Abstract
Microplastic pollution, or commonly known as “White Pollution,” has been drawing great interest as a new omnipresent environmental pollution. Plastic is potentially used in all kinds of industries, and their irresponsible waste disposal is the reason behind both aquatic and terrestrial pollution. The degradation period of plastic is a long-term process and occurs due to solar radiation, thermal oxidation, thermolysis, photo-oxidation, etc. The end result is tiny particles known as “microplastics” (diameter less than 5 mm) and “nanoplastics” (diameter less than 100 nm). MPs and NPs act as carriers for various chemical and biological toxins and are ingested by zooplankton, bivalves, echinoderms—these move through the food web and damage the internal digestive system of living organisms. To eliminate the ecological threats, a bioremediation system is urgently required. Microorganisms like Ideonella sakaiensis are known to have enzymes that degrade plastic and can be useful as remediators. This chapter discusses the sources and transports routes of microplastics in the aquatic environment and their ecotoxicological effects on living organisms. Moreover, it highlights the bioremediation of environmental microplastics, its mechanism, and some necessary environmental policies to prevent it.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Plastic (from Greek “Plastikos”) means malleable or moldable. It is considered an integral material at present due to its durability as a key attribute (Sharma and Chatterjee 2017). It is a versatile material due to its strength, low price, lightweight, and resistance to corrosion. They are known to have higher electrical and thermal insulation values. Currently, the most popular synthetic plastic materials are polypropylene (PP), high- and low-density polyethylene (PE), polystyrene (PS), polyvinyl chloride (PVC), and polyethylene terephthalate (PET)—all together, they contribute to 90% of total production (Do Sul and Costa 2014).
Since the middle of the nineteenth century, several tonnes of plastics have been manufactured. Fast-forwarding to this day, plastic has gained a prominent place in the consumer market and is a ubiquitous component of modern life. According to the current trends, 25 billion of global plastic production has been predicted by 2050 (Yang et al. 2015). Tons of discarded plastics reach the environment through recycling, landfilling, or incineration and accumulate in the ecosystem. Eventually, plastic loses its mechanical integrity through biotic and abiotic pathways, and “microplastic” (MP) is introduced into the environment (Xu et al. 2020).
Microplastic is a predominant pollutant in both terrestrial and aquatic environments. This contaminant ends up in the environment and suffers fragmentation and degradation. Microplastic (MP) is defined by size ranging from 100 nm to 5 mm, and the size of nanoplastic (NP) is below 100 nm (Tang et al. 2021). Evidence of microplastic ingestion by marine organisms and subsequent transfer through trophic levels has been reported (Setälä et al. 2016; Pirsaheb et al. 2020). Nanoplastics are more hazardous in comparison as they are permeable to biological membranes.
This chapter will summarize the multidisciplinary overview of microplastic pollution in the aquatic environment. Though available literature relevant to this topic is vast, we will focus on some particular aspects such as classification and sources of microplastic and its toxicity in the environment.
2 Classification of Microplastics
2.1 Based on Size
Although Thompson et al. (2004) were the first to report microplastics, they did not specify the size based on which microplastic could be identified. However, several authors have set limits to distinguish between microplastic and macroplastic. Macroplastics are discarded plastic chunks visible to the naked eye among other sand and gravel (Malankowska et al. 2021). This criterion is of importance, because it helps differentiate between small macroplastic (one that can be detected using simple techniques) and microplastics (due to their much smaller size, it requires detection through optical instruments) (Costa et al. 2010). Collection, estimation of prevalence in the environment, and characterization of microplastics have been challenging due to their minute dimensions.
According to IUPAC (International Union for Pure and Applied Chemistry), the dimension of microparticles ranges between 0.1 and 100 μm (Vert et al. 2012). Nevertheless, in recent years, researchers have agreed that plastic particles having the longest dimension below 5 mm will be considered microplastic. Subsequently, countries of the Asia-pacific region, the European Union, the European Chemical Industry, and the EPA (United Stated Environment Protection Agency) collectively identify this criterion to be specific for microplastic based on various scientific reports (Wright et al. 2013). Along with microplastics, which are extensively explored as ocean contaminants, Andrady (2011) introduced a new concept, namely “nanoplastic,” in 2011, which is identified as a product of the fragmentation of microplastic. After a few years, the upper limit for the size of nanoparticles was set to be 100 nm (Jambeck et al. 2015). This particular restriction is significant as plastic particles below this limit could have the potential to disrupt the cell membrane, which is not possible in the case of microplastics (Nguyen et al. 2019). With time and more investigation, many scientific communities have agreed on this size limit for nanoplastics. However, due to the scarcity of reliable literature, it is quite challenging to establish a perfect size limit and the hazardous effects of nanoplastics on living organisms.
2.2 Classification According to Origin
2.2.1 Primary Microplastics
Primary microplastics are defined as those which were originally fabricated as microplastics (Boucher and Friot 2017). These particles are added to various products such as air blasting technology, cosmetics, and scrubbers for facial cleansing (Derraik 2002). Other products like plastic capsules and vectors for medicines are primary microplastics themselves (Lindeque et al. 2020). Auta et al. (2017) gave other examples of products containing microplastic like cleanser, peelings, shower gels, eye shadow, foundation, mascara, blush powder, nail polish, hair colors, deodorants, shaving creams, bath lotions, baby products, sunscreen, mosquito repellants, etc. This depicts that personal hygiene and beauty-care industry, followed by the detergent industry, are the prominent manufacturers of products with added microplastic. According to a report, polyurethane is one of the common polymers that is manufactured as microplastic and it contributes to almost half of the total microliter coming from the cosmetic industry (Lei et al. 2017). These products are used by the population at large and lead to ultimate release into the environment. Other than this, microplastics are released into the environment during fabrication and maintenance of larger plastic products, that is, tires, synthetic textiles, paint, etc. (Shahnawaz et al. 2019).
2.2.2 Secondary Microplastics
Small plastic particles generated due to the fragmentation of discarded microplastic through biological, chemical, and mechanical processes are defined as secondary microplastics (Shahnawaz et al. 2019). The degradation process involved are biodegradation, thermo-oxidative degradation, photodegradation, mechanical weathering, etc. (Avio et al. 2017) (discussed in this chapter in another section). The breakdown of macroplastics increases their amount in the aquatic environment and becomes detrimental to aquatic organisms. Due to mass reduction in size, secondary microplastics get easily ingested in living organisms, making them susceptible to hazardous consequences (Law and Thompson 2014).
3 Distribution of Microplastic in Aquatic Environment
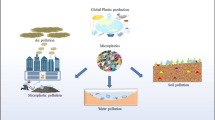
Microplastic pollution has heavily affected the aquatic environment. Reports of its presence in rivers, lakes, lagoons, coastal areas, and estuaries are available in the literature (Jiang et al. 2019; He et al. 2021; Sighicelli et al. 2018; Free et al. 2014; Quesadas-Rojas et al. 2021; Edo et al. 2020; Hantoro et al. 2019; Zhao et al. 2015). Microplastics are transported through the actions of rivers, wind, and ocean currents to beaches, shorelines, subsurface waters, and deep-sea sediments in remote locations (Eerkes-Medrano et al. 2015). The concentration of microplastics varies as per ocean currents distribution, depending on particle density and location of the sources. The consistent and buoyant nature of the particles helps them get dispersed widely through hydrodynamic processes (de Carvalho and Neto 2016). According to Cózar et al. (2014), the concentration was higher in the convergence zone of ocean surfaces. The sources and impacts of microplastics in aquatic environment are depicted in Fig. 13.1.
The abundance of microplastics in the aquatic ecosystem has been depicted through various surveys, including the polar region (Barnes et al. 2009), midocean islands (Martins et al. 2020), and deep-sea (Claessens et al. 2013). The omnipresent pollution by this contaminant in the Northeast Atlantic Ocean was demonstrated by Lusher et al. (2014). The average concentration of plastic was 2.46 particles m−3. According to Desforges et al. (2014), the subsurface water layers of the north-eastern Pacific Ocean contained microplastics with an average concentration of 4600 particle m−3. According to their study, off-shore Pacific waters had microplastics in density ranging from 8 to 9200 particles m−2, which was found to be increased by sixfold on West Coast Vancouver Island. The average abundance of the contaminating particles were in the range of 0–1.31 particles m−3 and 0–11.5 particles m−3 in the pelagic water layer of the Arctic Sea and south of Norway, respectively (Lusher et al. 2015a, b). The analysis of the particle composition depicted that they might be end- products from macroplastic degradation or wastewater effluent. Isobe et al. (2015) recorded a total particle density of about 1.72 million pieces km−2 in seawaters around Japan and East Asia (10 times and 27 times greater than the North Pacific and world oceans, respectively).
From the polluted subsurface waters, these particles accumulate in the sediments due to sinking. Various literatures support this theory and have reported high concentrations of plastic particles (in the range of 770–3300 plastic items kg−1) in the sediments of the Rhine estuary and Wadden Sea (Cole et al. 2011). Microplastics concentration was denser in beach sediments (340.7–4757 particles m−2) in comparison to the water column (204.5 and 1491.7 particles m−3) on the south-east coast of South Africa (Nel and Froneman 2015). Fauziah et al. (2015) conducted a study at the sand beaches in peninsular Malaysia to quantify microplastic debris and found 2542 pieces (265.30 g−2), which is an alarming number from a total of six beaches. Estuarine sediments from Vembanad lake of India (which is also a Ramsar site) were investigated to quantify microplastic particles by Sruthy and Ramasamy (2017). The range was 96–496 particles m−2 and the dominant polymer component for the pollution was low-density polyethylene.
In comparison to the marine environment, information regarding microplastic pollution in the freshwater environment is quite scarce. The source and transportation routes of this pollutant in this environment are diverse. Terrestrial, glacial, lacustrine, and marine environments are connected through the fluvial system, comprised of running water bodies. Rivers and streams play a highly dynamic role in accumulation and transportation of microplastics. They are connected with various pathways and release routes for the contaminant and are responsible for subsequent pollution. Jiang et al. (2019) investigated the pollution level in five rivers on the Tibet plateau. The surface water had a higher concentration of microplastics (483–967 items m−3) than the sediment samples (50–195 items kg−1). Similar reports have been found in Indian rivers like Nethravati and Alakananda stretch, where sediments had less concentration of microplastics (Amrutha and Warrier 2020; Chauhan et al. 2021). On the other hand, Li et al. (2020) have depicted that the flowing water in the river might have less concentration of microplastic than the sediments; furthermore, sediments act as a temporary storage space for the contaminants. For example, during a study in Pearl river, China, it was found that sediments contained a higher density of microplastics, that is, 685 items kg−1, in comparison to the column water (Fan et al. 2019). Hence, it is quite difficult to correlate the microplastic concentration in flowing water and static sediment. Lakes are another important component of the freshwater system. They receive microplastics through industrial effluent, surface runoffs, wastewater plants, etc., and rivers are the main carrier route of it. Lakes are complex systems that can potentially transport, disperse, or accumulate contaminants. Su et al. (2016) reported that the concentration of microplastics in Taihu lake, China, was 3.4–25.8 items L−1 and 11.0–234.6 items L−1 in surface water and sediments, respectively. Reports of heavily contaminated Lake Victoria, Africa, have been published (Egessa et al. 2020). The major pollutant resulted from microplastics’ fragmentation, and the prime contributor was polyethylene (60%). Several studies have depicted a higher level of pollution closer to the population centers and have pointed out surface currents as the reason behind the dispersion of microplastics in the surface waters (Eriksen et al. 2013; Fischer et al. 2016; Dusaucy et al. 2021).
