Abstract
To evaluate the efficacy of group embryo culture under low-oxygen tension in benchtop incubators on human embryo development in vitro. The study was designed as a prospective, patient blind, randomized, controlled trial of a complex intervention. One hundred forty-eight women undergoing IVF were recruited in our fertility practice and randomized into two groups: intervention group (study culture strategy) or control group (control culture strategy). Intervention group embryos were cultured grouped under low-oxygen tension in benchtop incubators while control group embryos were cultured individually under atmospheric oxygen tension in large-box incubators. Using the study culture strategy, there were a significantly higher implantation rate (65.1% vs 49.2%; RR, 1.42; 95% CI, 1.17–1.73) and live birth delivery rate per embryo transfer (52.7% vs 39.5%; RR, 1.33; 95% CI, 1.02–1.75) with the first fresh embryo transfer. Cumulative implantation rate (56.7% vs 43.6%; RR, 1.30; 95% CI, 1.05–1.62) and cumulative live birth rate per embryo transfer (47.4% vs 36.2%; RR, 1.31; 95% CI, 1.01–1.69) were also statistically significantly increased in the study culture strategy. Human embryos exposed to our study culture condition strategy had statistically significant increased cumulative implantation rate and cumulative live birth rate per embryo transferred. Our findings suggest that this strategy specially favours poor quality embryos. Clinical Trial Registration Number: NCT 01904006
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infertility is a common condition with a global prevalence of 9% [1] and most cases must undergo an in vitro fertilization treatment [2]. However, the delivery rate per oocyte retrieval is still low: 22.3% in IVF cycles and 20.1% in ICSI cycles according to the ESHRE 2014 register [3] or 33.3% according to the SART 2016 register (SART, 2016). A recommended standard clinical practice in IVF should be single-embryo transfer in blastocyst stage. Embryos that are developed in vitro until blastocyst stage seem to have other benefits: blastocysts have successfully surpassed the period of embryo division arrest. There is a better synchronization between embryo and endometrium and embryo selection is improved [4].
There has been an effort to improve embryo quality in order to increase the chances of achieving a pregnancy after transferring a single embryo. In vitro embryo culture exposes the embryos to some stress factors that have negative consequences in the embryo development and, therefore, in the later implantation potential of those embryos. Different aspects of the IVF laboratory have been improved: culture media, incubators, quality management, etc. [5]. These improvements have led to different changes in the strategy of culturing embryos until blastocyst stage in the laboratories although there is not yet a universal standard single culture system applied in them.
Among these improvements, three particular factors seem to show evidence of improving reproductive results: embryo density, low-oxygen tension and stability of embryo culture parameters.
Regarding embryo density, human embryos have been frequently cultured individually, in medium droplets with volumes ranging from 50 to 80 μl, approximately. Individual culture has been promoted mainly due to the need of selecting the best embryo for transfer or biopsy based in their morphology on the first 3 days of development. However, several studies have been performed in human embryos comparing individual versus group culture to the blastocyst stage. The majority of published works agrees on obtaining better quality embryos and higher blastocyst and pregnancy rates when performing grouped embryo culture [6,7,8,9] and lower apoptosis rates [10]. Other authors point out the possible negative effects of group embryo culture. The accumulation of toxic compounds secreted by the embryos or caused by the media break down of its components is the major concern, being ammonium one of the widely studied waste compounds [11, 12].
In order to improve the blastocyst culture conditions, it seems essential to optimize the oxygen concentration. Although several studies performed in different mammal species have shown that oxygen concentration in the oviduct fluid varies between 2 and 8% [5], mammal embryos are able to develop in vitro under atmospheric oxygen tension (~20%). In this sense, many researchers wondered if using low-oxygen tension in the incubator could improve in vitro embryo culture in comparison with atmospheric oxygen tension. In fact, according to a large meta-analysis, human embryo culture at low-oxygen tension increases 1.1 times the implantation rate when embryos are cultured up to the blastocyst stage [13]. In 2012, a Cochrane systematic review and meta-analysis of seven studies came to the conclusion that culturing embryos under low-oxygen tension results in a 2–13% improvement in live birth rate [14]. A more recent meta-analysis of 21 studies yielded similar results, reporting a small increase (~ 5%) in live birth/ongoing pregnancy, but specifying that the evidence was of very low quality [15].
