Abstract
Pre-eclampsia (PE) is a disorder of pregnancy characterized by proteinuria and high blood pressure, affecting 2–8% of pregnancies worldwide. Previous studies have shown that PE is closely associated with trophoblast cell dysfunction. Here, we investigated the role of tissue factor pathway inhibitor-2 (TFPI-2) in regulating the biological processes of trophoblast cells. The TFPI-2 levels in plasma samples and placental tissues were tested by ELISA, immunohistochemistry, qRT-PCR, and western blot. HTR8/Svneo cell line was used to simulate the primary trophoblast cells and H/R culture was applied to mimic the oxidative stress state of PE. MTT assay, Annexin V/propidium iodide (PI) apoptosis assay, and transwell assay were used to determine the cell proliferation, apoptosis, and invasion. The expression levels of matrix metalloproteinases (MMPs) were evaluated by western blot. The expression of TFPI-2 was remarkably up-regulated in both the serum and placenta of PE patients. Hypoxia/reoxygenation increased the expression of TFPI-2 in HTR‐8/SVneo cell line. TFPI-2 promoted that cell proliferation and inhibited the cell apoptosis of HTR8/SVneo cells in H/R condition. In addition, downregulation of TFPI-2 increased the cell invasion and the expression of MMP2 and MMP9. This study reveals that TFPI-2 plays a crucial role in monitoring the biological function of trophoblast cells, which might provide theoretical basis and therapeutic targets for the treatment of PE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preeclampsia (PE) is a special type of hypertensive disorders complicating pregnancy (HDCP) [1]. The main features conclude high blood pressure, proteinuria (24-hour quantitative urine protein ≥ 2 g) and edema after 20 weeks of pregnancy, and blood pressure returns to normal after delivery [2]. The symptoms of hypertension, edema, and proteinuria occur before seizures, so they are called preeclampsia [3]. It was later recognized that hypertension and proteinuria were closely related to maternal and child mortality even without seizures [4]. Therefore, PE is identified as a unique disease. Due to differences in geography, society, economy, ethnicity, and culture, PE has different incidence rates in different regions [5, 6]. At present, PE can be divided into early-onset PE (before the 34th week of pregnancy) and late-onset PE (after the 34th week of pregnancy) according to the onset time [7]. Generally, the earlier the disease, the more serious the condition [8]. At present, the preventive measures are mainly applied for treating PE clinically, such as aspirin treatment, low-dose calcium intake, etc., but production is still the most effective approach to alleviate PE [9, 10].
The pathogenesis of PE is complex and may be related to various factors including placental ischemia and hypoxia, immune factors, vascular endothelial cell injury, and genetic causes, etc. [11]. However, the exact pathogenesis mechanism of PE is still not clear and needs to be further elucidated. Previous studies have found that PE is associated with trophoblast cell dysfunction, including low trophoblast cell invasion, abnormal expression of adhesion factors and cytokines, immune dysfunction, excessive cell apoptosis, and abnormal secretion of vasoactive substances [12, 13]. Therefore, identifying the differentially expressed proteins in placental trophoblast cells of patients with PE and analyzing the possible relationship between differential proteins and disease occurrence might lay a foundation for further understanding of the complex molecular mechanisms that trigger PE.
Tissue factor pathway inhibitor-2 (TFPI-2) was originally found in placental tissue and is called matrix-associated serine protein inhibitor [14]. Later studies found that it plays an important role in biological behaviors such as cell proliferation, differentiation, and apoptosis [15]. As a suppressor gene, TFPI-2 is abnormally activated in various diseases including preeclampsia and tumors [16]. However, there is no uniform report on how TFPI-2 works in the development of PE.
In the present study, we explored how TFPI-2 affects the function of trophoblasts, thereby further affecting the pathogenesis and development of PE.
Methods
Patients and samples
The experiments were approved by the Ethical Committee of the Second Hospital of Hebei Medical University. All volunteers who agreed to participate in the research signed written informed consent following careful explanation. The information of the participants was recorded, including the gestational age at birth, maternal proteinuria, systolic blood pressure, diastolic blood pressure, and fetal birth weight. This information was listed in Table 1. We recruited 60 pregnant women with normal pregnancy, 60 pregnant women with early-onset PE, and 60 pregnant women with late-onset PE to obtain the maternal peripheral blood samples.