4 Degradation of Plastic in Environment
One of the prime features responsible for the versatility of a few synthetic polymers is resistance to environmental processes. However, this particular fact is the reason behind the very slow degradation process and longer residual periods for plastics in the environment. Plastic has hundreds or even more years of longevity depending on its properties and surrounding environmental factors (Anderson et al. 2016). Degradation is a physical or chemical transformation resulting in reduced molecular weight of polymer under environmental factors such as heat, light, moisture, or biological activity (Shah et al. 2008). The physical integrity of a polymer is affected by its molecular weight, and significant degradation of a polymer can weaken the material due to bond scission. Extensive degradation of plastic can even fragment it into powder form upon handling. Even these fragments can undergo further degradations through biological processes and result in “complete mineralization” (Urbanek et al. 2018). The degradation process is categorized into abiotic and biotic processes based on the nature of the causative agents.
4.1 Abiotic Degradation of Plastic
Abiotic degradation refers to changes in physical or chemical functionality of plastic due to the action of abiotic parameters such as air, water, light, heat, and mechanical processes. Usually, abiotic degradation occurs prior to biodegradation due to the scarce bioavailability of macroplastics (Andrady 2011).
4.1.1 Photo-oxidation of Plastic
Photo-oxidation is defined as the process of degradation of polymer by the activity of light. It is one of the primary factors that cause damage to the polymer in ambient conditions. Many synthetic polymers are susceptible to decomposition initiated by visible light and UV rays. Mainly, UV-A rays having medium energy (wavelength: 315–400 nm) and UV-B rays having high energy (wavelength: 290–315 nm) determine the lifespan of plastic in the outdoor environment (Jensen and Kops 1980). Most synthetic polymers are prone to absorption of high-energy radiation coming from the UV section of the spectrum, leading to activation of electrons and resulting in cleavage, oxidation, and decomposition. Degradation of microplastic occurs in the ether portions of soft fragments, and products with ester, formate, aldehyde, and propyl groups are formed (Singh and Sharma 2008). Photodegradation can alter plastic’s physical, optical, and visual (yellowing) properties (Martin et al. 2003).
Ultraviolet radiation can successfully cause the cleavage of C–C bonds. The most damaging wavelength of UV radiation for a particular polymer depends upon its bond structure, and therefore, the value varies for different polymers. The required wavelengths for degrading polyethylene (PE) and polypropylene (PP) are 300 nm and 370 nm, respectively (Fernando et al. 2007). PE and PP absorb radiation, generating free radicals, which form hydroperoxides, the formation of which leads to the breakage of double bonds present in the backbone chain and the production of smaller degradation end-products (Yang et al. 2018). Phenyl rings present in polystyrene (PS) get excited upon absorption of UV radiation and form a triple-state, making it susceptible to decomposition. Alternating subunits of terephthalate and ethylene glycolate present in polyethylene terephthalate (PET) are linked through ester bonds. Upon photo-oxidation, the ester bonds cleave to form carbon dioxide, carbon monoxide, terephthalic acids, carboxylic acids, anhydrides, and esters (Fairbrother et al. 2019).
Decomposition initiated by UV irradiation is more effective in plastic exposed to air or the surface of a beach in comparison to plastic floating in the water. The reason behind the retarded degradation of microplastic in aquatic environment is the reduced temperature and oxygen concentration. Furthermore, the process is hindered due to fouling effects. Floating plastics develop surface fouling quickly; layers consist of biofilms of debris, algal mat, and colonies of invertebrates, respectively (Muthukumar et al. 2011).
4.1.2 Thermal Degradation of Macroplastics
Thermal degradation is the breakdown process of macroplastics by the action of energy that is generated from elevated temperatures. At high temperatures, plastic can undergo thermo-oxidative degradation. Long and complex polymer chains break when polymers absorb sufficient energy to surmount the energy barrier, generating reactive free radicals (Pirsaheb et al. 2020). They react with available oxygen and subsequently, hydroperoxides are formed, which in turn produce alkoxy radicals and hydroxyl free radicals in a process quite similar to the photodegradation of macroplastics. It is a self-propagating reaction that keeps on repeating unless the source of energy is cut off or inert end-products are produced by the collision of radicals. A vital difference between the photodegradation and thermo-degradation of plastics lies in the steps leading to the auto-oxidation cycle; another difference is that the former process occurs only on the surface of the polymer, whereas the latter process occurs throughout its structure (Tyler 2004).
The precise temperature required for this process varies among different polymers and is affected by the thermal characteristics (melting point and glass transition temperature) of plastic and the availability of oxygen in the environment (Crawford and Quinn 2016). The occurrence of exothermic oxidation in the environment is unlikely as the key requirement is high temperature. However, slowly progressing thermal oxidation of synthetic polymers may occur along with photodegradation in areas that are directly exposed to the sunlight. Kamweru et al. (2011) stated that temperature and ultraviolet radiation act synergistically on plastic decomposition. In another study, Kotoyori (1972) found that increasing humidity lowered the activation energy bar for the thermal decomposition of plastics.
4.1.3 Chemical Degradation of Macroplastics
Pollutants present in the atmosphere, such as ozone (O3), nitrogen dioxide (NO2), sulfur dioxide (SO2), and volatile organic compounds (VOCs), either directly cleave the chemical bonds within the structure of polymers or act as catalyzing agents in the formation of radicals by photochemical processes leading to degradation (Cooper and Corcoran 2010).
Ozone (O3) is produced from oxygen (O2) under the activity of ultraviolet rays and lightning. Its concentration is low in the atmosphere compared to the ground, where its concentration increases due to pollution by SO2, NO2, and VOCs (Placet et al. 2000). Even at lower concentrations, O3 actively attacks the unsaturated double bonds present in the polymer structure, causing bond scission in the chain. In contrast to unsaturated polymers, O3 reacts quite slowly with saturated polymers (Mohan et al. 2019). Sulfur dioxide (SO2) can be excited upon absorbing UV irradiation, producing a singlet or triplet state that either readily reacts with unsaturated C–C bonds or produces O3 from oxygen (O2) through photochemical reactions. Nitrogen dioxide (NO2) is also very reactive due to the presence of odd electrons in the molecular structure; hence, it reacts with unsaturated C–C double bonds in the polymer chain. Similar to SO2, NO2 is capable of producing O3 from O2 during the photochemical reaction (Min et al. 2020).
pH and salinity are the two most influential chemical parameters affecting the decomposition of macroplastics in the aquatic environment. A denser concentration of H+ (responsible for lower pH) or OH− (responsible for higher pH) can catalyze the hydrolysis of some particular synthetic polymers such as polyamides (PAs) (Wadsö and Karlsson 2013). These two parameters can also alter the surface properties of other macro- and microplastics and regulate their behavior in an aquatic environment and toward other pollutants present in water (Liu et al. 2019).
4.1.4 Mechanical Degradation of Macroplastics
The decomposition of plastics due to the activity of external forces is known as mechanical degradation. Examples of external forces are abrasion and collision of plastics with rocks or sands due to the activity of winds or waves. Freezing-thawing cycle of plastics in water can lead to mechanical degradation (Pastorelli et al. 2014). The mechanical properties of plastic determine the effect of outdoor forces. Among them, an important feature is “elongation at break,” which is defined by the ability of plastic to resist changes of shape without any tear or formation of crack (Krasilnikov et al. 2005). Plastics with lower elongation values at break are more prone to fragment under external tensile forces. Constant application of stress on plastics ultimately results in the breakage of chains in polymers (Sugimoto et al. 2020).
This kind of degradation is important for synthetic polymers. One of the major contributors of microplastics has been domestic washing due to the abrasion, shear, and stress on fibers during laundry (Cesa et al. 2020). Another noticeable source is abrasion of tire against road, which leads to scratching or microcutting of the tire and creating microplastics (Corcoran 2021).
Bond scissions and breakage of chains during decomposition by light, temperature, and chemical components affect the mechanical characteristics of macroplastics and precisely their tensile strength (Andrady 2017). O’Brine and Thompson (2010) found that environmental degradation processes reduced elongation values at the break of plastics, which subsequently decreased the need for external forces to fragment the plastics and enable the mechanical decomposition of macroplastics.
4.2 Biotic Degradation of Macroplastics
Biodegradation is a biochemical process that transforms compounds through complete mineralization by microbes. The end-products of the process are water and carbon dioxide under aerobic conditions and methane and carbon dioxide under anaerobic conditions. Microorganisms such as bacteria, fungi, insects, etc., are the prime agents for the biological decomposition of plastics (Crawford and Quinn 2016). Abiotic degradation processes, producing low- molecular- weight compounds, aid in eventual biotic degradation, as macromolecules could not be taken up and metabolized directly by microbes (Chen et al. 2019). Metabolites produced in this process are nontoxic to the ecosystem and are redistributed via nitrogen, carbon, and sulfur cycles (Cau et al. 2020). Though the source of biological degradation is microorganisms, nature is chemical; these chemicals are enzymes aiding in catalysis. The success of microbial action on polymers depends upon the availability of enzymes, the existence of a substrate site in the polymer for the enzymes to act upon, the specificity of the enzyme, and the availability of coenzyme if required (Reich and Stivala 1971).
Macroplastics are categorized into hydrolyzable and nonhydrolyzable based on the absence or presence of amide and ester functional groups, which can be attacked by extracellular hydrolases. Polymers like PE, PP, and PVC are identified as non-hydrolyzable, and the action of extracellular enzymes on them is complex. Santo et al. (2013) found the enzyme laccase released by actinomycete Rhodococcus ruber to be able to degrade PE. Hydroquinone peroxidase secreted by Azotobacter beijerinckii could degrade PS (Nakamiya et al. 1997). Hydrolyzable polymers like PET, PA, and polyurethane (PUR) are susceptible to biodegradation as extracellular hydrolases can act upon them (Chen et al. 2019). According to De Sá et al. (2018), PETase found in Ideonella sakiensis could hydrolyze PET in the environment. Enzymes like cutinase, serine esterase, lipase, and nitro-benzyl esterase are capable of hydrolyzing PET; on the other hand, cutinase, hydrolase, and amidase are involved in PA hydrolysis (Guebitz and Cavaco-Paulo 2008).
Oxidative and hydrolytic decomposition of plastics by different extracellular enzymes leads to chain breakage, generating polymers and fragments of short-chains (e.g., monomers, dimers, and oligomers). When the molecular weight of these fragments is small enough, they are taken up by microorganisms, assimilated, and subjected to subsequent intracellular metabolism (Wilkes and Aristilde 2017). Eventually, after both extracellular and intracellular pathways, mineralization results in the formation of carbon dioxide and water under aerobic conditions and in the formation of carbon dioxide, methane, water, organic acids, and ammonia under anaerobic conditions. Nonetheless, anaerobic biodegradation requires more time for complete mineralization than aerobic biodegradation (Gu 2003).
