Abstract
The tyrosine metabolism pathway serves as a starting point for the production of a variety of structurally diverse natural compounds in plants, such as tocopherols, plastoquinone, ubiquinone, betalains, salidroside, benzylisoquinoline alkaloids, and so on. Among these, tyrosine-derived metabolites, tocopherols, plastoquinone, and ubiquinone are essential to plant survival. In addition, this pathway provides us essential micronutrients (e.g., vitamin E and ubiquinone) and medicine (e.g., morphine, salidroside, and salvianolic acid B). However, our knowledge of the plant tyrosine metabolism pathway remains rudimentary, and genes encoding the pathway enzymes have not been fully defined. In this review, we summarize and discuss recent advances in the tyrosine metabolism pathway, key enzymes, and important tyrosine-derived metabolites in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In plants, tyrosine is synthesized de novo via the shikimate pathway, which also gives rise to the other two aromatic amino acids, phenylalanine and tryptophan (Maeda and Dudareva 2012). Besides proteinogenic, tyrosine is a biosynthetic precursor of tocopherols, plastoquinone, and ubiquinone that are essential to plant itself. Furthermore, tyrosine is used as a substrate to synthesize numerous specialized metabolites in different groups of plants, such as rosmarinic acid (RA) and its derivatives in the families of Lamiaceae and Boraginaceae (Petersen and Simmonds 2003), dhurrin in Sorghum, salidroside in Rhodiola, betalains in the Caryophyllales order, benzylisoquinoline alkaloids in the Ranunculales order (Liscombe et al. 2005), amaryllidaceae alkaloids in the family of Amaryllidaceae (Kilgore and Kutchan 2016), emetine alkaloids in the families of Alangiaceae, Icacinaceae, and Rubiaceae (Wiegrebe et al. 1984), phenylethylamines like mescaline in cactus species (Cassels and Saez-Briones 2018), phenethylisoquinoline alkaloids like colchicine in Colchicum (Rinner and Waser 2016), and catecholamines in a long list of plant species (Kulma and Szopa 2007). The importance of tyrosine-derived plant metabolites for both plant survival and human health has attracted great interests in elucidation of their biosynthetic pathways.
In plants, tyrosine can be modified by different enzymes to yield specific types of the tyrosine-derived metabolites, of which the distributions, functions, and practical uses have been recently reviewed (Schenck and Maeda 2018). In this short review, we focus on the biosynthetic pathways of tyrosine-derived metabolites in plants. We propose a concept of the general tyrosine metabolism pathway, which is common in plants. We also discuss key enzymes of tyrosine metabolism, which catalyze committed steps in the biosynthesis of tyrosine-derived specialized metabolites.
The general tyrosine metabolism pathway
The amino acid tyrosine provides the core cyclic scaffold to tocopherols, plastoquinone, and ubiquinone, which are synthesized in all plants (Fig. 1). Tocopherols, together with tocotrienols, form a group of lipid soluble antioxidants termed tocochromanols, which play a number of physiological roles in plants beyond antioxidation (Munné-Bosch and Alegre 2002; Falk and Munne-Bosch 2010). Tocochromanols are commonly known as vitamin E and are essential components of our nutrition. Unlike tocopherols, the tocotrienols are not as widespread as tocopherols (Horvath et al. 2006), and contain three double bonds in the isoprenoid side chain. Plastoquinone is found in all green lineages, ranging from cyanobacteria and algae to land plants, and acts as electron transporter in photophosphorylation, thus is indispensable to plant growth. Ubiquinone, also known as Coenzyme Q (CoQ), is an isoprenoid quinone produced in almost all living organisms and functions as electron transporter in the respiratory chain. Ubiquinone, which is now widely used as a food supplement, is often termed vitamin Q (Shukla and Dubey 2018; Pravst et al. 2010). The benzene quinone ring of ubiquinone is thought to be derived from 4-hydroxybenzoic acid (4-HB), whereas the ring precursor for tocopherols and plastoquinone is homogentisic acid (HGA). The remaining steps for the biosynthesis of tocopherols, plastoquinone, and ubiquinone have been reviewed elsewhere (Liu and Lu 2016; Mene-Saffrane 2017; Fritsche et al. 2017); this section will summarize metabolic origins of the HGA and 4-HB, which are key intermediates of the general tyrosine metabolism pathway.
