Abstract
The issue of environmental pollution caused by the widespread presence of microplastics (MPs) in environmental media has garnered significant attention. However, research on MPs pollution has mainly focused on aquatic ecosystems in recent years. The sources and pollution characteristics of MPs in the environment, especially in solid waste, have not been well-described. Additionally, there are few reports on the ecotoxicity of MPs, which highlights the need to fill this gap. This review first summarizes the occurrence characteristics of MPs in water, soil, and marine environments, and then provides an overview of their toxic effects on organisms and the relevant mechanisms. This paper also provides an outlook on the hotspots of research on pollution characterization and ecotoxicity of MPs. Finally, this review aims to provide insights for future ecotoxicity control of MPs. Overall, this paper expands our understanding of the pollution characteristics and ecological toxicity of MPs in current environmental media, providing forward-looking guidance for future research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
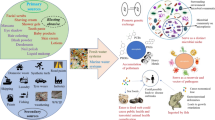
Microplastics (MPs) refer to plastic particles or fragments with a diameter of less than 5 mm, including primary MPs and secondary MPs [1]. They are widely distributed in environmental media such as soil, oceans, rivers, and lakes and have even been detected in remote areas like polar regions, high mountains, and deep-sea sediments [2, 3]. The production, transportation, use, and storage of plastic products are the main sources of MPs, and the widespread use of disposable masks during the COVID-19 pandemic has also increased pollution from MPs in the environment [4]. The main types of polymers for MPs include polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), and polyethylene terephthalate (PET). It has been reported about 3 million tons of MPs were lost into the environment in 2015 alone, and it is estimated that by 2060, MPs will account for 13.2% of the total weight of plastic waste worldwide [5, 6]. Due to their small particle size, high specific surface area and difficulty in degradation, MPs can be transported long distances in the environment and persist for a long time. They are easily ingested by aquatic or terrestrial organisms and accumulate through food chains. Despite lacking evidence to show that MPs are toxic to human bodies, the toxicological test results on organisms, cells, and mammals may provide certain clues. For instance, MPs could cause pulmonary diseases [7], inducing disorder of hepatic lipid metabolism and dysbiosis of gut microbiota and disorder in mice [8], and so forth. PS particles have been demonstrated to induce inflammatory and cytotoxic effects in epithelial cells of human lung, ultimately posing potential hazards to human health (Fig. 1). Therefore, it is necessary to pay attention to the pollution characteristics and ecological toxicity effects caused by MPs in the environment [9]. However, previous reviews on environmental pollution characteristics and ecological toxicity effects related to MPs have mostly focused on specific aspects, lacking a systematic overview of MP's pollution characteristics across water environments, soil, and oceans along with its ecological toxicity effects. In order to better understand the behavior of MPs in the environment and their pollution control, this paper first systematically summarizes the sources and occurrence characteristics of MPs in wastewater treatment plants, marine environments, soil environments, and urban solid waste. Secondly, this work analyzes the toxic effects of MPs on aquatic ecosystems, terrestrial organisms, and human health, and reveals the relevant mechanisms. Finally, this paper looks forward to the research hotspots and urgent environmental issues related to MPs. This paper will help clarify the pollution characteristics of MPs in different environmental media, understand the mechanisms of MPs-induced ecological toxicity effects, and provide a theoretical basis for the deep pollution control of MPs.
Pollution Characteristics of MPs in Environmental Media
Due to their stable structure and strong hydrophobicity, MPs can be transported by external forces, such as wind, rivers, and ocean currents, in the environment. Their presence has been found all over the world, including rarely visited places like Antarctica [9]. Organisms in the environment may ingest MPs and become carriers, leading to biomagnification through the food chain. This not only causes ecological pollution but also poses a threat to human health [10]. Currently, research on MP pollution mainly focuses on terrestrial and aquatic environments. MPs in water mainly originate from sewage treatment plant discharge, marine fishing activities, and ship transportation emissions [11], while those on land primarily come from plastic products breaking down at landfill sites or being used for agricultural purposes, as well as MPs present in soil [12, 13].
Pollution Characteristics of MPs in Wastewater Treatment Plants
MPs in urban wastewater treatment plants mainly come from urban sewage, which is a type of mixed sewage that usually includes domestic and industrial wastewater, with the former accounting for the largest proportion [14].
Pollution Characteristics of MPs in Domestic Sewage
The primary sources of MPs in domestic sewage are plastic microbeads in personal care products and fibers released during clothing washing. Fabric fibers become pollutants in laundry wastewater during the cleaning process as they enter the water [15, 16]. Each piece of clothing washed can produce thousands of fibers that eventually reach the wastewater treatment plant via the sewer system [17]. The amount of fiber released depends on washing methods, textile properties, and detergents used [18]. Textile fibers can break down under UV radiation, mechanical wear, or biological decomposition, making clothes and fabrics an essential source of MP pollution in the environment [19]. Fiber MPs account for 50% of all MPs found in urban wastewater treatment plants, making laundry wastewater one of their main sources [20].
Moreover, plastic microbeads are commonly used in cosmetics and personal care products, including facial cleansers, bath gels, toothpaste, and makeup, to enhance cleansing ability or adjust viscosity [21]. These microbeads are spherical or irregularly shaped and can be used for exfoliation or cleansing. A single use of a microbead-containing product can release up to 94,500 microbeads into the drainage system [21]. A survey conducted at major supermarkets in Beijing showed that 7.1% of facial cleansers and 2.2% of bath products contained plastic microbeads (with an average size range between 313±130 μm and an average addition amount ranging between 25.04±10.69 mg/g for facial cleansers and between 422±185 μm and an average addition amount ranging between 17.80±7.50 mg/g for bath products) [22]. These microbeads are released during use, enter the wastewater from bathing, and become another significant source of MPs in urban domestic sewage.
MPs in Industrial Wastewater
MPs found in industrial wastewater originate from the processing and manufacturing of plastic products. For instance, textile mills and printing and dyeing factories produce significant amounts of fiber MPs during their production processes. The production of fibers occurs during spinning, weaving, cutting, and other manufacturing processes, and traditional cutting methods can extract up to 31 times more fiber MPs than laser cutting [23, 24]. The concentration of microfibers in textile dyeing wastewater can reach up to 54,100 MFs/L, and the average concentration of microfibers is 537.5 MFs/L in treated effluent from sewage treatment plants [24].
Another significant contributor to the presence of MPs in the environment is tire wear, road abrasion, and the shipping industry. Research estimates that tire wear alone releases 63 tons of MPs annually in the UK, 125 tons in Germany, and 240 tons in Japan [25]. These tiny particles are also present in air pollution and can be washed out by rain, snow, and dew condensation, eventually making their way into wastewater treatment plants via run-off. As a result, the presence of MPs in industrial wastewater contributes significantly to their presence in wastewater treatment plants and the environment as a whole.
The Occurrence Characteristics of MPs in Urban Sewage
Wastewater containing MPs undergoes three stages of treatment when entering a wastewater treatment plant, including primary treatment such as grid screens and primary sedimentation tanks, secondary biological purification, and advanced treatment [26]. However, part of the incoming MPs is discharged into the environment with treated effluent after various processes, while another part is enriched in sludge through settling and flocculation, affecting the subsequent processing and disposal of sludge [27].
Wastewater treatment plants can be a significant source of MP-containing sludge and water pollution (Table 1), as large amounts of sewage enter the plant and MPs become trapped in the sludge. The methods used to treat sludge, such as anaerobic digestion, thickening, lime stabilization, and thermal dehydration, are inadequate for removing MPs from it [27]. After sludge processing, 69 to 80% of MPs remain in the produced biological solids, which are often used as soil fertilizers for agricultural land use, leading to a significant accumulation of MPs in soil [12]. Therefore, wastewater effluent and sludge from wastewater treatment plants need to be treated for MP removal before being released into aquatic or soil environments.