Embryos require specific conditions of temperature, oxygen tension and pH in order to develop properly [16]. Besides, these conditions must remain stable to avoid additional stress for embryos. This required stability is closely related to the volume inside the incubator and the number of door openings. Incubator volume directly determines the gas equilibration and the recovery rate of the gas concentrations after each opening. Traditionally, IVF laboratories have used ‘large-box’ incubators designed originally for flasks cell culture. These incubators need more time to refill the different gases and re-establish the optimal culture conditions. Furthermore, more than one patient has been usually placed per incubator. Benchtop incubators have been specifically designed for culturing the embryos of a single patient in each. These incubators, due to their lesser volume, recover fast the inside atmosphere after each opening, considerably reducing the stress for the embryos and improving embryo development [17].
As there is evidence of benefits for each of these strategies, in our study, we wanted to measure the impact of a combined culture strategy in our laboratory in 2013 (benchtop incubator with less doors openings, low oxygen and group culture) versus the conventional culture strategy (big incubator, standard culture in single droplets, and daily entry assessment, atmospheric oxygen tension).
Materials and Methods
Patients
This prospective randomized complex intervention study was approved by the institutional Ethics Committee Hospital Universitario Virgen Macarena (Seville, Spain) and all patients signed an informed consent form in order to participate in the study. It was registered in Clinical Trials as NCT 01904006. A total of 164 patients undergoing an IVF treatment at the fertility clinic IVI Sevilla (Seville, Spain) were recruited between July 2013 and November 2015. The inclusion criteria were (1) to be less than 38 years old and undergoing their first IVF treatment, or patients using donor oocytes, (2) to observe at least 10 ovarian follicles of 10 mm of diameter or higher on the day when ovulation is induced. Patients with criptozoospermia or patients using testicular sperm cells were excluded from the study.
The day before the oocyte retrieval, the patient was randomized into one of the two treatment groups: CoC (control culture conditions group) or StC (study culture conditions group). The randomisation scheme was generated by one of the authors (NP) using a web tool (http://www.randomization.com, [Accessed Jul 15, 2013]) with randomly permuted blocks of four subjects per block. The list was kept in a locked drawer in the administration office, to which the clinical staff who enrolled the participants (MR, ES and VB) in the study had no access. Group allocation was requested by telephone. Physicians and patients were blinded to the assigned study intervention. After embryo transfer, they knew the allocation group. In CoC, the embryos were cultured in individual medium droplets inside incubators at 20% oxygen; while in StC, embryos were cultured in groups of up to 5 embryos inside incubators at 5% of oxygen tension. We define this study as a complex intervention [18] as there are changes in more than one variable in the study group.
Ovarian Stimulation Protocol
Patients using their own oocytes for the IVF treatment were subjected to controlled ovarian hyperstimulation in a GnRH antagonist protocol. The patients self-administered daily doses of FSH (Fostipur® [Angelini Farmacéutica, Barcelona, Spain] or Gonal-F® [Merk Serono, London, UK]) and hMG (Menopur®, Ferring Pharmaceuticals, Saint-Prex, Switzerland), starting on day 3 of the menstrual cycle in a combined regimen, following the routine practice of the centre. During this period, ultrasound scans were performed periodically to monitor the growth of the ovarian follicles. When at least one follicle with a diameter of 14 mm or more was detected, the administration of the GnRH antagonist began (0.25 mg of Ganirelix daily, Orgalutran®, MSD, Hertfordshire, UK) together with the previously described stimulation drugs. Once that three or more follicles with a diameter of 17 mm or more were detected, ovulation was artificially induced with the administration of a single dose of 6500 IU of hCG (Ovitrelle®, Merk Serono, London, UK).
IVF Treatment
Thirty-six hours after the administration of the hCG, the ovarian puncture was performed to retrieve the cumulus-oocyte complexes. In the IVF laboratory, the complexes were washed and cultured in gamete culture medium (Sydney IVF Fertilization Medium®, Cook Medical, Limerick, Ireland). Four hours and a half after oocyte retrieval, ICSI was performed to fertilize the oocytes in all patients. From this moment onwards, the treatment differed regarding the study group in which the patient was allocated.
In CoC, the microinjected oocytes were cultured individually in cleavage medium droplets (Cook Medical, Limerick, Ireland) covered with mineral oil (LifeGuard® Oil, LifeGlobal Europe, Brussels, Belgium) and observed once at 18, 44, 68 and 144 h (± 1 h) after ICSI [19]. The embryo culture took place inside Heracell® 150i incubators (Thermo Fisher Scientific, Waltham, USA) at 37 °C, 6% CO2 and an atmospheric tension of O2 (20%).
In StC, the microinjected oocytes were cultured in groups of up to 5 oocytes, in Cleavage medium droplets covered with mineral oil. Embryos were scored at 18, 68 and 144 (± 1 h) after ICSI. In this group, the embryo culture took place inside K-MINC® incubators (Cook Medical, Limerick, Ireland) at 37 °C, 6% CO2 and 5% O2.
