Abstract
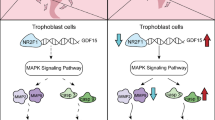
Preeclampsia (PE) is a leading cause of perinatal and maternal mortality. Considering that Nesfatin-1 was reported to be downregulated in serum of PE patients, we aimed to explore the functional role of Nesfatin-1 in trophoblast cells. Cell transfection was conducted to overexpress or inhibit Nesfatin-1, and its expression was measured by quantitative PCR. Cell proliferation, migration, and invasion abilities were determined employing CCK-8, flow cytometry, wound-healing, and transwell assays. Immunofluorescence assay was performed to detect E-cadherin and vimentin. ROS, MDA, and SOD levels were measured using their corresponding commercial kits. Western blot was used to identify the expression of vital kinases in PI3K/AKT/mTOR or GSK3β pathway and invasion-related proteins in trophoblast cells. Nesfatin-1 knockdown significantly suppressed proliferation, migration, and invasion and increased cell arrest in G1 phase, as well as downregulated expressions of MMP2/9 in HTR-8/SVneo cells. Besides, Nesfatin-1 knockdown promoted the expression of E-cadherin and reduced the expression of vimentin. Additionally, the levels of ROS, MDA, and SOD were elevated upon Nesfatin-1 knockdown. On the contrary, Nesfatin-1 overexpression exerted the opposite effects. Nesfatin-1 promoted the activation of PI3K/AKT/mTOR or GSK3β pathway, blocking of which reversed the promotive effects on trophoblast invasion and the inhibitory effects on oxidative stress of Nesfatin-1 in HTR-8/SVneo cells. In short, this study revealed that Nesfatin-1 promoted trophoblast cell proliferation, migration, invasion, and EMT and suppressed oxidative stress by activating PI3K/AKT/mTOR and AKT/GSK3β signaling pathway, laying the foundation for the development of therapeutic strategy for PE by targeting Nesfatin-1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preeclampsia (PE) is a disease among pregnant women characterized by high maternal blood pressure, proteinuria (24-h quantitative urine protein ≥ 2 g), and edema following 20 weeks of gestation, threatening maternal and fetal health [1]. The morbidity of PE in China has achieved 2.4~4.2%, which still fluctuates greatly in economic less-developed regions due to the lack of prevention and treatment [2]. PE is associated with intrauterine growth restriction (IUGR), placental abruption, fetal neurotubule malformation, preterm birth, and perinatal deaths [3]. Furthermore, PE is linked with an increased long-term risk of developing cardiovascular disease and metabolic disease in life [4, 5]. Although the exact etiology of PE remains elusive, it is generally believed that impaired spiral artery remodeling caused by inadequate extra-villous trophoblasts (EVTs) invasion takes the main responsibility for the diseases [6]. The epithelial-mesenchymal transition (EMT), a phenomenon whereby epithelial cells acquire a mesenchymal phenotype, has been reported to occur during trophoblasts invasion because cytotrophoblasts differentiate into EVTs, and defective EMT in placental trophoblast is one of the pathologies associated with PE [7, 8]. Besides, various treatment, such as 5-Aza-dC and vitamin D, can induce trophoblasts invasion by promoting EMT, contributing to the prevention of pregnancy complications [9, 10]. Thus, factors affecting the invasion of EVTs may result in abnormal placentation, which also open new perspectives into the pathogenesis of PE.
Nesfatin-1 is an 82-amino acid polypeptide derived from the precursor protein nucleobindin-2 (NUCB2) whose sequence is highly conserved from fish to mammals [11]. Nasfatin-1 was first found in the rat hypothalamus by Oh-1 and his collaborators for its anorexigenic property [12], and subsequent studies have been conducted to explore more potential properties of nesfatin-1, such as lipid metabolism, cardiovascular effects, reproduction functions, and sleep and emotion-associated functions [13,14,15,16]. Numerous evidence demonstrated the beneficial effects of Nesfatin-1. For example, Nesfatin-1 can alleviate necrotizing enterocolitis-induced oxidative injury in intestines and brain partly depending on afferent innervation [17]; Nesfatin-1 can alleviate lung injury through reducing inflammation and oxidative stress [18]. Furthermore, the hemochorial mammalian placenta has been reported to be one of the source of Nesfatin-1 which may has potential roles in glucose homeostasis and/or nutrient sensing [19]. Serum Nesfatin-1 was detected to be reduced in patients with polycystic ovary syndrome or patients with gestational diabetes mellitus, indicating that low level of serum Nesfatin-1 is associated with different gynecological diseases [20, 21]. Of note, serum nesfatin-1 level was also associated with the presence and severity of PE, as serum nesfatin-1 levels were significantly decreased in patients with PE compared with healthy controls and were decreased more in severe PE than that in mild PE [22], indicating that Nesfatin-1 might be an important biomarker for PE. However, there is no more information concerning about Nesfatin-1 in PE. Increasing evidence demonstrated an accelerated ability of Nesfatin-1 on cell proliferation, migration, and invasion in bladder cancer and colon cancer [23, 24]. Given that EVTs have the characteristics of tumor invasion and migration, we hypothesize that Nesfatin-1 might promote invasion of EVTs to accelerate spiral artery remodeling in PE.