Studies have shown that wastewater treatment plants in China have lower interception rates for incoming MPs compared to foreign counterparts, with removal rates ranging from 35.99% for primary-treated effluent to 80.97% for secondary-treated effluent [28]. This indicates the need for improving waste management systems to control sources contributing to environmental pollution by MPs. Domestic or industrial wastewater containing large amounts of plastic and other pollutants should not be discharged without treatment. Sustainable wastewater infrastructure should be developed, especially in developing countries where people lack access to waste treatment facilities. More effective technologies are needed to eradicate MPs from sewage and sludge at the source for more efficient source control.
Characteristics of MPs Pollution in Marine Environment
Since the 1970s, a growing number of studies have reported the ubiquitous presence of MPs in seawater, sediments, and marine ecosystems, drawing increasing attention to their pollution of the ocean [33]. It is estimated that more than 10 million tons of plastic enter the ocean each year on a global scale, with a cumulative total of 250 million tons of plastic projected to accumulate in marine environments by 2025 [34, 35]. The high mobility of MPs enables them to permeate the global marine system ubiquitously [12]. Pollution characteristics of MPs in different sea areas are presented in Table 2, which demonstrates that the marine environment has become a significant source of MPs pollution.
Coastal waters are the interface of marine and terrestrial systems, closely linked to human activities [48]. MPs enter the ocean and are transported by wind, waves, suspended sand, and ocean currents before eventually accumulating in beaches and nearshore sediments [49]. From a global perspective, coastal areas are heavily contaminated with MPs, especially bays, estuaries, islands, and other densely populated areas. The characteristics of marine MP pollution mainly stem from the analysis of surface seawater. The abundance of MPs collected from small volumes (2.2–2.8 L) of micro-surface seawater off the southern coast of Korea reached 1.63 × 104 items·m−3, according to studies by Song et al. [50] Antunes et al. [51] detected MP content at 362 items·m−3 along Portugal’s coastline. Vibhatabandhu et al. [52] found an abundance level as high as 9.97×103 items·m−3 in Thailand’s Gulf region, which is currently one of the most heavily polluted coastal regions globally. Surface seawater samples taken from China’s East Sea, South Sea, and Bohai Sea also demonstrate evidence of MP pollution. This may be related to China’s booming coastal economy, where industrialization, fishing industry development, and tourism activity are relatively dense, resulting in higher levels of MP abundance [53,54,55,56].
According to numerous studies, MPs have become a ubiquitous presence in the deep sea and their distribution and transfer depend on several factors. Sedimentation or gravity-driven transport plays a significant role in the distribution of MPs, as particles with greater density than seawater sink more easily into deeper waters [57]. Biological transportation is another factor contributing to the spread of MPs in the deep sea [58]. While most studies exploring deep-sea MP pollution focus primarily on abundance levels, rather than spatial distribution patterns, the abundance of MPs in deep-sea sediments is lower compared to nearshore sediments, as they tend to remain suspended in the water for longer periods due to surface tension effects and ocean currents [59].
Studies investigating MP abundance levels in deep-sea sediments have shown that their presence is relatively low. MP abundance levels ranging between 2×10 4–4×10 4 items·m−3 were detected in sediment samples from the Southern Ocean and North Atlantic deep sea (water depth > 4000 m) [59]. Low concentrations of MPs were also found in sediment samples taken from the Mediterranean Sea (water depth 3500 m), Pacific Ocean (water depth 2200 m), and Indian Ocean (water depth 1000 m), with values ranging between 1.2×10 5–8×10 5 items·m−3 and between 3×10 4–6×10 4 items·m−3, respectively [60, 61]. However, a continuous increase in the concentration of MPs in garbage collected near the Polar Hasgatang Observatory, located near a deep-sea area with a water depth of up to 5570 m, has been observed from 2002 to 2014. The highest concentration level found among sediment samples collected in 2015 reached as high as 6595 items·kg−1, which some researchers attribute to melting ice leading to higher MPs sources [62]. Few studies have explored MPs abundance levels within deep-sea waters. However, Courtene-Jones et al. [58] detected an MPs concentration level of 70.8 items·m−3 in seawater sampled from the Roccal Sea Trench, located at depths of 2227 m off the Northeastern Atlantic coast. Overall, the widespread presence of MPs in various marine environments and sediment layers worldwide suggests that MP pollution cannot be ignored.
Characteristics of MPs Pollution in Soil
The pathways for MPs entering soil include degradation of agricultural plastic films, irrigation using industrial wastewater or sludge application along with organic fertilizers [63]. Once they enter soil environments, it becomes easier for MPs to be ingested by organisms, altering soil properties and affecting soil biological functions and diversity [64].
Agricultural Pollution
With the widespread adoption of modern agricultural practices, plastic products have become essential tools for planting, fertilization, and mulching. However, the degradation of plastics in agricultural soils increases the number of MPs in soils and even within crops themselves [65]. Among these sources, plastic films are the largest proportion and are widely used in cold, arid regions worldwide to maintain suitable temperatures for crop growth, resulting in increased yields [66]. Exposure to light or mechanical forces from tillage operations can cause these films to break down into MPs that enter agricultural soils, especially in regions where film recovery rates are low [67]. For example, Table 3 shows high levels of MPs in agricultural soils from Xinjiang (40.35 items·kg−1), Shanghai vegetable fields (78.00 ± 12.91 items·kg−1), and Wuhan (320–12560 items·kg−1) [68, 69]. In addition to plastic films, compost is also widely applied to improve soil fertility; however, high concentrations of MPs have been detected within compost as well.
Industrial Pollution
The textile industry is a major contributor to soil MPs. Microfibers, originating from textile outerwear and synthetic fur, were found to be present in soil [72]. Factors such as fabric structure, yarn type, twist level, and clothing cutting patterns also affect the release of microfibers from textiles [73]. Effective intervention measures at the source can help reduce the emission of MP fibers. Illegal dumping, plastic coating shedding, plastic sandblasting during industrial processes, and tire wear are other sources of MPs that can directly increase MP levels in soil [74]. MPs can be released into the environment from tire wear on cars during braking and acceleration periods, which cause mechanical abrasion [19]. Protective plastic coatings or paints used in shipbuilding undergo weathering, scraping, and polishing during use, leading to the production of MPs [75]. In summary, there are diverse industrial sources of MPs, and it is necessary to standardize production sources and control the use of MP fibers to reduce possible pathways for their entry into soils. It is believed that tire wear emissions account for about 67% of all MP emissions originating from tire wear, which ultimately settle in soils [75].
Characteristics of MPs Pollution in Urban Solid Waste
Landfills are a complex system that contains large amounts of plastic waste materials; however, there is limited research on associated MPs. Detection and characterization methods for landfill-associated MPs mainly focus on leachate samples or garbage piles within landfills due to a lack of standardized detection methods. He et al. [13] measured the MPs abundance was 0.42–24.58 MPs per liter in leachate from southern China . It was 0–4.51 MPs per liter in the leachates from Nordic countries and the mean quantities of MPs released to environment was 334.8 thousand per year [76]. For instance, Su et al. [77] collected garbage samples with different burial times as well as leachate samples from the Shanghai Laogang Landfill site and detected MPs in all samples. They used saturated NaCl solution as a density flotation agent, and H2O2 solution to improve the purification efficiency of the sample digestion time. The results showed that the abundance of MPs in leachate samples ranged from 400 to 1300 items·m−3 with an average size of 0.07–3.67 mm. In contrast, for garbage samples, MP abundance ranged from 20 to 91 items·g−1 with an average size of 0.23–4.97 mm. Given the limited research on landfill-associated MP pollution characteristics, further studies should be conducted to investigate their presence and control measures within landfills.