The expression of TFPI-2 in placental tissues was evaluated in 20 pregnant women with normal pregnancy, 20 pregnant women with early-onset PE, and 20 pregnant women with late-onset PE. Placentas were collected by elective cesarean section, in the absence of induced labor, for all groups studied. Placental tissues were obtained by cutting a vertical plane through the full thickness of a central and apparently normal area, including both the fetal and maternal surfaces. Tissues with calcification or clots were avoided.
Cell culture
HTR‐8/SVneo cell line was used in this paper, which possesses similar biological functions and traits with primary trophoblasts. HTR8/SVneo cells display trophoblast progenitor cell-like characteristics indicative of self-renewal, repopulation activity, and expression of "stemness-" associated transcription factors [17]. H/R culture (two 24-h cycles of 8 h of hypoxia and 16 h of reoxygenation) was used to simulate the oxidative stress state of PE. HTR‐8/SVneo cell line was purchased from American Type Culture Collection (ATCC, Manassas, VA). HTR‐8/SVneo cell line was cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) with 5% fetal bovine serum (FBS, Gibco). Cells were maintained at 37 °C and under 5% carbon dioxide (CO2). For cell transfection, Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA USA) was used according to manufacturer’s instructions. The siRNA sequences were listed as follows:
5′-AGCCCAUACAAGUAGCUUCAUCUGG-3′ and 5′-CCAGAUGAAGCUACUUGUAUGGGCU-3′ for siTFPI-2;
5′-UGCACAUGCACGUUUGCAAUCCUCC-3′ and 5′GGAGGAUUGCAAACGUGCAUGUGCA-3′ for siTFPI-2/2.
Enzyme-linked immunosorbent assay (ELISA)
The Human TFPI2 ELISA kit (ab213836) purchased from Abcam was used for the quantitative measurement of human TFPI2 in serum according to manufacturer’s instructions.
Immunohistochemistry
The immunohistochemistry method uses routine procedures. Anti-TFPI2 (ab86933) was purchased from Abcam.
Western blot
The following antibodies were used: anti-TFPI2 (ab186747), anti-β-actin (ab115777), and anti-MMP2 (ab37150); anti-MMP9 (ab38898) were purchased from Abcam. Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), and p44/42 MAPK (Erk1/2) were purchased from Cell Signaling Technology.
Reverse transcription-quantitative PCR (RT-qPCR)
TRIzol Reagent (Thermo Fisher Scientific) was used to extract total RNA from tissues. SuperScript™ II Reverse Transcriptase (Invitrogen, Waltham, MA USA) was used to synthesize first-strand cDNA. Fast SYBR™ Green Master Mix (Applied Biosystems, Waltham, MA, USA) was used for real-time quantification analysis. The following primers were used: forward primer 5′-GTCGATTCTGCTGCTTTTCC-3′ and reverse primer 5′-CAGCTCTGCGTGTACCTGTC-3′ for TFPI-2; forward primer 5′-CAGCCTCAAGATCATCAGCA-3′ and reverse primer 5′-TGTGGTCATGAGTCCTTCCA-3′ for GAPDH. GAPDH was used as an internal control.
Cell proliferation
Cell proliferation was tested by -(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Six groups were designed, which were standard culture conditions (Standard group), control group under H/R conditions, transfected siNC, siTFPI-2, pcDNA and pcDNA-TFPI-2 groups. The log phase cells were collected, and the cell suspension concentration was adjusted. 100 μl of the cell suspension was added to each well in a 96-well plate, and the cells to be tested were adjusted to a density of 3500 cells per well. Cells were incubated at 5% CO2 at 37 °C and cell proliferation was detected by MTT assay after 24, 48, and 72 h. For the measurement, 20 μl of MTT solution (5 mg/ml, i.e., 0.5% MTT) was added to each well, and incubation was continued for 4 h. The culture was then terminated and the culture medium in the well was carefully aspirated. 150 μl of dimethyl sulfoxide was added to each well and shaken on a shaker at low speed for 0.5 min. The absorbance of each well was measured at OD 560 nm of the enzyme-linked immunosorbent detector. Each group has three repetitions.
Cell apoptosis
Annexin V/propidium iodide (PI) apoptosis assay was used for assessing cell apoptosis. Six groups were designed, which were standard culture conditions (Standard group), control group under H/R conditions, and transfected with siNC, siTFPI-2, pcDNA and pcDNA-TFPI-2 groups. After 24 h of transfection, the cells were plated into 24-well plates (50,000 cells per well), and apoptosis was detected by FITC Annexin V/Dead Cell Apoptosis Kit with FITC annexin V and PI (Thermo Fisher Scientific) 24 h later. Cell apoptosis was determined by flow cytometry according to manufacturer’s instructions.