Embryo transfer was initially scheduled for day 5. Our standard protocols at the time established a minimum number of two good-quality embryos to continue the culture of the embryos from days 3 to 5. Good quality was defined as an A or B embryo according to ASEBIR guidelines (Spanish Society of Reproductive Biology) (Alpha and ESHRE, 2011). Embryo transfer was performed using a soft catheter (Soft 23 cm Classic Wallace®, Smiths Medical, London, UK). Though single-embryo transfer was recommended, patients had the last choice of transferring 1 or 2 embryos.
Luteal Support
Luteal support consisted on 200 mg vaginal micronised progesterone (Utrogestan capsules 200 mg, Seid, Barcelona, Spain; or Progeffik capsules 200 mg, Effik, Madrid, Spain) every 12 h until the day of pregnancy test. In case of pregnancy, luteal support was maintained until 6th–8th week once heart beating was checked by vaginal ultrasound.
For the frozen-thawed cycles, luteal phase support, depending on the developmental stage of the frozen embryo, 200 mg vaginal micronised progesterone (Utrogestan capsules 200 mg, Seid, Barcelona, Spain; or Progeffik capsules 200 mg, Effik, Madrid, Spain) was added every 12 h either 2 or 4 days before ET (in the case of cleavage and blastocyst stage embryos, respectively), as previously described [19].
For the cryotransfer, hormonal replacement therapy (HRT) was performed with a 200 mg oestradiol transdermal patch (Estraderm matrix 100 mg; Novartis, Basel, Switzerland) every 2–3 days for 10–12 days, starting on the first or second day of the cycle, once the ovarian basal state was confirmed by transvaginal ultrasound, as previously described [19]. In these cases, no GnRH agonist was used during the preparation. Additionally, intravaginal micronised progesterone (Utrogestan capsules 200 mg, Seid, Barcelona, Spain; or Progeffik capsules 200 mg, Effik, Madrid, Spain) was started at doses of 400 mg/12 h after transvaginal ultrasound confirming adequate endometrium development (trilaminar morphology, at least 7 mm diameter) after those 10–12 days of oestradiol treatment. One or two embryos were transferred the same day of thawing after confirming their survival (50% live surviving cells). The number of embryos transferred (single-embryo transfer or double-embryo transfer) depended on the patient’s decision and availability after thawing.
Pregnancy and Live Birth
The main outcomes measured were implantation rate, clinical pregnancy rate and live birth per embryo transferred of fresh embryo transfer. In addition to the standard parameters, we also analysed fertilization rate, blastocyst rate, birth rate, multiple pregnancy rate and clinical miscarriage rate. Success rates from vitrified-warmed embryo transfer cycles (cryotransfers) were also measured. We defined the cumulative rates as the resulting from the cycle when fresh embryos were transferred and from subsequent cryotransfers. The last retrieval was performed on November 2015. The data on live birth and cryotransfers were collected until October 2016.
Statistical Analysis
Statistical analysis was performed according intention-to-treat principle (ITT) and stratifying for the day of the transfer, day 3 (D3) or day 5 (D5), and for oocyte origin, standard patients or oocyte recipients. For descriptive statistics, data are presented as the means and the 95% IC of their difference. Each comparison generated a different p value. In all cases, p value ≤ 0.05 was considered significant. The χ2 test was used for non-parametric analysis and the t test for parametric analysis (two-sided). We calculated a sample size of 182 patients assuming a pregnancy rate of 50% for the CoC group and 70% for StC group with and an α-risk of 5% and power of 80% using a web resource (https://www.sealedenvelope.com/power/binary-equivalence/ [Accessed Apr 15, 2013]). This paper was written according to the updated guidelines for reporting parallel group randomized trials (CONSORT statement) [20].
Results
Patient Enrolment
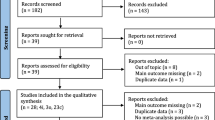
Before the start of stimulation, 164 patients of 1527 potential subjects who met the initial inclusion criteria agreed to participate in the study. Sixteen of these patients were excluded after signing the study informed consent form because they did not meet the follicle count inclusion criterion. Thus, 148 patients were randomly assigned to one of the two groups: 73 patients to CoC group and 75 patients to StC group (Fig. 1). We recruited patients between July 2013 and November 2015. During follow-up period, the data on live birth and cryotransfers were collected until October 2016. We stopped the recruitment after 28 months even though we had not reached the 182 patients, as the multiple pregnancy rate was too high. The proportion of standard patients in each group was similar (46.6% in CoC group vs 38.7% in StC group, p = 0.331). There were no significant differences in the characteristics of the patients assigned to each group, such as female, BMI, infertility duration, ovarian hyperstimulation days, rFSH total dose, hMG total dose and number of oocytes retrieved (Table 1).