In the present study, the potential role of nesfatin-1 in the development of PE was investigated via up- or downregulation of its expression in HTR-8/SVneo trophoblast cells in vitro, clarifying the specific mechanism of Nesfatin-1 during impaired spiral artery remodeling.
Materials and Methods
Cell Culture and Transfection
Human HTR-8/SVneo trophoblast cell line was obtained from the American Type Culture Collection (Rockville, MD, USA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin. Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2.
For transfection, pcDNA-nesfatin-1 and pcDNA-NC (pcDNA 3.1 expression vector, lentivirus packaged), short hairpin RNA for targeting nesfatin-1 (shRNA-nesfatin-1), and shRNA-NC (GenePharma; Shanghai, China) were transfected into HTR-8/SVneo cells using Lipofectamine 3000 transfection reagent (Life Technologies, Inc., Carlsbad, CA, USA). After transfection for 48 h, cells were harvested to determine the transfection efficiency by quantitative real-time reverse transcription PCR (qRT-PCR).
RNA Extraction and qRT-PCR
Total RNA was extracted with TRIzol reagents (Invitrogen, CA, USA) according to the manufacturer’s protocol. The first-strand cDNA was synthesized using a Reverse Transcriptase Kit (Takara, China). qRT-PCR was performed in triplicate on ABI Prism 7900HT sequence detection system (Applied Biosystems) using SYBR Green PCR Master mix (QIAGEN, China) according to the manufacturer’s protocol. Gene expressions were measured relative to the endogenous reference gene GAPDH using the comparative CT method described previously [25].
Cell Proliferation Assay
Cell proliferation ability was determined using Cell Counting Kit-8 (CCK-8) assay according to the manufacturer’s instructions. Briefly, cells were plated in 96-well plates and incubated for 24 h, 48 h, and 72 h, respectively. Ten microliters of CCK-8 solution was added to each well, and cells were incubated for another 3 h. The absorbance of each well at 450 nm was measured using a microplate reader (Multiscan FC Microplate Reader, Fisher Scientific Inc., Pittsburgh, PA).
Migration and Invasion Assays
For wound-healing migration assay, cells were cultured in 6-well plates for 24 h. Straight lines were drawn by scraping the confluent cells with a 20-μl pipette tip and ruler. Then cells were washed with PBS and incubated with fresh medium free of FBS. Cells were imaged at 0 h using a phase contrast microscope (Nikon Instrument Inc., Melville, NY, USA). After 24 h, scratch wounds were imaged again in the same position. The results were analyzed using ImageJ software.
For transwell invasion assay, the BD BioCoat Matrigel invasion chambers (8-μm pore size, BD, USA) were used. Cells were seeded into the upper chambers pre-coated with Matrigel. The complete medium with 10% FBS was added to the lower chamber. After 24 h, the invading cells attached to the membrane of the lower chamber were fixed in methanol, stained in crystal violet, and observed under microscope.
Western Blotting
Total protein was extracted with RIPA lysis buffer (Beyotime Institute of Biotechnology, Jiangsu, China) for 30 min and centrifuged at 12,000 rpm for 30 min at 4 °C. After determining the protein concentration using Pierce BCA Protein Assay Kit (Pierce, Rockford, IL, USA), equal amount of protein was separated on 8–12% SDS-PAGE and transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, USA). Subsequently, the membrane was blocked with skim milk (5%) Tris-buffered saline with 0.1% Tween-20 (TBST), washed, and incubated with primary antibodies at 4 °C overnight. Next, the membrane was incubated with the diluted secondary antibody at room temperature for 2 h. After washed with TBST, the membrane was visualized using enhanced chemiluminescence, and the protein expression levels were defined as gray value by the use of ImageJ version 1.38 (National Institutes of Health, USA).
Measurement of Reactive Oxygen Species (ROS)
The transfected cells were collected and suspended in 10 μM DCFH-DA (Merck, Shanghai, China) at 37 °C for 30 min. At the end of the incubation, the cells were washed three times with PBS to remove the free DCFH-DA molecules. The production of ROS was measured by a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, USA).
Immunofluorescence Assay
Cells were grown in the cover slips in 24-well plates for 24 h. After treatment, cells were washed with PBS and blocked using PBS containing 2% BSA at room temperature for 1 h. Then, cells were washed and incubated with primary antibodies against E-cadherin and vimentin at 4 °C overnight. After the subsequent incubation was performed using FITC-labeled secondary antibody in the dark place for 1 h, cells were washed with PBS and mounted in dark onto the glass slide using DAB solution (Sangon Biotechnology, Co. Ltd., Shanghai, China). Slides were observed under a fluorescent phase contrast microscope (Nikon).
