Abstract
Patients with metabolic syndrome (MetS) often exhibit generalized endothelial and cardiac dysfunction with decreased nitric oxide (NO) production and/or bioavailability. Since phosphodiesterase 5 (PDE5) inhibitors restore NO signaling, we hypothesized that chronic treatment with long-acting PDE5 inhibitor tadalafil may enhance plasma NO levels and reduce cardiac dysfunction following ischemia/reperfusion (I/R) injury in C57BL/6NCrl-Leprdb−lb/Crl mice with MetS phenotypes. Adult male MetS mice were randomized to receive vehicle solvent or tadalafil (1 mg/kg,i.p.) daily for 28 days and C57BL/6NCrl mice served as healthy wild-type controls. After 28 days, cardiac function was assessed by echocardiography and hearts from a subset of mice were isolated and subjected to 30 min of global ischemia followed by 60 min of reperfusion (I/R) in ex vivo Langendorff mode. Body weight, blood lipids, and glucose levels were elevated in MetS mice as compared with wild-type controls. The dyslipidemia in MetS was ameliorated following tadalafil treatment. Although left ventricular (LV) systolic function was minimally altered in the MetS mice, there was a significant diastolic dysfunction as indicated by reduction in the ratio of peak velocity of early to late filling of the mitral inflow, which was significantly improved by tadalafil treatment. Post-ischemic cardiac function, heart rate, and coronary flow decreased significantly in MetS mice compared to wild-type controls, but preserved by tadalafil treatment. Myocardial infarct size was significantly smaller following I/R, which was associated with higher plasma levels of nitrate and nitrite in the tadalafil-treated MetS mice. In conclusion, tadalafil induces significant cardioprotective effects as shown by improvement of LV diastolic function, lipid profile, and reduced infarct size following I/R. Tadalafil treatment enhanced NO production, which may have contributed to the cardioprotective effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MetS) is a cluster of risk factors characterized by abdominal obesity, dyslipidemia, hypertension, and insulin resistance [1, 2]. It is associated with increased risk of multiple chronic diseases, including cardiovascular disease, type 2 diabetes, arthritis, chronic kidney disease, cancer, and all-cause mortality [3,4,5,6]. The main causes of MetS are nutrition transition in the modern generations including consumption of high calorie-low fiber fast food, sugar-sweetened beverage consumption leading to obesity, predisposed genetics, reduced physical activity or sedentary lifestyle, disrupted sleep, mood disorders, stress, smoking/alcohol abuse, and use of certain medications like psychotropic drugs [7,8,9,10,11,12,13,14].
Nitric oxide (NO) plays a crucial role in the pathogenesis of MetS. Defective endogenous synthesis and bioavailability of NO has been suggested as a molecular mechanism contributing to the features of MetS [15]. NO plays an important role in the development of MetS components, such as insulin resistance, endothelial dysfunction, hypertriglyceridemia, and chronic adipose tissue inflammation. Endothelial dysfunction, increases vascular resistance, and hypertension normally observed in MetS patients may further lead to LV hypertrophy and cardiomyopathy. Likewise, dyslipidemia associated with MetS can also cause atherosclerotic process leading to symptomatic ischemic heart disease [16]. In fact, endothelial nitric oxide synthase (eNOS)-deficient mice display many of the defining features of MetS, including hypertension, dyslipidemia, insulin resistance, and increased weight gain [17, 18]. Consistent with this, polymorphism in eNOS gene is associated with MetS in human beings [19, 20]. Previous studies had demonstrated that dietary inorganic nitrate or nitrite reversed several features of MetS in eNOS-deficient mice [15], diet-induced obese hypertensive rats [21], and high-fat-diet-fed and ovariectomized mice [22]. Moreover, long-term deficiency of dietary nitrate/nitrite caused MetS and cardiovascular dysfunction and death of mice [23]. Recent clinical trial also indicated enhanced levels of plasma nitrate and antioxidant capability in the MetS patients consuming Mediterranean diets [24].
Sildenafil (Viagra®), vardenafil (Levitra®), and tadalafil (Cialis®) represent class of PDE5 inhibitors approved for erectile dysfunction (ED). PDE5 inhibitors act by correcting the damage to the penile vascular endothelium by upregulating eNOS, iNOS, and increased NO production [25]. The constellation of risk factors that make up MetS is also linked to erectile dysfunction, which is up to three times more prevalent in men with MetS [26]. Approximately 79–96% of patients with MetS present with ED and 29–66% of patients with ED have MetS [27, 28]. Endothelial dysfunction is a common denominator between ED and cardiovascular disease. In MetS and dyslipidemia patients, LDL production causes destruction of eNOS [29, 30]. Bioavailability of NO on the endothelial surface is reduced, early in the pathogenesis of atherosclerosis, causing either local or circulating vasoconstrictor factors to become dominant, leading to damaged endothelium-based vasodilatation [31, 32]. Thus, the damage to the vascular and cavernosal endothelium leads to ED in the MetS patients. PDE5 inhibitors are therefore commonly used among MetS patient population to treat ED.