Yang et al. [78] found that the abundance of MPs in bottom ash from waste incineration was significantly higher than that in contaminated agricultural soil. And 593 items·kg−1 of MPs were found in 26 floodplain soil samples in Switzerland [79], which is of the same order of magnitude as bottom ash. Therefore, the bottom ash generated by MSW incinerators is a potential source of MPs. In addition, some studies have estimated the amount of MPs in the composting process: 12 million metric tons of biowaste translated into more than 5 million metric tons of compost in Germany in 2013, and 1 kg compost contains approximately 50% dry weight content conservatively, with 14–895 MP particles per kilogram dry weight. Each MT of biowaste will produce 2900 to 186,000 MPs [80, 81].
At present, there are fewer studies on the pollution characteristics of MPs in urban solid waste, and now there is still a lack of systematic analysis and summarization, which should be a key research area of concern in the future.
For instance, discharging of domestic and industrial wastewater can release MPs into the aquatic and terrestrial environment. Sludge from water treatment plants can transfer significant amounts of MPs to the soil for application as fertilizer. Surface run-off from contaminated soils and landfills results in the MPs input to freshwater and marine environments. Freshwaters like rivers and lakes can transport MPs by eventually arriving in the oceans. Still, the presence of MPs in different environments remains a complex global problem, and in order to deal with this issue, a better understanding will be essential regarding their sources, transport and dynamic way, as well as how they may affect each unique ecosystem.
Ecotoxicological Effects of MPs
The toxicity and harm of MPs to natural ecosystems and human health are closely related to their types, surface properties, and behavior in different environmental compartments. MPs can act as source pollutants that cause physical damage to organisms by ingestion or release of toxic chemicals. Additives used in MPs like plasticizers, antioxidants, phthalates, bisphenol A-based polycarbonates, polybrominated diphenyl ethers (PBDEs) , bisphenols, clarifiers, flame retardants, colorants [80, 82, 83]. Under different environmentally linked conditions in water or soil, oligopolymers, additives, chemical byproducts, and their intermediates may leach from plastics in diverse degradation processes [84]. Studies have found that plastic leachate impacts cellular immune response, chemicals in plastic can cause mitochondrial dysfunction to cause immune suppression [85]. The organisms attached to the surface of the plastic are more sensitive to the chemicals and additives on the surface of the plastic, and complex substances released from plastic leachate can therefore significantly affect the settling, attachment and growth of organisms in the environment [8]. Also, phthalates have been shown to act as endocrine disruptors, causing endocrine disruption, metabolic disorders [86] and PBDEs affect hormone levels in organisms [87]. As well as form complex pollutants with toxic contaminants that affect chemical toxicity, posing a threat to both biological organisms and humans.
MPs can entangle organisms, restrict their behavior, and cause physical damage by blocking intestines, affecting growth and development, and altering gene expression. Qiu et al. [88] found that nanopolystyrene at high concentrations reduced nematode lifespan, and PS accumulates in large quantities in the nematode gut, leading to alterations in the nematode’s innate immune response. This was confirmed by significant decreases in the intestinal immune response genes lys-1, lys-7, lys-8 and spp-1. Qu et al. [89] investigated the role of ERK MAPK signaling pathway modulation in neurons on nano-PS response. The results showed that nano PS significantly increased the expression of genes encoding the ERK MAPK signaling pathway (lin-45, mek-2, mpk-1) within a certain range. Li et al. [90] found that PS can disrupt polysaccharide synthesis and detoxification systems on the cell surface of Chlamydomonas reinhardtii and inhibited the expression of genes related to growth. Additionally, MPs can directly threaten human health through food chain transmission. In aquatic ecosystems, MPs can affect the behavior, growth, and development of a variety of organisms, from plankton to fish, and even cause death. Terrestrial organisms, such as insects and earthworms, can also be affected by MPs. In humans, ingestion of contaminated seafood or drinking water containing MPs can lead to various health risks, including reproductive disorders and inflammation. Fig. 2 illustrates the various toxic effects of MPs on living beings. Therefore, it is crucial to investigate the toxic effects of MPs on different organisms and develop measures to reduce their presence in the environment.
Ecotoxicological Studies of MPs on Aquatic Systems
The main focus of ecotoxicological studies on MPs in aquatic organisms is on micro and small-sized organisms. Currently, short-term exposure experiments are mainly used to evaluate the toxic effects of MPs on aquatic organisms, with indicators including behavior, feeding rate, growth status, oxidative stress, inflammatory response and mortality rate. These different effects are interrelated. Murphy et al. [91] investigated the impact of MPs ingestion by freshwater caddisfly larvae on feeding habits morphology reproduction, etc., finding that caddisfly larvae ingesting MPs experienced reduced reproductive capacity, delayed growth and even death. Kim et al. [92] found that the presence of MPs decreases nematode locomotor behavior and reproductive rate, affects metabolism, and induces oxidative stress. Besides, when mistaken for food after ingestion by an organism it creates a false sense of satiety thereby reducing food intake affecting its growth development ultimately leading to starvation causing death. Fiber and film-type MPs can easily entangle with the organism’s body, restrict its movement or even cause death; most MPs primarily exhibit physical damage by blocking the organism’s intestinal tract and reducing nutrient absorption which affects their growth and development leading to death [93]. Table 4 summarizes the toxic effects of MPs on aquatic organisms. Purple clams and herbivorous crabs can ingest polyethylene (<80 μm) and polystyrene microspheres (10 μm) through gill respiration which damages their intestines [94, 95]. Polystyrene can adsorb onto algae or plankton surfaces via electrostatic attraction that hinders sunlight and air flow affecting photosynthesis and respiration resulting in inhibited growth reproduction [96]. Experiments exposing freshwater animals such as Daphnia magna to polypropylene fibers or polyethylene particles show that MP ingestion impairs digestion function leading to reduced growth rates [97]. The experiment also found that fiber toxicity was greater than particle toxicity possibly due to longer residence time in the intestine [97]. In addition, accumulation of MP particles in zebrafish or nematodes causes intestinal damage including villi cracking and intestinal cell division [98]. The toxicity of MPs is closely related to their size, type, shape and exposure dose; most MPs are excreted with feces after ingestion. Shang et al. [99] also found that MPs with particle sizes of 1 μm and 5 μm reduced nematode lifespan, and 1 μm had a greater effect on lifespan at the same concentration. Lei et al. [100] found that common types of MPs particles such as PA, PE, PP and PS also all affect nematode survival at different concentrations. And in the same concentration series of 0.001–10.0 mg·L−1, 10 mg·L−1 PP resulted in a 27.1% reduction in zebrafish survival rate. Goldstein et al. [101] found that MPs accumulation in the gut was highest in goose-neck barnacles living on the water surface. When MP sizes decrease to the nanoscale level, their invasiveness and toxicity increase further. Kashiwada et al. [102] detected polystyrene in the brain testis liver and blood of adult bluegill indicating that nanoscale MPs can penetrate through the blood-brain barrier into brain tissue. The combined pollution caused by MPs and pollutants has become a key issue for studying its ecotoxicological effects. Studies have shown that short-term exposure to composite pollution caused by MPs and mercury affects swimming speed and retention time of European sea bass juveniles [103]. Adsorption of pyrene onto MPs causes greater pyrene metabolite accumulation changes in mortality rate, etc., among common prawn fish [104]. Research on freshwater animals shows that combined pollution from MPs and organic pollutants not only damages liver cells but also affects gene expression at a genetic level among bighead carp [102]. Combined pollution from MPs and phenanthrene may even affect protein synthesis among sharp-tooth catfish affecting normal life activities [105]. In addition, MP may release different additive chemicals acting as carriers for other harmful microorganisms making biological toxic effects more complex under composite stress from MP-pollutant combinations.