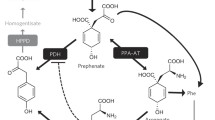
Tyrosine metabolism in plants. The general tyrosine metabolism pathway is given in orange. Arrows with dashed lines designate multiple enzymes steps. TAT tyrosine aminotransferase, HPPD hydroxyphenylpyruvate dioxygenase, HPPR hydroxyphenylpyruvate reductase, TAL tyrosine ammonia-lyase, TYDCl-tyrosine decarboxylase, 4HPAAS 4-hydroxyphenylacetaldehyde synthase, PPO polyphenol oxidase, l-DOPAl-3,4-dihydroxyphenylalanine, pHPP 4-hydroxyphenylpyruvic acid, pHPL 4-hydroxyphenyllactic acid, 4-HB 4-hydroxybenzoic acid, 4-HPAA 4-hydroxyphenylacetaldehyde, HGA homogentisic acid
Tyrosine aminotransferase (TAT; EC 2.6.1.5) catalyzes the reversible transamination from tyrosine to form 4-hydroxyphenylpyruvic acid (pHPP), an initial step of the tyrosine conversion. The aminotransferase activity depends on pyridoxal-5′-phosphate. TAT homologs are likely widely distributed in plants (Fig. 2A). In most plant species, TAT catalyzes the removal of the amino group of tyrosine (Wang et al. 2016, 2019; Wang and Maeda 2018). An exception is that in legumes, which have a non-plastidic prephenate dehydrogenase (PDH, EC 1.3.1.13) that converts prephenate into pHPP, TAT is assumed to catalyze the transamination of pHPP to synthesize tyrosine (Schenck et al. 2015). However, the in planta role of TAT in tyrosine biosynthesis has not been clearly demonstrated (Maeda and Dudareva 2012). In Arabidopsis thaliana, there are at least two genes encoding TAT (Table 1), tat1 mutants have decreased tocopherol levels (Riewe et al. 2012), and a tat1 tat2 double mutant accumulates less tocopherols than the tat1 mutant under high-light stress (Wang et al. 2019), indicating that both TAT1 and TAT2 contribute to the biosynthesis of tocopherols. TAT is also involved in the biosynthesis of many other tyrosine-derived metabolites. For instance, an opium poppy (Papaver somniferum) TAT was shown to participate in the production of benzylisoquinoline alkaloids, including morphine and codeine (Lee and Facchini 2011). In Prunella vulgaris, a Traditional Chinese Medicinal (TCM) plant in the family of Lamiaceae, TAT is involved in the biosynthesis of RA (Ru et al. 2017).
Phylogenetic analysis of plant tyrosine aminotransferase (A) and 4-hydroxyphenylpyruvate dioxygenase (B). Amino acid sequences were aligned by ClustalX (Thompson et al. 1997) and the trees were generated in PhyML (Guindon et al. 2010) using maximum-likelihood method (1000 bootstrap replication). Bootstrap values less than 500 are not shown. The sequences of Homo sapiens and Mus musculus were used as outgroups. (A) The TAT sequences of Homo sapiens, Mus musculus, and Selaginella moellendorffii were obtained from NCBI, the protein sequences of Picea abies were from the PLAZA project (Van Bel et al. 2018), and the others were obtained via Phytozome v12 (Goodstein et al. 2012). (B) The HPPD sequences of Homo sapiens and Mus musculus were obtained from NCBI, the protein sequences of Picea abies were from the PLAZA project, and the others were obtained via Phytozome v12
Following the formation by TAT, pHPP can be converted to HGA by 4-hydroxyphenylpyruvate dioxygenase (HPPD, EC 1.13.11.27), a member of the large family of non-heme iron α-ketoglutarate-dependent dioxygenases. This complex reaction involves decarboxylation, aromatic hydroxylation, and substituent migration in a single catalytic cycle (Fig. 1). This transformation seems unique in nearly all aerobic organisms (Moran 2005), and HPPD homologs were found in a wide range of plant species (Fig. 2B). In animals, HPPD is required to modulate blood tyrosine levels, while in plants, it plays a key role in production of the aromatic precursor of tocopherols and plastoquinone. As plastoquinone is essential to photosynthetic organisms (Munné-Bosch and Alegre 2002; Norris et al. 1995; Amesz 1973), HPPD is a target for the development of herbicides (Beaudegnies et al. 2009; Ndikuryayo et al. 2017). In Arabidopsis, the loss-of-function mutation of HPPD led to tocopherol and plastoquinone deficiency (Norris et al. 1995, 1998). The availability of HGA limits tocochromanol production at least in some plant species and organs. For example, de-regulated HGA accumulation by co-overexpression of Arabidopsis HPPD and microbial enzymes such as Escherichia coli bifunctional chorismate mutase/prephenate dehydrogenase (TyrA) or Saccharomyces cerevisiae prephenate dehydrogenase (TYR1) in tobacco and Arabidopsis resulted in high levels of tocochromanol accumulation (Rippert et al. 2004; Zhang et al. 2013). In soybean, the mutant of HGO1, which encodes a homogentisate dioxygenase that breaks down HGA, overaccumulated HGA and tocochromanol (Stacey et al. 2016).