Analysis per ITT
Main results of the study are summarized in Table 2. To analyse the real impact on success rates of StC group, we calculated the different outcomes according to the ITT independently of the day of transfer. The fertilization rate was similar in both groups (74.5% vs 71.1%, p = 0.10). There was a significantly higher percentage of cycles meeting criteria for blastocyst culture on StC group than on CoC group (88.0% vs 56.2%, p < 0.001). The mean number of viable embryos (transferred and vitrified) was significantly lower on CoC group than on StC group (3.55 vs 5.37, p < 0.001). There was no significant difference in one of the main outcomes measured (fresh transfer clinical pregnancy rate). There were no significant differences either in the live birth rate or miscarriage rate. StC group, compared with the CoC group, resulted in a higher implantation rate (65.1% vs 49.2%, p = 0.01) and live birth rate per embryo transfer (52.7% vs 39.5%, p = 0.04). The multiple pregnancy rate was more than twice higher in the StC group (55.6% vs 27.1%, p = 0.004).
More cryotransfer cycles were performed in the StC group compared with the CoC group (2.35 vs 1.71, p = 0.05). No significant differences were obtained for the remaining outcomes analysed from cryotransfers.
Although the difference in cumulative pregnancy rate and cumulative birth rate were not significant, the cumulative implantation rate was significantly higher in the StC group (56.7% vs 43.6%, p = 0.02), as the cumulative live birth rate per embryo transfer (47.4% vs 36.2%, p = 0.04).
D3 and D5 Group Analysis
In addition, we analysed the previous parameters stratifying by the day of the transfer (Table 3). Although fewer cycles in the StC group meet the criteria for D3 transfer, no significant differences were found between both groups in any of the variables comparing day 3 transfers.
In day 5 transfers, StC group, compared with the CoC group, resulted in a significantly higher blastocyst rate (57.2% vs 45.5%, p < 0.001). The mean number of viable embryos (transferred and vitrified) was significantly lower on CoC group than on StC group (4.41 vs 5.71, p = 0.02). No significant differences were obtained for the outcomes analysed from cryotransfers. When cumulative results were studied, also in day 5 transfers, compared with the CoC group, the cumulative implantation rate (44.0% vs 57.6%, p = 0.03) was significantly different.
Analysis per Type of Patient
Results stratified by type of patients are summarized in Table 4. We have separated the patients depending on the origin of the oocyte. The benefit of the study culture conditions was much clearer in the standard patients than in oocyte recipients. In standard patients, the implantation rate (66.0% vs 43.9%, p = 0.02) and live birth rate per embryo transfer (60.4% vs 35.1%, p = 0.008) were significantly improved in the StC group. Multiple pregnancy rate (52.2% vs 19.0%, p = 0.02) was also significantly higher in StC group. Regarding cumulative rates, cumulative birth rate (82.8% vs 58.8%, p = 0.04), cumulative implantation rate (66.7% vs 37.2%, p = 0.001) and cumulative live birth rate per embryo transfer (64.9% vs 32.1%, p < 0.001) were significantly higher in the StC group.
In oocyte recipients, the results were similar between CoC and StC groups. StC patients showed a significant increase in the mean number of viable embryos, the blastocyst rate and the number of cryotransfers. Cumulative results were also similar between both groups (Table 4).
Discussion
Assisted reproduction techniques (ART) have evolved greatly during the last decades but the efficiency of this technology is still not optimal. Most advances have been focused in embryo selection (as preimplantational genetic testing or time lapse morphokinetics). Improvements in embryo culture systems are also necessary in order to increase the success rates of the IVF cycles and reduce the number of cycles required to get a healthy baby at home. Some improvements have been introduced in culture media composition (for example, in single-step media). Fresh blastocyst stage transfer seems to be associated with higher live birth and clinical pregnancy rates than fresh cleavage stage transfer [21]. A wide number of trials have been published regarding this issue. Most of them agree to argue in favour of blastocyst stage strategy as the best approach in order to improve the implantation rate and to reduce the necessity to transfer more than one embryo [22]. Less attention has been paid to other crucial aspects of embryo culture systems. In this study, we have focused on three specific characteristics of the embryo culture protocols: embryo density in the culture media, oxygen concentration inside the incubator and stability of the culture conditions. In this way, we have compared two different culture strategies.