Our prior work showed that NO signaling was significantly restored by treatment with PDE5 inhibitors, which led to significant attenuation in myocardial ischemia/reperfusion (I/R) injury [33,34,35]. Moreover, both short-acting PDE5 inhibitors, sildenafil and vardenafil, have been shown to cause vasorelaxation through enhanced endogenous NO signaling in diabetic rats [36, 37]. Similarly, endothelial function was reported to be improved following chronic administration of tadalafil in men with ED [38] and in patients with type 2 diabetes [39, 40]. In our earlier studies, we demonstrated that tadalafil treatment ameliorated pro-inflammatory cytokines, reversed the maladaptive alterations of cytoskeletal/contractile proteins, and increased expression of eNOS, Sirt1, and PGC-1α signaling, which was associated with significant improvement of mitochondrial dysfunction following I/R injury in Type 2 diabetic hearts [41]. Based on these background studies, we hypothesized that chronic treatment with long-acting PDE5 inhibitor, tadalafil, would enhance circulating NO levels and reduce myocardial I/R injury in MetS mice.
Methods
Animals
Adult male mice (C57BL/6NCrl-Leprdb−lb/Crl strain), which were established as a mouse model fulfilling all the MetS criteria in a single animal were obtained from Charles River (Wilmington, MA). More detailed information about the MetS mice and their controls can be found at the following link: https://www.criver.com/sites/default/files/resources/rm_rm_r_POUND_MOUSE_fact_sheet.pdf.
These MetS mice and their non-MetS control mice (C57BL/6NCrl background) were housed within School of Medicine Animal Facility, Virginia Commonwealth University. The animals were fed with rodent chow diet ad libitum and are characterized by obesity at 1 month of age, hyperinsulinemia and hyperglycemia at 18 weeks of age, as well as increased cholesterol levels at 14 weeks of age [42]. All animal experiments were conducted under the guidelines on humane use and care of laboratory animals for biomedical research published by the National Institutes of Health (No. 85-23, Revised 1996). The animal care and experimental protocols were approved by Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Protocol for animal experiments
Eighteen- to twenty-week--sold MetS mice (n = 12/group) were randomized to receive daily intraperitoneal (i.p.) injections of DMSO (10% DMSO in 0.9% NaCl, 0.2 mL) or tadalafil (1 mg/kg in 10% DMSO) daily for 4 weeks (Fig. 1). The tadalafil dose (1 mg/kg) was chosen based on interspecies dose extrapolation scaling to result in plasma concentrations equivalent to those found in humans receiving an oral dose of 20 mg/day. Metabolic parameters including fasting blood glucose levels and body weight were monitored weekly during the treatment period. LV function was assessed by echocardiography before and after the treatments. Upon completion of the 4 weeks of tadalafil therapy, the mice were sacrificed; anesthetized with pentobarbital sodium (70 mg/kg, i.p.) and blood samples were taken by cardiac puncture via thoracotomy for assessment of biochemical parameters and hearts were collected for further experimental analysis.
Experimental design and protocol. The diagram shows the sequence of various experimental procedures. The mice were treated with tadalafil (TAD) or vehicle (DMSO) for 28 days. Body weights and blood glucose levels were monitored to confirm hyperglycemia. After drug treatment period, oral glucose tolerance test (OGTT), insulin tolerance test (ITT), as well as echocardiography were performed and mice were sacrificed to collect plasma and hearts. The isolated hearts were subjected to 20 min stabilization followed by 30 min of ischemia and 60 min of reperfusion in Langendorff model and infarct size was measured by tetrazolium staining. Plasma was used for biochemical measurements
Measurement of plasma glucose and insulin
Weekly blood glucose concentrations were obtained using a handheld glucometer (Bayer, PA) using 5 µL of blood obtained from tail vein from 12 h fasted mice and applied directly to glucose test strips to measure fasting glucose levels to monitor hyperglycemia. After the animals were sacrificed, blood was collected and centrifuged at 1000 g for 10 min at 4 °C to collect plasma, which was stored at − 80 °C. The plasma samples were assayed for glucose, using commercially available colorimetric assay kits (Cayman Chemicals, Ann Arbor, MI). Plasma insulin concentrations were determined by ELISA method following the manufacturer’s instructions (Crystal Chem, Downers Grove, IL).
Analysis of plasma lipids
The analysis of lipoprotein profiles was performed as previously reported [43]. Briefly, plasma lipoprotein was separated by using AB SCIEX 4000 QTRAP 4000 Series high-performance liquid chromatography tandem mass spectrometry (LC–MS/MS) HPLC system (Shimadzu, Japan). Total cholesterol levels in the column effluent were continuously measured using in-line mixture with an enzymatic and colorimetric cholesterol detection reagent (Total Cholesterol E, Wako Chemicals USA). HDL (high-density lipoproteins), LDL (low-density lipoproteins), and VLDL (very low-density lipoproteins) were eluted from the column. The concentration for each lipoprotein fraction was calculated by multiplying the ratio of the corresponding peak area to total peak area by the total cholesterol concentration for each sample, which was measured using a plate reader with 1:1 mixture of cholesterol E reagent and diluted plasma against the standard curve generated using the cholesterol standard solution (200 mg/dL) provided in the commercial kit following a serial dilution.
Glucose and insulin tolerance test
For oral glucose tolerance test (OGTT), mice were fasted overnight and glucose (3 g/kg body weight) was given via oral gavage. A drop of blood was then drawn via the tail vein and read by a glucometer (Bayer, PA). The glucose levels were measured before and 15, 30, 45, 60, 75, 90, 120, 150, and 180 min after glucose administration. For insulin tolerance test (ITT), long-acting insulin (Novo Nordisk, Mainz, Germany) was injected (0.15 IU/kg body weight, i.p), and blood glucose levels were determined 0, 15, 30, 45, 60, and 90 min post injection, as stated above.