The 1,4-benzoquinone ring precursor for ubiquinone 4-HB is derived from tyrosine and phenylalanine in plants (Block et al. 2014). Significant progresses have been made recently in characterization of the biosynthesis of 4-HB from phenylalanine (Block et al. 2014; Soubeyrand et al. 2018); by contrast, much less is known about the generation of 4-HB from tyrosine. In S. cerevisiae, the first and last reactions of this pathway, namely the deamination of tyrosine to pHPP and the oxidation of 4-hydroxybenzaldehyde to 4-HB, have been characterized recently (Payet et al. 2016; Stefely et al. 2016); however, the evidence that TAT is involved in 4-HB formation is still lacking in plants. The endogenous levels of 4-HB may limit the ubiquinone production, since elevation in its pool has reported to have a positive effect on ubiquinone biosynthesis in Arabidopsis and tomato (Block et al. 2014; Soubeyrand et al. 2018). Further investigation of the tyrosine-derived metabolites and their connection to the phenylpropanoid pathway would provide new insights into the ubiquinone biosynthesis and help to improve nutritional value of crop products.
Biosynthesis of tyrosine-derived specialized metabolites
Hydroxyphenylpyruvate reductase (HPPR, EC 1.1.1.237) catalyzes the reduction of pHPP to 4-hydroxyphenyllactic acid (pHPL), the precursor to rosmarinic acid (RA) (Petersen and Alfermann 1988; Häusler et al. 1991), which is frequently found in plants of the families Lamiaceae and Boraginaceae, with random reports of its presence in other families (Petersen and Simmonds 2003; Petersen 2013; Petersen et al. 2009). Chemically, RA is an ester of caffeic acid and 3,4-dihydroxyphenyllactic acid. More complex derivatives of RA have been identified, e.g., salvianolic acid B and other salvianolic acids from Salvia (Wu et al. 2012), and rabdosiin from Rabdosia japonica. The HPPR enzyme was first isolated and characterized in Coleus blumei (Plectranthus scutellarioides) (Petersen and Alfermann 1988; Häusler et al. 1991). Recently, HPPRs from Arabidopsis were characterized (Xu et al. 2018), although RA is undetectable in this species (Petersen et al. 2009). Since HPPR is widely distributed in land plants rather than specific to RA-accumulating plants, pHPL could be converted into different types of natural products depending on the plant taxa, which deserves further investigation.
A subset of the phenylalanine ammonia-lyase (PAL, EC 4.3.1.24) enzymes in monocots also possess tyrosine ammonia-lyase (TAL, EC 4.3.1.23) activity, leading to the non-oxidative deamination of tyrosine to yield 4-coumaric acid. The TAL activity was found thus far particularly in monocots (Rosler et al. 1997; Barros et al. 2016; Jun et al. 2018; Cass et al. 2015; Beaudoin-Eagan and Thorpe 1985). In Brachypodium distachyon, a single bifunctional phenylalanine/tyrosine ammonia-lyase (PTAL) displays both PAL and TAL activities, the latter leading to the conversion of tyrosine to 4-coumaric acid, which is ultimately integrated into the phenylpropanoid-derived compounds such as lignin and flavonoids (Barros et al. 2016). The discovery of TAL provides an alternative approach to optimize the production of phenylpropanoid compounds and tyrosine-derived metabolites. Enzymes that demonstrate specificity for tyrosine are referred to as TAL, which has been found in a number of microorganisms, such as Rhodobacter capsulatus (Kyndt et al. 2002), Rhodobacter sphaeroides (Watts et al. 2006), and Saccharothrix espanaensis (Berner et al. 2006). Replacing the active site residue His 89 with Phe can transform the Rhodobacter sphaeroides TAL into a highly active PAL and vice versa (Watts et al. 2006; Louie et al. 2006). The corresponding His residue is also critical for TAL activity in plants (Jun et al. 2018).
CYP79A1 catalyzes the multistep conversion of tyrosine into (E)-p-hydroxyphenylacetaldoxime (Koch et al. 1995; Sibbesen et al. 1995; Clausen et al. 2015), which is subsequently modified by CYP71E1 and a UDP (uridine diphosphate)-glucosyltransferase (UGT85B1) to produce dhurrin (Bak et al. 1998; Kahn et al. 1997; Jones et al. 1999; Laursen et al. 2016). Dhurrin, a defense cyanogenic glycoside mainly found in sorghum, has a strong insecticidal activity (Tattersall et al. 2001). Notably, enzymes of the dhurrin biosynthetic pathway are assembled in a metabolon (Laursen et al. 2016; Nielsen et al. 2008).
Hydroxylation of tyrosine at 3-position leads to the formation of 3,4-dihydroxy-l-phenylalanine (l-DOPA). This reaction can be catalyzed by the tyrosinase (EC 1.14.18.1) activity of polyphenol oxidases (PPOs), which are widely distributed in microorganisms, animals, and plants. Interestingly, PPOs are absent from the genus of Arabidopsis (Tran et al. 2012). In walnut (Juglans regia), the PPO-silenced transgenic plant displayed a decrease in l-DOPA-derived metabolites and an increases in tyramine, demonstrating that the walnut PPO catalyzes the 3-hydroxylation of tyrosine (Araji et al. 2014). l-DOPA is also a precursor for the biosynthesis of betalains, however, whether PPO is involved in betalain biosynthesis remains an open question. It seems more likely that in betalain-producing plants, the 3-hydroxylation of tyrosine is catalyzed by a P450 monooxygenase, CYP76AD1, CYP76AD5, or CYP76AD6 (Sunnadeniya et al. 2016; Polturak et al. 2016; Hatlestad et al. 2012). The biosynthesis of betalain pigments has been recently reviewed (Polturak and Aharoni 2018).