Main results of our study show better embryo quality with the StC. This effect is visible even on day 3 (more patients meets the blastocyst culture criteria). There is also an increase in the number of embryos available for transfer or vitrification.
Although the mean number of embryos transferred was similar in both groups, embryos from StC group implanted more, resulting in a much higher multiple pregnancy rate and live birth rate per embryo transferred (Table 2). We found no significant differences in the clinical pregnancy rate or in the birth rate. We think that this is a consequence of the limited number of patients and the high number of double-embryo transfers performed. The rest of variables show a much higher quantity and quality of embryos in the StC. Patients from StC group underwent a higher number of cryotransfers cycles because blastocyst rate was increased, and they had more viable embryos. These facts made that cumulative implantation rate and cumulative live birth rate per embryo transferred were both superior in StC group. The cumulative pregnancy rate and cumulative birth rate were not significantly different. It has been described that different settings can affect the aneuploidy rates in embryos from donor oocytes [23]. Temperature is one of the main parameters to set. The optimal temperature assumed for in vitro embryo culture is still 37 °C. There have been some efforts to optimize this temperature, but the latest Cochrane Review concludes that there is no evidence for lowering the temperature [24]. The only study that compares live birth rates (37 °C vs 36 °C) did not find any advantage in lowering the temperature [25]. There is no data on the effect of lowering the temperature of human embryo culture more 1°. In mouse embryos, zygotes exposed to room temperature for 5 min inhibited cleavage rates. When the authors increased the exposure time to 10 or 15 min, the cleavage rates were further decreased and blastocyst development was reduced to half of the control after 15 min at room temperature [26].
When comparing results stratified by the day of the transfer (D3 vs D5) (Table 3), mean number of viable embryos is significantly higher in the StC D5 group. This implies that the effect on the embryo starts just after ICSI since there are fewer cases of D3 transfer in the StC group. The StC strategy seems to improve the embryo quality of all patients: there are more viable embryos with higher implantation rate. When comparing results stratified by the oocytes origin (Table 4), this effect is not evident in oocyte recipients. Our data suggest that embryos with high developmental potential are less affected by the culture conditions. The best embryos reach blastocyst stage independently of the culture conditions, while embryos of lesser quality are favoured by the StC strategy. Although an IVF program can have satisfactory results in good prognosis patients, the culture conditions may affect negatively the rest of the patients.
The different culture conditions introduced in the StC group have been widely discussed in previous studies, although in an independent manner. However, we have observed additional benefits for each upgrading. There are studies in mice [27, 28] and humans [7, 8, 29] describing new advances in culture systems that did not achieve the same good results that those obtained by the group culture. It is worth highlighting the work of Ebner and colleagues, who conducted a large prospective study with human embryos comparing individual and grouped culture [7]. In this case, group embryo culture was associated with a higher level of compaction and blastulation and a better blastocyst quality, concluding that embryo density should be increased while medium culture volume should be reduced [7, 29]. Certain paracrine signals, which are present in the uterus and fallopian tubes, are lacking in commercial culture media [30,31,32]. During preimplantation period, the embryos produce soluble ligands and receptors for paracrine (maternal ligands) and autocrine mediators (embryo-produced ligands) [33]. These signalling molecules secreted by the embryos could partially compensate the absence of signals in the culture media that are present in the reproductive tract [34, 35]. Another group of investigators focused on the necessary distance between embryos in order to observe paracrine effects. They used a culture device to prove that porcine embryos exhibited an optimal development when cultured with a distance of 81–160 μm between embryos. Beyond this distance, embryos exhibited a lower blastocyst rate as the distance increased [36]. The same experiment was replicated using bovine embryos, finding similar results [37]. Other authors suggest that bad-quality embryos may exert a negative influence over the surrounding good-quality embryos [38].Another indirect advantage of group embryo culture is the less amount of time needed to evaluate and manipulate the embryos outside the incubator. A research group performed a prospective randomized study comparing two culture systems: a microwell group culture dish (Primo Vision, Vitrolife) and an individual culture system in microdroplets. Among their findings, they observed that microwell group culture implies a shorter time for embryo evaluation, around 30 s less [39]. There has also been a wide discussion about the optimal oxygen concentration for human embryo development in vitro. Most authors have focused on comparing atmospheric oxygen level (20–21%) versus 5% oxygen level [14]. These benefits of low-oxygen embryo culture are concordant to the results obtained in the studies performed in embryos from other mammal species. Besides, according to some of the previously mentioned studies, it seems that the highest benefit is obtained when culturing embryos through the complete period of culture, from day 0 to day 5 or 6 [40,41,42].