Measurement of LV function by echocardiography
Doppler echocardiography was performed under light anesthesia (pentobarbital 30 mg/kg, i.p.), using the Vevo 770™ imaging system (VisualSonics, Inc., Toronto, Canada), which is equipped with a 30-MHz mechanical scan probe to obtain high resolution two-dimensional images. B-mode images were obtained in the plane containing aortic and mitral valves, while M-mode images were obtained from parasternal short-axis view at the level of papillary muscles and the apical four-chamber view. Fractional shortening (FS), ejection fraction (EF), and LV wall parameters were calculated using Vevo Analysis software. M-mode measurements of LV dimensions were averaged from three cycles. LV diastolic function was quantified by measuring the flow patterns across the mitral wave using Pulse Wave Doppler [41]. Two waves are characteristically seen in the Doppler of the transmitral inflow, which are used for the evaluation of LV diastolic function: one representing passive filling of the ventricle (early [E] wave) and one consistent with active and late filling with atrial systole (atrial [A] wave). The ratio of peak velocity of early to late filling of the mitral inflow (E/A) was calculated and used for the evaluation of LV diastolic function.
Global ischemia/reperfusion injury in Langendroff mode
We have previously reported methodology of isolated perfused mouse heart [44]. In brief, following removal of the heart from the thorax, the aortic opening was rapidly cannulated that was connected to the Langendorff perfusion system. The heart was retrogradely perfused at a constant pressure of 55 mmHg at 37 °C with modified Krebs–Henseleit solution containing (in mM) 118 NaCl, 24 NaHCO3, 2.5 CaCl2, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 11 glucose, and 0.5 EDTA. The perfusion solution was continuously gassed with 95% O2-5% CO2 (pH 7.34–7.49). The heart apex was attached to a Grass FT03 force–displacement transducer (Grass Technologies, West Warwick, RI) with surgical thread and a rigid metal hook for measuring ventricular contractile force. The beat-by-beat cardiac contractile signals were continuously recorded with a PowerLab/8sp system (AD Instruments Inc., Colorado Springs, CO). After 20 min of stabilization period, the hearts were subjected to a 30 min of global ischemia and 60 min of reperfusion (Fig. 1). Coronary flow was measured at 15 min before ischemia and 15 min after reperfusion. At the end of the experiment, the hearts were collected, weighed, and stored at − 20 °C for further analysis.
Measurement of myocardial infarct size
Following reperfusion, the heart was immediately removed from the Langendorff apparatus, weighed, and frozen at − 20 °C. The frozen heart was cut into seven to eight transverse slices of approximately equal thickness (~ 0.8 mm) and stained by incubation in 10 mM triphenyltetrazolium chloride (TTC) for 30 min at room temperature (22 °C). TTC buffer was then replaced with 10% formaldehyde, and the slices were fixed for 4–6 h before the infarct area and risk zone were measured using computer morphometry [44]. The risk area was calculated as total ventricular area minus the area of cavities. The infarct size was calculated as a percentage of the risk area.
Measurement of plasma nitrate and nitrite
Blood samples were collected from the mice that underwent treatment conditions (n = 6 per group) and centrifuged to obtain the supernatant plasma. Following the previously reported methods [41, 45], plasma samples were subsequently centrifuged using Amicon Ultra-4 centrifugal filter devices at 7500 g in 4 °C to eliminate large molecules (molecular weight > 30 kDa) from the plasma. The plasma nitrate and nitrite were measured with a SIEVERS nitric oxide analyzer (model 280NOA). The reducing agents used were either vanadium (III) chloride (VCl3) in 1 M HCl (for nitrate) or 1% sodium iodide (NaI) in glacial acetic acid (for nitrite). Five mL of a reagent and 100 µL of 1:30 diluted anti-foaming agent were loaded into the purge vessel for analysis. These reducing agents converted nitrite and nitrate, respectively, to gaseous NO at 90 °C, which was quantified by the analyzer.
Statistical analysis
All parameters assessed in this study were analyzed using one-way ANOVA followed by Student–Newman–Keuls post hoc test for pair-wise comparison. Data were represented as mean ± S.E.M. p < 0.05 was considered significant.
Results
Effect of tadalafil on the basic metabolic characteristics
The metabolic health status of the mice was evaluated in terms of body weight, plasma glucose, insulin, and lipid profile as shown in Table 1. The MetS mice showed significant increase in the body weight, plasma glucose, and insulin levels when compared to their wild-type non-MetS controls. The body weight (65.2 ± 1.0 versus 63.6 ± 1.2 g) and fasting glucose levels (236 ± 12 mg/dL versus 214 ± 23 mg/dL) in MetS mice did not reduce significantly following treatment with tadalafil while the plasma insulin levels (461 ± 22 ng/mL versus 196 ± 20 ng/mL; p < 0.02) were significantly reduced upon tadalafil treatment as compared to the untreated MetS mice. MetS mice had significantly higher total cholesterol, triglycerides, VLDL, LDL, non-HDL, and total cholesterol/HDL ratio compared to their controls. Treatment with tadalafil demonstrated favorable effect on the lipid profile with significant reduction of triglycerides, non-HDL, and VLDL in tadalafil-treated MetS mice compared to untreated MetS mice.
