Abstract
Purpose
To investigate the possible protective role of syringic acid on torsion/detorsion-induced testicular injury using biochemical and histopathological approaches for the first time.
Methods
A total of 24 rats were divided into 4 groups: sham control, torsion/detorsion, torsion/detorsion + syringic acid (50 mg/kg and 100 mg/kg). Tissue malondialdehyde, total oxidant status and total antioxidant status levels were determined using colorimetric methods. Tissue 8-hydroxy-2′-deoxyguanosine, superoxide dismutase, catalase, high mobility group box 1, nuclear factor kappa B protein 65, tumor necrosis factor-alpha, interleukin-6, myeloperoxidase, 78-kDa glucose-regulated protein, activating transcription factor-6, C/EBP homologous protein and caspase-3 levels were determined using commercial enzyme-linked immunosorbent assay kits. Johnsen’s testicle scoring system was used for histological evaluation.
Results
Compared with the control group, the levels of oxidative stress, inflammation, endoplasmic reticulum stress and apoptosis were significantly increased in the torsion/detorsion group (p < 0.05). Syringic acid administrations statistically significantly restored these damage in a dose dependent manner (p < 0.05). Moreover, it was found that the results of histological examinations supported the biochemical results to a statistically significant extent.
Conclusion
The overall results suggest that syringic acid emerges as a potential compound for the treatment of testicular torsion and may be subject to clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Testicular torsion (TT) is one of the most serious surgical emergencies caused by the rotating of the spermatic cord around own axis, resulting in reduced blood flow to the affected testicle and ultimately testicular atrophy [1]. When the duration of ischemia is prolonged, the affected testicle may be irreversibly damaged, usually within a time frame of 4–8 h [2]. The incidence of TT in men under the age of 25 is 1 in 4000 [3]. The only treatment for TT is surgical detorsion [1]. Therefore, early diagnosis and detorsion of acute pathology is vital importance. While surgery to be performed within 4–6 h can save the testicles with a probability of 90%, this rate decreases to 50% at the end of 12 h and to 10% at the end of 24 h [3]. Rescue detorsion intervention following torsion paradoxically causes greater tissue damage, known as ischemia/reperfusion injury (IRI) [1]. IRI is the structural and functional damage that occurs as a result of temporary interruption of blood flow to the tissue and delayed restoration. A complex inflammatory state occurs as a result of I/R, including ATP depletion, reactive oxygen species (ROS) accumulation, pro-inflammatory cytokine production and apoptotic pathway activation [4]. In the light of this information, it is emphasized that the use of molecules with antioxidant and anti-inflammatory properties is inevitable for TT [3]. Determination of the testicular protective effects of compounds with antioxidant and anti-inflammatory activities applied following torsion/detorsion (T/D) in experimental models has accelerated the studies in this area [5].
Syringic acid (4-hydroxy-3,5-dimethoxybenzoic acid, SA) is a phenolic compound frequently found in a variety of dried fruits (olives, dates), spices, pumpkin, grapes, acai palm, honey and red wine [6]. Syringin, syringaldehyde and sinapic acid are derivatives of naturally occurring SA [7]. It is known to have various beneficial biological activities, such as antioxidant, antidiabetic, hepatoprotective, antihyperlipidemic, cardioprotective, neuroprotective and anti-inflammatory [6, 7]. These findings have increased the use of SA in the pharmaceutical and cosmetic industries [7].
The endoplasmic reticulum (ER) is a highly dynamic organelle and involved in various processes, including calcium homeostasis, synthesis and distribution of lipids, as well as synthesis, folding, modification and transport of proteins [8]. Various processes or stimuli, such as ischemia, hyperglycemia, exposure to heavy metals, chemotherapeutic agents or antibiotics can disrupt ER homeostasis and trigger a condition called “ER stress” [9]. In the case of ER stress, cells activate the unfolded protein response (UPR) to increase the ER protein folding capacity or to ensure the elimination of misfolded proteins through ER-associated protein degradation (ERAD) [11]. While the UPR is a pro-survival mechanism against moderate ER stress levels, it becomes pro-apoptotic against severe ER stress levels. It has been emphasized in recent years that ER stress associated with increased oxidative stress is involved in the etiopathogenesis of IRI [8]. Although previous studies have shown that SA can abolish I/R-induced brain, kidney and heart injury [4, 11, 12], to our best knowledge, there is no study demonstrating the efficacy of SA on T/D-induced testicular injury. The aim of this study was therefore to evaluate the protective effects of SA against T/D-induced testicular damage in terms of oxidative stress, inflammation, apoptosis, and especially ER stress, for the first time.