Ecotoxicological Studies of MPs on Terrestrial Systems
The large residues and accumulation of MPs in terrestrial ecosystems pose a threat to biodiversity, sustainability, climate change and food security. For example, due to the similarity of colored MPs to animal feed, they can be ingested by terrestrial animals such as chickens and thus have an effect on them [105]. Soil animals, such as earthworms, filter feeders, ciliates and nematodes, can crush fragile plastic debris and unconsciously ingest MPs, with deleterious effects on their intestines, neurons or kinases [108, 109]. Table 5 describes the toxic effects of MPs on terrestrial organisms. Several studies have shown that the intake of MPs reduces the growth rate of earthworms and leads to weight loss, decreases nematode survival and length, and results in reduced earthworm growth [110]. The reason may be due to the obstruction of the digestive tract by MPs in the intestine of the animal population, which reduces food intake and nutrient absorption, and even some sharp MPs cause damage to both the digestive tract and the skin area [100]. MPs have also been shown to be effective in enriching bacterial diversity and reducing the rate of colonization by harmful pathogenic microorganisms. Zhu et al. [111] reported that MPs increased the intestinal bacterial diversity of earthworms and decreased the reproduction rate of nematodes. In addition, MPs had no significant effect on the reproduction of epibiotic earthworms [112]. The reasons for these differences have not been confirmed, but it is thought to be related to the feeding selectivity of the animals and the nature and exposure concentration of the MPs [113]. Rodriguez-Seijo et al. [112] study also showed that polyethylene particles (250–1000 μm) had no effect on the survival, growth and reproduction of the Andersen earthworm, but caused histopathological damage and immune response, and that MPs were found in the intestine and midgut. In addition, Zhu et al. [111] found that polyvinyl chloride particles (80–250 μm) can affect the growth and reproduction of soil hoppers and intestinal flora, and that MPs and the toxic contaminants they carry may also cause changes in soil microorganisms and their ecological processes when they enter the soil. The toxic effects of MPs on terrestrial organisms are closely related to the type of organism, type of MPs and dose, therefore, the evaluation of the toxic effects of MPs on terrestrial organisms should be based on the actual situation Table 6.
MPs can act as a habitat for microorganisms in the soil and influence bacterial communities. These microbial communities, such as bacteria and fungi, can significantly contribute to the degradation process of plastics, releasing toxic chemicals and intermediates into the soil [123]. The additives in MPs can interfere with the nervous system, affect animal reproduction, cause genetic mutations, and even adversely affect human endocrine function and reproductive development. MPs tend to release polymer monomers during decomposition, which have a disruptive effect on the endocrine secretion of water fleas and reduce its reproduction rate [124]. In addition, when organisms ingest MPs carrying other toxic contaminants into the body, the contaminants interact with the additives, and the contaminants may affect the release of the additives, and the additives may have an effect on the accumulation of the contaminants in the organisms, or the toxic effects of both are in equilibrium, which should be the focus of future research.
Research on the Ecotoxicity of MPs to Human Ecology
Typically, MPs enter the body through three routes, namely ingestion (through food and water), inhalation (indoor and outdoor air) and dermal contact (through personal care products, dust and textiles), thereby affecting human health [125]. Differences in the type, content and size of plastic particles can lead to a variety of health problems, including oxidative stress, immune disease and cancer risk [126]. MPs have been found in different aquatic biota such as corals, phytoplankton and other marine animals. Exposure to MPs from toxic algae can produce algal toxins, which may have indirect negative effects on human health and the marine economy [127]. Due to their small size, MPs exist in pelagic and benthic ecosystems and are easily ingested by aquatic organisms, thus entering the marine food chain and eventually entering humans, causing harm to human health [128]. Meanwhile, several studies investigating drinking water around the world, including bottled water and tap water, have shown the presence of MPs in tap water [128, 129]. Bottled water, which is generally considered safe, has also been reported to contain contaminants in MPs [130, 131]. Samples from 11 popular mineral water brands in Iran were studied and MPs contaminants were detected in 9 samples with an average concentration of 8.5×103±10.2×103 items·m−3 [132]. MPs contaminate water sources, transporting MPs into the human food chain. Also, humans may accumulate MPs in their bodies through diet, such as consumption of fish and seafood contaminated with MPs [133]. Researchers at the American Chemical Society believe that plastic microbeads can trigger necrosis, inflammation and tissue laceration in human cells when fish that have ingested them are consumed by humans [134]. The potential threat of MPs-induced toxicity to human health is also a key area of research interest in the future.
Because of the ecotoxicity of MPs, in many countries government policies have been effective in reducing plastic consumption. For example, some countries have imposed bans, taxes or pricing on plastic carrier bags to encourage the public to use reusable bags and significantly reduce plastic consumption. In China, the use of plastic bags decreased by 49% following the introduction of a plastic bag ban [135]. Similarly, Denmark achieved a 66% reduction in plastic bag use after implementing a plastic bag tax [136], and Portugal saw a 74% reduction after introducing a plastic bag tax [137]. Through these measures, the use of plastic products has been greatly reduced, leading to the development of new biodegradable materials and promoting socio-economic development.
Conclusion and Outlook
This work summarizes the ecotoxic impacts of MPs on aquatic systems, terrestrial systems and humans based on the analysis of the pollution characteristics of MPs in environmental media. MPs in the environment can be transported within the environmental medium through external forces such as wind, rivers, and ocean currents, and in addition, organisms in the environment may ingest MPs and become carriers, producing a biomagnification effect through the transmission of the food chain, which will not only cause ecological pollution, but even threaten human health. The size, shape and composition, sorption properties and distribution of MPs throughout the environment depend on uncertainties such as the natural environment, human activities, etc., which leads to great challenges in the development of research methods and classification criteria related to MPs. The current research system for MPs is not sound, and there is a lack of holistic thinking about their pollution processes in the environment. The ecotoxic effects of MPs on aquatic systems, terrestrial systems and humans should be given sufficient attention. Regarding the investigation of the pollution characteristics and ecotoxic effects of MPs in environmental media, the following points need special attention in the future:
-
In-depth study of the final destination, effects and detailed toxicological mechanisms of MPs in the human body. The threat to food safety, food security and human health from contamination of food materials and additives by MPs is of particular concern. Therefore, it is important to understand the extent of environmental contamination by MPs and the details of the toxicological effects of MPs on humans.
-
Depending on the different properties of different MPs, the mechanisms of their toxic effects on organisms in the environment should be studied not only at the level of individual organisms, but also at the cellular and genetic levels of organisms. The effect of environmental factors on the toxicity of MPs should also be considered, such as temperature, pH, light intensity, etc.
-
For the pollution characteristics and ecotoxicity of MPs, source analysis of MPs in the environment should be carried out, and traceability techniques such as isotope tracing and fingerprinting should be applied to the source analysis study of MPs. Generation and use of MPs should be reduced at source. For example, relevant policies and regulations should be formulated and misuse of plastics should be banned, etc.
-
Furthermore, efforts should be made to improve the quality and efficiency of plastic alternatives, such as bioplastics, and to integrate MPs treatment technologies to enhance their removal efficiency and minimise negative impacts.
Data Availability
The authors confirm that data supporting the literature of this review article are available within the article.
References
Luo, H., Liu, C., He, D., Xu, J., Sun, J., Li, J., & Pan, X. (2022). Environmental behaviors of microplastics in aquatic systems: a systematic review on degradation, adsorption, toxicity and biofilm under aging conditions. Journal of Hazardous Materials, 423, 126915.