Tadalafil attenuates LV diastolic dysfunction
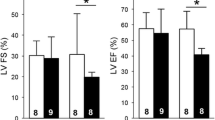
Heart rate, LV, EF, and FS decreased slightly but not significantly in MetS group compared with the non-MetS control group (Fig. 2a–c). Tadalafil treatment in MetS mice slightly enhanced the heart rate, EF, and FS; however, there was no significant difference in these parameters among the groups. There were no significant changes in the LV wall thickness between the groups (Fig. 2e–h). The MetS mice developed diastolic dysfunction while maintaining a normal systolic function. Mitral tissue Doppler E/A ratio was measured as the index of diastolic function. A significant decrease in the E/A ratio was found in MetS mice compared to the non-MetS control group demonstrating diastolic dysfunction. Tadalafil treatment prevented the diastolic dysfunction in MetS mice (Fig. 2d).
Echocardiographic assessment of cardiac contractile function in MetS mice. The averaged data of heart rate (a), ejection fraction (b), fractional shortening (c), E/A ratio (d), anterior wall diastolic thickness (e), anterior wall systolic thickness (f), posterior wall diastolic thickness (g), and posterior wall systolic thickness (h). Data are expressed as mean ± SE (n = 6 per group), *p < 0.05 vs. Control, #p < 0.05 vs. MetS
Tadalafil treatment improves insulin sensitivity
The OGTT revealed no significant difference in glucose uptake between the MetS and MetS + TAD groups (Fig. 3a, b). Treatment with tadalafil prevented larger decline in blood glucose levels following insulin injection (Fig. 3c, d) as shown by approximately 20% drop in the area under the curve.
Effect of tadalafil (TAD) on oral glucose tolerance test (OGTT) and insulin tolerance test (ITT). a Data are expressed as mean ± S.E.M. (n = 6/group), of glucose concentrations following oral glucose gavage (3 mg/kg) in Control (open circle), MetS (dot) and MetS + TAD (filled triangle) groups. b Area under the curve of OGTT. c Data are mean ± S.E.M (n = 6/group) of glucose concentration following intraperitoneal insulin injection (0.15 U/kg body weight) in Control (open circle), MetS (dot) and MetS + TAD (filled triangle) groups. d Area under the curve of ITT. Symbols *p < 0.05 vs. Control, #p < 0.05 vs. MetS
Tadalafil protects against global I/R injury
Myocardial infarction was evident among the groups following I/R injury protocol as shown in Fig. 4a. Treatment with tadalafil for 28 days attenuated the infarct size in MetS mice compared to untreated MetS mice (16.2 ± 2.1% versus 31.0 ± 2.0%; p < 0.01). The area at risk of the globally ischemic hearts was not different between the groups (Fig. 4b). There was no difference in the cardiac contractile function based on ventricular developed force and rate–force product before ischemia. Heart rate and coronary flow rate also did not differ among the groups before ischemia. However, following ischemia, the heart rate, coronary flow rate, and rate-force product decreased significantly in the MetS group compared to the non-MetS mice as shown in Fig. 5b–d. Tadalafil treatment improved the post-ischemic cardiac function in MetS mice by restoring the heart rate, coronary flow rate, and rate–force product (Fig. 5b–d).
Effect of tadalafil (TAD) on myocardial infarct size (% of Risk Area; a) after 30 min of ex vivo global no-flow ischemia followed by 60 min of reperfusion in Langendorff mode. The area at risk, expressed as % of total left ventricular area (b). Data are expressed as mean ± S.E.M. (n = 6/group). Symbols *p < 0.05 vs. Wild-type Control group; # indicates p < 0.05 vs. MetS
Effect of tadalafil (TAD) on cardiac function and coronary flow. The post-ischemic cardiac ventricular developed force (a), heart rate (b), rate-force product (c), and coronary flow rate (d) are presented as a percentage of pre-ischemia baseline. Data are expressed as mean ± S.E.M. (n = 6/group).*p < 0.05 vs. Control, #p < 0.05 vs MetS
Tadalafil treatment enhances plasma nitrate and nitrite levels
Since MetS is known to impair NO bioavailability [46], we therefore measured plasma levels of nitrate and nitrite in these mice. The MetS mice showed only a slight reduction in the nitrate levels while the plasma nitrite levels remained unchanged compared to non-MetS controls (Fig. 6b). Tadalafil treatment significantly enhanced plasma nitrate and nitrite levels in MetS mice compared to both the control group and the untreated MetS groups, most likely through enhanced NOS-dependent NO synthesis triggered by tadalafil (Fig. 6a). Overall NO oxidation products (NOx) were significantly increased in tadalafil-treated MetS mice compared to the non-MetS control and untreated MetS groups (Fig. 6c).
Discussion
In the present study, we demonstrated that chronic treatment with a long-acting PDE5 inhibitor, tadalafil, protects against cardiac I/R injury in the mice with MetS. The MetS mouse model displayed several key features of metabolic syndrome including obesity, hyperinsulinemia, hypertriglyceridemia, and high cholesterol levels. Metabolic syndrome subjects have consistently high glucose levels reflecting either prediabetic/diabetic conditions. In addition, their postprandial glucose surges are very high as compared to normal subjects due to profound insulin resistance. High levels of postprandial blood glucose are linked to microvascular damage, which leads to macrovascular complications and organ failures. The treatment regimen with tadalafil exhibited beneficial effects on the systemic abnormalities induced by MetS. Tadalafil treatment was effective in improving the metabolic status of mice as shown by slight decrease in body weights and blood glucose coupled with significant reductions in hyperinsulinemia and hypertriglyceridemia. Tadalafil treatment also reduced total cholesterol, VLDL significantly, and improved insulin sensitivity as evidenced by a significant decrease in the area under the curve following insulin tolerance test in the treated MetS mice. We have previously reported that tadalafil decreased plasma glucose levels, inflammatory profile, and oxidative stress in Type 2 diabetic db/db mice [41, 47,48,49]. Mechanistically, PDE5 inhibitors are known to improve NO bioavailability and vasodilatation, which is associated with increased blood flow for muscle glucose utilization, resulting in increased glucose uptake and thereby leading to decrease in circulating glucose levels. In fact, a previous study demonstrated that cardiac NO production regulates myocardial glucose uptake via a cGMP-dependent mechanism [50]. This study suggested the role of NO derived from eNOS in regulation of glucose uptake. Nevertheless, the role of Tadalafil in glucose uptake in the hearts from MetS mice needs to be elucidated. We speculate that the moderate alterations in terms of body weight, blood glucose and lipid profile observed in treated MetS mice group would be augmented if tadalafil treatment is combined with other anti-diabetic drugs and lipid lowering drugs in MetS mice.