Materials and methods
Chemicals and drugs
Phosphate buffered saline (PBS) tablet, phosphoric acid, thiobarbituric acid (TBA), 1,1,3,3-tetramethoxypropane, dimethyl sulfoxide (DMSO), SA and hematoxylin and eosin (H&E) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ketamine hydrochloride and xylazine were purchased from Vem Pharmaceuticals (Ankara, Turkey) and Bayer (Leverkusen, Germany), respectively.
Animals
The experiment was compliant with World Medical Association Declaration of Helsinki, and the animal experiment was approved by the Animal Experiments Local Ethics Committee of Karadeniz Technical University (Protocol number: 2021/65). 24 male adult Sprague–Dawley rats weighing 200–225 g were included in this study. The sample size was calculated using G*Power Software 3.1.9.2 (Kiel University, Kiel, Germany). The minimum number of samples in each required group was calculated as 6 by taking the effect size of Cohen’s d = 2, alpha = 0.05, power = 0.90 (1- β) and a sample size ratio = 1. This study was conducted at the Surgical Practice Research Center of Karadeniz Technical University (Trabzon, Turkey). All experimental protocols were performed according to the Guide for the Care and Use of Laboratory Animals.
Experimental design
Rats were acclimated two weeks before the experiments. All drug and solvent applications to the rats were done by intraperitoneal (ip) route. All surgical procedures were performed in a sterile environment and general anesthesia was provided with ketamine hydrochloride (50 mg/kg, ip) and xylazine (10 mg/kg, ip). The 24 animals were randomly divided into four groups and each group consisted of six rats: sham control, T/D, T/D + SA (50 mg/kg) and T/D + SA (100 mg/kg). Group I (sham control): A middle scrotal incision was made by shaving the scrotal region, and the left testicle was mobilized with the spermatic cord, and the scrotum was closed with sutures. 10% DMSO application (ip) was performed 210 min after the start. Group II (T/D): To provide IRI in rats in this group, T/D model was created according to the model suggested by Turner et al. [13]. The left testicle was visualized with a left inguinoscrotal incision. In the next step, the left testicle was fixed to the scrotum with a suture by turning 720◦ in a clockwise direction. In this way, the left testicle was kept in the torsion position for a total of 240 min. 10% DMSO application (ip) was performed 210 min after the torsion. At the end of the period, the suture was released and the left testicle was detorsioned in its normal position for 120 min. The 4 h torsion and 2 h detorsion times were determined based on previous studies [14, 15]. Group III and IV (T/D + SA [50 and 100 mg/kg]): As in Group II, a testicular T/D procedure was performed in the rats of these groups. Unlike other groups, the rats in these groups were administered two doses of SA (50 mg/kg and 100 mg/kg, respectively) 30 min before the detorsion procedure (Table 1). SA doses selected considering previous studies and was prepared by dissolving in 10% DMSO [12, 16]. At the end of the experiment, all rats underwent left orchiectomy. The removed left testicles were divided longitudinally into two pieces. One half was preserved in Bouin's solution for histological analysis, while the other half was placed in micro-volume tubes to be used in biochemical analyzes and stored at -80 °C.
Biochemical analysis
Tissue samples were homogenized using a homogenizer (Ultra-Turrax T25, IKA, Staufen, Germany) in 2 mL of ice-cold PBS. Then the homogenates were centrifuged at 1800xg for 15 min at 4 °C. The supernatants were then collected, the protein contents determined using a commercial kit (Pierce BCA Protein Assay Kit, Thermo Scientific, Rockford, IL) according to the manufacturer's instructions and used for biochemical analysis.
Malondialdehyde (MDA) levels of tissue samples were determined according to the method developed by Mihara and Uchiyama [17]. 1,1,3,3-tetramethoxypropane was used as a standard and tissue MDA levels were expressed as nmol/mg protein.