l-Tyrosine decarboxylase (TYDC, EC 4.1.1.25) catalyzes the pyridoxal-5′-phosphate dependent decarboxylation of tyrosine and l-DOPA to yield tyramine and dopamine, respectively (Facchini and De Luca 1994, 1995). TYDC has been characterized from a variety of plant species (Facchini and De Luca 1994, 1995; Lehmann and Pollmann 2009; Torrens-Spence et al. 2013; Kang et al. 2007) and is assumed to be nearly ubiquitous among plants (Lehmann and Pollmann 2009). Notably, tyramine exhibits toxicity toward plants (Negrel et al. 1993; Christou and Barton 1989). For example, overexpression of TYDC in rice led to tyramine accumulation and stunted growth (Kang et al. 2007). Similarly, treatment of walnut leaves with exogenous tyramine induced the development of necrotic lesions (Araji et al. 2014). Tyramine is a precursor for the biosynthesis of hydroxycinnamic acid amides (e.g., feruloyltyramine) (Facchini et al. 2002) and amaryllidaceae alkaloids (e.g., lycorine) (Kilgore and Kutchan 2016).
Dopamine is an important neurotransmitter in the brain, and in plants, it is a precursor of numerous specialized metabolites, including phenethylisoquinoline alkaloids (e.g., colchicine) (Polturak and Aharoni 2018; Ehrenworth and Peralta-Yahya 2017), emetine alkaloids (e.g., emetine) (Nomura and Kutchan 2010), benzylisoquinoline alkaloids (e.g., morphine) (Schlager and Drager 2016; Liu et al. 2017), catecholamines (e.g., epinephrine), and phenylethylamines (e.g., mescaline) (Rinner and Waser 2016). Dopamine can be synthesized via 3-hydroxylation of tyramine or decarboxylation of l-DOPA, but enzymes responsible for the 3-hydroxylation of tyrosine or tyramine are still unclear in plants. In walnut, PPO is involved in the biosynthesis of dopamine (Araji et al. 2014); however, whether PPO plays a similar role in other plants awaits investigation. The predominant biosynthetic pathway of dopamine has not been identified, since TYDC has been shown to accept both tyrosine and l-DOPA as substrates. In human hydroxylation precedes decarboxylation in the major biosynthetic pathway of dopamine (Meiser et al. 2013).
Tyrosine is also converted to 4-hydroxyphenylacetaldehyde (4-HPAA) through decarboxylation–oxidative deamination, which is catalyzed by 4-hydroxyphenylacetaldehyde synthase (4HPAAS, EC 4.1.1.108) (Torrens-Spence et al. 2012, 2018a). 4-HPAA is a key intermediate in the biosynthesis of benzylisoquinoline alkaloids (e.g., morphine in Opium poppy) and tyrosol-derived specialized metabolites (e.g., salidroside in Rhodiola). In the biosynthesis of benzylisoquinoline alkaloids, 4-HPAA is proposed to be generated from tyrosine via TAT and an unidentified 4-hydroxyphenylpyruvate decarboxylase, respectively (Lee and Facchini 2011). Whether 4HPAAS, which directly converts tyrosine to 4-HPAA in Rhodiola, functions similarly in the benzylisoquinoline alkaloid metabolism is unclear. 4HPAAS belongs to the plant aromatic amino acid decarboxylase (AAAD) family (Facchini et al. 2000), of which other members include TYDC, tryptophan decarboxylase (TDC), and phenylacetaldehyde synthase (PAAS) (Torrens-Spence et al. 2018b). A single amino acid substitution of plant AAADs is capable of impacting substrate selectivity or altering catalytic reactions (Torrens-Spence et al. 2013, 2014, 2018b).
Conclusion and perspectives
Tyrosine serves as a biosynthetic precursor of a wide range of metabolites, many of which are of great nutritional, pharmacologic, and economic importance. Tocopherols and ubiquinone are vitamins essential to nearly all domains of life. Many benzylisoquinoline alkaloids possess potent pharmacological activities, including morphine and codeine (narcotic analgesics), noscapine (antitussive drug), papaverine (antispasmodic drug), and so on. RA and its derivatives have health-promoting properties, such as cardioprotection, antioxidant, antibacterial, and antiinflammatory activities (Bulgakov et al. 2012).
Considering the great diversity of tyrosine-related natural products in plants, the recent significant achievements in the biosynthetic pathways of the tyrosine-originated metabolites are just new beginnings of further investigation. Many questions and knowledge gaps remain. For one example, none of the enzymes responsible for 4-HB production from tyrosine has been identified in the plant general tyrosine metabolism pathway. Definitely, better understanding of the tyrosine metabolism pathways will facilitate the breeding of high nutritional crop varieties and improving the production of valuable natural metabolites in plants.