Biochemical Assays
Assessment of malondialdehyde (MDA) content and measurement of superoxide dismutase (SOD) activity were carried out using kits from CELL BIOLABS INC (San Diego, CA, USA) in accordance with the manufacturer’s instructions. Nesfatin-1 level in cell culture supernatant was measured using an enzyme-linked immunosorbent assay (Phoenix Pharmaceuticals, Inc., Burlingame, CA).
Flow Cytometry Assay
After transfection for 48 h, cells were typsinized and washed with PBS three times and fixed in 70% ice-cold ethanol overnight. Then, cells were incubated with 1 mg/ml RNase A at 37 °C for 30 min and stained with PI for 1 h in the dark. Cell cycle distribution analysis was conducted with a BD FACS Caliber flow cytometer (BD Biosciences, San Jose, USA).
Statistical Analysis
All experiments were repeated three times, and the data were presented as mean ± SD. Data analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, USA). The statistical significance of the difference was analyzed by ANOVA followed by Tukey’s post hoc test. P < 0.05 was considered to indicate a statistically significant difference.
Results
Nesfatin-1 Knockdown Suppressed HTR-8/SVneo Cell Proliferation, Migration, and Invasion
HTR-8/SVneo cells were transfected with shRNA-Nesfatin-1-1 and shRNA-Nesfatin-1-2, and both of the mRNA level and protein expression of Nesfatin-1 were significantly decreased (Fig. 1a–b). In addition, the Nesfatin-1 level was also detected from the cell culture supernatants (Fig. 1c), indirectly confirming the clinical findings of Nesfatin-1 expression in the serum of PE patients [22]. Due to a higher transfection efficacy, shRNA-Nesfatin-1-1 was used for further investigation. Then, inhibition of Nesfatin-1 exhibited an obvious decrease on cell proliferation ability by CCK-8 assay (Fig. 1d). Flow cytometry assay showed that Nesfatin-1 knockdown significantly promoted cell arrest in G1 phase and decreased cell amount in S phase (Fig. 1e–f). Considering a well cell growth and a relatively high cell viability (over 70%) upon inhibition of Nesfatin-1 from CCK-8 assay, inhibition of Nesfation-1 was considered noncytotoxic on HTR-8/SVneo cells, and inhibition of Nesfation-1 might exert its suppressive function in cell proliferation ability through inhibiting cell cycle progression. Wound-healing assay revealed a decreased migration rate after Nesfatin-1 knockdown, and transwell assay also revealed a decreased invasion rate after Nesfatin-1 knockdown (Fig. 1g–j). In addition, Nesfatin-1 knockdown significantly reduced the protein expression of matrix metalloproteinase-2 (MMP2) and MMP9, two important markers for cell invasion (Fig. 1k). These results indicated that Nesfatin-1 knockdown could suppress HTR-8/SVneo cell proliferation, migration, and invasion.
Nesfatin-1 knockdown suppressed HTR-8/SVneo cell migration and invasion. (a) HTR-8/SVneo cells were transfected with shRNA-Nesfatin-1-1 and shRNA-Nesfatin-1-2, and the mRNA level of Nesfatin-1 was measured using qRT-PCR. (b) After transfection, protein expression of Nesfatin-1 was measured using western blotting assay. (c) After transfection, the expression level of Nesfatin-1 from cell culture supernatants was measured using the ELISA kit. (d)After transfection, the OD value of HTR-8/SVneo cells at 24 h, 48 h, and 72 h was measured, respectively, by CCK-8 assay to determine cell proliferation ability. (e, f) Cell cycle distribution was detected using flow cytometry. (g, h) Wound-healing assay was performed to determine cell migration ability. (i, j) Transwell assay was performed to determine cell invasion ability. (k) The protein expressions of MMP2 and MMP9 were measured using western blotting assay, and the band intensities were quantified. **, ***p < 0.01 and 0.001 vs shRNA-NC
Nesfatin-1 Knockdown Suppressed HTR-8/SVneo Cell EMT
Western blotting analysis was used to determine EMT of HTR-8/SVneo cell. As shown in Fig. 2a, Nesfatin-1 increased the protein expression of E-cadherin whereas reduced the protein expression of vimentin. Furthermore, this result was also verified by immunofluorescence assay. As shown in Fig. 2b, the fluorescence intensity of E-cadherin was enhanced after Nesfatin-1 knockdown (Fig. 2b), whereas vimentin was reduced after Nesfatin-1 knockdown (Fig. 2c). These results indicated that Nesfatin-1 knockdown significantly inhibited EMT.