Tissue total oxidant status (TOS) and total antioxidant status (TAS) levels were measured using colorimetric kits (Rel Assay Diagnostics, Gaziantep, Turkey) according to manufacturer's instructions. The ratio of TOS to TAS was accepted as oxidative stress index (OSI) and was calculated according to the following formula [18]:
Tissue 8-hydroxy-2′-deoxyguanosine (8-OHdG), superoxide dismutase (SOD), catalase (CAT), high mobility group box 1 (HMGB1), nuclear factor kappa B protein 65 (NF-κB p65), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), myeloperoxidase (MPO), 78-kDa glucose-regulated protein (GRP78), activating transcription factor 6 (ATF6), C/EBP homologous protein (CHOP) and caspase-3 levels in tissue samples were determined using enzyme-linked immunosorbent assay (ELISA) kits (Finetest, Wuhan, China) according to the manufacturer's instructions.
Histological analysis
Fixed tissues were treated with graded alcohol series and then embedded in paraffin. Sections of 5 μm thickness were taken from paraffin blocks with an automatic microtome and stained with the H&E and examined under a light microscope equipped with a camera (Olympus BX50, Tokyo, Japan) [14]. Later, seminiferous tubule architecture, spermatogenesis processes and germ cell maturation were graded with the scoring system defined by Johnsen [19]. Scoring and histological evaluation were performed blindly by a histologist unaware of the groups.
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics for Windows version 23.0 (SPSS Inc, Chicago, IL). The compliance of the data to normal distribution was evaluated with the Shapiro–Wilk test. Results were expressed as median and interquartile range [IQR (25th and 75th percentiles)]. The median of groups were compared using Kruskal Wallis and followed by Mann–Whitney U test were used for the statistical assessment of differences among the individual groups. For all parameters, significance was set at p < 0.05.
Results
The levels of biochemical parameters and histological Johnsen’s scores of all groups are presented in Table 2.
Oxidative stress parameters
The MDA, TOS, OSI and 8-OHdG levels of the T/D group was significantly increased compared to the control group (all p = 0.004). SA (50 mg/kg) treatment decreased the levels of MDA, TOS, OSI and 8-OHdG significantly (p = 0.004, p = 0.01, p = 0.004 and p = 0.016, respectively) compared with T/D group. Similarly, SA (100 mg/kg) treatment decreased the levels of MDA, TOS, OSI and 8-OHdG significantly compared with T/D group (all p = 0.004).
The levels of TAS, SOD and CAT were found to be significantly decreased in the testicular tissues of the T/D group compared to the control group of rats (all p = 0.004). SA (50 mg/kg) treatment increased the levels of TAS, SOD and CAT significantly compared with T/D group (p = 0.045, p = 0.006 and p = 0.03, respectively). Similarly, SA (100 mg/kg) treatment increased the levels of TAS, SOD and CAT significantly compared with T/D group (all p = 0.004).
Inflammation parameters
The HMGB1, NF-κB p65, TNF-α, IL-6 and MPO levels of the T/D group was significantly increased compared to the control group (all p = 0.004). SA (50 mg/kg) treatment decreased the levels of HMGB1, NF-κB p65, TNF-α, IL-6 and MPO significantly (p = 0.004, p = 0.004, p = 0.006, p = 0.006 and p = 0.004, respectively) compared with T/D group. Similarly, SA (100 mg/kg) treatment decreased the levels of HMGB1, NF-κB p65, TNF-α, IL-6 and MPO significantly compared with T/D group (all p = 0.004).
ER stress and apoptosis parameters
The GRP78, ATF6, CHOP and caspase-3 levels of the T/D group was significantly increased compared to the control group (all p = 0.004). SA (50 mg/kg) treatment decreased the levels of GRP78, ATF6, CHOP and caspase-3 significantly (p = 0.025, p = 0.004, p = 0.004 and p = 0.045, respectively) compared with T/D group. Similarly, SA (100 mg/kg) treatment decreased the levels of GRP78, ATF6, CHOP and caspase-3 significantly compared with T/D group (all p = 0.004).
Furthermore, no significant difference was found between the T/D + SA (100 mg/kg) group and the control group in terms of biochemical parameters (p > 0.05).
Histological parameters
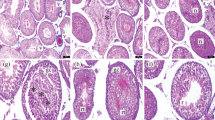
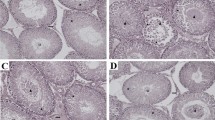
Microscopic images of testicular tissues belonging to the groups are presented in Fig. 1. The histopathological Johnsen score was significantly lower in the T/D group compared to the control group (p = 0.003). Although the Johnsen score was found to be increased in the T/D + SA (50 mg/kg) group compared to the T/D group significantly (p = 0.008), this score was still higher compared with control group significantly (p = 0.004). Nevertheless, SA (100 mg/kg) treatment increased the Johnsen score significantly compared with T/D group (p = 0.003). Furthermore, no significant difference was found between the T/D + SA (100 mg/kg) group and the control group in terms of Johnsen scores (p > 0.05).