References
Amesz J (1973) The function of plastoquinone in photosynthetic electron transport. Biochim Biophys Acta 301:35–51
Araji S, Grammer TA, Gertzen R, Anderson SD, Mikulic-Petkovsek M, Veberic R, Phu ML, Solar A, Leslie CA, Dandekar AM, Escobar MA (2014) Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut. Plant Physiol 164:1191–1203
Bak S, Kahn RA, Nielsen HL, Moller BL, Halkier BA (1998) Cloning of three A-type cytochromes P450, CYP71E1, CYP98, and CYP99 from Sorghum bicolor (L.) Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome P450 in the biosynthesis of the cyanogenic glucoside dhurrin. Plant Mol Biol 36:393–405
Barros J, Serrani-Yarce JC, Chen F, Baxter D, Venables BJ, Dixon RA (2016) Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nat Plants. 2:16050
Beaudegnies R, Edmunds AJ, Fraser TE, Hall RG, Hawkes TR, Mitchell G, Schaetzer J, Wendeborn S, Wibley J (2009) Herbicidal 4-hydroxyphenylpyruvate dioxygenase inhibitors—a review of the triketone chemistry story from a Syngenta perspective. Bioorg Med Chem 17:4134–4152
Beaudoin-Eagan LD, Thorpe TA (1985) Tyrosine and phenylalanine ammonia lyase activities during shoot initiation in tobacco callus cultures. Plant Physiol 78:438–441
Berner M, Krug D, Bihlmaier C, Vente A, Muller R, Bechthold A (2006) Genes and enzymes involved in caffeic acid biosynthesis in the actinomycete Saccharothrix espanaensis. J Bacteriol 188:2666–2673
Block A, Widhalm JR, Fatihi A, Cahoon RE, Wamboldt Y, Elowsky C, Mackenzie SA, Cahoon EB, Chapple C, Dudareva N, Basset GJ (2014) The origin and biosynthesis of the benzenoid moiety of ubiquinone (Coenzyme Q) in Arabidopsis. Plant Cell 26:1938–1948
Bulgakov VP, Inyushkina YV, Fedoreyev SA (2012) Rosmarinic acid and its derivatives: biotechnology and applications. Crit Rev Biotechnol 32:203–217
Cass CL, Peraldi A, Dowd PF, Mottiar Y, Santoro N, Karlen SD, Bukhman YV, Foster CE, Thrower N, Bruno LC, Moskvin OV, Johnson ET, Willhoit ME, Phutane M, Ralph J, Mansfield SD, Nicholson P, Sedbrook JC (2015) Effects of PHENYLALANINE AMMONIA LYASE (PAL) knockdown on cell wall composition, biomass digestibility, and biotic and abiotic stress responses in Brachypodium. J Exp Bot 66:4317–4335
Cassels BK, Saez-Briones P (2018) Dark classics in chemical neuroscience: mescaline. ACS Chem Neurosci 9:2448–2458
Christou P, Barton KA (1989) Cytokinin antagonist activity of substituted phenethylamines in plant cell culture. Plant Physiol 89:564–568
Clausen M, Kannangara RM, Olsen CE, Blomstedt CK, Gleadow RM, Jorgensen K, Bak S, Motawie MS, Moller BL (2015) The bifurcation of the cyanogenic glucoside and glucosinolate biosynthetic pathways. Plant J 84:558–573
Ehrenworth AM, Peralta-Yahya P (2017) Accelerating the semisynthesis of alkaloid-based drugs through metabolic engineering. Nat Chem Biol 13:249–258
Facchini PJ, De Luca V (1994) Differential and tissue-specific expression of a gene family for tyrosine/dopa decarboxylase in opium poppy. J Biol Chem 269:26684–26690
Facchini PJ, De Luca V (1995) Expression in Escherichia coli and partial characterization of two tyrosine/dopa decarboxylases from opium poppy. Phytochemistry 38:1119–1126
Facchini PJ, Huber-Allanach KL, Tari LW (2000) Plant aromatic l-amino acid decarboxylases: evolution, biochemistry, regulation, and metabolic engineering applications. Phytochemistry 54:121–138
Facchini PJ, Hagel J, Zulak KG (2002) Hydroxycinnamic acid amide metabolism: physiology and biochemistry. Can J Bot 80:577–589
Falk J, Munne-Bosch S (2010) Tocochromanol functions in plants: antioxidation and beyond. J Exp Bot 61:1549–1566
Fritsche S, Wang X, Jung C (2017) Recent advances in our understanding of tocopherol biosynthesis in plants: an overview of key genes, functions, and breeding of vitamin E improved crops. Antioxidants (Basel) 6:99
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40:D1178–1186
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Hatlestad GJ, Sunnadeniya RM, Akhavan NA, Gonzalez A, Goldman IL, McGrath JM, Lloyd AM (2012) The beet R locus encodes a new cytochrome P450 required for red betalain production. Nat Genet 44:816–820