The incidence and severity of myocardial infarction and ischemic injury are greater in the diabetic and MetS population compared to the non-diabetic and non-MetS populations as indicated in several clinical studies [51,52,53]. In the present study, we challenged the MetS hearts to 30 min global ischemia followed by 1 h reperfusion. Our results showed no significant difference in the infarct sizes of non-MetS and MetS mice hearts. These differing results could be partially due to different durations and severity of MetS and differences between species [54, 55]. It is also notable that previous experimental data reveal both higher and lower vulnerability of heart to ischemia in diabetic models [56]. The present study shows that tadalafil treatment in MetS mice resulted in significant reduction of infarct size as compared with the vehicle (DMSO)-treated MetS mice (Fig. 4), which was associated with improved post-ischemic cardiac function in tadalafil-treated MetS mice (Fig. 5). These results are in agreement with our previous report on tadalafil-induced cardioprotective effects against in vivo I/R injury in normoglycemic mice [35]. Despite the protective effect of Tadalafil against acute I/R injury in MetS mice, its effect in chronic heart failure in this model is yet to be investigated.
In the present study, we also performed echocardiography to examine alterations of LV function in MetS mice. LV systolic function remained unchanged among the groups as evidenced by no significant alterations in the heart rate, ejection fraction, and fractional shortening. LV wall parameters were also not significantly different among the groups. However, the MetS mice exhibited significant reduction in E/A ratio indicating decreased diastolic function, which was improved upon tadalafil treatment. It is noteworthy that the LV systolic function remained unaltered in the experimental groups (20–24-week-old mice), which could be manifested in aged mice [48]. Heart failure is commonly associated with impaired LV systolic function. However, nearly 30–40% of the patients have LV diastolic dysfunction while the ejection fraction (the typical symptom of congestive heart failure) is either normal or slightly reduced [57, 58]. Diastolic dysfunction is typically seen in patients with hypertension and hypertrophic or restrictive cardiomyopathy. It is more prevalent in the elderly population since tachycardia, hypertension, and ischemia contributing to impaired diastolic function are often seen in elderly patients [59]. So far, there is no specific therapy to improve LV diastolic function directly. In this regard, chronic treatment with tadalafil could be a novel and safe therapeutic option in preventing the deteriorated LV diastolic dysfunction and ischemic heart diseases in MetS patients.
We have earlier shown that chronic treatment with tadalafil for 4 weeks in type 2 diabetic db/db mice resulted in the reduction of blood glucose and triglyceride levels, attenuated oxidative stress, and reduced infarct size thus protecting against I/R injury in type 2 diabetic conditions [47, 48]. We also reported that PDE5 inhibition by sildenafil triggered a signaling cascade including upregulation of eNOS/inducible NO synthase (iNOS) [34, 60], resulting in enhanced activation of cGMP-dependent protein kinase (PKG) levels in mouse models of cardiac injury suggesting a critical role of NO-cGMP-PKG signaling pathway in cardioprotection with this drug. MetS is known to cause endothelial and cardiac dysfunction primarily due to impaired NO bioavailability. Variations at the eNOS gene are known to influence energy expenditure, severity of glucose intolerance, risk of developing type II diabetes, and associated cardiovascular complications [61]. In addition, eNOS and iNOS expressions are decreased in the type 2 diabetic and MetS patients [62]. In the present study, MetS mice did not show significant decrease in NO levels compared to the controls, albeit there is a tendency of decrease in the nitrate and total NOx levels. The tadalafil-treated MetS mice had a significant increase in plasma nitrate, nitrite, and total NOx levels. Therefore, tadalafil treatment improved bioavailability of NO as evidenced by enhanced circulating NO levels (Fig. 6c) and improved cardiac function in MetS mice (Fig. 5).
In conclusion, the present study demonstrated that tadalafil had a positive effect on the metabolic health status by improving insulin sensitivity, lowering circulating lipids, and protecting against I/R injury via enhanced NO production in MetS mice. Tadalafil treatment also improved LV diastolic dysfunction in MetS mice. This study is particularly important since the favorable effects of tadalafil treatment in MetS mice could clinically benefit MetS patients who are at high risk for cardiovascular diseases. As Tadalafil is approved by FDA for treatment of ED, pulmonary hypertension, and enlarged prostate, prescribing tadalafil to MetS patients may turn out to be promising therapeutic strategy providing dual benefit of treating ED as well as reducing cardiovascular injury in MetS patients.