Cell invasion
Cell invasion was examined by transwell assay. Matrigel (Becton Dickinson) stored at − 20 °C was thawed overnight on ice at 2 °C to 8 °C. 100 μl of Matrigel was pipetted on ice with a pre-cooled tip and added to ice-cold 300 μl of serum-free medium for thorough mixing. 25 μl of the above diluted Matrigel was placed in a transwell plate (Costar), covering the entire polycarbonate film at 37 °C for 30 min to polymerize Matrigel into a gel. Six groups were designed, which were standard culture conditions (Standard group), control group under H/R conditions, and transfected with siNC, siTFPI-2, pcDNA, and pcDNA-TFPI-2 groups. Each group of cells was rinsed with PBS. Single cell suspensions were prepared using serum-free medium: add 200 μl of cell suspension (50,000 cells) to the upper chamber of the transwell plate. 500 μl of medium containing 20% FBS was added to the lower chamber of the transwell plate. Incubate at 37 °C, 5% CO2 for 24 h. Gently wipe the cells on the upper surface of the Matrigel gel and polycarbonate film with a damp cotton swab. The upper chamber was carefully removed, lined up, marked, incubated with ice-cold methanol for 30 min and stained with hematoxylin for 1 min, dehydrated with gradient ethanol (80%, 95%, 100%), and made transparent with xylene. Carefully cut the polycarbonate film from the upper chamber substrate and place a neutral resin seal on the slide. The cells attached to the lower surface of the polycarbonate membrane were randomly counted 6 times under high magnification (×400), and the average was taken. Each group has three repetitions.
Statistical analysis
All data were shown as the mean ± SD. Differences between groups were analyzed using the one-way ANOVA or two-tailed Student’s t-test. Statistical significance was accepted at P < 0.05.
Results
TFPI-2 is up-regulated in the serum and placenta of pregnant woman with PE
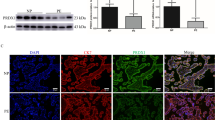
To illustrate whether the expression of TFPI-2 was changed in the PE patients, we first recruited 60 pregnant women with normal pregnancy, 60 pregnant women with early-onset PE, and 60 pregnant women with late-onset PE. Table 1 shows the clinical characteristics including the gestational age at birth, maternal proteinuria, systolic blood pressure, diastolic blood pressure, and fetal birth weight. We then collected the maternal peripheral blood samples from the three groups and measured the plasma concentration of TFPI-2 by ELISA. Our results proved that the protein levels of TFPI-2 in serum were significantly increased in both the early-onset and late-onset PE compared with the NC group (Fig. 1a). We next collected the placenta samples and detected the expression of TFPI-2 by immunohistochemistry. As shown in Fig. 1b, TFPI-2 was highly expressed in both the early-onset and late-onset PE compared with the NC group. To further confirm these results, the mRNA and the protein levels of TFPI-2 in placenta tissues were examined by qPCR analysis and western blot. As shown in Fig. 1c and d, both the mRNA and the protein levels of TFPI-2 were significantly higher in both the early-onset and late-onset PE than that in the NC group. Therefore, our results demonstrated that the expression of TFPI-2 was remarkably up-regulated in both the serum and placenta of PE patients.
Plasma and placental levels of TFPI-2 in pregnant woman with early, late PE and normal pregnancy. a The TFPI-2 levels in maternal plasma samples in early and late PE before delivery, compared to that in normal pregnancy (normal control group, NC). b The representative images of TFPI-2 expression in human placentas tissue were detected by immunohistochemistry. Scale bar, 50 μm. The expression of TFPI-2 in placental tissues from women with early, late PE, and normal pregnancy examined by real-time PCR (c) and Western blot (d), respectively. β-actin was set as loading protein. Data are shown as means ± SD, n = 60. ***p < 0.001, ****p < 0.0001 (versus normal control)
Hypoxia/reoxygenation promotes the expression of TFPI-2 in HTR‐8/SVneo cell line
To further explore the expression of TFPI-2 in placental trophoblast cells, we used the HTR‐8/SVneo cell line, which possesses similar biological functions and traits with primary trophoblasts. H/R culture (two 24-h cycles of 8 h of hypoxia and 16 h of reoxygenation) was used to simulate the oxidative stress state of PE. Western blot results revealed that the protein levels of TFPI-2 in the cells under H/R culture were significantly higher than the control group (Fig. 2a). Next, we down-regulated or overexpressed TFPI-2 by transfecting siTFPI-2 and pcDNA-TFPI-2. Our results showed that transfection with siTFPI-2 effectively decreased the protein level of TFPI-2 in HTR‐8/SVneo cell line in H/R condition (Fig. 2b). In addition, the expression of TFPI-2 was significantly up-regulated by the transfection of pcDNA-TFPI-2 in HTR-8/SVneo cells in H/R condition (Fig. 2c). Taken together, our results proved that hypoxia/reoxygenation treatment promoted the expression of TFPI-2 in HTR‐8/SVneo cell line, suggesting that the oxidative stress state of PE might also trigger the expression of TFPI-2.