There is evidence of the impact of an atmospheric oxygen tension (20%) on embryos during the preimplantation period, causing DNA fragmentation, changes in the DNA expression profile, aneuploidies and organelle and membrane disturbances [41, 43,44,45,46,47,48]. In addition, it has been proved in bovine embryos that oxidative stress can also induce changes in the epigenome, specifically in the methylation pattern [5]. Similarly, other authors have also found alterations in the proteome [49], metabolome [50] and secretome [51] of embryos cultured under a 20% oxygen tension.
Our results show a positive effect beginning right after the ICSI procedure, as most patients met the criteria for blastocyst culture. It has been proposed that 2% should be theoretically the best oxygen level to promote embryo viability from day 3 to day 5 [52]. In addition, in the StC group of our study, we have reduced the number of times we open the incubator because there is one patient per incubator and embryos are not evaluated in day 2. All these aspects contribute to reduce the embryo stress and maintain the stability of the culture system.
The observed differences between the two culture strategies might be due to a combination of the changes made in the oxygen level inside the incubator, the embryo density in the culture droplets and the incubator type. However, we cannot know the weight of each modification in the resulting rates. Still, many previous studies have found similar improvements when studying individually these culture parameters.
Limitations
One limitation of this study was the option of transferring two embryos. We stopped the recruitment as it was taking too long to reach the calculated number of patients and the multiple pregnancy rate was too high. In this study, the improvement of embryo quality without changing the single-embryo transfer policy has raised the multiple pregnancy rate to an unacceptable level for a standard IVF program. This improvement in embryo culture can force the implantation of strict single-embryo transfer policies.
This is a complex intervention study where there is more than one variable different in both groups. Although this makes difficult to distinguish which is the actual impact in the results of each single variable studied, we considered appropriate the trial design as we wanted to assay the impact of changing all the conditions at the same time in an IVF laboratory, comparing the conventional routinely practice versus an optimized one that involves these parameters simultaneously. This may seem less “academic” but in fact is closer to the real practice and, therefore, more useful to be transferred to real life’s routine in an IVF laboratory. When we designed this study, we believed there was enough evidence to apply these different strategies in all patients as standard culture and our aim was to show the practical impact of this decision in an IVF setting.
After our results, it seems to be promising to move to this combined strategy in a modern IVF laboratory.
Conclusions
In our study, we have found that cumulative implantation rate and cumulative live birth rate per embryo transferred were significantly higher using a culture strategy combining low-oxygen tension, grouped embryo culture and benchtop stable incubators, favouring specially those oocytes and embryos with poor quality. With this study, we show the impact of using three changes made in culture conditions that have been widely studied separately, but never as a group, to our knowledge. These findings made us change our clinical practice, so we now apply this StC strategy in all our patients, and we have decreased considerably the number of double-embryo transfers performed in order to decrease the multiple pregnancy rate.
References
Dyer SJ. International estimates on infertility prevalence and treatment seeking: potential need and demand for medical care. Hum Reprod. 2009;24(9):2379–80 author reply 2380-2373.
Mantikou E, Youssef MA, van Wely M, et al. Embryo culture media and IVF/ICSI success rates: a systematic review. Hum Reprod Update. 2013;19(3):210–20.
De Geyter C, Calhaz-Jorge C, Kupka MS, et al. ART in Europe, 2014: results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod. 2018;33(9):1586–601.
Martins WP, Nastri CO, Rienzi L, van der Poel SZ, Gracia C, Racowsky C. Blastocyst vs cleavage-stage embryo transfer: systematic review and meta-analysis of reproductive outcomes. Ultrasound Obstet Gynecol. 2017;49(5):583–91.
Wale PL, Gardner DK. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum Reprod Update. 2016;22(1):2–22.
Almagor M, Bejar C, Kafka I, Yaffe H. Pregnancy rates after communal growth of preimplantation human embryos in vitro. Fertil Steril. 1996;66(3):394–7.
Ebner T, Shebl O, Moser M, Mayer RB, Arzt W, Tews G. Group culture of human zygotes is superior to individual culture in terms of blastulation, implantation and life birth. Reprod BioMed Online. 2010;21(6):762–8.
Moessner J, Dodson WC. The quality of human embryo growth is improved when embryos are cultured in groups rather than separately. Fertil Steril. 1995;64(5):1034–5.
Rebollar-Lazaro I, Matson P. The culture of human cleavage stage embryos alone or in groups: effect upon blastocyst utilization rates and implantation. Reprod Biol. 2010;10(3):227–34.