References
Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH (2008) The metabolic syndrome. Endocr Rev 29:777–822. https://doi.org/10.1210/er.2008-0024
Huang PL (2009) A comprehensive definition for metabolic syndrome. Dis Model Mech 2:231–237. https://doi.org/10.1242/dmm.001180
Ford ES (2005) Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 28:1769–1778. https://doi.org/10.2337/diacare.28.7.1769
Hallajzadeh J, Safiri S, Mansournia MA, Khoramdad M, Izadi N, Almasi-Hashiani A, Pakzad R, Ayubi E, Sullman MJ, Karamzad N (2017) Metabolic syndrome and its components among rheumatoid arthritis patients: a comprehensive updated systematic review and meta-analysis. PLoS ONE 12:e0170361. https://doi.org/10.1371/journal.pone.0170361
Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT (2002) The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288:2709–2716. https://doi.org/10.1001/jama.288.21.2709
Stocks T, Bjorge T, Ulmer H, Manjer J, Haggstrom C, Nagel G, Engeland A, Johansen D, Hallmans G, Selmer R, Concin H, Tretli S, Jonsson H, Stattin P (2015) Metabolic risk score and cancer risk: pooled analysis of seven cohorts. Int J Epidemiol 44:1353–1363. https://doi.org/10.1093/ije/dyv001
Alkerwi A, Boutsen M, Vaillant M, Barre J, Lair ML, Albert A, Guillaume M, Dramaix M (2009) Alcohol consumption and the prevalence of metabolic syndrome: a meta-analysis of observational studies. Atherosclerosis 204:624–635. https://doi.org/10.1016/j.atherosclerosis.2008.10.036
Bouchard C (1995) Genetics and the metabolic syndrome. Int J Obes Relat Metab Disord 19(Suppl 1):S52–S59
Edwardson CL, Gorely T, Davies MJ, Gray LJ, Khunti K, Wilmot EG, Yates T, Biddle SJ (2012) Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS ONE 7:e34916. https://doi.org/10.1371/journal.pone.0034916
He D, Xi B, Xue J, Huai P, Zhang M, Li J (2014) Association between leisure time physical activity and metabolic syndrome: a meta-analysis of prospective cohort studies. Endocrine 46:231–240. https://doi.org/10.1007/s12020-013-0110-0
Katzmarzyk PT, Leon AS, Wilmore JH, Skinner JS, Rao DC, Rankinen T, Bouchard C (2003) Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc 35:1703–1709. https://doi.org/10.1249/01.MSS.0000089337.73244.9B
Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB (2010) Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 33:2477–2483. https://doi.org/10.2337/dc10-1079
Pollex RL, Hegele RA (2006) Genetic determinants of the metabolic syndrome. Nat Clin Pract Cardiovasc Med 3:482–489. https://doi.org/10.1038/ncpcardio0638
Xi B, He D, Zhang M, Xue J, Zhou D (2014) Short sleep duration predicts risk of metabolic syndrome: a systematic review and meta-analysis. Sleep Med Rev 18:293–297. https://doi.org/10.1016/j.smrv.2013.06.001
Carlstrom M, Larsen FJ, Nystrom T, Hezel M, Borniquel S, Weitzberg E, Lundberg JO (2010) Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc Natl Acad Sci USA 107:17716–17720. https://doi.org/10.1073/pnas.1008872107
Ctoi AF, Parvu AE, Andreicut AD, Mironiuc A, Crciun A, Ctoi C, Pop ID (2018) Metabolically healthy versus unhealthy morbidly obese: chronic inflammation, nitro-oxidative stress, and insulin resistance. Nutrients. https://doi.org/10.3390/nu10091199
Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U (2001) Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 104:342–345. https://doi.org/10.1161/01.cir.104.3.342
Huang PL (2009) eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab 20:295–302. https://doi.org/10.1016/j.tem.2009.03.005
Fernandez ML, Ruiz R, Gonzalez MA, Ramirez-Lorca R, Couto C, Ramos A, Gutierrez-Tous R, Rivera JM, Ruiz A, Real LM, Grilo A (2004) Association of NOS3 gene with metabolic syndrome in hypertensive patients. Thromb Haemost 92:413–418. https://doi.org/10.1160/TH04-02-0103
Monti LD, Barlassina C, Citterio L, Galluccio E, Berzuini C, Setola E, Valsecchi G, Lucotti P, Pozza G, Bernardinelli L, Casari G, Piatti P (2003) Endothelial nitric oxide synthase polymorphisms are associated with type 2 diabetes and the insulin resistance syndrome. Diabetes 52:1270–1275. https://doi.org/10.2337/diabetes.52.5.1270
Bhaswant M, Brown L, McAinch AJ, Mathai ML (2017) Beetroot and sodium nitrate ameliorate cardiometabolic changes in diet-induced obese hypertensive rats. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201700478
Ohtake K, Ehara N, Chiba H, Nakano G, Sonoda K, Ito J, Uchida H, Kobayashi J (2017) Dietary nitrite reverses features of postmenopausal metabolic syndrome induced by high-fat diet and ovariectomy in mice. Am J Physiol Endocrinol Metab 312:E300–E308. https://doi.org/10.1152/ajpendo.00360.2016
Kina-Tanada M, Sakanashi M, Tanimoto A, Kaname T, Matsuzaki T, Noguchi K, Uchida T, Nakasone J, Kozuka C, Ishida M, Kubota H, Taira Y, Totsuka Y, Kina SI, Sunakawa H, Omura J, Satoh K, Shimokawa H, Yanagihara N, Maeda S, Ohya Y, Matsushita M, Masuzaki H, Arasaki A, Tsutsui M (2017) Long-term dietary nitrite and nitrate deficiency causes the metabolic syndrome, endothelial dysfunction and cardiovascular death in mice. Diabetologia 60:1138–1151. https://doi.org/10.1007/s00125-017-4259-6