Downregulation and overexpression of TFPI-2 in HTR8/SVneo cells in hypoxia–reoxygenation (H/R) condition. a TFPI-2 expression in HTR8/SVneo cells in normal and H/R condition examined by real-time PCR and WB. b The suppression of TFPI-2 levels by siTFPI-2 in HTR-8/SVneo cells. siNC represents the nonsense siRNA. c The overexpression of TFPI-2 levels by pcDNA-TFPI-2 in HTR-8/SVneo cells. pcNC represents the control plasmid. Data are shown as means ± SD. Independent experiments were repeated in triplicate. ***p < 0.001
TFPI-2 regulates the proliferation and apoptosis of HTR8/SVneo cells in H/R condition
To investigate the potential biological function of TFPI-2 in placental trophoblast cells in H/R condition, we performed MTT assay to estimate cell growth ability in HTR8/SVneo cells. Our results showed that H/R culture significantly suppressed the placental trophoblast cell proliferation, as expected. Transfection of siTFPI-2 rescued the inhibitory effects in H/R condition, whereas transfection of pcDNA-TFPI-2 showed a reverse trend (Fig. 3a). We also selected another siRNA sequence and confirmed that this siTFPI-2/2 could also inhibit the proliferation of HTR8/SVneo cells (Fig. S1). Next, we analyzed the function of TFPI-2 in cell apoptosis of in HTR8/SVneo cells in H/R condition by Annexin V/PI apoptosis assay. In H/R condition, the increased cell apoptosis in HTR8/SVneo cells was enhanced by the overexpression of TFPI-2 and suppressed by the downregulation of TFPI-2 expression (Fig. 3b and c). Next, we further explored the mechanisms by which TFPI-2 influences cellular proliferation and apoptosis. Caspases are interleukin-1β (IL-1β)-converting enzyme (ICE)-family proteases that initiate apoptosis in mammalian cells. Here, we observed decreased protein levels of cleaved (active) caspases 10, 9, 8, 7, 6, and 3 after siRNA-mediated downregulation of TFPI-2 in HTR8/SVneo cells (Fig. S2). These results indicated that overexpression of TFPI-2 triggers caspase-mediated pathway and apoptosis, and TFPI-2 suppression, in turn, decreases cell apoptosis and promotes cell proliferation. Based on these data, we demonstrated that TFPI-2 promoted the cell proliferation and decreased the cell apoptosis of HTR8/SVneo cells in H/R condition, suggesting that TFPI-2 might function in regulating the proliferation and apoptosis in placental trophoblast cells.