Nesfatin-1 knockdown suppressed HTR-8/SVneo cell epithelial-to-mesenchymal transition (EMT). (a) After transfection, the protein expressions of E-cadherin and vimentin were measured using western blotting assay, and the band intensities were quantified. (b) The expression level of E-cadherin in HTR-8/SVneo cell was detected using immunofluorescence assay. (c) The expression level of vimentin in HTR-8/SVneo cell was detected using immunofluorescence assay. *, **p < 0.05 and 0.01 vs shRNA-NC
Nesfatin-1 Knockdown Suppressed HTR-8/SVneo Cell Oxidative Stress
The ROS level was assayed by DCFH-DA, and the results showed that Nesfatin-1 knockdown significantly increased the level of ROS (Fig. 3a–b). Besides, MDA was significantly elevated by Nesfatin-1 knockdown, while the level of SOD was significantly reduced by Nesfatin-1 (Fig. 3c–d). These results indicated that Nesfatin-1 could trigger oxidative stress of HTR-8/SVneo cells.
Nesfatin-1 Regulated the Activation of PI3K/AKT/mTOR and AKT/GSK3β Signaling
To understand how Nesfatin-1 exerted its function on cell migration, invasion, and oxidative stress of HTR-8/SVneo cells, some proteins related to PI3K/AKT/mTOR and AKT/GSK3β signaling were detected. As shown from Fig. 4a, the protein expressions of p-PI3K, p-AKT, p-mTOR, and p-GSK3β were markedly downregulated when Nesfatin-1 was inhibited, indicating that Nesfatin-1 knockdown inhibited the activation of PI3K/AKT/mTOR and AKT/GSK3β signaling (Fig, 4a). On the contrary, these protein expressions were markedly upregulated when Nesfatin-1 was overexpressed, which was then reversed by PI3K inhibitor LY294002 or AKT inhibitor MK-22-6 2HCl or GSK3β inhibitor SB21676310 to some degree (Fig. 4b). These results indicated that Nefatin-1 might exert its function by regulating the activation of PI3K/AKT/mTOR and AKT/GSK3β signaling.
Nesfatin-1 regulated the activation of PI3K/AKT/mTOR and AKT/GSK3β signaling. (a) HTR-8/SVneo cells were transfected with shRNA-Nesfatin-1 and its negative control, and the expression of proteins related to PI3K/AKT/mTOR and AKT/GSK3β signaling, including p-PI3K/PI3K, p-AKT/AKT, p-mTOR/mTOR, and p-GSK3β/GSK3β, was measured using western blotting. (b) HTR-8/SVneo cells were transfected with pcDNA-Nesfatin-1 and its negative control, and the transfected cells were treated with PI3K inhibitor LY294002 or AKT inhibitor MK-22-6 2HCl or GSK3β inhibitor SB21676310. Then, the expression of proteins related to PI3K/AKT/mTOR and AKT/GSK3β signaling was measured using western blotting
Nesfatin-1 Promoted HTR-8/SVneo Cell Migration and Invasion and EMT Through Activating AKT-Mediated Signaling Pathway
To further investigate the role of PI3K/AKT/mTOR and AKT/GSK3β signaling in Nesfatin-1-affected HTR-8/SVneo cell, cells were first transfected with pcDNA-Nesfatin-1, and the expression of Nesfatin-1 was significantly increased (Fig. 5a). Then, HTR-8/SVneo cells were treated with PI3K inhibitor LY294002 or AKT inhibitor MK-22-6 2HCl or GSK3β inhibitor SB21676310, respectively. A series of cell functional experiments showed that Nesfatin-1 overexpression significantly promoted cell proliferation, migration, and invasion abilities and promoted cell process from G1 phase to S phase; however, these effects were weakened by LY294002 or MK-22-6 2HCl or SB21676310 (Fig. 5b–h). Meanwhile, the protein expressions of MMP2 and MMP9 were increased after Nesfatin-1 overexpression but were then inhibited by LY294002 or MK-22-6 2HCl or SB21676310 (Fig. 5i). Furthermore, the expression of E-cadherin was inhibited by Nesfatin-1 overexpression, whereas vimentin was elevated by western blotting assay (Fig. 6a) or immunofluorescence assay (Fig. 6b–c), indicating Nesfatino-1 overexpression promoted EMT. However, the two detection methods also exhibited an upregulated E-cadherin and a downregulated vimentin after the treatment of LY294002 or MK-22-6 2HCl or SB21676310. These results indicated that Nesfatin-1 overexpression could promote HTR-8/SVneo cell proliferation, migration, invasion, and EMT, but blocking AKT-mediated signaling pathway was able to weaken these promotive effects caused by Nesfatin-1.