Sureda A, Bibiloni MD, Martorell M, Buil-Cosiales P, Marti A, Pons A, Tur JA, Martinez-Gonzalez MA, Investigators PS (2016) Mediterranean diets supplemented with virgin olive oil and nuts enhance plasmatic antioxidant capabilities and decrease xanthine oxidase activity in people with metabolic syndrome: the PREDIMED study. Mol Nutr Food Res 60:2654–2664. https://doi.org/10.1002/mnfr.201600450
Behr-Roussel D, Gorny D, Mevel K, Caisey S, Bernabe J, Burgess G, Wayman C, Alexandre L, Giuliano F (2005) Chronic sildenafil improves erectile function and endothelium-dependent cavernosal relaxations in rats: lack of tachyphylaxis. Eur Urol 47:87–91
Schulster ML, Liang SE, Najari BB (2017) Metabolic syndrome and sexual dysfunction. Curr Opin Urol 27:435–440. https://doi.org/10.1097/MOU.0000000000000426
Chaudhary RK, Shamsi BH, Tan T, Chen HM, Xing JP (2016) Study of the relationship between male erectile dysfunction and type 2 diabetes mellitus/metabolic syndrome and its components. J Int Med Res 44:735–741. https://doi.org/10.1177/0300060515623122
Gunduz MI, Gumus BH, Sekuri C (2004) Relationship between metabolic syndrome and erectile dysfunction. Asian J Androl 6:355–358
Kim SC (2000) Hyperlipidemia and erectile dysfunction. Asian J Androl 2:161–166
Vallance P, Chan N (2001) Endothelial function and nitric oxide: clinical relevance. Heart 85:342–350. https://doi.org/10.1136/heart.85.3.342
Sullivan ME, Thompson CS, Dashwood MR, Khan MA, Jeremy JY, Morgan RJ, Mikhailidis DP (1999) Nitric oxide and penile erection: is erectile dysfunction another manifestation of vascular disease? Cardiovasc Res 43:658–665. https://doi.org/10.1016/s0008-6363(99)00135-2
Wheatcroft SB, Williams IL, Shah AM, Kearney MT (2003) Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med 20:255–268
Ockaili R, Salloum F, Hawkins J, Kukreja RC (2002) Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol Heart Circ Physiol 283:H1263–H1269
Salloum F, Yin C, Xi L, Kukreja RC (2003) Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res 92:595–597
Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S, Kukreja RC (2009) Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation 120:S31–S36
Radovits T, Bomicke T, Kokeny G, Arif R, Loganathan S, Kecsan K, Korkmaz S, Barnucz E, Sandner P, Karck M, Szabo G (2009) The phosphodiesterase-5 inhibitor vardenafil improves cardiovascular dysfunction in experimental diabetes mellitus. Br J Pharmacol 156:909–919. https://doi.org/10.1111/j.1476-5381.2008.00098.x
Schafer A, Fraccarollo D, Pfortsch S, Flierl U, Vogt C, Pfrang J, Kobsar A, Renne T, Eigenthaler M, Ertl G, Bauersachs J (2008) Improvement of vascular function by acute and chronic treatment with the PDE-5 inhibitor sildenafil in experimental diabetes mellitus. Br J Pharmacol 153:886–893. https://doi.org/10.1038/sj.bjp.0707459
Aversa A, Greco E, Bruzziches R, Pili M, Rosano G, Spera G (2007) Relationship between chronic tadalafil administration and improvement of endothelial function in men with erectile dysfunction: a pilot study. Int J Impot Res 19:200–207
DeSouza C, Parulkar A, Lumpkin D, Akers D, Fonseca VA (2002) Acute and prolonged effects of sildenafil on brachial artery flow-mediated dilatation in type 2 diabetes. Diabetes Care 25:1336–1339
Deyoung L, Chung E, Kovac JR, Romano W, Brock GB (2012) Daily use of sildenafil improves endothelial function in men with type 2 diabetes. J Androl 33:176–180. https://doi.org/10.2164/jandrol.111.013367
Koka S, Aluri HS, Xi L, Lesnefsky EJ, Kukreja RC (2014) Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: potential role of NO/SIRT1/PGC-1alpha signaling. Am J Physiol Heart Circ Physiol 306:H1558–H1568. https://doi.org/10.1152/ajpheart.00865.2013
Wong SK, Chin KY, Suhaimi FH, Fairus A, Ima-Nirwana S (2016) Animal models of metabolic syndrome: a review. Nutr Metab (Lond) 13:65. https://doi.org/10.1186/s12986-016-0123-9
Ni YG, Condra JH, Orsatti L, Shen X, Di Marco S, Pandit S, Bottomley MJ, Ruggeri L, Cummings RT, Cubbon RM, Santoro JC, Ehrhardt A, Lewis D, Fisher TS, Ha S, Njimoluh L, Wood DD, Hammond HA, Wisniewski D, Volpari C, Noto A, Lo Surdo P, Hubbard B, Carfi A, Sitlani A (2010) A proprotein convertase subtilisin-like/kexin type 9 (PCSK9) C-terminal domain antibody antigen-binding fragment inhibits PCSK9 internalization and restores low density lipoprotein uptake. J Biol Chem 285:12882–12891. https://doi.org/10.1074/jbc.M110.113035
Xi L, Jarrett NC, Hess ML, Kukreja RC (1999) Essential role of inducible nitric oxide synthase in monophosphoryl lipid A-induced late cardioprotection: evidence from pharmacological inhibition and gene knockout mice. Circulation 99:2157–2163