Häusler E, Petersen M, Alfermann AW (1991) Hydroxyphenylpyruvate reductase from cell suspension cultures of Coleus blumei Benth. Zeitschrift für Naturforschung C 46:371–376
Horvath G, Wessjohann L, Bigirimana J, Jansen M, Guisez Y, Caubergs R, Horemans N (2006) Differential distribution of tocopherols and tocotrienols in photosynthetic and non-photosynthetic tissues. Phytochemistry 67:1185–1195
Jones PR, Moller BL, Hoj PB (1999) The UDP-glucose: p-hydroxymandelonitrile-O-glucosyltransferase that catalyzes the last step in synthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor. Isolation, cloning, heterologous expression, and substrate specificity. J Biol Chem 274:35483–35491
Jun SY, Sattler SA, Cortez GS, Vermerris W, Sattler SE, Kang C (2018) Biochemical and structural analysis of substrate specificity of a Phenylalanine Ammonia-Lyase. Plant Physiol 176:1452–1468
Kahn RA, Bak S, Svendsen I, Halkier BA, Moller BL (1997) Isolation and reconstitution of cytochrome P450ox and in vitro reconstitution of the entire biosynthetic pathway of the cyanogenic glucoside dhurrin from sorghum. Plant Physiol 115:1661–1670
Kang S, Kang K, Lee K, Back K (2007) Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta 227:263–272
Kilgore MB, Kutchan TM (2016) The Amaryllidaceae alkaloids: biosynthesis and methods for enzyme discovery. Phytochem Rev 15:317–337
Koch BM, Sibbesen O, Halkier BA, Svendsen I, Moller BL (1995) The primary sequence of cytochrome P450tyr, the multifunctional N-hydroxylase catalyzing the conversion of l-tyrosine to p-hydroxyphenylacetaldehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Arch Biochem Biophys 323:177–186
Kulma A, Szopa J (2007) Catecholamines are active compounds in plants. Plant Sci 172:433–440
Kyndt JA, Meyer TE, Cusanovich MA, Van Beeumen JJ (2002) Characterization of a bacterial tyrosine ammonia lyase, a biosynthetic enzyme for the photoactive yellow protein. FEBS Lett 512:240–244
Laursen T, Borch J, Knudsen C, Bavishi K, Torta F, Martens HJ, Silvestro D, Hatzakis NS, Wenk MR, Dafforn TR, Olsen CE, Motawia MS, Hamberger B, Moller BL, Bassard JE (2016) Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum. Science 354:890–893
Lee EJ, Facchini PJ (2011) Tyrosine aminotransferase contributes to benzylisoquinoline alkaloid biosynthesis in opium poppy. Plant Physiol 157:1067–1078
Lehmann T, Pollmann S (2009) Gene expression and characterization of a stress-induced tyrosine decarboxylase from Arabidopsis thaliana. FEBS Lett 583:1895–1900
Liscombe DK, Macleod BP, Loukanina N, Nandi OI, Facchini PJ (2005) Evidence for the monophyletic evolution of benzylisoquinoline alkaloid biosynthesis in angiosperms. Phytochemistry 66:1374–1393
Liu M, Lu S (2016) Plastoquinone and ubiquinone in plants: biosynthesis, physiological function and metabolic engineering. Front Plant Sci 7:1–18
Liu X, Liu Y, Huang P, Ma Y, Qing Z, Tang Q, Cao H, Cheng P, Zheng Y, Yuan Z, Zhou Y, Liu J, Tang Z, Zhuo Y, Zhang Y, Yu L, Huang J, Yang P, Peng Q, Zhang J, Jiang W, Zhang Z, Lin K, Ro DK, Chen X, Xiong X, Shang Y, Huang S, Zeng J (2017) The genome of medicinal plant Macleaya cordata provides new insights into benzylisoquinoline alkaloids metabolism. Mol Plant 10:975–989
Louie GV, Bowman ME, Moffitt MC, Baiga TJ, Moore BS, Noel JP (2006) Structural determinants and modulation of substrate specificity in phenylalanine-tyrosine ammonia-lyases. Chem Biol 13:1327–1338
Maeda H, Dudareva N (2012) The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu Rev Plant Biol 63:73–105
Meiser J, Weindl D, Hiller K (2013) Complexity of dopamine metabolism. Cell Commun Signal 11:34
Mene-Saffrane L (2017) Vitamin E biosynthesis and its regulation in plants. Antioxidants (Basel) 7:2
Moran GR (2005) 4-Hydroxyphenylpyruvate dioxygenase. Arch Biochem Biophys 433:117–128
Munné-Bosch S, Alegre L (2002) The function of tocopherols and tocotrienols in Plants. Crit Rev Plant Sci 21:31–57
Ndikuryayo F, Moosavi B, Yang WC, Yang GF (2017) 4-Hydroxyphenylpyruvate dioxygenase inhibitors: from chemical biology to agrochemicals. J Agric Food Chem 65:8523–8537