Nesfatin-1 promoted HTR-8/SVneo cell migration and invasion through activating AKT-mediated signaling pathway. (a) HTR-8/SVneo cells were transfected with pcDNA-Nesfatin-1 or its negative control, and the expression of Nesfatin-1 was measured by qRT-PCR. (b) After transfection, cells were treated with LY294002 or MK-22-6 2HCl or SB21676310. The OD value of HTR-8/SVneo cells at 24 h, 48 h, and 72 h was measured, respectively, by CCK-8 assay to determined cell proliferation ability. (c, d) Cell cycle distribution was detected using flow cytometry. (e, g) Wound-healing assay was performed to determine cell migration ability. (f, h) Transwell assay was performed to determine cell invasion ability. (i) The protein expressions of MMP2 and MMP9 were measured using western blotting assay, and the band intensities were quantified. ***p < 0.001 vs NC; #, ##, ###p < 0.05, 0.01, and 0.001 vs Nesfatin-1
Nesfatin-1 promoted HTR-8/SVneo EMT through activating AKT-mediated signaling pathway. (a) After treatment, the protein expressions of E-cadherin and vimentin were measured using western blotting assay, and the band intensities were quantified. (b) The expression level of E-cadherin in HTR-8/SVneo cell was detected using immunofluorescence assay. (c) The expression level of vimentin in HTR-8/SVneo cell was detected using immunofluorescence assay. ***p < 0.001 vs NC; #, ###p < 0.05 and 0.001 vs Nesfatin-1
Nesfatin-1 Promoted HTR-8/SVneo Cell Oxidative Stress Through Activating AKT-Mediated Signaling Pathway
Furthermore, we also found that cellular ROS level was significantly reduced after Nesfatin-1 overexpression, which was then reversed by LY294002 or MK-22-6 2HCl or SB21676310 (Fig. 7a). Meanwhile, the total ROS level and MDA level were also reduced after Nesfatin-1 overexpression, whereas total SOD level was elevated after Nesfatin-1 overexpression, indicating that Nesfatin-1 overexpression inhibited oxidative stress in HTR/SVneo cells. However, the inhibitory effect of Nesfatin-1 on oxidative stress was weakened by LY294002 or MK-22-6 2HCl or SB21676310 (Fig. 7b–d). These results indicated that Nesfatin-1 overexpression could inhibit oxidative stress in HTR-8/SVneo cells by activating AKT-mediated signaling pathway.
Nesfatin-1 promoted HTR-8/SVneo cell oxidative stress through activating AKT-mediated signaling pathway. (a–b) After treatment, intracellular ROS level was determined using DCFH-DA. (c–d) The MDA level and SOD level were detected using their corresponding commercial kits. ***p < 0.001 vs NC; #, ##, ###p < 0.05, 0.01, and 0.001 vs Nesfatin-1
Discussion
PE is one of the most frequently encountered complications in all pregnancies and also is the leading cause of prematurity and associated complications. Up to date, the therapeutic strategies for PE are lacking, and delivery of the placenta and fetus is currently the only effective way to relieve maternal symptoms [26]. The pathogenesis in early-onset PE was closely associated with the placental abnormality attributed to reduced invasion of trophoblast [27]. Abnormal expression of key genes in PE has been reported by researchers, and some of these genes participated into the development of PE by regulating trophoblast migration and invasion. For example, hsa_circ_0005243 was significantly reduced in the placenta of gestational diabetes mellitus patients, and knockdown of hsa_circ_0005243 in trophoblast cells suppressed cell proliferation and migration ability [28]. Tissue factor pathway inhibitor-2 (TFPI-2) was reported to be upregulated in both the placenta and serum of PE patients, and downregulation of TFPI-2 could promote HTR-8/SVneo cell invasion and inhibit cell apoptosis, demonstrating its critical role in monitoring the biological function of trophoblast cells [29]. Lower levels of Nesfatin-1 were reported in PE patients [22]; thus, decoding the role of Nesfatin-1 in trophoblast migration and invasion might reveal the molecular aspects of the above pregnancy complications.
Herein, we investigated the effect of Nesfatin-1 on human trophoblast using an in vitro model. HTR-8/SVneo cell line has been demonstrated to be appropriate and widely used for functional research on human trophoblast proliferation and invasion [30]. In the present study, we constructed a plasmid with high expression of Nesfatin-1 and a Nesfatin-1 shRNA fragment to transfect HTR-8/SVneo cells to verify the effect of abnormal expression of Nesfatin-1 on trophoblast cells’ function. The low expression of Nesfatin-1 inhibited proliferation, migration, and invasion activities of HTR-8/SVneo cells through inhibiting EMT, whereas the high expression of Nesfatin-1 had the opposite effects. These findings suggested that abnormal expression of Nesfatin-1 may contribute to the dysfunction of extravillous trophoblasts, and high expression of Nesfatin-1 might be involved in the pathological mechanism of PE.