Zhu SG, Kukreja RC, Das A, Chen Q, Lesnefsky EJ, Xi L (2011) Dietary nitrate supplementation protects against Doxorubicin-induced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol 57:2181–2189. https://doi.org/10.1016/j.jacc.2011.01.024
Frisbee JC (2005) Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 289:R307–R316. https://doi.org/10.1152/ajpregu.00114.2005
Koka S, Das A, Salloum FN, Kukreja RC (2013) Phosphodiesterase-5 inhibitor tadalafil attenuates oxidative stress and protects against myocardial ischemia/reperfusion injury in type 2 diabetic mice. Free Radic Biol Med 60:80–88. https://doi.org/10.1016/j.freeradbiomed.2013.01.031
Koka S, Xi L, Kukreja RC (2012) Chronic treatment with long acting phosphodiesterase-5 inhibitor tadalafil alters proteomic changes associated with cytoskeletal rearrangement and redox regulation in Type 2 diabetic hearts. Basic Res Cardiol 107:249. https://doi.org/10.1007/s00395-012-0249-5
Varma A, Das A, Hoke NN, Durrant DE, Salloum FN, Kukreja RC (2012) Anti-inflammatory and cardioprotective effects of tadalafil in diabetic mice. PLoS ONE 7:e45243. https://doi.org/10.1371/journal.pone.0045243
Tada H, Thompson CI, Recchia FA, Loke KE, Ochoa M, Smith CJ, Shesely EG, Kaley G, Hintze TH (2000) Myocardial glucose uptake is regulated by nitric oxide via endothelial nitric oxide synthase in Langendorff mouse heart. Circ Res 86:270–274. https://doi.org/10.1161/01.res.86.3.270
Gwilt DJ, Petri M, Lewis PW, Nattrass M, Pentecost BL (1985) Myocardial infarct size and mortality in diabetic patients. Br Heart J 54:466–472. https://doi.org/10.1136/hrt.54.5.466
Jaffe AS, Spadaro JJ, Schechtman K, Roberts R, Geltman EM, Sobel BE (1984) Increased congestive heart failure after myocardial infarction of modest extent in patients with diabetes mellitus. Am Heart J 108:31–37. https://doi.org/10.1016/0002-8703(84)90541-6
Stone PH, Muller JE, Hartwell T, York BJ, Rutherford JD, Parker CB, Turi ZG, Strauss HW, Willerson JT, Robertson T et al (1989) The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol 14:49–57. https://doi.org/10.1016/0735-1097(89)90053-3
Feuvray D, Lopaschuk GD (1997) Diabetes mellitus and the cardiovascular system. Cardiovasc Res 34:1–2. https://doi.org/10.1016/s0008-6363(97)00071-0
Liu Y, Thornton JD, Cohen MV, Downey JM, Schaffer SW (1993) Streptozotocin-induced non-insulin-dependent diabetes protects the heart from infarction. Circulation 88:1273–1278. https://doi.org/10.1161/01.cir.88.3.1273
Ravingerova T, Neckar J, Kolar F (2003) Ischemic tolerance of rat hearts in acute and chronic phases of experimental diabetes. Mol Cell Biochem 249:167–174
Bonow RO, Udelson JE (1992) Left ventricular diastolic dysfunction as a cause of congestive heart failure. Mech Manag Ann Intern Med 117:502–510. https://doi.org/10.7326/0003-4819-117-6-502
Cregler LL, Georgiou D, Sosa I (1991) Left ventricular diastolic dysfunction in patients with congestive heart failure. J Natl Med Assoc 83:49–52
Zabalgoitia M, Rahman SN, Haley WE, Mercado R, Yunis C, Lucas C, Yarows S, Krause L, Amarena J (1998) Comparison in systemic hypertension of left ventricular mass and geometry with systolic and diastolic function in patients %3c65 to %3e or = 65 years of age. Am J Cardiol 82:604–608. https://doi.org/10.1016/s0002-9149(98)00404-4
Das A, Xi L, Kukreja RC (2005) Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem 280:12944–12955
Franks PW, Luan J, Barroso I, Brage S, Gonzalez Sanchez JL, Ekelund U, Rios MS, Schafer AJ, O'Rahilly S, Wareham NJ (2005) Variation in the eNOS gene modifies the association between total energy expenditure and glucose intolerance. Diabetes 54:2795–2801. https://doi.org/10.2337/diabetes.54.9.2795
Torres SH, De Sanctis JB, Hernandez N, Finol HJ et al (2004) Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J Endocrinol 181:419–427. https://doi.org/10.1677/joe.0.1810419
Acknowledgements
This work was supported by grants from the National Institutes of Health (Grant Nos. CA221813, DK120866, HL118808, HL134366) to R.C.K. S.K. is supported by American Heart Association Institutional Research Enhancement Award (Grant No. 19AIREA34380223) and National Institutes of Health (Grant No. R56HL143809). L.X. is a recipient of Pauley Pilot Research Grant by Virginia Commonwealth University Pauley Heart Center.
Author information
Authors and Affiliations
Contributions
SK and LX performed the experiments and data analysis. SK wrote the manuscript; RCK and LX critically edited the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koka, S., Xi, L. & Kukreja, R.C. Chronic inhibition of phosphodiesterase 5 with tadalafil affords cardioprotection in a mouse model of metabolic syndrome: role of nitric oxide. Mol Cell Biochem 468, 47–58 (2020). https://doi.org/10.1007/s11010-020-03710-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03710-0