5 Sources of Microplastics in Aquatic Ecosystem and Transport Route
“Freshwater” indicates streams, rivers, lakes, and ponds, all of which have distinct features. The transport and retention of microplastics in the freshwater environment is a complex system. The freshwater environment acts as a conduit for microplastics entering into the system from the terrestrial region, acts as a hotspot for the production of microplastics through the degradation of macroplastics, and also acts as a basin retaining the plastic particles in sediments (Horton and Dixon 2018).
Disposal of waste in an inadequate manner leads to the release of macroplastics into the freshwater environment. The sources are general littering, wastes from landfills, or transportation from land via surface runoff. Apart from macroplastics, microplastics are significantly released into freshwater systems directly. Runoff and drainage from agricultural soils render ponds, lakes, and rivers contaminated with agricultural plastics, fibers, and microbeads (Steinmetz et al. 2016). Untreated and unfiltered urban runoff and storm drainage, containing road paint, particles of tires (generated from the collision between roads and tires), etc., pollutes the freshwater bodies (Treilles et al. 2021). Although wastewater treatment plants are used to remove microplastics, direct input of effluent-containing plastics into the water bodies can cause contamination (Murphy et al. 2016). During high flow, combined sewage overflows (CSOs) channel the untreated wastewater into nearby rivers to reduce the overpressure on drainage systems, inducing microplastic pollution in rivers. Studies suggest that due to the drainage systems, water bodies act as more of a hotspot for pollution than the surrounding urban areas; thus, storm drainage, surface runoffs, inputs from CSOs, etc. should be controlled, and proper care has to be taken for their disposal (Horton et al. 2017). Along with rivers, other freshwater bodies like lakes and ponds receive wind-blown debris and land runoffs as inputs, and it gets accumulated over time due to their standing nature leading to burial and preservation within the sediments for a long time (Vaughan et al. 2017).
After entering the rivers, the particle of plastics is subjected to the same transportation system as the other sediments like sand and silts for mobilization. The speed of the water flowing in the river gives it energy, leading to the transportation of a greater portion of the particles (Knighton 2014). In the sections of the rivers where the energy drops and the flowing speed reduces, it is more likely for the plastic particles to settle down into the sediments. Additionally, the sedimentation helps in the burial of microplastics (Corcoran et al. 2015). Thus, the sediments would retain the microplastics throughout their movement through the freshwater systems (Nizzetto et al. 2016).
For all the microplastic wastes released from the freshwater and terrestrial environment (horizontal transportation), the oceans represent an ultimate sink (Lechner et al. 2014). After it reaches the oceans, they are dispersed widely and rapidly. Additionally, microplastics move through a vertical transport system via biofouling, incorporation in the marine snow, and excretion through fecal pellets (Rummel et al. 2017). This is considered vertical transportation. Apart from receiving inputs from rivers, oceans are contaminated with plastic by mismanaged fishing, which involves accidental cargo loss, illegal dumping, abandoned fishing nets, sinking of crafts, etc. (Xue et al. 2020). The major pollutant in these cases would be macroplastics, which will transform to microplastics and accumulate in the sediments over the years.
6 Ecotoxicological Impacts of Microplastics on Aquatic Ecosystem
Although various attempts have been made to evaluate the toxicity of microplastics to aquatic creatures, the impacts and mechanisms involved are still unknown (Desforges et al. 2014). Microplastics’ potential toxicity can be attributed to three different mechanisms: (1) ingestion stress, such as physical blockage and expenditure of energy required for egestion, (2) additive leakage from plastic, such as plasticizers, and (3) exposure to toxicants involved with microplastics (persistent organic pollutants/POPs) (Cole et al. 2011; Andrady 2011). The consequences of microplastic exposure would be expected to differ depending on particle accumulation and translocation inside tissues, the organism’s ability to ingest the particles, and the possibility of trophic transmission.
6.1 Impact on Aquatic Organisms
Phytoplanktons
Any negative impact on the primary producers can eventually jeopardize a specific ecosystem’s whole food web and food chain. It has been reported that exposure of phytoplanktons to microplastics can result in a stunted growth rate (Besseling et al. 2014). The negative impact of microplastics on algal development appeared to diminish as particle size rose (Zhang et al. 2017a, b). Physical interaction was found to be the cause of the impact of nanoplastic beads on two algae species, Scenedesmus spp. and Chlorella spp. When Chlorella vulgaris, Dunaliella tertiolecta, and Thalassiosira pseudonana were exposed to polystyrene particles with sizes ranging from 0.05 to 6 m for 72 h, no changes in algal growth were observed, but photosynthesis was reduced by 2.5– 45%. Contrastingly, Besseling et al. (2013) found that in the Scenedesmus obliquus, nanosized polystyrene particle (0.22 and 103 mg/l) exposure influenced algal development and reduced chlorophyll concentration, resulting in lower photosynthesis.
The effects of MPs have been documented in the marine ecosystem, with most scientific data indicating a possible effect of MPs at the producer level. After 96 h, exposure to polyvinyl chloride (PVC) MP (1 m size) lowered the growth rate by 39.7%, but 1 mm PVC had no harmful effect on Skeletonema costatum (Zhang et al. 2017a, b). In contrast, when Tetraselmis chuii was exposed to fluorescent red polyethylene microspheres (1–5 m) in the presence and absence of copper, no significant growth rate suppression was detected, suggesting that particle size was inversely proportional to MP toxicity (Davarpanah and Guilhermino 2015).
Zooplanktons
Microplastics have been detected in rotifers, copepods, and cladocerans, which come in contact with microplastics through primary surface adherence and feeding habit (Jeong et al. 2016). Ingestion of 1 μm PE microplastics resulted in the immobility of the limnic Daphnia magna as concentration and time of exposure increased, according to Rehse et al. (2016), whereas the 100 μm that were not swallowed by Daphnia magna did not produce the physical impacts. Calanus helgolandicus copepods swallowed 11% fewer algae when exposed to 20 μm PS microbeads and cultivated algae, resulting in lower ingested carbon biomass and decreased fecundity (Cole et al. 2011). Differing sizes of microplastics can have significant size-related impacts on zooplankton, such as lower eating capacity, decreased fecundity and growth rate, increased mortality, lengthy reproduction time, and could even affect the next generation (Zheng et al. 2020). Smaller plastic particles, such as nanoplastics, are more toxic and damaging to zooplankton (Sun et al. 2017). Furthermore, the capacity of microplastics to be excreted may be directly proportional to their particle size. In brief, consumption of microplastics by zooplanktons revealed that primary consumers could directly interact with microplastics in the ecosystem.
Invertebrates
Aquatic invertebrates feed directly on primary producers and serve as a valuable food source for the carnivores, which are significant ecological players. Aquatic invertebrates are more prone to microplastic pollution due to their feeding habits and position in the food chain as primary predators. Several reports have identified arthropods (De Sá et al. 2018; Arias-Andres et al. 2019), mollusks (Abidli et al. 2019; Teng et al. 2019) and worms (Li et al. 2019; Lv et al. 2019) as potent species for accumulation and transfer of microplastics into the next trophic level. Because of the abundance and toxicity of microplastics in bivalves like clams and mussels, the species has been considered a useful bioindicator for aquatic microplastic contamination (Ward et al. 2019). Furthermore, different aquatic invertebrate species have varied life properties, which has an impact on microplastic uptake models and dispersion in invertebrates. For example, microplastic uptake into the nonfilter-feeder marine shore crab might be facilitated by respiratory exposure (Watts et al. 2014). Furthermore, Kolandhasamy et al. (2018) discovered that adherence of microplastics to soft tissues of mussels causes microplastic accumulation and subsequent ingestion. Various negative impacts of ingested microplastic particles on the growth, feeding, development, survival, and reproduction of the invertebrates have been documented (Huvet et al. 2016; Foley et al. 2018; Trestrail et al. 2020).
Fish
Microplastics can be ingested by fish either directly from the aquatic ecosystem or indirectly from their prey. Fish features (e.g., species, life phases, feeding behavior, and living habitat) influence microplastic intake the most, followed by exposure conditions, plastic qualities (e.g., kind, size, shape, color) and aging of microplastic biofilms (Neves et al. 2015; Ory et al. 2018; Collard et al. 2019). Lusher et al. (2013) investigated 504 fish from ten both pelagic and demersal species caught in the English Channel and discovered plastic waste (0.13–14.3 mm) in 36.5% of the fish digestive tracts, 92.4% of which were microplastics. As per a recent global analysis, 427 different fish species are present in all freshwater, brackishwater, and marine environments, while different food chain positions (i.e., herbivore, algivore, omnivore, carnivore, and detritivore) could ingest microplastics (Lima et al. 2021). Three distinct benthic fishes, that is, Cleisthenes herzensteini, Liparis tanakae, and Lophius litulon collected from 14 different spots of the South Yellow Sea, were found with microplastic concentrations of 19.2, 27.5, and 5.9 particles g−1, respectively (Wang et al. 2020). According to this finding, it can be concluded that microplastics had significantly contaminated the surface sediments and benthic species. Microplastics can interact with fish in various ways, including direct feeding, transfer in levels of the food chain, respiratory exposure and absorption through the skin, but their distribution in fish is difficult to predict. Hotspots for primary plastic accumulation are gastrointestinal tracts and gills (Barboza et al. 2020; Jaafar et al. 2020; Koongolla et al. 2020); especially nanoplastics are transported to various tissues and organs via complex mechanisms due to their smaller size and membrane permeability (Jacob et al. 2020; Guerrera et al. 2021; Ma et al. 2021). Microplastics in fish can affect the histological function and produce gastrointestinal obstruction, leading to reduced feeding. The very fine-sized microplastic may be absorbed through the intestinal lining as it passes through the gastrointestinal tract and eventually enters the bloodstream, translocates to other organs, and threatens survival (Barría et al. 2020). Other severe impacts are dysfunctionality of gills, disruption in neuromuscular functions, and rendering the fish vulnerable to plastic toxicity (Chen et al. 2021).
6.2 Toxicity from Contaminants Associated with Plastic
Microplastics’ enormous surface-to-volume ratio and hydrophobicity allow them to accumulate harmful chemicals in water (e.g., heavy metals and persistent organic pollutants) at concentrations far greater than in ambient water (Mato et al. 2001; Holmes et al. 2012). Furthermore, several additives, such as bisphenol A, alkylphenols, phthalates, and polybrominated diphenyl ethers, are commonly used in the manufacture of plastics to improve the performance of the final product (Barnes et al. 2009). These plastic additives may have hazardous effects on the aquatic biota after they have leached out. Colonization of the plastic by potentially hazardous microbes could endanger the aquatic food web (Zettler et al. 2013). Despite the fact that microplastics are biochemically inert, the leaching of plastic additives and the buildup of other toxicants and pathogenic bacteria turn them into a complex cocktail of dangerous compounds (Hossain et al. 2019). The ingestion of contaminated microplastics by aquatic creatures provides a viable route for these dangerous compounds to enter the aquatic food web. Microplastics, in combination with noxious chemicals, have the potential to cause neurotoxicity (Avio et al. 2015), organ disease (Bhatt et al. 2021; Du et al. 2021), metabolic disorders (Ye et al. 2021), and mortality in aquatic biota (Phothakwanpracha et al. 2021). However, whether ingesting microplastics promotes the transmission of toxicants to aquatic organisms is still debatable, especially when compared to other exposure paths.