Although the precise mechanism behind Nesfatin-1-mediated invasion is still unclear, it may be possible that the anti-oxidative activity of Nesfatin-1 contributes to the protection of trophoblast invasion. Placental oxidative stress might be involved in the pathophysiologic characteristics of PE, and oxidative stress induced by ischemic and hypoxic microenvironment of placental trophoblast during gestation results in impairment of trophoblast invasion and abnormalities in human placenta [31, 32]. Thus, antioxidant therapy has been applied during pregnancies to reduce oxidative stress and the occurrence of PE [33, 34]. Zhou Y et al. found that resveratrol reversed the oxidative stress reaction in pregnant rats with hypertension by decreasing the level of the oxidant MDA and increasing the antioxidant SOD, which demonstrated the protective role of resveratrol in PE-model rats due to its suppressive effect on oxidative stress [32]. Ebegboni VJ et al. reported that pretreatment with flavonoids or their metabolites exerted the cytoprotective effect against oxidative stress by reducing the generation of superoxide/hydrogen peroxide in trophoblasts, indicating that increasing the intake of in flavonoids-rich food might benefit early pregnancy [34]. In the present study, we confirmed that low expression of Nesfatin-1 in HTR-8/SVneo cells induced obvious oxidative stress presented as the elevated levels of ROS and MDA and the decreased activity of SOD, and overexpression of Nesfatin-1 exerted the opposite effects. The strong oxidative stress induced by Nesfatin-1 knockdown might be one of the reasons for impairment of trophoblast invasion, which is in line with the results that HTR-8/SVneo cell invasion ability was inhibited by Nefastin-1 knockdown.
Furthermore, human trophoblast invasion is precisely regulated by numerous signaling mediators, including JAK, STAT, and MAPK [35]. The balance between activating and inhibiting these proteins influences cell invasion activity. PI3K/AKT/mTOR axis transmits cell surface receptor signals and influences various tissue-dependent cellular functions, crucial to many aspects of cell growth and survival, in physiological as well as in pathological conditions [36]. Jin Xu et al. confirmed that PI3K/AKT/mTOR signaling pathway was involved in regulation of trophoblast viability and invasion by lncRNA-H19 [37]. Shen H et al. found that CD97 was demonstrated to be downregulated in PE placentas, and further mechanism investigation showed that CD97 promoted trophoblast invasion through PI3K/Akt signaling pathway [38]. In our study, the phosphorylation levels of PI3K, AKT, and mTOR were reduced by Nesfatin-1 downregulation whereas elevated by Nesfatin-1 overexpression, indicating that Nesfatin-1 promoted the activation of PI3K/AKT/mTOR pathway. Blocking of this pathway using LY294002 or MK-22-6 2HCl reversed the promotive effects of Nesfatin-1 on trophoblast invasion and reversed the inhibitory effects of Nesfatin-1 on oxidative stress in HTR-8/SVneo cells, suggesting that Nesfatin-1 exerted its function partly depending on the activation of PI3K/AKT/mTOR pathway. GSK3β, another downstream target of AKT, was investigated and found to be phosphorylated following Nesfatin-1 overexpression. AKT-mediated inhibition of GSK3β was found to be involved in regulating trophoblast apoptosis by hepatocyte growth factor [39]. The phosphorylation of AKT and GSK3β is regarded as an indicator of the activation of PI3K/AKT pathway, and PI3K/AKT/GSK3β signaling pathway has been demonstrated to be closely correlated with trophoblast proliferation [40]. Here, we found that GSK3β inhibitor SB21676310 also significantly affected the effects of Nesfatin-1 on trophoblast invasion and oxidative stress. Thus, it can be seen that PI3K/AKT/mTOR and AKT/GSK3β pathway is probably the key mechanism underlying deregulated Nesfatin-1 leading to PE.
Conclusions
In this study, we found that Nesfatin-1 knockdown suppressed trophoblast proliferation, migration, and invasion abilities by inhibiting EMT while driving oxidative stress, while overexpression of Nesfatin-1 exerted the opposite effects. Subsequent mechanistic studies showed that the expression level of Nesfatin-1 influenced the activation of PI3K/AKT/mTOR and AKT/GSK3β pathway, which might be the mechanism underlying deregulated Nesfatin-1 leading to PE. Although our study decodes the role and potential mechanism of Nesfatin-1 in trophoblast invasion and oxidative stress, additional in vivo and in vitro researches are recommended due to the complexity of the regulator mechanisms.
References
Leslie MS, Briggs LA. Preeclampsia and the risk of future vascular disease and mortality: a review. J Midwifery Womens Health. 2016;61(3):315–24. https://doi.org/10.1111/jmwh.12469.
Xiao J, Shen F, Xue Q, Chen G, Zeng K, Stone P, et al. Is ethnicity a risk factor for developing preeclampsia? An analysis of the prevalence of preeclampsia in China. J Hum Hypertens. 2014;28(11):694–8. https://doi.org/10.1038/jhh.2013.148.
Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46(6):1243–9. https://doi.org/10.1161/01.HYP.0000188408.49896.c5.
Behrens I, Basit S, Lykke JA, Ranthe MF, Wohlfahrt J, Bundgaard H, et al. Association between hypertensive disorders of pregnancy and later risk of cardiomyopathy. JAMA. 2016;315(10):1026–33. https://doi.org/10.1001/jama.2016.1869.
Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–44. https://doi.org/10.1016/S0140-6736(10)60279-6.
Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–4. https://doi.org/10.1126/science.1111726.