Guo, J. J., Huang, X. P., Xiang, L., Wang, Y. Z., Li, Y. W., Li, H., & Wong, M. H. (2020). Source, migration and toxicology of microplastics in soil. Environment international, 137, 105263.
Rochman, C. M. (2018). Microplastics research—from sink to source. Science, 360(6384), 28–29.
Fadare, O. O., & Okoffo, E. D. (2020). Covid-19 face masks: a potential source of microplastic fibers in the environment. The Science of the total environment, 737, 140279.
Ryberg, M. W., Hauschild, M. Z., Wang, F., Averous-Monnery, S., & Laurent, A. (2019). Global environmental losses of plastics across their value chains. Resources, Conservation and Recycling, 151, 104459.
Cox, K. D., Covernton, G. A., Davies, H. L., Dower, J. F., Juanes, F., & Dudas, S. E. (2019). Human consumption of microplastics. Environmental science & technology, 53(12), 7068–7074.
Pimentel, J. C., Avila, R., & Lourenco, A. G. (1975). Respiratory disease caused by synthetic fibres: a new occupational disease. Thorax, 30(2), 204–219.
Lu, L., Wan, Z., Luo, T., Fu, Z., & Jin, Y. (2018). Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Science of the total environment, 631, 449–458.
Zhang, Z., Deng, C., Dong, L., Liu, L., Li, H., Wu, J., & Ye, C. (2021). Microplastic pollution in the Yangtze River Basin: heterogeneity of abundances and characteristics in different environments. Environmental Pollution, 287, 117580.
Duan, J., Bolan, N., Li, Y., Ding, S., Atugoda, T., Vithanage, M., & Kirkham, M. B. (2021). Weathering of microplastics and interaction with other coexisting constituents in terrestrial and aquatic environments. Water Research, 196, 117011.
Tian, W., Song, P., Zhang, H., Duan, X., Wei, Y., Wang, H., & Wang, S. (2023). Microplastic materials in the environment: Problem and strategical solutions. Progress in Materials Science, 132, 101035.
Nizzetto, L., Futter, M., & Langaas, S. (2016). Are agricultural soils dumps for microplastics of urban origin?
He, P., Chen, L., Shao, L., Zhang, H., & Lü, F. (2019). Municipal solid waste (MSW) landfill: a source of microplastics? -Evidence of microplastics in landfill leachate. Water research, 159, 38–45.
Mintenig, S. M., Int-Veen, I., Löder, M. G., Primpke, S., & Gerdts, G. (2017). Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water research, 108, 365–372.
Fendall, L. S., & Sewell, M. A. (2009). Contributing to marine pollution by washing your face: microplastics in facial cleansers. Marine pollution bulletin, 58(8), 1225–1228.
Cheung, P. K., & Fok, L. (2017). Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water research, 122, 53–61.
Browne, M. A., Crump, P., Niven, S. J., Teuten, E., Tonkin, A., Galloway, T., & Thompson, R. (2011). Accumulation of microplastic on shorelines worldwide: sources and sinks. Environmental science & technology, 45(21), 9175–9179.
Cesa, F. S., Turra, A., & Baruque-Ramos, J. (2017). Synthetic fibers as microplastics in the marine environment: a review from textile perspective with a focus on domestic washings. Science of the total environment, 598, 1116–1129.
Enfrin, M., Dumée, L. F., & Lee, J. (2019). Nano/microplastics in water and wastewater treatment processes–origin, impact and potential solutions. Water research, 161, 621–638.
Tian, Y., Chen, Z., Zhang, J., Wang, Z., Zhu, Y., Wang, P., & Wang, L. (2021). An innovative evaluation method based on polymer mass detection to evaluate the contribution of microfibers from laundry process to municipal wastewater. Journal of Hazardous Materials, 407, 124861.
Napper, I. E., Bakir, A., Rowland, S. J., & Thompson, R. C. (2015). Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Marine pollution bulletin, 99(1-2), 178–185.
Lei, K., Qiao, F., Liu, Q., Wei, Z., Qi, H., Cui, S., & An, L. (2017). Microplastics releasing from personal care and cosmetic products in China. Marine pollution bulletin, 123(1-2), 122–126.
Cai, Y., Mitrano, D. M., Heuberger, M., Hufenus, R., & Nowack, B. (2020). The origin of microplastic fiber in polyester textiles: the textile production process matters. Journal of Cleaner Production, 267, 121970.
Zhou, H., Zhou, L., & Ma, K. (2020). Microfiber from textile dyeing and printing wastewater of a typical industrial park in China: occurrence, removal and release. Science of the Total Environment, 739, 140329.
Kole, P. J., Löhr, A. J., Van Belleghem, F. G., & Ragas, A. M. (2017). Wear and tear of tyres: a stealthy source of microplastics in the environment. International journal of environmental research and public health, 14(10), 1265.
Ziajahromi, S., Neale, P. A., Rintoul, L., & Leusch, F. D. (2017). Wastewater treatment plants as a pathway for microplastics: development of a new approach to sample wastewater-based microplastics. Water research, 112, 93–99.
Mahon, A. M., O’Connell, B., Healy, M. G., O’Connor, I., Officer, R., Nash, R., & Morrison, L. (2017). Microplastics in sewage sludge: effects of treatment. Environmental Science & Technology, 51(2), 810–818.
Long, Z., Pan, Z., Wang, W., Ren, J., Yu, X., Lin, L., & Jin, X. (2019). Microplastic abundance, characteristics, and removal in wastewater treatment plants in a coastal city of China. Water Research, 155, 255–265.
Murphy, F., Ewins, C., Carbonnier, F., & Quinn, B. (2016). Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environmental science & technology, 50(11), 5800–5808.
Akdemir, T., & Gedik, K. (2023). Microplastic emission trends in Turkish primary and secondary municipal wastewater treatment plant effluents discharged into the Sea of Marmara and Black Sea. Environmental Research, 231, 116188.
Tadsuwan, K., & Babel, S. (2022). Unraveling microplastics removal in wastewater treatment plant: a comparative study of two wastewater treatment plants in Thailand. Chemosphere, 307, 135733.
Park, H. J., Oh, M. J., Kim, P. G., Kim, G., Jeong, D. H., Ju, B. K., & Kwon, J. H. (2020). National reconnaissance survey of microplastics in municipal wastewater treatment plants in Korea. Environmental Science & Technology, 54(3), 1503–1512.
do Sul, J. A. I., & Costa, M. F. (2014). The present and future of microplastic pollution in the marine environment. Environmental pollution, 185, 352–364.
Lebreton, L. C., Van Der Zwet, J., Damsteeg, J. W., Slat, B., Andrady, A., & Reisser, J. (2017). River plastic emissions to the world’s oceans. Nature communications, 8(1), 15611.
Wright, S. L., & Kelly, F. J. (2017). Plastic and human health: a micro issue? Environmental science & technology, 51(12), 6634–6647.
Desforges, J. P. W., Galbraith, M., Dangerfield, N., & Ross, P. S. (2014). Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Marine pollution bulletin, 79(1-2), 94–99.
Pan, Z., Liu, Q., Sun, Y., Sun, X., & Lin, H. (2019). Environmental implications of microplastic pollution in the Northwestern Pacific Ocean. Marine pollution bulletin, 146, 215–224.
Qi, H., Fu, D., Wang, Z., Gao, M., & Peng, L. (2020). Microplastics occurrence and spatial distribution in seawater and sediment of Haikou Bay in the northern South China Sea. Estuarine, Coastal and Shelf Science, 239, 106757.