Downregulation and overexpression of TFPI-2 affect the proliferation and apoptosis of HTR8/SVneo cells in H/R condition. a MTT assays show that TFPI-2 suppression promotes the growth of trophoblast cells in H/R condition, whereas cell proliferation capacity was impaired after transfecting with pcDNA-TFPI-2. Data are shown as means ± SD. Independent experiments were repeated in triplicate. *p < 0.05 (pcDNA-TFPI-2 versus pcDNA group), **p < 0.01 (siTFPI-2 versus siNC group). b Representative images of flow cytometry demonstrating the apoptotic trophoblast cells after being transfected with siTFPI-2 or pcDNA-TFPI-2. c Quantitative analysis of apoptotic cells. Data are shown as means ± SD. Independent experiments were repeated in triplicate. **p < 0.005 (pcDNA-TFPI-2 versus pcDNA group), ***p < 0.001 (siTFPI-2 versus siNC group)
TFPI-2 regulates the invasion of HTR8/SVneo cells in H/R condition
To illustrate whether TFPI-2 regulates the cell invasion of placental trophoblast cells in H/R condition, the cell invasion of HTR8/SVneo cells was determined by Transwell assay. The results showed that H/R culture significantly inhibited the cell invasion in HTR8/SVneo cells, whereas downregulation of TFPI-2 by siTFPI-2 reversed this trend (Fig. 4a and b). In addition, the decreased cell invasion ability by H/R culture was enhanced by overexpression of TFPI-2 in HTR8/SVneo cells (Fig. 4a and b). Previous studies have shown that matrix metalloproteinases (MMPs) such as MMP2 and MMP9 play an important role in the invasion of trophoblast cells [18]. Therefore, we examined the protein levels of MMP2 and MMP9 in TFPI-2 silenced and overexpressed HTR8/SVneo cells by western blot. Our results revealed that H/R culture remarkably suppressed the expression of MMP2 and MMP9. Besides, downregulation of TFPI-2 by siTFPI-2 significantly enhanced the protein levels of MMP2 and MMP9, whereas overexpression of TFPI-2 showed a reverse effect (Fig. 4c). We also tested the effects of MMP inhibitor on the invasion of HTR8/SVneo cells, and our results confirmed that MMPs inhibition significantly suppresses invasion of HTR8/SVneo cells (Fig. S3). Next, we further explored the mechanism by which TFPI-2 modulated the expression of MMPs. Mitogen-activated protein kinase (MAPK) cascade is an important pathway that responds to extracellular stimuli and regulates MMP-2 or MMP-9 expression, and the extracellular signal regulated kinase-1/2 (ERK1/2) signaling pathway is preferentially activated. Therefore, the expression of p-ERK and total ERK after TFPI-2 down-regulation was examined by Western blot. Our results showed that there was a significant increase in phosphorylated of ERK1/2 in TFPI-2 silenced HTR8/SVneo cells, whereas there was no obvious alteration in total ERK (Fig. S4). These results indicated that TFPI-2 regulate the MMP2 and MMP9 expressions via MAPK/ERK cascade. Therefore, our data suggested that TFPI-2 might regulate the cell invasion of placental trophoblast cells under the oxidative stress state of PE.
Downregulation and overexpression of TFPI-2 affect the invasion of HTR8/SVneo cells in H/R condition. a Representative images of the invasion assays. Trans-well assays exhibit that TFPI-2 suppression induces the invasion of trophoblast cells in the H/R condition, whereas overexpression of TFPI-2 regress cell invasion in vitro. b Quantitative analysis of invasion potential of HTR8/SVneo cells after treatments. Data are shown as means ± SD. Independent experiments were repeated in triplicate. **p < 0.005 (pcDNA-TFPI-2 versus pcDNA group), ***p < 0.001 (siTFPI-2 versus siNC group). c MMP-2 and MMP-9 protein expression in TFPI-2 silenced and overexpressed HTR8/SVneo cells. β-actin was used as a loading control
Discussion
PE is a multisystem disorder during pregnancy which considered to be the second lethal disease in direct maternal mortality. Based on statistical data, PE causes nearly 500,000 perinatal and 70–80,000 maternal deaths each year all over the world [19]. PE is defined as proteinuric gestational hypertension 20 weeks after pregnancy based on clinical phenotypes including hypertension and proteinuria [20]. The origin of PE is confirmed to be triggered by the interaction between the maternal condition and the fetoplacental condition in early pregnancy [21]. Till now, the molecular mechanism during the pathogenesis of PE is still not be fully elucidated and the new therapeutic strategies for PE are needed urgently.
TFPI-2 has been reported to participate in the regulation of several different cell behaviors including cell proliferation, migration, apoptosis, and differentiation [22, 23]. Transcription of the TFPI-2 gene in umbilical vein endothelial cells, liver, and placenta is very active [24]. Previous studies have reported that TFPI-2 is localized in placental syncytiotrophoblasts and is expressed in the blood circulation of normal pregnant women [25]. However, there have been no reports on the changes in maternal serum and placental TFPI-2 levels during PE. It has been reported that serum concentrations of TFPI-2 increase in PE and intrauterine growth-restricted maternal bodies; however, other results did not suggest such changes. In the present study, we recruited 60 pregnant women with normal pregnancy, 60 pregnant women with early-onset PE, and 60 pregnant women with late-onset PE. Our results proved that the levels of TFPI-2 in the serum and placenta tissues were significantly increased in both the early-onset and late-onset PE compared with that in normal pregnancies. Therefore, we hypothesize that overexpression of TFPI-2 may be one of the causes of abnormal placental structure and function during PE.