Negrel J, Javelle F, Paynot M (1993) Biochemical basis of resistance of tobacco callus tissue cultures to hydroxyphenylethylamines. Plant Physiol 103:329–334
Nielsen KA, Tattersall DB, Jones PR, Moller BL (2008) Metabolon formation in dhurrin biosynthesis. Phytochemistry 69:88–98
Nomura T, Kutchan TM (2010) Three new O-methyltransferases are sufficient for all O-methylation reactions of ipecac alkaloid biosynthesis in root culture of Psychotria ipecacuanha. J Biol Chem 285:7722–7738
Norris SR, Barrette TR, DellaPenna D (1995) Genetic dissection of carotenoid synthesis in arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell 7:2139–2149
Norris SR, Shen X, DellaPenna D (1998) Complementation of the Arabidopsis pds1 mutation with the gene encoding p-hydroxyphenylpyruvate dioxygenase. Plant Physiol 117:1317–1323
Payet LA, Leroux M, Willison JC, Kihara A, Pelosi L, Pierrel F (2016) Mechanistic details of early steps in Coenzyme Q biosynthesis pathway in yeast. Cell Chem Biol 23:1241–1250
Petersen M (2013) Rosmarinic acid: new aspects. Phytochem Rev 12:207–227
Petersen M, Alfermann AW (1988) Two new enzymes of rosmarinic acid biosynthesis from cell cultures of Coleus blumei: hydroxyphenylpyruvate reductase and rosmarinic acid synthase. Zeitschrift für Naturforschung C 43:501–504
Petersen M, Simmonds MS (2003) Rosmarinic acid. Phytochemistry 62:121–125
Petersen M, Abdullah Y, Benner J, Eberle D, Gehlen K, Hucherig S, Janiak V, Kim KH, Sander M, Weitzel C, Wolters S (2009) Evolution of rosmarinic acid biosynthesis. Phytochemistry 70:1663–1679
Polturak G, Aharoni A (2018) “La Vie en Rose”: biosynthesis, sources, and applications of betalain pigments. Mol Plant 11:7–22
Polturak G, Breitel D, Grossman N, Sarrion-Perdigones A, Weithorn E, Pliner M, Orzaez D, Granell A, Rogachev I, Aharoni A (2016) Elucidation of the first committed step in betalain biosynthesis enables the heterologous engineering of betalain pigments in plants. New Phytol 210:269–283
Pravst I, Zmitek K, Zmitek J (2010) Coenzyme Q10 contents in foods and fortification strategies. Crit Rev Food Sci Nutr 50:269–280
Riewe D, Koohi M, Lisec J, Pfeiffer M, Lippmann R, Schmeichel J, Willmitzer L, Altmann T (2012) A tyrosine aminotransferase involved in tocopherol synthesis in Arabidopsis. Plant J 71:850–859
Rinner U, Waser M (2016) Tyrosine alkaloids. In: Zografos AL (ed) From biosynthesis to total synthesis: strategies and tactics for natural products, pp 431–472
Rippert P, Scimemi C, Dubald M, Matringe M (2004) Engineering plant shikimate pathway for production of tocotrienol and improving herbicide resistance. Plant Physiol 134:92–100
Rosler J, Krekel F, Amrhein N, Schmid J (1997) Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiol 113:175–179
Ru M, Wang K, Bai Z, Peng L, He S, Wang Y, Liang Z (2017) A tyrosine aminotransferase involved in rosmarinic acid biosynthesis in Prunella vulgaris L. Scientific Reports. 7:4892
Schenck CA, Maeda HA (2018) Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 149:82–102
Schenck CA, Chen S, Siehl DL, Maeda HA (2015) Non-plastidic, tyrosine-insensitive prephenate dehydrogenases from legumes. Nat Chem Biol 11:52–57
Schlager S, Drager B (2016) Exploiting plant alkaloids. Curr Opin Biotechnol 37:155–164
Shukla S, Dubey KK (2018) CoQ10 a super-vitamin: review on application and biosynthesis. 3 Biotech 8:249
Sibbesen O, Koch B, Halkier BA, Moller BL (1995) Cytochrome P-450TYR is a multifunctional heme-thiolate enzyme catalyzing the conversion of l-tyrosine to p-hydroxyphenylacetaldehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. J Biol Chem 270:3506–3511
Soubeyrand E, Johnson TS, Latimer S, Block A, Kim J, Colquhoun TA, Butelli E, Martin C, Wilson MA, Basset GJ (2018) The peroxidative cleavage of kaempferol contributes to the biosynthesis of the benzenoid moiety of ubiquinone in plants. Plant Cell 30:2910–2921
Stacey MG, Cahoon RE, Nguyen HT, Cui Y, Sato S, Nguyen CT, Phoka N, Clark KM, Liang Y, Forrester J, Batek J, Do PT, Sleper DA, Clemente TE, Cahoon EB, Stacey G (2016) Identification of homogentisate dioxygenase as a target for vitamin E biofortification in oilseeds. Plant Physiol 172:1506–1518