Akindele, E. O., & Alimba, C. G. (2021). Plastic pollution threat in Africa: current status and implications for aquatic ecosystem health. Environmental Science and Pollution Research, 28, 7636–7651.
Gambardella, C., Morgana, S., Ferrando, S., Bramini, M., Piazza, V., Costa, E., & Faimali, M. (2017). Effects of polystyrene microbeads in marine planktonic crustaceans. Ecotoxicology and environmental safety, 145, 250–257.
Ma, H., Pu, S., Liu, S., Bai, Y., Mandal, S., & Xing, B. (2020). Microplastics in aquatic environments: toxicity to trigger ecological consequences. Environmental Pollution, 261, 114089.
Cole, M., Lindeque, P., Halsband, C., & Galloway, T. S. (2011). Microplastics as contaminants in the marine environment: a review. Marine pollution bulletin, 62(12), 2588–2597.
Provencher, J. F., Bond, A. L., Avery-Gomm, S., Borrelle, S. B., Rebolledo, E. L. B., Hammer, S., & Van Franeker, J. A. (2017). Quantifying ingested debris in marine megafauna: a review and recommendations for standardization. Analytical Methods, 9(9), 1454–1469.
Ding, L., Zhang, S., Wang, X., Yang, X., Zhang, C., Qi, Y., & Guo, X. (2020). The occurrence and distribution characteristics of microplastics in the agricultural soils of Shaanxi Province, in north-western China. Science of the Total Environment, 720, 137525.
Huntington, A., Corcoran, P. L., Jantunen, L., Thaysen, C., Bernstein, S., Stern, G. A., & Rochman, C. M. (2020). A first assessment of microplastics and other anthropogenic particles in Hudson Bay and the surrounding eastern Canadian Arctic waters of Nunavut. Facets, 5(1), 432–454.
de Carvalho, D. G., & Neto, J. A. B. (2016). Microplastic pollution of the beaches of Guanabara Bay, Southeast Brazil. Ocean & coastal management, 128, 10–17.
Matsuguma, Y., Takada, H., Kumata, H., Kanke, H., Sakurai, S., Suzuki, T., & Newman, B. (2017). Microplastics in sediment cores from Asia and Africa as indicators of temporal trends in plastic pollution. Archives of environmental contamination and toxicology, 73, 230–239.
De-la-Torre, G. E., Dioses-Salinas, D. C., Castro, J. M., Antay, R., Fernández, N. Y., Espinoza-Morriberón, D., & Saldaña-Serrano, M. (2020). Abundance and distribution of microplastics on sandy beaches of Lima, Peru. Marine Pollution Bulletin, 151, 110877.
Zhang, H. (2017). Transport of microplastics in coastal seas. Estuarine Coastal and Shelf Science, 199, 74–86.
Song, Y. K., Hong, S. H., Jang, M., Kang, J. H., Kwon, O. Y., Han, G. M., & Shim, W. J. (2014). Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer. Environmental science & technology, 48(16), 9014–9021.
Antunes, J. C., Frias, J. G. L., Micaelo, A. C., & Sobral, P. (2013). Resin pellets from beaches of the Portuguese coast and adsorbed persistent organic pollutants. Estuarine, Coastal and Shelf Science, 130, 62–69.
Vibhatabandhu, P., & Srithongouthai, S. (2022). Abundance and characteristics of microplastics contaminating the surface water of the inner Gulf of Thailand. Water, Air, & Soil Pollution, 233(2), 50.
Zhao, S., Zhu, L., Wang, T., & Li, D. (2014). Suspended microplastics in the surface water of the Yangtze Estuary System, China: first observations on occurrence, distribution. Marine pollution bulletin, 86(1-2), 562–568.
Zhao, S., Zhu, L., & Li, D. (2015). Characterization of small plastic debris on tourism beaches around the South China Sea. Regional Studies in Marine Science, 1, 55–62.
Zhao, S., Zhu, L., & Li, D. (2015). Microplastic in three urban estuaries, China. Environmental pollution, 206, 597–604.
Yu, X., Peng, J., Wang, J., Wang, K., & Bao, S. (2016). Occurrence of microplastics in the beach sand of the Chinese inner sea: the Bohai Sea. Environmental pollution, 214, 722–730.
Kane, I. A., & Clare, M. A. (2019). Dispersion, accumulation, and the ultimate fate of microplastics in deep-marine environments: a review and future directions. Frontiers in earth science, 7, 80.
Courtene-Jones, W., Quinn, B., Gary, S. F., Mogg, A. O., & Narayanaswamy, B. E. (2017). Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the Rockall Trough, North Atlantic Ocean. Environmental pollution, 231, 271–280.
Van Cauwenberghe, L., Vanreusel, A., Mees, J., & Janssen, C. R. (2013). Microplastic pollution in deep-sea sediments. Environmental pollution, 182, 495–499.
Woodall, L. C., Sanchez-Vidal, A., Canals, M., Paterson, G. L., Coppock, R., Sleight, V., & Thompson, R. C. (2014). The deep sea is a major sink for microplastic debris. Royal Society open science, 1(4), 140317.
Fischer, V., Elsner, N. O., Brenke, N., Schwabe, E., & Brandt, A. (2015). Plastic pollution of the Kuril–Kamchatka Trench area (NW pacific). Deep Sea Research Part II: Topical Studies in Oceanography, 111, 399–405.
Lusher, A. L., Tirelli, V., O’Connor, I., & Officer, R. (2015). Microplastics in Arctic polar waters: the first reported values of particles in surface and sub-surface samples. Scientific reports, 5(1), 14947.
Rillig, M. C., Ingraffia, R., & de Souza Machado, A. A. (2017). Microplastic incorporation into soil in agroecosystems. Frontiers in plant science, 8, 1805.
Jâms, I. B., Windsor, F. M., Poudevigne-Durance, T., Ormerod, S. J., & Durance, I. (2020). Estimating the size distribution of plastics ingested by animals. Nature Communications, 11(1), 1594.
Zhang, B., Yang, X., Chen, L., Chao, J., Teng, J., & Wang, Q. (2020). Microplastics in soils: a review of possible sources, analytical methods and ecological impacts. Journal of Chemical Technology & Biotechnology, 95(8), 2052–2068.
Liu, Y., Shao, H., Liu, J., Cao, R., Shang, E., Liu, S., & Li, Y. (2021). Transport and transformation of microplastics and nanoplastics in the soil environment: a critical review. Soil use and management, 37(2), 224–242.
Steinmetz, Z., Wollmann, C., Schaefer, M., Buchmann, C., David, J., Tröger, J., & Schaumann, G. E. (2016). Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Science of the total environment, 550, 690–705.
Liu, M., Lu, S., Song, Y., Lei, L., Hu, J., Lv, W., & He, D. (2018). Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environmental pollution, 242, 855–862.
Zhou, B., Wang, J., Zhang, H., Shi, H., Fei, Y., Huang, S., & Barceló, D. (2020). Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: multiple sources other than plastic mulching film. Journal of Hazardous Materials, 388, 121814.
Nor, N. H. M., & Obbard, J. P. (2014). Microplastics in Singapore’s coastal mangrove ecosystems. Marine pollution bulletin, 79(1-2), 278–283.
Helcoski, R., Yonkos, L. T., Sanchez, A., & Baldwin, A. H. (2020). Wetland soil microplastics are negatively related to vegetation cover and stem density. Environmental Pollution, 256, 113391.
Belzagui, F., Crespi, M., Álvarez, A., Gutiérrez-Bouzán, C., & Vilaseca, M. (2019). Microplastics' emissions: microfibers’ detachment from textile garments. Environmental Pollution, 248, 1028–1035.