7 Bioremediation Aspects of Microplastics
Biodegradation is the breakdown of organic substances by living organisms, and this process is referred to as environmental remediation or bioremediation when it occurs in conjunction with the ecosystem and waste management (Masiá et al. 2020). The type of polymer, its characteristics, the type of organisms used, and the pretreatment form are the prime influencers of the biodegradation process. In the degradation process, the molecular weight of the polymer and the additives coated with the polymer play a major role (Artham and Doble 2008). The rate of degradation is reciprocally propositional to the molecular weight of the polymer. The degradation rate for some plastics, such as polyhydroxyalkanoates (PHA), polyhydroxybutyrate (PHB) and polylactic acid (PLA), is higher in comparison to synthetic polymers, such as polyethylene (PE), polycaprolactone (PCL), and polystyrene (PS) (Sivan 2011).
Biodeterioration, biofragmentation, assimilation, and mineralization are the four phases in the biodegradation process. The biodeterioration process begins with creating a biofilm surrounding the plastic polymer, signaling the start of the degradation process. Microbes manufacture the extracellular enzyme in the second stage, which acts on the polymer, converting it into an oligomer, dimer, or monomer, and preparing it for easy ingestion. In the third step, the oligomer/dimer/monomer assemblage on the surface of microorganisms is absorbed by microbial cells by simple diffusion or enhanced diffusion. The formation of daughter metabolites such as CO2, H2O, and CH4 is the final stage (Lugauskas et al. 2003; Tokiwa et al. 2009). Each microorganism has the ability to destroy microplastics through the techniques described above. However, each microbe has the ability to release a unique enzyme for microplastic breakdown. It differs for microorganisms depending on their environment. Tanasupawat et al. (2016) reported the isolation of Ideonella sakaiensis bacteria from a PET-polluted environment. Palm et al. (2019) have revealed that this bacterium secretes the two enzymes responsible for PET breakdown: PETase and MHETase. Some bacteria can multiply on the flat substrate of plastic items, using them as a carbon source and forming a biofilm around them (Zettler et al. 2013). These bacteria use specific enzymes to break down the synthetic polymer into monomers. Skariyachan et al. (2021) recently reported the use of a bacterial consortium (Pseudomonas and Enterococcus sp.) to decompose microfragments of LDPE and PP.
Alcaligenes faecalis, Pseudomonas stutzeri, and Streptomyces sp.—all have polyhydroxyalkanoate (PHA) depolymerase (Jendrossek and Handrick 2002; Kadouri et al. 2005). Basidiomycetes, Deuteromycetes (Penicillium and Aspergillus), and Ascomycetes are the most common PHA-degrading fungus isolated from soil and marine habitats (Egbeocha et al. 2018). Polycaprolactone (PCL) is synthetic polyester that is quickly destroyed by the bacteria Alcaligenes faecalis and Clostridium botulinum and the fungus Fusarium (Grigore 2017; Egbeocha et al. 2018; Li 2018). Polylactic acid (PLA) is a polymer commonly used in biodegradable plastics; it has been shown to be degraded by a thermophilic bacteria (Bacillus brevis) (Duis and Coors 2016), as well as by only two Fusarium moniliforme fungus strains and Penicillium roqueforti (Egbeocha et al. 2018).
Other than microbes, the potential of various plants and animals is being researched for bioremediation of microplastics. Higher eukaryotes such as vertebrates, cephalopods, and decapods, which have been reported to be vulnerable under stress, should not be used in bioremediation. Second, microplastics should be captured, retained, and filtered/ingested at higher rates, as should their digestion/elimination; they should not be released into the environment. Species should only be used within their indigenous range, as geographical migrations must be avoided for the sake of biodiversity conservation (Molnar et al. 2008). Species with a wider distribution and easier control and management are considered potential candidates.
Microplastic retention appears to be a possibility for filter-feeding organisms. Mytilus mussels are pollutant-retention organisms that help bioremediation in natural habitats (Broszeit et al. 2016). Microfragments of plastics can be kept in their circulatory system for 48 days (Browne et al. 2008); nevertheless, most microplastic fibers are expelled after 24 h, compromising their elimination efficiency (Chae and An 2020). Adherence to the coral surface appears to be an effective strategy for MPs retention in other filter-feeders such as cnidarians (ingestion rates were 0.251–14.810 particles/h, whereas surface adhesion was 40 times larger; Martin et al. 2019). The sandworm Arenicola marina has a lifetime retention rate of 240–700 MPs (1.2 ± 2.8 particles/g), which appears to have no effect on its metabolism (Bansal et al. 2021); it could be a candidate for environmental remediation in oceanic and brackish waters, because it can tolerate salinities as low as 12 ppt. The echinoderm sea cucumber (recommended for pollution monitoring) is another intriguing organism that may be appropriate for eliminating PCB-contaminated plastic, since it preferentially ingests microplastics over other sediment particles (Alava 2019).
Higher plants have the greatest advantage over animals in that there is no sign of suffering. The bioremediation capability of algae, specifically microalgae, has been investigated in water. Roccuzzo et al. (2021) depicted that unicellular microalgae may digest endocrine-disrupting substances in wastewaters when used alone or in combination with bacteria. Seagrasses, which can grow in marine and brackish habitats, are of interest in cleaning effluents near the sea. The smooth ribbon seagrass Cymodocea rotundata was proposed by Huang et al. (2021a, b) for bioremediation of textile dye wastewater. Microplastics can be retained in a variety of ways in seagrasses and higher plants, with the particles collecting on the blades along with their associated bacteria. Plastic particles are found in epibiont communities on the blades in the seaweed Thalassia testudinum (Goss et al. 2018), but synthetic microfibers have been identified adhering to blades without epibiont communities in the seaweed Fucus vesiculosus (Goss et al. 2018; Gutow et al. 2016). Ali et al. (2020) recommended many freshwater Magnoliophyta (having heavy metal removing capability) for removing microplastics from WWTPs, including water hyacinth (Eichhornia crassipes), water lettuce (Pistia stratiotes), and duckweed (Lemna minor). Nano- and microplastics do not appear to constitute an ecological concern to aquatic macrophytes in environmentally realistic quantities. Some macrophytes, including Egeria densa, and their microbiomes have been shown to collect and convert gold nanoparticles (Avellan et al. 2018); these systems could be studied for MPs bioremediation.
8 Development of Regulations and Policies
Plastic production has been increasing exponentially for decades, and it appears that the number of microplastic particles will continue to rise in the coming years. The original sources and classifications of plastics and microplastics entering the marine environment must be recognized to reduce the introduction of microplastics into the aquatic ecosystem. Also, raising public awareness about microplastics through education in the public, private, and government sectors will go a long way. Raw water, groundwater, bottled drinking water, and food items have all been discovered to contain microplastics (Koelmans et al. 2019; Rainieri and Barranco 2019; Jadhav et al. 2021). However, no microplastic contamination criteria have been established, and no parameters have been designed to assess the microplastic limit in drinking water. As a result, a number of organizations, government agencies, institutes, and authorities working on new pollutants must concentrate on determining microplastic limits in various resources and their potential repercussions. Microfibers and microbeads are predominantly secondary microplastics produced by washing garments; as a result, policy implications must be established to filter and catch these microplastics before they contaminate the natural ecosystem (McDevitt et al. 2017). Aside from that, corrective methods to limit and control plastic and microplastic debris with public participation must be implemented. To reduce microplastic and plastic pollution in the natural environment, comprehensive and effective measures must be implemented.
Concerns about microplastics have prompted numerous groups to propose management standards. The United Nations Environmental Programme’s (UNEP) Expert Panel has called for prompt action to clear the oceans of microplastics, citing that microplastics are swallowed by a huge number of marine organisms, causing them physical and chemical harm (Wu et al. 2017). Similarly, the UNEP-MAP (United Nations Environment Program/Mediterranean Action Plan), OSPAR (the Oslo/Paris Convention—for the Protection of the Marine Environment of the North-East Atlantic), and HELCOM (Baltic Marine Environment Protection Commission—Helsinki Commission) have developed guidelines for assessing marine litter, including microplastics (Karbalaei et al. 2018). Nongovernmental organizations (NGOs) have also presented initiatives to raise awareness and assist in quantifying the extent of MPs pollution and its consequences on a national and international scale.
Many regions have established or implemented regulations prohibiting the manufacture and use of primary MPs, such as microbeads, which could minimize MPs entering the aquatic system (CEPA (Canadian Environmental Protection Act) 2016; Beat the Microbead 2016; United Kingdom Department for Environment, Food and Rural Affairs 2016) (Wu et al. 2017), as well as restrictions on the use of single-use macroplastics (i.e., bottles, carrier bags). The Netherlands was the first country to declare its intention to create microbead-free cosmetics, with a 2016 deadline (Ogunola et al. 2018). According to Kamat and Kamat, China has outlawed non-biodegradable bags and single-use straws in all cities. Korea, meanwhile, prohibited microplastic-based cosmetics in 2021. Some nongovernmental organizations (NGOs) and businesses identified microplastic-free products and promoted the certificates “Good Scrub Guid” (Flora and Fauna) and “Zero plastic within” (product label) (Jeyavani et al. 2021). Bio-based plastics have recently been employed by China and American (USA) customers in place of plastic products. According to Kamat and Kamat, the Indian government announced in 2018 that single-use plastics would be phased out by 2020. One-time used plastic bags, cutlery, and some PET bottles were all banned in India in June 2019. The airport administration certified 55 of the country’s 134 airports to be plastic-free. The government of Himachal Pradesh has outlawed the disposal of plastic products (plastic cups and plates) since 2017, resulting in a significant reduction in plastic pollution (Kamat and Kamat 2021).
9 Conclusions and Future Perspectives
Because of their longevity and slow rate of deterioration, plastic materials survive in the environment. It has been changed into microplastics due to natural forces, which easily enter all ecosystems and, as a result, into living beings, where it accumulates, magnifies, and causes damaging consequences. Microplastics are easily swallowed by marine organisms due to their small size and have been reported to concentrate in tissues, the circulatory system, and the brain. This chapter highlights these important aspects of microplastics in an effort to promote coherent literature and future research among scientists interested in this field. Microplastics cannot be reduced without the general population’s participation, the socioeconomic sectors, tourism, and waste management companies.
Microplastics are predicted to have a greater negative impact, because most countries lack sewage treatment infrastructure and have yet to minimize, repurpose, and recycle plastic products. Appropriate technological solutions must be implemented, and knowledge of waste segregation at the source and the need for plastic recycling must be raised. Strategies to limit the input of plastic into the biosphere should be included in the management protocol. Specific approaches should be used to treat primary and secondary microplastics and keep them out of the ecosystem. Furthermore, there are little research on the prevalence, fate, toxicology, and reduction of MPs in India, necessitating the scientific community’s attention for further investigation.
Flaws in waste management must be addressed in order to improve current procedures and reduce the risks connected with them. Furthermore, several bacteria are being tested with properties that could degrade microplastics of aquatic origin. These microorganisms might potentially be used to clean up contaminated settings. The use of microbes for microplastic degradation is a potential and environment-friendly action plan that will allow for the management of microplastics without negative consequences and eventually support the natural clean-up of contaminated areas.
