induced polycystic ovary syndrome in rats. Cukurova Med J 46(2), 508–515. https://doi.org/10.17826/cumj.850380.
Wang H, Yuan J, Hu X, Tao K, Liu J, Hu D. The effectiveness and safety of avanafil for erectile dysfunction: a systematic review and meta-analysis. Curr Med Res Opin. 2014;30:1565–71. https://doi.org/10.1185/03007995.2014.909391.
Palit V, Eardley I. An update on new oral PDE5 inhibitors for the treatment of erectile dysfunction. Nat Rev Urol. 2010;7:603–9. https://doi.org/10.1038/nrurol.2010.165.
Mostafa T. Useful Implications of Low-dose Long-term Use of PDE-5 Inhibitors. Sex Med Rev. 2016;4:270–84. https://doi.org/10.1016/j.sxmr.2015.12.005.
Gasanov F, Aytac B, Vuruskan H. The effects of tadalafil on renal ischemia reperfusion injury: an experimental study. Bosn J Basic Med Sci 2011;11:158. https://doi.org/10.17305/bjbms.2011.2567.
Shih P-K, Cheng C-M, Li H-P, Huang M-F, Chiu C-W, Chen J-X, et al. Pretreatment with sildenafil alleviates early lung ischemia-reperfusion injury in a rat model. J Surg Res. 2013;185:e77-83. https://doi.org/10.1016/j.jss.2013.07.010.
Koka S, Xi L, Kukreja RC. Chronic inhibition of phosphodiesterase 5 with tadalafil affords cardioprotection in a mouse model of metabolic syndrome: role of nitric oxide. Mol Cell Biochem. 2020;468:47–58. https://doi.org/10.1007/s11010-020-03710-0.
Ozdegirmenci O, Kucukozkan T, Akdag E, Topal T, Haberal A, Kayir H, et al. Effects of sildenafil and tadalafil on ischemia/reperfusion injury in fetal rat brain. J Matern Fetal Neonatal Med. 2011;24:317–23. https://doi.org/10.3109/14767058.2010.492061.
Ghasemnejad-Berenji M, Ghazi-Khansari M, Yazdani I, Nobakht M, Abdollahi A, Ghasemnejad-Berenji H, et al. Effect of metformin on germ cell-specific apoptosis, oxidative stress and epididymal sperm quality after testicular torsion/detorsion in rats. Andrologia. 2018;50: e12846. https://doi.org/10.1111/and.12846.
Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–28. https://doi.org/10.1016/j.numecd.2005.05.003.
Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017;524:13–30. https://doi.org/10.1016/j.ab.2016.10.021.
Ali SS, Ahsan H, Zia MK, Siddiqui T, Khan FH. Understanding oxidants and antioxidants: Classical team with new players. J Food Biochem 2020;44. https://doi.org/10.1111/jfbc.13145.
Ozbal S, Ergur BU, Erbil G, Tekmen I, Bagrıyanık A, Cavdar Z. The Effects of α -Lipoic Acid against Testicular Ischemia-Reperfusion Injury in Rats. Sci World J. 2012;2012:1–8. https://doi.org/10.1100/2012/489248.
Beheshtian A, Salmasi AH, Payabvash S, Kiumehr S, Ghazinezami B, Rahimpour S, et al. Protective effects of sildenafil administration on testicular torsion/detorsion damage in rats. World J Urol. 2008;26:197–202. https://doi.org/10.1007/s00345-008-0243-6.
Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 2009;19:455–64. https://doi.org/10.1016/j.tcb.2009.06.002.
Shao B-Z, Xu Z-Q, Han B-Z, Su D-F, Liu C. NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol 2015;6. https://doi.org/10.3389/fphar.2015.00262.
Perri A, Bossio S, Rago V, Greco EA, Lofaro D, A LAR, et al. NLRP3-inflammasome activation in male reproductive system diseases. Minerva Endocrinol (Torino). 2022;. https://doi.org/10.23736/s2724-6507.22.03918-5
Ördek E, Kati B, Koyuncu İ, Demir M, Yağmur İ, Pelit ES, et al. What is the impact of inflammasome mechanisms on male infertility? Turk J Med Sci. 2023;53(3):685–91 https://doi.org/10.55730/1300-0144.5631
Minutoli L, Antonuccio P, Irrera N, Rinaldi M, Bitto A, Marini H, et al. NLRP3 Inflammasome Involvement in the Organ Damage and Impaired Spermatogenesis Induced by Testicular Ischemia and Reperfusion in Mice. J Pharmacol Exp Ther. 2015;355:370–80. https://doi.org/10.1124/jpet.115.226936.
Reyes JG, Farias JG, Henríquez-Olavarrieta S, Madrid E, Parraga M, Zepeda AB, et al. The Hypoxic Testicle: Physiology and Pathophysiology. Oxid Med Cell Longev. 2012;2012:1–15. https://doi.org/10.1155/2012/929285.
Minutoli L, Antonuccio P, Squadrito F, Bitto A, Nicotina PA, Fazzari C, et al. Effects of polydeoxyribonucleotide on the histological damage and the altered spermatogenesis induced by testicular ischaemia and reperfusion in rats. Int J Androl. 2012;35:133–44. https://doi.org/10.1111/j.1365-2605.2011.01194.x.
Acknowledgements
We thank the Clinical Research, Development, and Design Center of Atatürk University for their kind support in our academic studies.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
The Local Ethics Council of Animal Experiments of Atatürk University (approval number: 31.05.2022/2022–6/106). The study was conducted in accordance with national and Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines accepted and adopted by the relevant Ethics Committee.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Celik, M., Aydin, P., Civelek, M.S. et al. Avanafil Mitigates Testicular Ischemia/Reperfusion Injury via NLRP3 Pathway Modulation in Rats. Reprod. Sci. (2024). https://doi.org/10.1007/s43032-024-01696-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43032-024-01696-4