Zhang, L., Xie, Y., Liu, J., Zhong, S., Qian, Y., & Gao, P. (2020). An overlooked entry pathway of microplastics into agricultural soils from application of sludge-based fertilizers. Environmental Science & Technology, 54(7), 4248–4255.
Karbalaei, S., Hanachi, P., Walker, T. R., & Cole, M. (2018). Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environmental science and pollution research, 25, 36046–36063.
Sobhani, Z., Lei, Y., Tang, Y., Wu, L., Zhang, X., Naidu, R., & Fang, C. (2020). Microplastics generated when opening plastic packaging. Scientific reports, 10(1), 4841.
Piehl, S., Leibner, A., Löder, M. G., Dris, R., Bogner, C., & Laforsch, C. (2018). Identification and quantification of macro-and microplastics on an agricultural farmland. Scientific reports, 8(1), 17950.
Su, Y., Zhang, Z., Wu, D., Zhan, L., Shi, H., & Xie, B. (2019). Occurrence of microplastics in landfill systems and their fate with landfill age. Water research, 164, 114968.
Yang, Z., Lü, F., Zhang, H., Wang, W., Shao, L., Ye, J., & He, P. (2021). Is incineration the terminator of plastics and microplastics? Journal of Hazardous Materials, 401, 123429.
Scheurer, M., & Bigalke, M. (2018). Microplastics in Swiss floodplain soils. Environmental science & technology, 52(6), 3591–3598.
He, W., Song, P., Yu, B., Fang, Z., & Wang, H. (2020). Flame retardant polymeric nanocomposites through the combination of nanomaterials and conventional flame retardants. Progress in Materials Science, 114, 100687.
Weithmann, N., Möller, J. N., Löder, M. G., Piehl, S., Laforsch, C., & Freitag, R. (2018). Organic fertilizer as a vehicle for the entry of microplastic into the environment. Science advances, 4(4), eaap8060.
Brooke, R., Cottis, P., Talemi, P., Fabretto, M., Murphy, P., & Evans, D. (2017). Recent advances in the synthesis of conducting polymers from the vapour phase. Progress in Materials Science, 86, 127–146.
Xu, S., Shi, X. L., Dargusch, M., Di, C., Zou, J., & Chen, Z. G. (2021). Conducting polymer-based flexible thermoelectric materials and devices: from mechanisms to applications. Progress in Materials Science, 121, 100840.
Suhrhoff, T. J., & Scholz-Böttcher, B. M. (2016). Qualitative impact of salinity, UV radiation and turbulence on leaching of organic plastic additives from four common plastics—a lab experiment. Marine pollution bulletin, 102(1), 84–94.
Barrick, A., Champeau, O., Chatel, A., Manier, N., Northcott, G., & Tremblay, L. A. (2021). Plastic additives: challenges in ecotox hazard assessment. PeerJ, 9, e11300.
Giulivo, M., de Alda, M. L., Capri, E., & Barceló, D. (2016). Human exposure to endocrine disrupting compounds: their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environmental research, 151, 251–264.
Costa, L. G., & Giordano, G. (2011). Is decabromodiphenyl ether (BDE-209) a developmental neurotoxicant? Neurotoxicology, 32(1), 9–24.
Qiu, Y., Luo, L., Yang, Y., Kong, Y., Li, Y., & Wang, D. (2020). Potential toxicity of nanopolystyrene on lifespan and aging process of nematode Caenorhabditis elegans. Science of the Total Environment, 705, 135918.
Qu, M., Li, D., Qiu, Y., & Wang, D. (2020). Neuronal ERK MAPK signaling in response to low-dose nanopolystyrene exposure by suppressing insulin peptide expression in Caenorhabditis elegans. Science of the Total Environment, 724, 138378.
Li, S., Wang, P., Zhang, C., Zhou, X., Yin, Z., Hu, T., et al. (2020). Influence of polystyrene microplastics on the growth, photosynthetic efficiency and aggregation of freshwater microalgae Chlamydomonas reinhardtii. Science of the Total Environment, 714, 136767.
Murphy, F., & Quinn, B. (2018). The effects of microplastic on freshwater Hydra attenuata feeding, morphology & reproduction. Environmental pollution, 234, 487–494.
Kim, H. M., Lee, D. K., Long, N. P., Kwon, S. W., & Park, J. H. (2019). Uptake of nanopolystyrene particles induces distinct metabolic profiles and toxic effects in Caenorhabditis elegans. Environmental pollution, 246, 578–586.
Li, J., Liu, H., & Chen, J. P. (2018). Microplastics in freshwater systems: a review on occurrence, environmental effects, and methods for microplastics detection. Water research, 137, 362–374.
Von Moos, N., Burkhardt-Holm, P., & Köhler, A. (2012). Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environmental science & technology, 46(20), 11327–11335.
Watts, A. J., Lewis, C., Goodhead, R. M., Beckett, S. J., Moger, J., Tyler, C. R., & Galloway, T. S. (2014). Uptake and retention of microplastics by the shore crab Carcinus maenas. Environmental science & technology, 48(15), 8823–8830.
Nolte, T. M., Hartmann, N. B., Kleijn, J. M., Garnæs, J., Van De Meent, D., Hendriks, A. J., & Baun, A. (2017). The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness and cellular adsorption. Aquatic toxicology, 183, 11–20.
Au, S. Y., Bruce, T. F., Bridges, W. C., & Klaine, S. J. (2015). Responses of Hyalella azteca to acute and chronic microplastic exposures. Environmental toxicology and chemistry, 34(11), 2564–2572.
Lei, L., Liu, M., Song, Y., Lu, S., Hu, J., Cao, C., & He, D. (2018). Polystyrene (nano) microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects in Caenorhabditis elegans. Environmental Science: Nano, 5(8), 2009–2020.
Shang, X., Lu, J., Feng, C., Ying, Y., He, Y., Fang, S., & Ju, J. (2020). Microplastic (1 and 5 μm) exposure disturbs lifespan and intestine function in the nematode Caenorhabditis elegans. Science of the Total Environment, 705, 135837.
Lei, L., Wu, S., Lu, S., Liu, M., Song, Y., Fu, Z., & He, D. (2018). Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Science of the total environment, 619, 1–8.
Goldstein, M. C., Rosenberg, M., & Cheng, L. (2012). Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect. Biology letters, 8(5), 817–820.
Kashiwada, S. (2006). Distribution of nanoparticles in the see-through medaka (Oryzias latipes). Environmental health perspectives, 114(11), 1697–1702.
Barboza, L. G. A., Vieira, L. R., & Guilhermino, L. (2018). Single and combined effects of microplastics and mercury on juveniles of the European seabass (Dicentrarchus labrax): changes in behavioural responses and reduction of swimming velocity and resistance time. Environmental Pollution, 236, 1014–1019.
Oliveira, M., Ribeiro, A., Hylland, K., & Guilhermino, L. (2013). Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecological indicators, 34, 641–647.
Karami, A., Romano, N., Galloway, T., & Hamzah, H. (2016). Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environmental research, 151, 58–70.
Lee, K. W., Shim, W. J., Kwon, O. Y., & Kang, J. H. (2013). Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environmental science & technology, 47(19), 11278–11283.
Anbumani, S., & Kakkar, P. (2018). Ecotoxicological effects of microplastics on biota: a review. Environmental Science and Pollution Research, 25, 14373–14396.
Peng, J., Wang, J., & Cai, L. (2017). Current understanding of microplastics in the environment: occurrence, fate, risks, and what we should do. Integrated environmental assessment and management, 13(3), 476–482.
de Souza Machado, A. A., Kloas, W., Zarfl, C., Hempel, S., & Rillig, M. C. (2018). Microplastics as an emerging threat to terrestrial ecosystems. Global change biology, 24(4), 1405–1416.