Stefely JA, Kwiecien NW, Freiberger EC, Richards AL, Jochem A, Rush MJP, Ulbrich A, Robinson KP, Hutchins PD, Veling MT, Guo X, Kemmerer ZA, Connors KJ, Trujillo EA, Sokol J, Marx H, Westphall MS, Hebert AS, Pagliarini DJ, Coon JJ (2016) Mitochondrial protein functions elucidated by multi-omic mass spectrometry profiling. Nat Biotechnol 34:1191–1197
Sunnadeniya R, Bean A, Brown M, Akhavan N, Hatlestad G, Gonzalez A, Symonds VV, Lloyd A (2016) Tyrosine hydroxylation in betalain pigment biosynthesis is performed by cytochrome P450 enzymes in beets (Beta vulgaris). PLoS One 11:1–16
Tattersall DB, Bak S, Jones PR, Olsen CE, Nielsen JK, Hansen ML, Hoj PB, Moller BL (2001) Resistance to an herbivore through engineered cyanogenic glucoside synthesis. Science 293:1826–1828
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Torrens-Spence MP, Gillaspy G, Zhao B, Harich K, White RH, Li J (2012) Biochemical evaluation of a parsley tyrosine decarboxylase results in a novel 4-hydroxyphenylacetaldehyde synthase enzyme. Biochem Biophys Res Commun 418:211–216
Torrens-Spence MP, Liu P, Ding H, Harich K, Gillaspy G, Li J (2013) Biochemical evaluation of the decarboxylation and decarboxylation-deamination activities of plant aromatic amino acid decarboxylases. J Biol Chem 288:2376–2387
Torrens-Spence MP, Lazear M, von Guggenberg R, Ding H, Li J (2014) Investigation of a substrate-specifying residue within Papaver somniferum and Catharanthus roseus aromatic amino acid decarboxylases. Phytochemistry 106:37–43
Torrens-Spence MP, Pluskal T, Li FS, Carballo V, Weng JK (2018a) Complete pathway elucidation and heterologous reconstitution of rhodiola salidroside biosynthesis. Mol Plant 11:205–217
Torrens-Spence MP, Chiang Y-C, Smith T, Vicent MA, Wang Y, Weng J-K (2018b) Structural basis for independent origins of new catalytic machineries in plant AAAD proteins. bioRxiv 404970
Tran LT, Taylor JS, Constabel CP (2012) The polyphenol oxidase gene family in land plants: lineage-specific duplication and expansion. BMC Genom 13:395
Van Bel M, Diels T, Vancaester E, Kreft L, Botzki A, Van de Peer Y, Coppens F, Vandepoele K (2018) PLAZA 4.0: an integrative resource for functional, evolutionary and comparative plant genomics. Nucleic Acids Res 46:D1190–D1196
Wang MM, Maeda HA (2018) Aromatic amino acid aminotransferases in plants. Phytochem Rev 17:131–159
Wang M, Toda K, Maeda HA (2016) Biochemical properties and subcellular localization of tyrosine aminotransferases in Arabidopsis thaliana. Phytochemistry 132:16–25
Wang M, Toda K, Block A, Maeda HA (2019) TAT1 and TAT2 tyrosine aminotransferases have both distinct and shared functions in tyrosine metabolism and degradation in Arabidopsis thaliana. J Biol Chem 294:3563–3576
Watts KT, Mijts BN, Lee PC, Manning AJ, Schmidt-Dannert C (2006) Discovery of a substrate selectivity switch in tyrosine ammonia-lyase, a member of the aromatic amino acid lyase family. Chem Biol 13:1317–1326
Wiegrebe W, Kramer WJ, Shamma M (1984) The emetine alkaloids. J Nat Prod 47:397–408
Wu YB, Ni ZY, Shi QW, Dong M, Kiyota H, Gu YC, Cong B (2012) Constituents from Salvia species and their biological activities. Chem Rev 112:5967–6026
Xu JJ, Fang X, Li CY, Zhao Q, Martin C, Chen XY, Yang L (2018) Characterization of Arabidopsis thaliana hydroxyphenylpyruvate reductases in the tyrosine conversion pathway. Front Plant Sci 9:1305
Zhang C, Cahoon RE, Hunter SC, Chen M, Han J, Cahoon EB (2013) Genetic and biochemical basis for alternative routes of tocotrienol biosynthesis for enhanced vitamin E antioxidant production. Plant J 73:628–639
Acknowledgements
This project was supported by the Special Fund for Shanghai Landscaping Administration Bureau Program (Grant nos. G192416 and G192419).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, JJ., Fang, X., Li, CY. et al. General and specialized tyrosine metabolism pathways in plants. aBIOTECH 1, 97–105 (2020). https://doi.org/10.1007/s42994-019-00006-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42994-019-00006-w