References
Abidli S, Lahbib Y, El Menif NT (2019) Microplastics in commercial molluscs from the lagoon of Bizerte (Northern Tunisia). Mar Pollut Bull 142:243–252. https://doi.org/10.1016/j.marpolbul.2019.03.048
Alava JJ (2019) Ocean pollution and warming oceans: toward ocean solutions and natural marine bioremediation. In: Predicting future oceans. Elsevier, Amsterdam, pp 495–518. https://doi.org/10.1016/B978-0-12-817945-1.00046-0
Ali S, Abbas Z, Rizwan M, Zaheer IE, Yavaş İ, Ünay A, Abdel-Daim MM, Bin-Jumah M, Hasanuzzaman M, Kalderis D (2020) Application of floating aquatic plants in phytoremediation of heavy metals polluted water: a review. Sustain For 12(5):1927. https://doi.org/10.3390/su12051927
Amrutha K, Warrier AK (2020) The first report on the source-to-sink characterization of microplastic pollution from a riverine environment in tropical India. Sci Total Environ 739:140377. https://doi.org/10.1016/j.scitotenv.2020.140377
Anderson JC, Park BJ, Palace VP (2016) Microplastics in aquatic environments: implications for Canadian ecosystems. Environ Pollut 218:269–280. https://doi.org/10.1016/j.envpol.2016.06.074
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62(8):1596–1605. https://doi.org/10.1016/j.marpolbul.2011.05.030
Andrady AL (2017) The plastic in microplastics: a review. Mar Pollut Bull 119(1):12–22. https://doi.org/10.1016/j.marpolbul.2017.01.082
Arias-Andres M, Rojas-Jimenez K, Grossart HP (2019) Collateral effects of microplastic pollution on aquatic microorganisms: an ecological perspective. Trends Anal Chem 112:234–240. https://doi.org/10.1016/j.trac.2018.11.041
Artham T, Doble M (2008) Biodegradation of aliphatic and aromatic polycarbonates. Macromol Biosci 8(1):14–24. https://doi.org/10.1002/mabi.200700106
Auta HS, Emenike CU, Fauziah SH (2017) Distribution and importance of microplastics in the marine environment: a review of the sources, fate, effects, and potential solutions. Environ Int 102:165–176. https://doi.org/10.1016/j.envint.2017.02.013
Avellan A, Simonin M, McGivney E, Bossa N, Spielman-Sun E, Rocca JD, Bernhardt ES, Geitner NK, Unrine JM, Wiesner MR, Lowry GV (2018) Gold nanoparticle biodissolution by a freshwater macrophyte and its associated microbiome. Nat Nanotechnol 13(11):1072–1077. https://doi.org/10.1038/s41565-018-0231-y
Avio CG, Gorbi S, Milan M, Benedetti M, Fattorini D, d’Errico G, Pauletto M, Bargelloni L, Regoli F (2015) Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ Pollut 198:211–222. https://doi.org/10.1016/j.envpol.2014.12.021
Avio CG, Gorbi S, Regoli F (2017) Plastics and microplastics in the oceans: from emerging pollutants to emerged threat. Mar Environ Res 128:2–11. https://doi.org/10.1016/j.marenvres.2016.05.012
Bansal M, Santhiya D, Sharma JG (2021) Behavioural mechanisms of microplastic pollutants in marine ecosystem: challenges and remediation measurements. Water Air Soil Pollut 232(9):1–22. https://doi.org/10.1007/s11270-021-05301-1
Barboza LGA, Lopes C, Oliveira P, Bessa F, Otero V, Henriques B, Raimundo J, Caetano M, Vale C, Guilhermino L (2020) Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci Total Environ 717:134625. https://doi.org/10.1016/j.scitotenv.2019.134625
Barnes DK, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc B Biol Sci 364(1526):1985–1998. https://doi.org/10.1098/rstb.2008.0205
Barría C, Brandts I, Tort L, Oliveira M, Teles M (2020) Effect of nanoplastics on fish health and performance: a review. Mar Pollut Bull 151:110791. https://doi.org/10.1016/j.marpolbul.2019.110791
Besseling E, Wegner A, Foekema EM, Van Den Heuvel-Greve MJ, Koelmans AA (2013) Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ Sci Technol 47(1):593–600. https://doi.org/10.1021/es302763x
Besseling E, Wang B, Lürling M, Koelmans AA (2014) Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol 48(20):12336–12343. https://doi.org/10.1021/es503001d
Bhatt P, Pathak VM, Bagheri AR, Bilal M (2021) Microplastic contaminants in the aqueous environment, fate, toxicity consequences, and remediation strategies. Environ Res 200:111762. https://doi.org/10.1016/j.envres.2021.111762
Boucher J, Friot D (2017) Primary microplastics in the oceans: a global evaluation of sources, vol 43. IUCN, Gland
Broszeit S, Hattam C, Beaumont N (2016) Bioremediation of waste under ocean acidification: reviewing the role of Mytilus edulis. Mar Pollut Bull 103(1-2):5–14. https://doi.org/10.1016/j.marpolbul.2015.12.040
Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC (2008) Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ Sci Technol 42(13):5026–5031. https://doi.org/10.1016/j.marpolbul.2015.12.040
Cau A, Avio CG, Dessì C, Moccia D, Pusceddu A, Regoli F, Cannas R, Follesa MC (2020) Benthic crustacean digestion can modulate the environmental fate of microplastics in the deep sea. Environ Sci Technol 54(8):4886–4892. https://doi.org/10.1021/acs.est.9b07705
Cesa FS, Turra A, Checon HH, Leonardi B, Baruque-Ramos J (2020) Laundering and textile parameters influence fibers release in household washings. Environ Pollut 257:113553. https://doi.org/10.1016/j.envpol.2019.113553
Chae Y, An YJ (2020) Effects of food presence on microplastic ingestion and egestion in Mytilus galloprovincialis. Chemosphere 240:124855. https://doi.org/10.1016/j.chemosphere.2019.124855
Chauhan JS, Semwal D, Nainwal M, Badola N, Thapliyal P (2021) Investigation of microplastic pollution in river Alaknanda stretch of Uttarakhand. Environ Dev Sustain 23(11):16819–16833. https://doi.org/10.1007/s10668-021-01388-y
Chen X, Xiong X, Jiang X, Shi H, Wu C (2019) Sinking of floating plastic debris caused by biofilm development in a freshwater lake. Chemosphere 222:856–864. https://doi.org/10.1016/j.chemosphere.2019.02.015
Chen G, Li Y, Wang J (2021) Occurrence and ecological impact of microplastics in aquaculture ecosystems. Chemosphere 274:129989. https://doi.org/10.1016/j.chemosphere.2021.129989
Claessens M, Van Cauwenberghe L, Vandegehuchte MB, Janssen CR (2013) New techniques for the detection of microplastics in sediments and field collected organisms. Mar Pollut Bull 70(1-2):227–233. https://doi.org/10.1016/j.marpolbul.2013.03.009
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62(12):2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
Collard F, Gasperi J, Gabrielsen GW, Tassin B (2019) Plastic particle ingestion by wild freshwater fish: a critical review. Environ Sci Technol 53(22):12974–12988. https://doi.org/10.1021/acs.est.9b03083
Cooper DA, Corcoran PL (2010) Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar Pollut Bull 60(5):650–654. https://doi.org/10.1016/j.marpolbul.2009.12.026
Corcoran PL (2021) Degradation of microplastics in the environment. In: Rocha-Santos T, Costa M, Mouneyrac C (eds) Handbook of microplastics in the environment. Springer, Cham, pp 1–12. https://doi.org/10.1007/978-3-030-10618-8_10-1
Corcoran PL, Norris T, Ceccanese T, Walzak MJ, Helm PA, Marvin CH (2015) Hidden plastics of Lake Ontario, Canada and their potential preservation in the sediment record. Environ Pollut 204:17–25. https://doi.org/10.1016/j.envpol.2015.04.009
Costa MF, Ivar Sul JA, Silva-Cavalcanti JS, Araújo MCB, Spengler Â, Tourinho PS (2010) On the importance of size of plastic fragments and pellets on the strandline: a snapshot of a Brazilian beach. Environ Monit Assess 168(1):299–304. https://doi.org/10.1007/s10661-009-1113-4
Cózar A, Echevarría F, González-Gordillo JI, Irigoien X, Úbeda B, Hernández-León S, Palma ÁT, Navarro S, García-de-Lomas J, Ruiz A, Fernández-de-Puelles ML, Duarte CM (2014) Plastic debris in the open ocean. Proc Natl Acad Sci 111(28):10239–10244. https://doi.org/10.1073/pnas.1314705111
Crawford CB, Quinn B (2016) Microplastic pollutants. Elsevier, Amsterdam
Davarpanah E, Guilhermino L (2015) Single and combined effects of microplastics and copper on the population growth of the marine microalgae Tetraselmis chuii. Estuar Coast Shelf Sci 167:269–275. https://doi.org/10.1016/j.ecss.2015.07.023
de Carvalho DG, Neto JAB (2016) Microplastic pollution of the beaches of Guanabara Bay, Southeast Brazil. Ocean Coast Manag 128:10–17. https://doi.org/10.1016/j.ocecoaman.2016.04.009
De Sá LC, Oliveira M, Ribeiro F, Rocha TL, Futter MN (2018) Studies of the effects of microplastics on aquatic organisms: what do we know and where should we focus our efforts in the future? Sci Total Environ 645:1029–1039. https://doi.org/10.1016/j.scitotenv.2018.07.207
Derraik JG (2002) The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull 44(9):842–852. https://doi.org/10.1016/S0025-326X(02)00220-5
Desforges JPW, Galbraith M, Dangerfield N, Ross PS (2014) Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar Pollut Bull 79(1-2):94–99. https://doi.org/10.1016/j.marpolbul.2013.12.035
Do Sul JAI, Costa MF (2014) The present and future of microplastic pollution in the marine environment. Environ Pollut 185:352–364. https://doi.org/10.1016/j.envpol.2013.10.036
Du J, Zhou Q, Li H, Xu S, Wang C, Fu L, Tang J (2021) Environmental distribution, transport and ecotoxicity of microplastics: A review. J Appl Toxicol 41(1):52–64. https://doi.org/10.1002/jat.4034
Duis K, Coors A (2016) Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ Sci Eur 28(1):1–25. https://doi.org/10.1186/s12302-015-0069-y
Dusaucy J, Gateuille D, Perrette Y, Naffrechoux E (2021) Microplastic pollution of worldwide lakes. Environ Pollut 284:117075. https://doi.org/10.1016/j.envpol.2021.117075
Edo C, González-Pleiter M, Tamayo-Belda M, Ortega-Ojeda FE, Leganés F, Fernández-Piñas F, Rosal R (2020) Microplastics in sediments of artificially recharged lagoons: case study in a Biosphere Reserve. Sci Total Environ 729:138824. https://doi.org/10.1016/j.scitotenv.2020.138824
Eerkes-Medrano D, Thompson RC, Aldridge DC (2015) Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res 75:63–82. https://doi.org/10.1016/j.watres.2015.02.012