Schöpfer, L., Menzel, R., Schnepf, U., Ruess, L., Marhan, S., Brümmer, F., & Kandeler, E. (2020). Microplastics effects on reproduction and body length of the soil-dwelling nematode Caenorhabditis elegans. Frontiers in Environmental Science, 8, 41.
Zhu, D., Chen, Q. L., An, X. L., Yang, X. R., Christie, P., Ke, X., & Zhu, Y. G. (2018). Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biology and Biochemistry, 116, 302–310.
Rodriguez-Seijo, A., Lourenço, J., Rocha-Santos, T. A. P., Da Costa, J., Duarte, A. C., Vala, H., & Pereira, R. (2017). Histopathological and molecular effects of microplastics in Eisenia andrei Bouché. Environmental Pollution, 220, 495–503.
Hodson, M. E., Duffus-Hodson, C. A., Clark, A., Prendergast-Miller, M. T., & Thorpe, K. L. (2017). Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environmental Science & Technology, 51(8), 4714–4721.
Browne, M. A., Niven, S. J., Galloway, T. S., Rowland, S. J., & Thompson, R. C. (2013). Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Current biology, 23(23), 2388–2392.
Song, Y., Qiu, R., Hu, J., Li, X., Zhang, X., Chen, Y., & He, D. (2020). Biodegradation and disintegration of expanded polystyrene by land snails Achatina fulica. Science of the Total Environment, 746, 141289.
Song, Y., Cao, C., Qiu, R., Hu, J., Liu, M., Lu, S., & He, D. (2019). Uptake and adverse effects of polyethylene terephthalate microplastics fibers on terrestrial snails (Achatina fulica) after soil exposure. Environmental Pollution, 250, 447–455.
Isobe, A., Uchida, K., Tokai, T., & Iwasaki, S. (2015). East Asian seas: a hot spot of pelagic microplastics. Marine pollution bulletin, 101(2), 618–623.
Tsang, Y. Y., Mak, C. W., Liebich, C., Lam, S. W., Sze, E. T., & Chan, K. M. (2017). Microplastic pollution in the marine waters and sediments of Hong Kong. Marine Pollution Bulletin, 115(1-2), 20–28.
Absher, T. M., Ferreira, S. L., Kern, Y., Ferreira, A. L., Christo, S. W., & Ando, R. A. (2019). Incidence and identification of microfibers in ocean waters in Admiralty Bay, Antarctica. Environmental Science and Pollution Research, 26, 292–298.
Cincinelli, A., Scopetani, C., Chelazzi, D., Lombardini, E., Martellini, T., Katsoyiannis, A., & Corsolini, S. (2017). Microplastic in the surface waters of the Ross Sea (Antarctica): occurrence, distribution and characterization by FTIR. Chemosphere, 175, 391–400.
Nel, H. A., & Froneman, P. W. (2015). A quantitative analysis of microplastic pollution along the south-eastern coastline of South Africa. Marine pollution bulletin, 101(1), 274–279.
Nel, H. A., Dalu, T., & Wasserman, R. J. (2018). Sinks and sources: assessing microplastic abundance in river sediment and deposit feeders in an Austral temperate urban river system. Science of the Total Environment, 612, 950–956.
De Tender, C. A., Devriese, L. I., Haegeman, A., Maes, S., Ruttink, T., & Dawyndt, P. (2015). Bacterial community profiling of plastic litter in the Belgian part of the North Sea. Environmental science & technology, 49(16), 9629–9638.
Iguchi, T., Watanabe, H., & Katsu, Y. (2006). Application of ecotoxicogenomics for studying endocrine disruption in vertebrates and invertebrates. Environmental health perspectives, 114(Suppl 1), 101–105.
Prata, J. C., da Costa, J. P., Lopes, I., Duarte, A. C., & Rocha-Santos, T. (2020). Environmental exposure to microplastics: an overview on possible human health effects. Science of the total environment, 702, 134455.
Sharma, S., & Chatterjee, S. (2017). Microplastic pollution, a threat to marine ecosystem and human health: a short review. Environmental Science and Pollution Research, 24, 21530–21547.
Thompson, R. C., Moore, C. J., Vom Saal, F. S., & Swan, S. H. (2009). Plastics, the environment and human health: current consensus and future trends. Philosophical transactions of the royal society B: biological sciences, 364(1526), 2153–2166.
Kosuth, M., Mason, S. A., & Wattenberg, E. V. (2018). Anthropogenic contamination of tap water, beer, and sea salt. PloS one, 13(4), e0194970.
Panno, S. V., Kelly, W. R., Scott, J., Zheng, W., McNeish, R. E., Holm, N., & Baranski, E. L. (2019). Microplastic contamination in karst groundwater systems. Groundwater, 57(2), 189–196.
Kankanige, D., & Babel, S. (2020). Smaller-sized micro-plastics (MPs) contamination in single-use PET-bottled water in Thailand. Science of the total environment, 717, 137232.
Oßmann, B. E., Sarau, G., Holtmannspötter, H., Pischetsrieder, M., Christiansen, S. H., & Dicke, W. (2018). Small-sized microplastics and pigmented particles in bottled mineral water. Water research, 141, 307–316.
Makhdoumi, P., Amin, A. A., Karimi, H., Pirsaheb, M., Kim, H., & Hossini, H. (2021). Occurrence of microplastic particles in the most popular Iranian bottled mineral water brands and an assessment of human exposure. Journal of water process engineering, 39, 101708.
Liboiron, M., Liboiron, F., Wells, E., Richárd, N., Zahara, A., Mather, C., & Murichi, J. (2016). Low plastic ingestion rate in Atlantic cod (Gadus morhua) from Newfoundland destined for human consumption collected through citizen science methods. Marine pollution bulletin, 113(1-2), 428–437.
Rochman, C. M., Kross, S. M., Armstrong, J. B., Bogan, M. T., Darling, E. S., Green, S. J., & Veríssimo, D. (2015). Scientific evidence supports a ban on microbeads. Environmental science & technology, 49(18), 10759–10761.
He, H. (2012). Effects of environmental policy on consumption: lessons from the Chinese plastic bag regulation. Environment and Development Economics, 17(4), 407–431.
Dikgang, J., Leiman, A., & Visser, M. (2012). Analysis of the plastic-bag levy in South Africa. Resources, Conservation and Recycling, 66, 59–65.
Martinho, G., Balaia, N., & Pires, A. (2017). The Portuguese plastic carrier bag tax: the effects on consumers’ behavior. Waste management, 61, 3–12.
Funding
This work was financially supported by the project of Taishan Scholar Engneering Program (NO. tsqn202306235), China Postdoctoral Science Foundation (No. 2023T160349 and No. 2019M660162), Shanghai Key Lab for Urban Ecological Processes and Eco-Restoration (SHUES2022A09), Shanghai Engineering Research Center of Biotransformation of Organic Solid Waste (SERC2020C05).
Author information
Authors and Affiliations
Contributions
Yuxin Wang: Writing—original draft. Zhou Fu: Writing—review & editing. Dezheng Guan: Methodology, Writing—review & editing. Jianwei Zhao: Project administration, Supervision, Funding acquisition. Qi Zhang: Methodology. Qingxin Liu and Liang Guo: Writing—review & editing. Jingliang Xie and Yingjie Sun: Project administration.
Corresponding authors
Ethics declarations
Ethics Approval
This is a review study.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
The authors provided informed consent for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Fu, Z., Guan, D. et al. Occurrence Characteristics and Ecotoxic Effects of Microplastics in Environmental Media: a Mini Review. Appl Biochem Biotechnol 196, 5484–5507 (2024). https://doi.org/10.1007/s12010-023-04832-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04832-z