PE occurs more commonly after 20 weeks of gestation, that is, after the placental function is mature [26]. After 2–3 days after delivery, the placenta is delivered, and the symptoms and signs disappear automatically without treatment [26]. These clinical features suggest that the incidence of PE is closely related to the placenta. The placenta is a natural barrier to pregnancy and plays a vital role in the process of pregnancy [27]. The placental vascular bed is mainly composed of trophoblast cells, which are the main functional cells of the placenta [28]. During pregnancy, trophoblast cells produce cell adhesion molecules, chemokines, hormones, enzymes, etc. to ensure normal pregnancy [29]. The trophoblast cells are members of the innate immune system of the pregnant mother, protecting the fetus from the immune damage of the mother, and also one of the main sources of oxygen free radicals in the mother [30]. Therefore, trophoblast cells have always been the focus and hotspot in the study of pathogenesis of PE.
Previous studies have found that trophoblast cell dysfunction occurs in PE, manifested as low invasiveness, abnormal expression of adhesion and cytokines, immune dysfunction, excessive cell apoptosis, vasoactive substances and abnormal secretion of antioxidants, viral infections, etc.[31]. Here, we used the HTR‐8/SVneo cell line, which possessed similar biological functions and traits with primary trophoblasts. In addition, H/R culture was applied to mimic the oxidative stress state of PE. We found that hypoxia/reoxygenation treatment promoted the expression of TFPI-2 in HTR‐8/SVneo cell line, suggesting that the oxidative stress state of PE might also trigger the expression of TFPI-2. To further confirm the potential biological function of TFPI-2 in placental trophoblast cells, we performed MTT assay to estimate cell growth ability in HTR8/SVneo cells under various conditions. Our results showed that downregulation of TFPI-2 increased the cell proliferation. In addition, our results demonstrated that overexpression of TFPI-2 enhanced cell apoptosis. Taken together, we proved that TFPI-2 increased the cell proliferation and decreased the cell apoptosis of HTR8/SVneo cells in H/R condition, which might be one of the causes of PE.
It has been previously reported that up-regulation of TFPI-2 can inhibit the invasion of lung cancer, liver cancer, pancreatic cancer, and esophageal cancer [32], but there is no specific report on the specific effects of TFPI-2 on trophoblast invasion. Therefore, we speculate that TFPI-2 expression has an inhibitory effect on trophoblast invasion. Previous studies have suggested that TFPI-2 affects cell migration, invasion, growth, and other functions mainly through interaction with extracellular matrix [33]. Through the inhibition of various proteases (such as MMP-1, MMP-2, MMP-3, MMP-9, MMP-13, etc.) by the arginine pathway, TFPI-2 can cause matrix barrier degradation and structural rearrangement, thereby playing a key role in inhibiting the extracellular matrix degradation [34,35,36,37]. The invasiveness of trophoblasts is critical during the implantation of placenta. Therefore, we speculate that TFPI-2 also has a similar effect on trophoblast cells, causing a decrease in matrix degradation, implantation disorders, and further aggravation of placental hypoxia–ischemia. Our results showed that downregulation of TFPI-2 promoted the cell invasion of HTR8/SVneo cells and enhanced the protein levels of MMP2 and MMP9. Therefore, our data suggest that TFPI-2 regulates the cell invasion of trophoblasts by monitoring the expression of MMP2 and MMP9.
Conclusion
In conclusion, our present study proved that the levels of TFPI-2 in the serum and placenta tissues were significantly increased in pregnant women with PE compared with that in normal pregnancies. In addition, TFPI-2 plays an important role in trophoblast proliferation, apoptosis, and invasion. Therefore, TFPI-2 might serve as a clinical diagnostic indicator and a target gene for treatment in PE.
References
Jim B, Karumanchi SA. Preeclampsia: pathogenesis, prevention, and long-term complications. Semin Nephrol. 2017;37(4):386–97. https://doi.org/10.1016/j.semnephrol.2017.05.011.
Leslie MS, Briggs LA. Preeclampsia and the risk of future vascular disease and mortality: a review. J Midwifery Womens Health. 2016;61(3):315–24. https://doi.org/10.1111/jmwh.12469.
Balci S, Bodur T, Tohma YA, Okyay RE, Saatli B, Altunyurt S. Do preeclampsia symptoms resolve after intrauterine death of a fetus? Turk J Obstet Gynecol. 2016;13(2):103–5. https://doi.org/10.4274/tjod.84770.
van Esch JJA, van Heijst AF, de Haan AFJ, van der Heijden OWH. Early-onset preeclampsia is associated with perinatal mortality and severe neonatal morbidity. J Matern Fetal Neonatal Med. 2017;30(23):2789–94. https://doi.org/10.1080/14767058.2016.1263295.