Histopathological images of testicular tissues of groups (× 100, H&E staining). Control Group (A) SZ: spermatozoon, black star: seminiferous tubule germinal epithelium, black arrow: intertubular area. T/D Group (B) VC: vasocongestion in the intertubular area, V: vacuolization in tubule epithelium, DT: degenerative seminiferous tubule structure, black star: seminiferous tubule germinal epithelium, black triangle: edema in the intertubular area. T/D + 50 mg/kg SA (C) SZ: spermatozoon, black star: seminiferous tubule germinal epithelium, black arrow: intertubular area. T/D + 100 mg/kg SA (D) SZ: spermatozoon, black star: seminiferous tubule germinal epithelium, black arrow: intertubular area
Discussion
Cumulative evidence suggests that oxidative stress is the most fundamental mechanism of IRI [5]. Excessive ROS generated during the I/R process react with polyunsaturated fatty acids and cause lipid peroxidation [11]. MDA is the end product of lipid peroxidation, and an increase in MDA level indicates an increase in the amount of ROS and/or a decrease in the activities of antioxidant enzymes [18]. TOS reflects the level of total oxidants in a biological sample, while TAS is a cumulative indicator of total antioxidant power in a biological sample. More importantly, the ratio of TOS to TAS is considered the OSI, which is an indicator of oxidative stress [4]. 8-OHdG is a widely used biomarker of ROS-induced DNA damage and it is known that there is a positive correlation between the amount of ROS and the levels of 8-OHdG [18]. SOD and CAT are vital antioxidant enzymes that remove superoxide and hydrogen peroxide, respectively, to prevent the formation of more reactive hydroxyl radicals. Therefore, these two antioxidant enzymes form the first line of cellular defense against oxidative damage [20]. In this study, increased MDA, TOS, OSI and 8-OHdG levels and decreased TAS, SOD and CAT levels in the T/D group showed that I/R-induced testicular damage is mediated by oxidative stress. The attenuation of the levels of these parameters by treatments with SA in this study may be due to the free radical scavenging potential of SA. Similar to our results, previous studies have shown that MDA, TOS, OSI and DNA damage levels increase and TAS, SOD and CAT levels decrease in IRI and that SA treatment restores the levels of these parameters and is protective in various tissues, such as brain and kidney [4, 11].
It is known that inflammation is the another main pathway that play a role in the pathophysiology of T/D-induced testicular injury [5]. I/R-mediated inflammation and activation of pro-inflammatory mediators has been the focus of recent research in this area, and the HMGB1, a typical injury-associated protein, has received special attention [21]. HMGB-1 binds to its receptors, such as toll like receptor 4 (TLR4) and receptor for advanced glycation endproducts (RAGE) and subsequently activates NF-κB, which targets several pro-inflammatory genes, including TNF-α and IL-6, and eventually mediates inflammation. Emerging studies have shown that HMGB1 and NF-κB are also involved in oxidative stress after I/R injury [22]. In this study, increased levels of HMGB1, NF-κB p65, TNF-α, IL-6 and MPO in the T/D group showed that the testicular damage caused by I/R is mediated by inflammation. Treatments with SA significantly reduced inflammation parameters compared to the T/D group. Consistent with our results, previous studies have shown that SA can show protective activity against I/R-induced brain and heart damage by reducing the levels of inflammation [20, 23].