Brison DR, Schultz RM. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor alpha. Biol Reprod. 1997;56(5):1088–96.
Lane M, Gardner DK. Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol Reprod. 2003;69(4):1109–17.
Orsi NM, Leese HJ. Ammonium exposure and pyruvate affect the amino acid metabolism of bovine blastocysts in vitro. Reproduction. 2004;127(1):131–40.
Gomes Sobrinho DB, Oliveira JB, Petersen CG, et al. IVF/ICSI outcomes after culture of human embryos at low oxygen tension: a meta-analysis. Reprod Biol Endocrinol. 2011;9:143.
Bontekoe S, Mantikou E, van Wely M, Seshadri S, Repping S, Mastenbroek S. Low oxygen concentrations for embryo culture in assisted reproductive technologies. Cochrane Database Syst Rev. 2012;7:CD008950.
Nastri CO, Nobrega BN, Teixeira DM, et al. Low versus atmospheric oxygen tension for embryo culture in assisted reproduction: a systematic review and meta-analysis. Fertil Steril. 2016;106(1):95–104 e117.
Swain JE, Carrell D, Cobo A, Meseguer M, Rubio C, Smith GD. Optimizing the culture environment and embryo manipulation to help maintain embryo developmental potential. Fertil Steril. 2016;105(3):571–87.
Swain JE. Decisions for the IVF laboratory: comparative analysis of embryo culture incubators. Reprod BioMed Online. 2014;28(5):535–47.
Council MR. A framework for the development and evaluation of randomised controlled trials for complex interventions to improve health. MRC. 2006.
Prados N, Quiroga R, Caligara C, et al. Elective single versus double embryo transfer: live birth outcome and patient acceptance in a prospective randomised trial. Reprod Fertil Dev. 2015;27(5):794–800.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1(2):100–7.
Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016;(6):CD002118.
Gardner DK. The impact of physiological oxygen during culture, and vitrification for cryopreservation, on the outcome of extended culture in human IVF. Reprod BioMed Online. 2016;32(2):137–41.
Munne S, Alikani M, Ribustello L, Colls P, Martinez-Ortiz PA, McCulloh DH. Euploidy rates in donor egg cycles significantly differ between fertility centers. Hum Reprod. 2017;32(4):743–9.
Baak NA, Cantineau AE, Farquhar C, Brison DR. Temperature of embryo culture for assisted reproduction. Cochrane Database Syst Rev. 2019;9:CD012192.
Hong KH, Lee H, Forman EJ, Upham KM, Scott RT Jr. Examining the temperature of embryo culture in in vitro fertilization: a randomized controlled trial comparing traditional core temperature (37 degrees C) to a more physiologic, cooler temperature (36 degrees C). Fertil Steril. 2014;102(3):767–73.
FitzGerald-Scott L, Sundaram SG, Smith S. The relevance and use of mouse embryo bioassays for quality control in an assisted reproductive technology program. Fertil Steril. 1993;60(3):559–68.
Kelley RL, Gardner DK. In vitro culture of individual mouse preimplantation embryos: the role of embryo density, microwells, oxygen, timing and conditioned media. Reprod BioMed Online. 2017;34(5):441–54.
Vutyavanich T, Saeng-Anan U, Sirisukkasem S, Piromlertamorn W. Effect of embryo density and microdrop volume on the blastocyst development of mouse two-cell embryos. Fertil Steril. 2011;95(4):1435–9.
Sun B, Yu W, Wang F, Song W, Jin H, Sun Y. Effects of group culture on the development of discarded human embryos and the construction of human embryonic stem cell lines. J Assist Reprod Genet. 2014;31(10):1369–76.
Aviles M, Gutierrez-Adan A, Coy P. Oviductal secretions: will they be key factors for the future ARTs? Mol Hum Reprod. 2010;16(12):896–906.
Hannan NJ, Paiva P, Meehan KL, Rombauts LJ, Gardner DK, Salamonsen LA. Analysis of fertility-related soluble mediators in human uterine fluid identifies VEGF as a key regulator of embryo implantation. Endocrinology. 2011;152(12):4948–56.
Robertson SA, Chin PY, Schjenken JE, Thompson JG. Female tract cytokines and developmental programming in embryos. Adv Exp Med Biol. 2015;843:173–213.
Richter KS. The importance of growth factors for preimplantation embryo development and in-vitro culture. Curr Opin Obstet Gynecol. 2008;20(3):292–304.
Thouas GA, Dominguez F, Green MP, Vilella F, Simon C, Gardner DK. Soluble ligands and their receptors in human embryo development and implantation. Endocr Rev. 2015;36(1):92–130.