Blechschmidt K, Mylonas I, Mayr D, Schiessl B, Schulze S, Becker KF, et al. Expression of E-cadherin and its repressor snail in placental tissue of normal, preeclamptic and HELLP pregnancies. Virchows Arch. 2007;450(2):195–202. https://doi.org/10.1007/s00428-006-0343-x.
Davies JE, Pollheimer J, Yong HE, Kokkinos MI, Kalionis B, Knofler M, et al. Epithelial-mesenchymal transition during extravillous trophoblast differentiation. Cell Adhes Migr. 2016;10(3):310–21. https://doi.org/10.1080/19336918.2016.1170258.
Chen Y, Wang K, Leach R. 5-Aza-dC treatment induces mesenchymal-to-epithelial transition in 1st trimester trophoblast cell line HTR8/SVneo. Biochem Biophys Res Commun. 2013;432(1):116–22. https://doi.org/10.1016/j.bbrc.2013.01.075.
Kim RH, Ryu BJ, Lee KM, Han JW, Lee SK. Vitamin D facilitates trophoblast invasion through induction of epithelial-mesenchymal transition. Am J Reprod Immunol. 2018;79(2). https://doi.org/10.1111/aji.12796.
Dore R, Levata L, Lehnert H, Schulz C. Nesfatin-1: functions and physiology of a novel regulatory peptide. J Endocrinol. 2017;232(1):R45–65. https://doi.org/10.1530/JOE-16-0361.
Oh IS, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443(7112):709–12. https://doi.org/10.1038/nature05162.
Angelone T, Filice E, Pasqua T, Amodio N, Galluccio M, Montesanti G, et al. Nesfatin-1 as a novel cardiac peptide: identification, functional characterization, and protection against ischemia/reperfusion injury. Cell Mol Life Sci. 2013;70(3):495–509. https://doi.org/10.1007/s00018-012-1138-7.
Prinz P, Stengel A. Nesfatin-1: current status as a peripheral hormone and future prospects. Curr Opin Pharmacol. 2016;31:19–24. https://doi.org/10.1016/j.coph.2016.08.011.
Gao X, Zhang K, Song M, Li X, Luo L, Tian Y, et al. Role of Nesfatin-1 in the reproductive axis of male rat. Sci Rep. 2016;6:32877. https://doi.org/10.1038/srep32877.
Wei Y, Li J, Wang H, Wang G. NUCB2/nesfatin-1: expression and functions in the regulation of emotion and stress. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;81:221–7. https://doi.org/10.1016/j.pnpbp.2017.09.024.
Karadeniz Cerit K, Koyuncuoglu T, Yagmur D, Peker Eyuboglu I, Sirvanci S, Akkiprik M, et al. Nesfatin-1 ameliorates oxidative bowel injury in rats with necrotizing enterocolitis: the role of the microbiota composition and claudin-3 expression. J Pediatr Surg. 2020. https://doi.org/10.1016/j.jpedsurg.2020.02.025.
Wang ZZ, Chen SC, Zou XB, Tian LL, Sui SH, Liu NZ. Nesfatin-1 alleviates acute lung injury through reducing inflammation and oxidative stress via the regulation of HMGB1. Eur Rev Med Pharmacol Sci. 2020;24(9):5071–81. https://doi.org/10.26355/eurrev_202005_21200.
Legg-St Pierre CB, Mackova M, Miskiewicz EI, Hemmings DG, Unniappan S, MacPhee DJ. Insulinotropic nucleobindin-2/nesfatin-1 is dynamically expressed in the haemochorial mouse and human placenta. Reprod Fertil Dev. 2018;30(3):519–32. https://doi.org/10.1071/RD16486.
Kucukler FK, Gorkem U, Simsek Y, Kocabas R, Gulen S, Guler S. Low level of Nesfatin-1 is associated with gestational diabetes mellitus. Gynecol Endocrinol. 2016;32(9):759–61. https://doi.org/10.1080/09513590.2016.1180679.
Alp E, Gormus U, Guducu N, Bozkurt S. Nesfatin-1 levels and metabolic markers in polycystic ovary syndrome. Gynecol Endocrinol. 2015;31(7):543–7. https://doi.org/10.3109/09513590.2015.1024219.
Zhang C, Wang Y, Wang Y, Li J, Liu R, Liu H. Decreased levels of serum nesfatin-1 in patients with preeclampsia. Biomarkers. 2014;19(5):402–6. https://doi.org/10.3109/1354750X.2014.919027.
Kan JY, Yen MC, Wang JY, Wu DC, Chiu YJ, Ho YW, et al. Nesfatin-1/nucleobindin-2 enhances cell migration, invasion, and epithelial-mesenchymal transition via LKB1/AMPK/TORC1/ZEB1 pathways in colon cancer. Oncotarget. 2016;7(21):31336–49. https://doi.org/10.18632/oncotarget.9140.
Liu GM, Xu ZQ, Ma HS. Nesfatin-1/nucleobindin-2 is a potent prognostic marker and enhances cell proliferation, migration, and invasion in bladder cancer. Dis Markers. 2018;2018:4272064–9. https://doi.org/10.1155/2018/4272064.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–8. https://doi.org/10.1006/meth.2001.1262.