Kongwattanakul K, Saksiriwuttho P, Chaiyarach S, Thepsuthammarat K. Incidence, characteristics, maternal complications, and perinatal outcomes associated with preeclampsia with severe features and HELLP syndrome. Int J Womens Health. 2018;10:371–7. https://doi.org/10.2147/IJWH.S168569.
Lin S, Leonard D, Co MA, Mukhopadhyay D, Giri B, Perger L, et al. Pre-eclampsia has an adverse impact on maternal and fetal health. Transl Res. 2015;165(4):449–63. https://doi.org/10.1016/j.trsl.2014.10.006.
Ibanoglu MC, Ozgu-Erdinc AS, Uygur D. Maternal placi protein levels in early- and late-onset preeclampsia. Ginekol Pol. 2018;89(3):147–52. https://doi.org/10.5603/GP.a2018.0025.
Kornacki J, Skrzypczak J. Results of Doppler examinations in fetuses of mothers with early- and late-onset preeclampsia. Ginekol Pol. 2014;85(7):504–8.
Palomaki GE, Martin JN Jr, Karumanchi SA, Poon LC. Updates on screening, prevention, treatment, and genetic markers for preeclampsia. Clin Chem. 2018;64(12):1684–9. https://doi.org/10.1373/clinchem.2018.289801.
Myatt L, Redman CW, Staff AC, Hansson S, Wilson ML, Laivuori H, et al. Strategy for standardization of preeclampsia research study design. Hypertension. 2014;63(6):1293–301. https://doi.org/10.1161/HYPERTENSIONAHA.113.02664.
Phipps E, Prasanna D, Brima W, Jim B. Preeclampsia: updates in pathogenesis, definitions, and guidelines. Clin J Am Soc Nephrol. 2016;11(6):1102–13. https://doi.org/10.2215/CJN.12081115.
Song X, Luo X, Gao Q, Wang Y, Gao Q, Long W. Dysregulation of LncRNAs in placenta and pathogenesis of preeclampsia. Curr Drug Targets. 2017;18(10):1165–70. https://doi.org/10.2174/1389450118666170404160000.
Schneider H. Placental dysfunction as a key element in the pathogenesis of preeclampsia. Dev Period Med. 2017;21(4):309–16.
Ali MN, Kasetty G, Elven M, Alyafei S, Jovic S, Egesten A, et al. TFPI-2 Protects against gram-negative bacterial infection. Front Immunol. 2018;9:2072. https://doi.org/10.3389/fimmu.2018.02072.
Wang G, Huang W, Li W, Chen S, Chen W, Zhou Y, et al. TFPI-2 suppresses breast cancer cell proliferation and invasion through regulation of ERK signaling and interaction with actinin-4 and myosin-9. Sci Rep. 2018;8(1):14402. https://doi.org/10.1038/s41598-018-32698-3.
Xu C, Wang H, He H, Zheng F, Chen Y, Zhang J, et al. Low expression of TFPI-2 associated with poor survival outcome in patients with breast cancer. BMC Cancer. 2013;13:118. https://doi.org/10.1186/1471-2407-13-118.
Weber M, Knoefler I, Schleussner E, Markert UR, Fitzgerald JS. HTR8/SVneo cells display trophoblast progenitor cell-like characteristics indicative of self-renewal, repopulation activity, and expression of "stemness-" associated transcription factors. Biomed Res Int. 2013;2013:243649. https://doi.org/10.1155/2013/243649.
Cohen M, Bischof P. Factors regulating trophoblast invasion. Gynecol Obstet Invest. 2007;64(3):126–30. https://doi.org/10.1159/000101734.
Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–403. https://doi.org/10.1016/j.bpobgyn.2011.01.006.
Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–44. https://doi.org/10.1016/S0140-6736(10)60279-6.
von Dadelszen P, Magee LA. Pre-eclampsia: an update. Curr Hypertens Rep. 2014;16(8):454. https://doi.org/10.1007/s11906-014-0454-8.
Feng C, Ho Y, Sun C, Xia G, Ding Q, Gu B. TFPI-2 expression is decreased in bladder cancer and is related to apoptosis. J BUON. 2016;21(6):1518–23.