Wydooghe E, Vandaele L, Heras S, de Sutter P, Deforce D, Peelman L, et al. Autocrine embryotropins revisited: how do embryos communicate with each other in vitro when cultured in groups? Biol Rev Camb Philos Soc. 2017;92(1):505–20.
Stokes PJ, Abeydeera LR, Leese HJ. Development of porcine embryos in vivo and in vitro; evidence for embryo 'cross talk' in vitro. Dev Biol. 2005;284(1):62–71.
Gopichandran N, Leese HJ. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction. 2006;131(2):269–77.
Tao T, Robichaud A, Mercier J, Ouellette R. Influence of group embryo culture strategies on the blastocyst development and pregnancy outcome. J Assist Reprod Genet. 2013;30(1):63–8.
Fancsovits P, Pribenszky C, Lehner Á, et al. Oral communication O-007: prospective randomized study comparing human embryo development in a microwell group culture dish (primo vision dish) or in a standard dish with individual droplets. European Society of Human Reproduction and Embryology Annual Meeting (Helsinki) 2016.
Kovacic B, Vlaisavljevic V. Influence of atmospheric versus reduced oxygen concentration on development of human blastocysts in vitro: a prospective study on sibling oocytes. Reprod BioMed Online. 2008;17(2):229–36.
Meintjes M, Chantilis SJ, Douglas JD, Rodriguez AJ, Guerami AR, Bookout DM, et al. A controlled randomized trial evaluating the effect of lowered incubator oxygen tension on live births in a predominantly blastocyst transfer program. Hum Reprod. 2009;24(2):300–7.
Waldenstrom U, Engstrom AB, Hellberg D, Nilsson S. Low-oxygen compared with high-oxygen atmosphere in blastocyst culture, a prospective randomized study. Fertil Steril. 2009;91(6):2461–5.
Arias ME, Sanchez R, Felmer R. Evaluation of different culture systems with low oxygen tension on the development, quality and oxidative stress-related genes of bovine embryos produced in vitro. Zygote. 2012;20(3):209–17.
Bean CJ, Hassold TJ, Judis L, Hunt PA. Fertilization in vitro increases non-disjunction during early cleavage divisions in a mouse model system. Hum Reprod. 2002;17(9):2362–7.
Catt JW, Henman M. Toxic effects of oxygen on human embryo development. Hum Reprod. 2000;15(Suppl 2):199–206.
Kang JT, Atikuzzaman M, Kwon DK, Park SJ, Kim SJ, Moon JH, et al. Developmental competence of porcine oocytes after in vitro maturation and in vitro culture under different oxygen concentrations. Zygote. 2012;20(1):1–8.
Kwon HC, Yang HW, Hwang KJ, Yoo JH, Kim MS, Lee CH, et al. Effects of low oxygen condition on the generation of reactive oxygen species and the development in mouse embryos cultured in vitro. J Obstet Gynaecol Res. 1999;25(5):359–66.
Rinaudo PF, Giritharan G, Talbi S, Dobson AT, Schultz RM. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril. 2006;86(4 Suppl):1252–65 1265 e1251–1236.
Katz-Jaffe MG, Linck DW, Schoolcraft WB, Gardner DK. A proteomic analysis of mammalian preimplantation embryonic development. Reproduction. 2005;130(6):899–905.
Wale PL, Gardner DK. Oxygen regulates amino acid turnover and carbohydrate uptake during the preimplantation period of mouse embryo development. Biol Reprod. 2012;87(1):24 21-28.
Kubisch HM, Johnson KM. The effects of blastomere biopsy and oxygen tension on bovine embryo development, rate of apoptosis and interferon-tau secretion. Reprod Domest Anim. 2007;42(5):509–15.
Morin SJ. Oxygen tension in embryo culture: does a shift to 2% O2 in extended culture represent the most physiologic system? J Assist Reprod Genet. 2017;34(3):309–14.
Acknowledgements
The authors thank all the patients for participating in the study. The authors also thank all the staff at IVIRMA Seville (Seville, Spain) for their contribution to this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest. Manuel Fernández-Sánchez has participated in Advisory Boards and received speaker fees and research support from Ferring Pharmaceuticals, Merck Serono, MSD, IBSA, Finox, SEID and Angelini.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ruíz, M., Santamaría-López, E., Blasco, V. et al. Effect of Group Embryo Culture under Low-Oxygen Tension in Benchtop Incubators on Human Embryo Culture: Prospective, Randomized, Controlled Trial. Reprod. Sci. 27, 1522–1533 (2020). https://doi.org/10.1007/s43032-020-00150-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00150-5