The accumulation of unfolded/misfolded proteins in the ER triggers an evolutionarily conserved pathway called UPR. There are three proteins in ER that act as stress sensors and activate the UPR pathway: ATF6, protein kinase RNA- like endoplasmic reticulum kinase (PERK) and inositol-requiring enzyme 1 (IRE1) [24]. In the physiological state, these sensor proteins are kept inactive state via bound to GRP78. Under conditions that cause impairment of ER functions, GRP78 releases these sensor proteins that will initiate UPR [25]. Under stress conditions, ATF6 transfers from ER to Golgi and is cleaved by site-1 and site-2 proteases to form active fragment. Activated ATF6 then moves to the nucleus to mediate the expression of various proteins, including CHOP. Therefore, there is no doubt that ATF6 is an important mediator during ischemia [15]. The first cellular response that occurs as a result of UPR activation is the global inhibition of protein synthesis. In the second stage, it is aimed to reduce the proteotoxic stress caused by misfolded/unfolded proteins by increasing chaperone synthesis and activating the ERAD pathway. In the third stage, if the proteotoxic environment cannot be eliminated, apoptotic cell death is activated [24]. CHOP is a highly inducible gene that is expressed at very low levels in physiological conditions and is remarkably up-regulated in response to stress. CHOP is the first protein identified that promotes apoptosis under conditions of ER stress [25]. It is known that increased apoptosis plays an important role in I/R-induced tissue damage and the apoptotic process is mediated by caspase-3 [5, 11]. Therefore, GRP78, ATF6 and CHOP levels were determined in testicular tissues to reveal ER stress, and caspase-3 levels were determined to show the level of apoptosis in this study. The results showed that GRP78, ATF6, CHOP and caspase-3 levels were increased in the T/D group compared to the control group, while SA treatments protected the testicular tissue against IRI by suppressing ER stress and apoptosis. These results are thought to be important as they reveal ER stress inhibitory activity for the first time in an experimental IRI model of SA. Consistent with our results, previous studies have shown that SA and its derivatives exhibit tissue protective effect in various different experimental models via suppressing the levels of ER stress and apoptosis [10, 26]
Histological evaluation using a microscopic Johnsen scoring system is considered the gold standard in the assessment of IRI [27, 28]. The statistically significant decrease in the Johnsen scores in the T/D group compared with the control group indicates that the I/R damage was successfully achieved. The statistically significant improvement of this decrease with the applications of SA showed the testicular protective effect of SA. In parallel with our results, Unsal et al. reported that sinapic acid protect testicular tissue against I/R-induced damage through decreasing MDA, TNF-α, IL-6 and IL-1β levels and increasing SOD and GPx levels. Again, it was shown that sinapic acid administration improved degenerative abnormalities, such as enlargement of the interstitial space, vascular dilatation, bleeding, obstruction and edema caused by T/D, and Johnsen scores in the same study [3].
SA is evaluated as a good antioxidant due to its strong free radical scavenging activity [6]. It is suggested that the antioxidant property of SA is due to its redox properties, which allow it to act as a hydrogen donor, free radical scavenger, metal chelator, and modulator of enzymatic activity, resulting from this structural property [29]. Therefore, we suggest that the tissue protective effect of SA against I/R-induced testicular damage is mainly due to its antioxidant potential.
Our study has a few limitations that need to be explored in detail in future studies. First, the testicle protective effect of SA can be re-evaluated by focusing on more detailed mechanisms. Second, the efficacy of only two doses of SA was evaluated in the study. Comprehensive studies should test the effect of different doses of SA. Third, we did not investigate the application of SA at different times or in prolonged I/R periods in this study. We believe that demonstrating the protective efficacy of SA against I/R-induced testicular damage, together with physiological fertility behavior experiments in long-term studies, will shed light on clinical stages.
Conclusion
Our study confirms that the testicular protective effect of SA occurs through suppression of oxidative stress, inflammation, ER stress and apoptosis in the rat TT model. Histopathological studies on testicular tissues have also confirmed that SA alleviates T/D-induced impaired spermatogenesis. Therefore, the overall results suggest that SA emerges as a potential compound for the treatment of TT and may be subject to clinical trials.
Data availability
The data presented in the current study are available from the corresponding author on reasonable request.