Zhao B, Luo X, Shi H, Ma D. Tissue factor pathway inhibitor-2 is downregulated by ox-LDL and inhibits ox-LDL induced vascular smooth muscle cells proliferation and migration. Thromb Res. 2011;128(2):179–85. https://doi.org/10.1016/j.thromres.2011.02.025.
Jia Y, Yang Y, Brock MV, Cao B, Zhan Q, Li Y, et al. Methylation of TFPI-2 is an early event of esophageal carcinogenesis. Epigenomics. 2012;4(2):135–46. https://doi.org/10.2217/epi.12.11.
Karaszi K, Szabo S, Juhasz K, Kiraly P, Kocsis-Deak B, Hargitai B, et al. Increased placental expression of placental protein 5 (PP5)/tissue factor pathway inhibitor-2 (TFPI-2) in women with preeclampsia and HELLP syndrome: relevance to impaired trophoblast invasion? Placenta. 2019;76:30–9. https://doi.org/10.1016/j.placenta.2019.01.011.
Staun-Ram E, Shalev E. Human trophoblast function during the implantation process. Reprod Biol Endocrinol. 2005;3:56. https://doi.org/10.1186/1477-7827-3-56.
Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med. 2000;6(5):589–93. https://doi.org/10.1038/75074.
Abbas Y, Oefner CM, Polacheck WJ, Gardner L, Farrell L, Sharkey A, et al. A microfluidics assay to study invasion of human placental trophoblast cells. J R Soc Interface. 2017. https://doi.org/10.1098/rsif.2017.0131.
Bertero MT, Camaschella C, Serra A, Bergui L, Caligaris-Cappio F. Circulating 'trophoblast' cells in pregnancy have maternal genetic markers. Prenat Diagn. 1988;8(8):585–90.
Wagner D, Schunck R, Isebarth H. Detection of trophoblast cells in the circulating blood of women with normal and complicated pregnancy. Gynaecologia. 1964;158:175–92.
Chen H, Zhou X, Han TL, Baker PN, Qi H, Zhang H. Decreased IL-33 production contributes to trophoblast cell dysfunction in pregnancies with preeclampsia. Mediat Inflamm. 2018;2018:9787239. https://doi.org/10.1155/2018/9787239.
Konduri SD, Tasiou A, Chandrasekar N, Rao JS. Overexpression of tissue factor pathway inhibitor-2 (TFPI-2), decreases the invasiveness of prostate cancer cells in vitro. Int J Oncol. 2001;18(1):127–31.
Udagawa K, Miyagi Y, Hirahara F, Miyagi E, Nagashima Y, Minaguchi H, et al. Specific expression of PP5/TFPI2 mRNA by syncytiotrophoblasts in human placenta as revealed by in situ hybridization. Placenta. 1998;19(2–3):217–23.
Iochmann S, Blechet C, Chabot V, Saulnier A, Amini A, Gaud G, et al. Transient RNA silencing of tissue factor pathway inhibitor-2 modulates lung cancer cell invasion. Clin Exp Metastasis. 2009;26(5):457–67. https://doi.org/10.1007/s10585-009-9245-z.
Zhai LL, Wu Y, Huang DW, Tang ZG. Increased matrix metalloproteinase-2 expression and reduced tissue factor pathway inhibitor-2 expression correlate with angiogenesis and early postoperative recurrence of pancreatic carcinoma. Am J Transl Res. 2015;7(11):2412–22.
Zhai LL, Wu Y, Cai CY, Tang ZG. Upregulated matrix metalloproteinase-2 and downregulated tissue factor pathway inhibitor-2 are risk factors for lymph node metastasis and perineural invasion in pancreatic carcinoma. Onco Targets Ther. 2015;8:2827–34. https://doi.org/10.2147/OTT.S90599.
Zhu B, Zhang P, Zeng P, Huang Z, Dong TF, Gui YK, et al. Tissue factor pathway inhibitor-2 silencing promotes hepatocellular carcinoma cell invasion in vitro. Anat Rec (Hoboken). 2013;296(11):1708–16. https://doi.org/10.1002/ar.22789.
Funding
The study was supported by the Hebei Science and Technology Support Project (17277739D).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
The experiments were approved by the Ethical Committee of the Second Hospital of Hebei Medical University.
Informed consent
All participants in this study were informed and gave written consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, L., Huang, J., Su, Y. et al. Overexpression of tissue factor pathway inhibitor 2 attenuates trophoblast proliferation and invasion in preeclampsia. Human Cell 33, 512–520 (2020). https://doi.org/10.1007/s13577-020-00322-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00322-0