Egbeocha CO, Malek S, Emenike CU, Milow P (2018) Feasting on microplastics: ingestion by and effects on marine organisms. Aquat Biol 27:93–106. https://doi.org/10.3354/ab00701
Egessa R, Nankabirwa A, Ocaya H, Pabire WG (2020) Microplastic pollution in surface water of Lake Victoria. Sci Total Environ 741:140201. https://doi.org/10.1016/j.scitotenv.2020.140201
Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, Farley H, Amato S (2013) Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar Pollut Bull 77(1-2):177–182. https://doi.org/10.1016/j.marpolbul.2013.10.007
Fairbrother A, Hsueh HC, Kim JH, Jacobs D, Perry L, Goodwin D, White C, Watson S, Sung LP (2019) Temperature and light intensity effects on photodegradation of high-density polyethylene. Polym Degrad Stab 165:153–160. https://doi.org/10.1016/j.polymdegradstab.2019.05.002
Fan Y, Zheng K, Zhu Z, Chen G, Peng X (2019) Distribution, sedimentary record, and persistence of microplastics in the Pearl River catchment, China. Environ Pollut 251:862–870. https://doi.org/10.1016/j.envpol.2019.05.056
Fauziah SH, Liyana IA, Agamuthu P (2015) Plastic debris in the coastal environment: the invincible threat? Abundance of buried plastic debris on Malaysian beaches. Waste Manag Res 33(9):812–821. https://doi.org/10.1177/0734242X15588587
Fernando SS, Christensen PA, Egerton TA, White JR (2007) Carbon dioxide evolution and carbonyl group development during photodegradation of polyethylene and polypropylene. Polym Degrad Stab 92(12):2163–2172. https://doi.org/10.1016/j.polymdegradstab.2007.01.032
Fischer EK, Paglialonga L, Czech E, Tamminga M (2016) Microplastic pollution in lakes and lake shoreline sediments–a case study on Lake Bolsena and Lake Chiusi (central Italy). Environ Pollut 213:648–657. https://doi.org/10.1016/j.envpol.2016.03.012
Foley CJ, Feiner ZS, Malinich TD, Höök TO (2018) A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Sci Total Environ 631:550–559. https://doi.org/10.1016/j.scitotenv.2018.03.046
Free CM, Jensen OP, Mason SA, Eriksen M, Williamson NJ, Boldgiv B (2014) High-levels of microplastic pollution in a large, remote, mountain lake. Mar Pollut Bull 85(1):156–163. https://doi.org/10.1016/j.marpolbul.2014.06.001
Goss H, Jaskiel J, Rotjan R (2018) Thalassia testudinum as a potential vector for incorporating microplastics into benthic marine food webs. Mar Pollut Bull 135:1085–1089. https://doi.org/10.1016/j.marpolbul.2018.08.024
Grigore ME (2017) Methods of recycling, properties and applications of recycled thermoplastic polymers. Recycling 2(4):24. https://doi.org/10.3390/recycling2040024
Gu JD (2003) Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int Biodeterior Biodegradation 52(2):69–91. https://doi.org/10.1016/S0964-8305(02)00177-4
Guebitz GM, Cavaco-Paulo A (2008) Enzymes go big: surface hydrolysis and functionalisation of synthetic polymers. Trends Biotechnol 26(1):32–38. https://doi.org/10.1016/j.tibtech.2007.10.003
Guerrera MC, Aragona M, Porcino C, Fazio F, Laurà R, Levanti M, Montalbano G, Germanà G, Abbate F, Germanà A (2021) Micro and nano plastics distribution in fish as model organisms: histopathology, blood response and bioaccumulation in different organs. Appl Sci 11(13):5768. https://doi.org/10.3390/app11135768
Gutow L, Eckerlebe A, Giménez L, Saborowski R (2016) Experimental evaluation of seaweeds as a vector for microplastics into marine food webs. Environ Sci Technol 50(2):915–923. https://doi.org/10.1021/acs.est.5b02431
Hantoro I, Löhr AJ, Van Belleghem FG, Widianarko B, Ragas AM (2019) Microplastics in coastal areas and seafood: implications for food safety. Food Addit Contam Part A 36(5):74–711. https://doi.org/10.1080/19440049.2019.1585581
He B, Smith M, Egodawatta P, Ayoko GA, Rintoul L, Goonetilleke A (2021) Dispersal and transport of microplastics in river sediments. Environ Pollut 279:116884. https://doi.org/10.1016/j.envpol.2021.116884
Holmes LA, Turner A, Thompson RC (2012) Adsorption of trace metals to plastic resin pellets in the marine environment. Environ Pollut 160:42–48. https://doi.org/10.1016/j.envpol.2011.08.052
Horton AA, Dixon SJ (2018) Microplastics: an introduction to environmental transport processes. Wiley Interdiscip Rev Water 5(2):e1268. https://doi.org/10.1002/wat2.1268
Horton AA, Svendsen C, Williams RJ, Spurgeon DJ, Lahive E (2017) Large microplastic particles in sediments of tributaries of the River Thames, UK–Abundance, sources and methods for effective quantification. Mar Pollut Bull 114(1):218–226. https://doi.org/10.1016/j.marpolbul.2016.09.004
Hossain MR, Jiang M, Wei Q, Leff LG (2019) Microplastic surface properties affect bacterial colonization in freshwater. J Basic Microbiol 59(1):54–61. https://doi.org/10.1002/jobm.201800174
Huang W, Song B, Liang J, Niu Q, Zeng G, Shen M, Deng J, Luo Y, Wen X, Zhang Y (2021a) Microplastics and associated contaminants in the aquatic environment: a review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J Hazard Mater 405:124187. https://doi.org/10.1016/j.jhazmat.2020.124187
Huang Y, Xiao X, Effiong K, Xu C, Su Z, Hu J, Jiao S, Holmer M (2021b) New insights into the microplastic enrichment in the blue carbon ecosystem: evidence from seagrass meadows and mangrove forests in coastal South China Sea. Environ Sci Technol 55(8):4804–4812. https://doi.org/10.1021/acs.est.0c07289
Huvet A, Paul-Pont I, Fabioux C, Lambert C, Suquet M, Thomas Y, Robbens J, Soudant P, Sussarellu R (2016) Reply to Lenz et al.: quantifying the smallest microplastics is the challenge for a comprehensive view of their environmental impacts. Proc Natl Acad Sci 113(29):E4123–E4124. https://doi.org/10.1073/pnas.1607221113
Isobe A, Uchida K, Tokai T, Iwasaki S (2015) East Asian seas: a hot spot of pelagic microplastics. Mar Pollut Bull 101(2):618–623. https://doi.org/10.1016/j.marpolbul.2015.10.042
Jaafar N, Musa SM, Azfaralariff A, Mohamed M, Yusoff AH, Lazim AM (2020) Improving the efficiency of post-digestion method in extracting microplastics from gastrointestinal tract and gills of fish. Chemosphere 260:127649. https://doi.org/10.1016/j.chemosphere.2020.127649
Jacob H, Besson M, Swarzenski PW, Lecchini D, Metian M (2020) Effects of virgin micro-and nanoplastics on fish: trends, meta-analysis, and perspectives. Environ Sci Technol 54(8):4733–4745. https://doi.org/10.1021/acs.est.9b05995
Jadhav EB, Sankhla MS, Bhat RA, Bhagat DS (2021) Microplastics from food packaging: an overview of human consumption, health threats, and alternative solutions. Environ Nanotechnol Monit Manage 16:100608. https://doi.org/10.1016/j.enmm.2021.100608
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL (2015) Plastic waste inputs from land into the ocean. Science 347(6223):768–771. https://doi.org/10.1126/science.1260352
Jendrossek D, Handrick R (2002) Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol 56(1):403–432. https://doi.org/10.1146/annurev.micro.56.012302.160838
Jensen JT, Kops J (1980) Photochemical degradation of blends of polystyrene and poly (2,6-dimethyl-1,4-phenylene oxide). J Polym Sci 18(8):2737–2746. https://doi.org/10.1002/pol.1980.170180830
Jeong CB, Won EJ, Kang HM, Lee MC, Hwang DS, Hwang UK, Zhou B, Souissi S, Lee SJ, Lee JS (2016) Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ Sci Technol 50(16):8849–8857. https://doi.org/10.1021/acs.est.6b01441
Jeyavani J, Sibiya A, Shanthini S, Ravi C, Vijayakumar S, Rajan DK, Vaseeharan B (2021) A review on aquatic impacts of microplastics and its bioremediation aspects. Curr Pollut Rep 7(3):286–299. https://doi.org/10.1007/s40726-021-00188-2
Jiang C, Yin L, Li Z, Wen X, Luo X, Hu S, Yang H, Long Y, Deng B, Huang L, Liu Y (2019) Microplastic pollution in the rivers of the Tibet Plateau. Environ Pollut 249:91–98. https://doi.org/10.1016/j.envpol.2019.03.022
Kadouri D, Jurkevitch E, Okon Y, Castro-Sowinski S (2005) Ecological and agricultural significance of bacterial polyhydroxyalkanoates. Crit Rev Microbiol 31(2):55–67. https://doi.org/10.1080/10408410590899228
Kamat N, Kamat PV (2021) Single-use plastics (SUPs) and marine pollution: challenges and perspectives in India. Indian J Democr Governance 2021:31
Kamweru PK, Ndiritu FG, Kinyanjui TK, Muthui ZW, Ngumbu RG, Odhiambo PM (2011) Study of temperature and UV wavelength range effects on degradation of photo-irradiated polyethylene films using DMA. J Macromol Sci Part B 50(7):1338–1349. https://doi.org/10.1080/00222348.2010.516172
Karbalaei S, Hanachi P, Walker TR, Cole M (2018) Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ Sci Pollut Res 25(36):36046–36063. https://doi.org/10.1007/s11356-018-3508-7
Knighton D (2014) Fluvial forms and processes: a new perspective. Routledge, London. https://doi.org/10.4324/9780203784662
Koelmans AA, Nor NHM, Hermsen E, Kooi M, Mintenig SM, De France J (2019) Microplastics in freshwaters and drinking water: critical review and assessment of data quality. Water Res 155:410–422. https://doi.org/10.1016/j.watres.2019.02.054
Kolandhasamy P, Su L, Li J, Qu X, Jabeen K, Shi H (2018) Adherence of microplastics to soft tissue of mussels: a novel way to uptake microplastics beyond ingestion. Sci Total Environ 610:635–640. https://doi.org/10.1016/j.scitotenv.2017.08.053
Koongolla JB, Lin L, Pan YF, Yang CP, Sun DR, Liu S, Xu XR, Maharana D, Huang JS, Li HX (2020) Occurrence of microplastics in gastrointestinal tracts and gills of fish from Beibu Gulf, South China Sea. Environ Pollut 258:113734. https://doi.org/10.1016/j.envpol.2019.113734
Kotoyori T (1972) Activation energy for the oxidative thermal degradation of plastics. Thermochim Acta 5(1):51–58. https://doi.org/10.1016/0040-6031(72)80018-2
Krasilnikov N, Lojkowski W, Pakiela Z, Valiev R (2005) Tensile strength and ductility of ultra-fine-grained nickel processed by severe plastic deformation. Mater Sci Eng A 397(1-2):330–337. https://doi.org/10.1016/j.msea.2005.03.001