Abbreviations
- 8-OHdG:
-
8-Hydroxy-2′-deoxyguanosine
- ATF6:
-
Activating transcription factor 6
- CAT:
-
Catalase
- CHOP:
-
C/EBP homologous protein
- DMSO:
-
Dimethyl sulfoxide
- ELISA:
-
Enzyme-linked immunosorbent assay
- ER:
-
Endoplasmic reticulum
- ERAD:
-
Endoplasmic reticulum-associated degradation
- GPx:
-
Glutathione peroxidase
- GRP78:
-
78-KDa glucose-regulated protein
- H&E:
-
Hematoxylin and eosin
- HMGB1:
-
High mobility group box 1
- IP:
-
Intraperitoneal
- I/R:
-
Ischemia/reperfusion
- IL-6:
-
Interleukin-6
- IRE1:
-
Inositol requiring enzyme 1
- IRI:
-
Ischemia/reperfusion injury
- IQR:
-
Interquantile range
- MDA:
-
Malondialdehyde
- MPO:
-
Myeloperoxidase
- NF-κB p65:
-
Nuclear factor kappa B protein 65
- OSI:
-
Oxidative stress index
- PBS:
-
Phosphate buffered saline
- PERK:
-
Protein kinase RNA-activated-like ER kinase
- ROS:
-
Reactive oxygen species
- SA:
-
Syringic acid
- SOD:
-
Superoxide dismutase
- TAS:
-
Total antioxidant status
- TBA:
-
Thiobarbituric acid
- T/D:
-
Torsion/detorsion
- TNF-α:
-
Tumor necrosis factor-alpha
- TOS:
-
Total oxidant status
- TT:
-
Testicular torsion
- UPR:
-
Unfolded protein response
References
Vaos G, Zavras N. Antioxidants in experimental ischemia-reperfusion injury of the testis: Where are we heading towards? World J Methodol. 2017;7(2):37–45.
Shunmugam M, Goldman RD. Testicular torsion in children. Can Fam Physician. 2021;67(9):669–71.
Unsal V, Kolukcu E, Gevrek F, Firat F. Sinapic acid reduces ischemia/reperfusion injury due to testicular torsion/detorsion in rats. Andrologia. 2021;53(8): e14117.
Sancak EB, Akbas A, Silan C, Cakir DU, Turkon H, Ozkanli SS. Protective effect of syringic acid on kidney ischemia-reperfusion injury. Ren Fail. 2016;38(4):629–35.
Karaguzel E, Kadihasanoglu M, Kutlu O. Mechanisms of testicular torsion and potential protective agents. Nat Rev Urol. 2014;11:391–9.
Li Y, Zhang L, Wang X, Wu W, Qin R. Effect of syringic acid on antioxidant biomarkers and associated inflammatory markers in mice model of asthma. Drug Dev Res. 2019;80(2):253–61.
Srinivasulu C, Ramgopal M, Ramanjaneyulu G, Anuradha CM, Kumar CS. Syringic acid (SA)-A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed Pharmacother. 2018;108:547–57.
Reyes-Fermín LM, Aparicio-Trejo OE, Avila-Rojas SH, Gómez-Sierra T, Martínez-Klimova E, Pedraza-Chaverri J. Natural antioxidants’ effects on endoplasmic reticulum stress-related diseases. Food Chem Toxicol. 2020;138: 111229.
Martucciello S, Masullo M, Cerulli A, Piacente S. Natural products targeting ER stress, and the functional link to mitochondria. Int J Mol Sci. 2020;21:1905.
Tungalag T, Yang DK. Sinapic acid protects SH-SY5Y human neuroblastoma cells against 6-hydroxydopamine-induced neurotoxicity. Biomedicines. 2021;9(3):295.
Güven M, Aras AB, Topaloğlu N, Özkan A, Şen HM, Kalkan Y, Okuyucu A, Akbal A, Gökmen F, Coşar M. The protective effect of syringic acid on ischemia injury in rat brain. Turk J Med Sci. 2015;45(1):233–40.
Liu G, Zhang BF, Hu Q, Liu XP, Chen J. Syringic acid mitigates myocardial ischemia reperfusion injury by activating the PI3K/Akt/GSK-3β signaling pathway. Biochem Biophys Res Commun. 2020;531(2):242–9.
Turner TT, Tung KS, Tomomasa H, Wilson LW. Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol Reprod. 1997;57(6):1267–74.
Demir S, Kazaz IO, Kerimoglu G, Demir EA, Colak F, Yilmaz S, Mentese A. Astaxanthin protects testicular tissue against torsion/detorsion-induced injury via suppressing endoplasmic reticulum stress in rats. J Invest Surg. 2022;35(5):1044–9.
Kazaz IO, Demir S, Kerimoglu G, Colak F, Alemdar NT, Dogan SY, Bostan S, Mentese A. Chlorogenic acid ameliorates torsion/detorsion-induced testicular injury via decreasing endoplasmic reticulum stress. J Pediatr Urol. 2022;18(3):289.e1-e7.