Correa PJ, Palmeiro Y, Soto MJ, Ugarte C, Illanes SE. Etiopathogenesis, prediction, and prevention of preeclampsia. Hypertens Pregnancy. 2016;35(3):280–94. https://doi.org/10.1080/10641955.2016.1181180.
Oudejans CB, van Dijk M, Oosterkamp M, Lachmeijer A, Blankenstein MA. Genetics of preeclampsia: paradigm shifts. Hum Genet. 2007;120(5):607–12. https://doi.org/10.1007/s00439-006-0259-1.
Wang H, Zhou W, She G, Yu B, Sun L. Downregulation of hsa_circ_0005243 induces trophoblast cell dysfunction and inflammation via the beta-catenin and NF-kappaB pathways. Reprod Biol Endocrinol. 2020;18(1):51. https://doi.org/10.1186/s12958-020-00612-0.
Zheng L, Huang J, Su Y, Wang F, Kong H, Xin H. Overexpression of tissue factor pathway inhibitor 2 attenuates trophoblast proliferation and invasion in preeclampsia. Hum Cell. 2020;33:512–20. https://doi.org/10.1007/s13577-020-00322-0.
Hannan NJ, Paiva P, Dimitriadis E, Salamonsen LA. Models for study of human embryo implantation: choice of cell lines? Biol Reprod. 2010;82(2):235–45. https://doi.org/10.1095/biolreprod.109.077800.
Negi R, Pande D, Karki K, Kumar A, Khanna RS, Khanna HD. Association of oxidative DNA damage, protein oxidation and antioxidant function with oxidative stress induced cellular injury in pre-eclamptic/eclamptic mothers during fetal circulation. Chem Biol Interact. 2014;208:77–83. https://doi.org/10.1016/j.cbi.2013.11.010.
Zou Y, Zuo Q, Huang S, Yu X, Jiang Z, Zou S, et al. Resveratrol inhibits trophoblast apoptosis through oxidative stress in preeclampsia-model rats. Molecules. 2014;19(12):20570–9. https://doi.org/10.3390/molecules191220570.
Ly C, Yockell-Lelievre J, Ferraro ZM, Arnason JT, Ferrier J, Gruslin A. The effects of dietary polyphenols on reproductive health and early development. Hum Reprod Update. 2015;21(2):228–48. https://doi.org/10.1093/humupd/dmu058.
Ebegboni VJ, Dickenson JM, Sivasubramaniam SD. Antioxidative effects of flavonoids and their metabolites against hypoxia/reoxygenation-induced oxidative stress in a human first trimester trophoblast cell line. Food Chem. 2019;272:117–25. https://doi.org/10.1016/j.foodchem.2018.08.036.
Gupta SK, Malhotra SS, Malik A, Verma S, Chaudhary P. Cell signaling pathways involved during invasion and syncytialization of Trophoblast cells. Am J Reprod Immunol. 2016;75(3):361–71. https://doi.org/10.1111/aji.12436.
Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–19. https://doi.org/10.1038/nrg1879.
Xu J, Xia Y, Zhang H, Guo H, Feng K, Zhang C. Overexpression of long non-coding RNA H19 promotes invasion and autophagy via the PI3K/AKT/mTOR pathways in trophoblast cells. Biomed Pharmacother. 2018;101:691–7. https://doi.org/10.1016/j.biopha.2018.02.134.
Shen H, Jin M, Gu S, Wu Y, Yang M, Hua X. CD97 is decreased in Preeclamptic placentas and promotes human Trophoblast invasion through PI3K/Akt/mTOR signaling pathway. Reprod Sci. 2020;27:1553–61. https://doi.org/10.1007/s43032-020-00183-w.
Dash PR, Whitley GS, Ayling LJ, Johnstone AP, Cartwright JE. Trophoblast apoptosis is inhibited by hepatocyte growth factor through the Akt and beta-catenin mediated up-regulation of inducible nitric oxide synthase. Cell Signal. 2005;17(5):571–80. https://doi.org/10.1016/j.cellsig.2004.09.015.
Astuti Y, Nakabayashi K, Deguchi M, Ebina Y, Yamada H. Human recombinant H2 relaxin induces AKT and GSK3beta phosphorylation and HTR-8/SVneo cell proliferation. Kobe J Med Sci. 2015;61(1):E1–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no competing interest.
Ethical Consents
This study is conducted based on cell experiments and is not involved in animal or human experiments, so ethical consents are not appropriate in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, T., Wei, S., Fan, C. et al. Nesfatin-1 Promotes Proliferation, Migration and Invasion of HTR-8/SVneo Trophoblast Cells and Inhibits Oxidative Stress via Activation of PI3K/AKT/mTOR and AKT/GSK3β Pathway. Reprod. Sci. 28, 550–561 (2021). https://doi.org/10.1007/s43032-020-00324-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00324-1