Law KL, Thompson RC (2014) Microplastics in the seas. Science 345(6193):144–145. https://doi.org/10.1126/science.1254065
Lechner A, Keckeis H, Lumesberger-Loisl F, Zens B, Krusch R, Tritthart M, Glas M, Schludermann E (2014) The Danube so colourful: a potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ Pollut 188:177–181. https://doi.org/10.1016/j.envpol.2014.02.006
Lei K, Qiao F, Liu Q, Wei Z, Qi H, Cui S, Yue X, Deng Y, An L (2017) Microplastics releasing from personal care and cosmetic products in China. Mar Pollut Bull 123(1-2):122–126. https://doi.org/10.1016/j.marpolbul.2017.09.016
Li WC (2018) The occurrence, fate, and effects of microplastics in the marine environment. In: Microplastic contamination in aquatic environments. Elsevier, Amsterdam, pp 133–173. https://doi.org/10.1016/B978-0-12-813747-5.00005-9
Li Q, Wu J, Zhao X, Gu X, Ji R (2019) Separation and identification of microplastics from soil and sewage sludge. Environ Pollut 254:113076. https://doi.org/10.1016/j.envpol.2019.113076
Li R, Tang X, Guo W, Lin L, Zhao L, Hu Y, Liu M (2020) Spatiotemporal distribution dynamics of heavy metals in water, sediment, and zoobenthos in mainstream sections of the middle and lower Changjiang River. Sci Total Environ 714:136779. https://doi.org/10.1016/j.scitotenv.2020.136779
Lima FP, Azevedo-Santos VM, Santos VM, Vidotto-Magnoni AP, Soares CL, Manzano FV, Nobile AB (2021) Plastic ingestion by commercial and non-commercial fishes from a Neotropical River Basin. Water Air Soil Pollut 232(1):1–8. https://doi.org/10.1007/s11270-020-04964-6
Lindeque PK, Cole M, Coppock RL, Lewis CN, Miller RZ, Watts AJ, Wilson-McNeal A, Wright SL, Galloway TS (2020) Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environ Pollut 265:114721. https://doi.org/10.1016/j.envpol.2020.114721
Liu FF, Liu GZ, Zhu ZL, Wang SC, Zhao FF (2019) Interactions between microplastics and phthalate esters as affected by microplastics characteristics and solution chemistry. Chemosphere 214:688–694. https://doi.org/10.1016/j.chemosphere.2018.09.174
Lugauskas A, Levinskait L, Pečiulyt D (2003) Micromycetes as deterioration agents of polymeric materials. Int Biodeterior Biodegrad 52(4):233–242. https://doi.org/10.1016/S0964-8305(03)00110-0
Lusher AL, Mchugh M, Thompson RC (2013) Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar Pollut Bull 67(1-2):94–99. https://doi.org/10.1016/j.marpolbul.2012.11.028
Lusher AL, Burke A, O’Connor I, Officer R (2014) Microplastic pollution in the Northeast Atlantic Ocean: validated and opportunistic sampling. Mar Pollut Bull 88(1-2):325–333. https://doi.org/10.1016/j.marpolbul.2014.08.023
Lusher AL, Hernandez-Milian G, O’Brien J, Berrow S, O’Connor I, Officer R (2015a) Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: the True’s beaked whale Mesoplodon mirus. Environ Pollut 199:185–191. https://doi.org/10.1016/j.envpol.2015.01.023
Lusher AL, Tirelli V, O’Connor I, Officer R (2015b) Microplastics in Arctic polar waters: the first reported values of particles in surface and sub-surface samples. Sci Rep 5(1):1–9. https://doi.org/10.1038/srep14947
Lv W, Zhou W, Lu S, Huang W, Yuan Q, Tian M, Lv W, He D (2019) Microplastic pollution in rice-fish co-culture system: a report of three farmland stations in Shanghai, China. Sci Total Environ 652:1209–1218. https://doi.org/10.1016/j.scitotenv.2018.10.321
Ma C, Chen Q, Li J, Li B, Liang W, Su L, Shi H (2021) Distribution and translocation of micro-and nanoplastics in fish. Crit Rev Toxicol 51(9):740–753. https://doi.org/10.1080/10408444.2021.2024495
Malankowska M, Echaide-Gorriz C, Coronas J (2021) Microplastics in marine environment: a review on sources, classification, and potential remediation by membrane technology. Environ Sci Water Res Technol 7(2):243–258. https://doi.org/10.1039/D0EW00802H
Martin JW, Chin JW, Nguyen T (2003) Reciprocity law experiments in polymeric photodegradation: a critical review. Prog Org Coat 47(3-4):292–311. https://doi.org/10.1016/j.porgcoat.2003.08.002
Martin C, Corona E, Mahadik GA, Duarte CM (2019) Adhesion to coral surface as a potential sink for marine microplastics. Environ Pollut 255:113281. https://doi.org/10.1016/j.envpol.2019.113281
Martins I, Rodríguez Y, Pham CK (2020) Trace elements in microplastics stranded on beaches of remote islands in the NE Atlantic. Mar Pollut Bull 156:111270. https://doi.org/10.1016/j.marpolbul.2020.111270
Masiá P, Sol D, Ardura A, Laca A, Borrell YJ, Dopico E, Laca A, Machado-Schiaffino G, Díaz M, Garcia-Vazquez E (2020) Bioremediation as a promising strategy for microplastics removal in wastewater treatment plants. Mar Pollut Bull 156:111252. https://doi.org/10.1016/j.marpolbul.2020.111252
Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T (2001) Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ Sci Technol 35(2):318–324. https://doi.org/10.1021/es0010498
McDevitt JP, Criddle CS, Morse M, Hale RC, Bott CB, Rochman CM (2017) Addressing the issue of microplastics in the wake of the microbead-free waters act a new standard can facilitate improved policy. Environ Sci Technol 51(12):6611–6617. https://doi.org/10.1021/acs.est.6b05812
Min K, Cuiffi JD, Mathers RT (2020) Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nat Commun 11(1):1–11. https://doi.org/10.1038/s41467-020-14538-z
Mohan S, Mamane H, Avisar D, Gozlan I, Kaplan A, Dayalan G (2019) Treatment of diethyl phthalate leached from plastic products in municipal solid waste using an ozone-based advanced oxidation process. Materials 12(24):4119. https://doi.org/10.3390/ma12244119
Molnar JL, Gamboa RL, Revenga C, Spalding MD (2008) Assessing the global threat of invasive species to marine biodiversity. Front Ecol Environ 6(9):485–492. https://doi.org/10.1890/070064
Murphy F, Ewins C, Carbonnier F, Quinn B (2016) Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ Sci Technol 50(11):5800–5808. https://doi.org/10.1021/acs.est.5b05416
Muthukumar T, Aravinthan A, Lakshmi K, Venkatesan R, Vedaprakash L, Doble M (2011) Fouling and stability of polymers and composites in marine environment. Int Biodeterior Biodegradation 65(2):276–284. https://doi.org/10.1016/j.ibiod.2010.11.012
Nakamiya K, Sakasita G, Ooi T, Kinoshita S (1997) Enzymatic degradation of polystyrene by hydroquinone peroxidase of Azotobacter beijerinckii HM121. J Ferment Bioeng 84(5):480–482. https://doi.org/10.1016/S0922-338X(97)82013-2
Nel HA, Froneman PW (2015) A quantitative analysis of microplastic pollution along the south-eastern coastline of South Africa. Mar Pollut Bull 101(1):274–279. https://doi.org/10.1016/j.marpolbul.2015.09.043
Neves D, Sobral P, Ferreira JL, Pereira T (2015) Ingestion of microplastics by commercial fish off the Portuguese coast. Mar Pollut Bull 101(1):119–126. https://doi.org/10.1016/j.marpolbul.2015.11.008
Nguyen B, Claveau-Mallet D, Hernandez LM, Xu EG, Farner JM, Tufenkji N (2019) Separation and analysis of microplastics and nanoplastics in complex environmental samples. Acc Chem Res 52(4):858–866. https://doi.org/10.1021/acs.accounts.8b00602
Nizzetto L, Bussi G, Futter MN, Butterfield D, Whitehead PG (2016) A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ Sci: Processes Impacts 18(8):1050–1059. https://doi.org/10.1039/C6EM00206D
O’Brine T, Thompson RC (2010) Degradation of plastic carrier bags in the marine environment. Mar Pollut Bull 60(12):2279–2283. https://doi.org/10.1016/j.marpolbul.2010.08.005
Ogunola OS, Onada OA, Falaye AE (2018) Mitigation measures to avert the impacts of plastics and microplastics in the marine environment (a review). Environ Sci Pollut Res 25(10):9293–9310. https://doi.org/10.1007/s11356-018-1499-z
Ory N, Chagnon C, Felix F, Fernández C, Ferreira JL, Gallardo C, Ordóñez OG, Henostroza A, Laaz E, Mizraji R, Mojica H (2018) Low prevalence of microplastic contamination in planktivorous fish species from the southeast Pacific Ocean. Mar Pollut Bull 127:211–216. https://doi.org/10.1016/j.marpolbul.2017.12.016
Palm GJ, Reisky L, Böttcher D, Müller H, Michels EA, Walczak MC, Berndt L, Weiss MS, Bornscheuer UT, Weber G (2019) Structure of the plastic-degrading Ideonella sakaiensis MHETase bound to a substrate. Nat Commun 10(1):1–10. https://doi.org/10.1038/s41467-019-09326-3
Pastorelli G, Cucci C, Garcia O, Piantanida G, Elnaggar A, Cassar M, Strlič M (2014) Environmentally induced colour change during natural degradation of selected polymers. Polym Degrad Stab 107:198–209. https://doi.org/10.1016/j.polymdegradstab.2013.11.007
Phothakwanpracha J, Lirdwitayaprasit T, Pairohakul S (2021) Effects of sizes and concentrations of different types of microplastics on bioaccumulation and lethality rate in the green mussel, Perna viridis. Mar Pollut Bull 173:112954. https://doi.org/10.1016/j.marpolbul.2021.112954
Pirsaheb M, Hossini H, Makhdoumi P (2020) Review of microplastic occurrence and toxicological effects in marine environment: experimental evidence of inflammation. Process Saf Environ Prot 142:1–14. https://doi.org/10.1016/j.psep.2020.05.050
Placet M, Mann CO, Gilbert RO, Niefer MJ (2000) Emissions of ozone precursors from stationary sources: a critical review. Atmos Environ 34(12-14):2183–2204. https://doi.org/10.1016/S1352-2310(99)00464-1
Quesadas-Rojas M, Enriquez C, Valle-Levinson A (2021) Natural and anthropogenic effects on microplastic distribution in a hypersaline lagoon. Sci Total Environ 776:145803. https://doi.org/10.1016/j.scitotenv.2021.145803
Rainieri S, Barranco A (2019) Microplastics, a food safety issue? Trends Food Sci Technol 84:55–57. https://doi.org/10.1016/j.tifs.2018.12.009
Rehse S, Kloas W, Zarfl C (2016) Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna. Chemosphere 153:91–99. https://doi.org/10.1016/j.chemosphere.2016.02.133