Manjunatha S, Shaik AH, Prasad EM, Omar SYA, Mohammad A, Kodidhela LD. Combined cardio-protective ability of syringic acid and resveratrol against isoproterenol induced cardio-toxicity in rats via attenuating NF-kB and TNF-α pathways. Sci Rep. 2020;10(1):3426.
Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–8.
Demir EA, Mentese A, Kucuk H, Alemdar NT, Demir S. p-Coumaric acid alleviates cisplatin-induced ovarian toxicity in rats. J Obstet Gynaecol Res. 2022;48(2):411–9.
Johnsen SG. Testicular biopsy score count-A method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25.
Shahzad S, Mateen S, Kausar T, Naeem SS, Hasan A, Abidi M, Nayeem SM, Faizy AF, Moin S. Effect of syringic acid and syringaldehyde on oxidative stress and inflammatory status in peripheral blood mononuclear cells from patients of myocardial infarction. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(4):691–704.
Ye Y, Zeng Z, Jin T, Zhang H, Xiong X, Gu L. The role of high mobility group box 1 in ischemic stroke. Front Cell Neurosci. 2019;13:127.
Zhang B, Zhong Q, Chen X, Wu X, Sha R, Song G, Zhang C, Chen X. Neuroprotective effects of celastrol on transient global cerebral ischemia rats via regulating HMGB1/NF-κB signaling pathway. Front Neurosci. 2020;14:847.
Kim IH, Yan BC, Park JH, Yeun GH, Yim Y, Ahn JH, Lee JC, Hwang IK, Cho JH, Kim YM, Lee YL, Park JH, Won MH. Neuroprotection of a novel synthetic caffeic acid-syringic acid hybrid compound against experimentally induced transient cerebral ischemic damage. Planta Med. 2013;79(5):313–21.
Lin CL. Attenuation of endoplasmic reticulum stress as a treatment strategy against ischemia/reperfusion injury. Neural Regen Res. 2015;10(12):1930–1.
Xin Q, Ji B, Cheng B, Wang C, Liu H, Chen X, Chen J, Bai B. Endoplasmic reticulum stress in cerebral ischemia. Neurochem Int. 2014;68:18–27.
Li X, Zhang X, Xing R, Qi F, Dong J, Li D, Yu B, Huang M, Zhang L, Yuan X, Yang Y, Wu H, Zang L, Mao X, Sui R. Syringic acid demonstrates promising protective effect against tau fibrillization and cytotoxicity through regulation of endoplasmic reticulum stress-mediated pathway as a prelude to Alzheimer’s disease. Int J Biol Macromol. 2021;192:491–7.
Kazaz IO, Demir S, Kerimoglu G, Colak F, Alemdar NT, Akman AU, Cekuc OC, Mentese A. Effect of chrysin on endoplasmic reticulum stress in a rat model of testicular torsion. J Invest Surg. 2022;35(5):1106–11.
Demir S, Kazaz IO, Aliyazicioglu Y, Kerimoglu G, Teoman AS, Yaman SO, Arslan A, Mentese A. Effect of ethyl pyruvate on oxidative state and endoplasmic reticulum stress in a rat model of testicular torsion. Biotech Histochem. 2020;95(4):317–22.
Cikman O, Soylemez O, Ozkan OF, Kiraz HA, Sayar I, Ademoglu S, Taysi S, Karaayvaz M. Antioxidant activity of syringic acid prevents oxidative stress in L-arginine-induced acute pancreatitis: An experimental study on rats. Int Surg. 2015;100:891–6.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
EAD: Conceptualization, data curation, validation, investigation, software, writing-original draft. SD: Conceptualization, methodology, formal analysis, writing-original draft. IOK: Conceptualization, methodology and writing-original draft. HK: Methodology, investigation. NTA: Methodology, Formal analysis. OFG: Methodology. AM: Data curation, validation, investigation, software. YA: Investigation, writing-original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
This study was approved by the Experimental Animal Local Ethics Committee of Karadeniz Technical University (Protocol no: 2021/65) and performed according to the animal research reporting of in vivo experiments (ARRIVE) guidelines.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Demir, E.A., Demir, S., Kazaz, I.O. et al. Syringic acid ameliorates ischemia/reperfusion-induced testicular injury in rats via suppressing of HMGB1/NF-κB axis and endoplasmic reticulum stress. Eur J Trauma Emerg Surg 49, 1595–1602 (2023). https://doi.org/10.1007/s00068-023-02227-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-023-02227-7