Reich L, Stivala S (1971) Elements of polymer degradation. McGraw Hill Book, New York. https://aiche.onlinelibrary.wiley.com/doi/10.1002/aic.690180242
Roccuzzo S, Beckerman AP, Trögl J (2021) New perspectives on the bioremediation of endocrine disrupting compounds from wastewater using algae-, bacteria-and fungi-based technologies. Int J Environ Sci Technol 18(1):89–106. https://doi.org/10.1007/s13762-020-02691-3
Rummel CD, Jahnke A, Gorokhova E, Kühnel D, Schmitt-Jansen M (2017) Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ Sci Technol Lett 4(7):258–267. https://doi.org/10.1021/acs.estlett.7b00164
Santo M, Weitsman R, Sivan A (2013) The role of the copper-binding enzyme–laccase–in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int Biodeterior Biodegradation 84:204–210. https://doi.org/10.1016/j.ibiod.2012.03.001
Setälä O, Norkko J, Lehtiniemi M (2016) Feeding type affects microplastic ingestion in a coastal invertebrate community. Mar Pollut Bull 102(1):95–101. https://doi.org/10.1016/j.marpolbul.2015.11.053
Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26(3):246–265. https://doi.org/10.1016/j.biotechadv.2007.12.005
Shahnawaz M, Sangale MK, Ade AB (2019) Bioremediation technology for plastic waste. Springer, Cham
Sharma S, Chatterjee S (2017) Microplastic pollution, a threat to marine ecosystem and human health: a short review. Environ Sci Pollut Res 24(27):21530–21547. https://doi.org/10.1007/s11356-017-9910-8
Sighicelli M, Pietrelli L, Lecce F, Iannilli V, Falconieri M, Coscia L, Di Vito S, Nuglio S, Zampetti G (2018) Microplastic pollution in the surface waters of Italian Subalpine Lakes. Environ Pollut 236:645–651. https://doi.org/10.1016/j.envpol.2018.02.008
Singh B, Sharma N (2008) Mechanistic implications of plastic degradation. Polym Degrad Stab 93(3):561–584. https://doi.org/10.1016/j.polymdegradstab.2007.11.008
Sivan A (2011) New perspectives in plastic biodegradation. Curr Opin Biotechnol 22(3):422–426. https://doi.org/10.1016/j.copbio.2011.01.013
Skariyachan S, Taskeen N, Kishore AP, Krishna BV, Naidu G (2021) Novel consortia of enterobacter and pseudomonas formulated from cow dung exhibited enhanced biodegradation of polyethylene and polypropylene. J Environ Manag 284:112030. https://doi.org/10.1016/j.jenvman.2021.112030
Sruthy S, Ramasamy EV (2017) Microplastic pollution in Vembanad Lake, Kerala, India: the first report of microplastics in lake and estuarine sediments in India. Environ Pollut 222:315–322. https://doi.org/10.1016/j.envpol.2016.12.038
Steinmetz Z, Wollmann C, Schaefer M, Buchmann C, David J, Tröger J, Muñoz K, Frör O, Schaumann GE (2016) Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci Total Environ 550:690–705. https://doi.org/10.1016/j.scitotenv.2016.01.153
Su L, Xue Y, Li L, Yang D, Kolandhasamy P, Li D, Shi H (2016) Microplastics in Taihu lake, China. Environ Pollut 216:711–719. https://doi.org/10.1016/j.envpol.2016.06.036
Sugimoto Y, Shimamoto D, Imai Y, Hotta Y (2020) Simultaneous evaluation of tensile strength and interfacial shear strength of short length carbon fibers using fragmentation test. Carbon 161:83–88. https://doi.org/10.1016/j.carbon.2019.12.089
Sun X, Li Q, Zhu M, Liang J, Zheng S, Zhao Y (2017) Ingestion of microplastics by natural zooplankton groups in the northern South China Sea. Mar Pollut Bull 115(1-2):217–224. https://doi.org/10.1016/j.marpolbul.2016.12.004
Tanasupawat S, Takehana T, Yoshida S, Hiraga K, Oda K (2016) Ideonella sakaiensis sp. nov., isolated from a microbial consortium that degrades poly (ethylene terephthalate). Int J Syst Evol Microbiol 66(8):2813–2818
Tang Y, Liu Y, Chen Y, Zhang W, Zhao J, He S, Yang C, Zhang T, Tang C, Zhang C, Yang Z (2021) A review: Research progress on microplastic pollutants in aquatic environments. Sci Total Environ 766:142572. https://doi.org/10.1016/j.scitotenv.2020.142572
Teng J, Wang Q, Ran W, Wu D, Liu Y, Sun S, Liu H, Cao R, Zhao J (2019) Microplastic in cultured oysters from different coastal areas of China. Sci Total Environ 653:1282–1292. https://doi.org/10.1016/j.scitotenv.2018.11.057
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AW, McGonigle D, Russell AE (2004) Lost at sea: where is all the plastic? Science 304(5672):838–838. https://doi.org/10.1126/science.1094559
Tokiwa Y, Calabia BP, Ugwu CU, Aiba S (2009) Biodegradability of plastics. Int J Mol Sci 10(9):3722–3742. https://doi.org/10.3390/ijms10093722
Treilles R, Gasperi J, Gallard A, Saad M, Dris R, Partibane C, Breton J, Tassin B (2021) Microplastics and microfibers in urban runoff from a suburban catchment of Greater Paris. Environ Pollut 287:117352. https://doi.org/10.1016/j.envpol.2021.117352
Trestrail C, Nugegoda D, Shimeta J (2020) Invertebrate responses to microplastic ingestion: reviewing the role of the antioxidant system. Sci Total Environ 734:138559. https://doi.org/10.1016/j.scitotenv.2020.138559
Tyler DR (2004) Mechanistic aspects of the effects of stress on the rates of photochemical degradation reactions in polymers. J Macromol Sci Part C Polym Rev 44(4):351–388. https://doi.org/10.1081/MC-200033682
Urbanek AK, Rymowicz W, Mirończuk AM (2018) Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl Microbiol Biotechnol 102(18):7669–7678. https://doi.org/10.1007/s00253-018-9195-y
Vaughan R, Turner SD, Rose NL (2017) Microplastics in the sediments of a UK urban lake. Environ Pollut 229:10–18. https://doi.org/10.1016/j.envpol.2017.05.057
Vert M, Doi Y, Hellwich KH, Hess M, Hodge P, Kubisa P, Rinaudo M, Schué F (2012) Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl Chem 84(2):377–410. https://doi.org/10.1351/PAC-REC-10-12-04
Wadsö L, Karlsson OJ (2013) Alkaline hydrolysis of polymers with ester groups studied by isothermal calorimetry. Polym Degrad Stab 98(1):73–78. https://doi.org/10.1016/j.polymdegradstab.2012.10.031
Wang W, Ge J, Yu X (2020) Bioavailability and toxicity of microplastics to fish species: a review. Ecotoxicol Environ Saf 189:109913. https://doi.org/10.1016/j.ecoenv.2019.109913
Ward JE, Zhao S, Holohan BA, Mladinich KM, Griffin TW, Wozniak J, Shumway SE (2019) Selective ingestion and egestion of plastic particles by the blue mussel (Mytilus edulis) and eastern oyster (Crassostrea virginica): implications for using bivalves as bioindicators of microplastic pollution. Environ Sci Technol 53(15):8776–8784. https://doi.org/10.1021/acs.est.9b02073
Watts AJ, Lewis C, Goodhead RM, Beckett SJ, Moger J, Tyler CR, Galloway TS (2014) Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ Sci Technol 48(15):8823–8830. https://doi.org/10.1021/es501090e
Wilkes RA, Aristilde L (2017) Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: capabilities and challenges. J Appl Microbiol 123(3):582–593. https://doi.org/10.1111/jam.13472
Wright SL, Thompson RC, Galloway TS (2013) The physical impacts of microplastics on marine organisms: a review. Environ Pollut 178:483–492. https://doi.org/10.1016/j.envpol.2013.02.031
Wu WM, Yang J, Criddle CS (2017) Microplastics pollution and reduction strategies. Front Environ Sci Eng 11(1):1–4. https://doi.org/10.1007/s11783-017-0897-7
Xu C, Zhang B, Gu C, Shen C, Yin S, Aamir M, Li F (2020) Are we underestimating the sources of microplastic pollution in terrestrial environment? J Hazard Mater 400:123228. https://doi.org/10.1016/j.jhazmat.2020.123228
Xue B, Zhang L, Li R, Wang Y, Guo J, Yu K, Wang S (2020) Underestimated microplastic pollution derived from fishery activities and “hidden” in deep sediment. Environ Sci Technol 54(4):2210–2217. https://doi.org/10.1021/acs.est.9b04850
Yang D, Shi H, Li L, Li J, Jabeen K, Kolandhasamy P (2015) Microplastic pollution in table salts from China. Environ Sci Technol 49(22):13622–13627. https://doi.org/10.1021/acs.est.5b03163
Yang H, Ma M, Thompson JR, Flower RJ (2018) Waste management, informal recycling, environmental pollution and public health. J Epidemiol Community Health 72(3):237–243. https://doi.org/10.1136/jech-2016-208597
Ye G, Zhang X, Liu X, Liao X, Zhang H, Yan C, Lin Y, Huang Q (2021) Polystyrene microplastics induce metabolic disturbances in marine medaka (Oryzias melastigmas) liver. Sci Total Environ 782:146885. https://doi.org/10.1016/j.scitotenv.2021.146885
Zettler ER, Mincer TJ, Amaral-Zettler LA (2013) Life in the “plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol 47(13):7137–7146. https://doi.org/10.1021/es401288x
Zhang C, Chen X, Wang J, Tan L (2017a) Toxic effects of microplastic on marine microalgae Skeletonema costatum: interactions between microplastic and algae. Environ Pollut 220:1282–1288. https://doi.org/10.1016/j.envpol.2016.11.005
Zhang W, Zhang S, Wang J, Wang Y, Mu J, Wang P, Lin X, Ma D (2017b) Microplastic pollution in the surface waters of the Bohai Sea, China. Environ Pollut 231:541–548. https://doi.org/10.1016/j.envpol.2017.08.058
Zhao S, Zhu L, Li D (2015) Microplastic in three urban estuaries, China. Environ Pollut 206:597–604. https://doi.org/10.1016/j.envpol.2015.08.027
Zheng S, Zhao Y, Liangwei W, Liang J, Liu T, Zhu M, Li Q, Sun X (2020) Characteristics of microplastics ingested by zooplankton from the Bohai Sea, China. Sci Total Environ 713:136357. https://doi.org/10.1016/j.scitotenv.2019.136357
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mukherjee, S., Ngasotter, S., Singh, S.K., Meitei, M.M. (2023). Microplastic Pollution in Aquatic Environment: Ecotoxicological Effects and Bioremediation Prospects. In: Soni, R., Suyal, D.C., Morales-Oyervides, L., Fouillaud, M. (eds) Current Status of Marine Water Microbiology. Springer, Singapore. https://doi.org/10.1007/978-981-99-5022-5_13
Download citation
DOI: https://doi.org/10.1007/978-981-99-5022-5_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-5021-8
Online ISBN: 978-981-99-5022-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)