Abstract
Soil salinity is considered as one of the major threats to rice production in coastal region of Bangladesh and rice plants are prone to salt stress at seedling stage. Therefore, this study was conducted to evaluate the performance of rice genotypes for salinity stress tolerance at five levels of salinity treatments i.e., control – nutrient solution without salt [EC (electrical conductivity): 0.0 dS m−1 (deciSiemens per meter)], EC: 08 dS m−1, EC: 10 dS m−1, EC: 13 dS m−1 and EC: 15 dS m−1; were used for the morphological screening at seedling stage. After initial screening of fifty-seven local rice landraces, thirty rice genotypes were selected based on their salt tolerance ability using a standard evaluation score (SES). The selected rice genotypes were further screened by morphological parameters and molecular markers. Binadhan-10 and Binadhan-17 were used as salt resistant and salt susceptible checks, respectively. The environmental effect had a weak involvement in the expression of morphological traits, which showed high heritability (in broad sense) and genetic advance as percentage of mean. Two molecular markers (RM7075 and RM10825) were used to identify the Saltol quantitative trait loci (QTL) associated with the genotypes. Based on morphological and molecular analyses, the thirty-two rice genotypes were grouped into different clusters and six genotypes (Pokkali, Holdegotal-2, Vojon, Blockkhira, Rajashail and Hatibejor) were selected as salt tolerant. The materials obtained from this study can be utilized for further breeding program and developing markers for salt tolerance in rice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice is the staple food of about 50% of the total world’s population, hence one of the most important cereals ensuring food security (FAOSTAT 2015). About 80% of the total cultivated land in Bangladesh is used for rice cultivation and total production is about more than 36 million metric tons (BBS 2020). Bangladesh holds the fourth position among the ninety-five rice-producing countries in terms of total rice production (FAOSTAT 2017; Schneider and Asch 2020). However, rice production is threatened due to the effect of many abiotic stress factors such as salinity, drought and flood (Gregorio et al. 2002). Among these, soil salinity is the most alarming due to the gradual extension of saline area around the globe for the higher use of groundwater and rising of the sea level due to global warming (Dasgupta et al. 2015; Brindha and Schneider 2019). A study by Climate Change Cell (2007) revealed that salinity is the most threatening stress factor that drastically reduces the yield of coastal agriculture. This study reported that varying degrees of soil salinity affected 830,000 million hectares of land in coastal areas of Bangladesh. Soil salinity affects 1.06 million hectares of arable land in Bangladesh including major coastal areas of Khulna, Satkhira, Bagerhat, Barguna, Patuakhali, Noakhali and Chattogram (SRDI 2010). Rice plants possess exertion with the normal growth due to its susceptibility nature to salt stress at both seedling and reproductive stages (Chowdhury et al. 2016; Razzaque et al. 2017). It has been shown that rice plant is more sensitive to salt stress at the seedling stage than when it is in the reproductive stage (Sahi et al. 2006) which affects its physiological, morphological and biochemical indices (Ali et al. 2014; Kordrostami et al. 2017). Salinity stress impairs rice seedling growth, shortens shoot lengths, root lengths and reduces leaf size, all of which contribute to the early mortality of the plant at seedling stage (Wankhade et al. 2013; Krishnamurthy et al. 2016). Injury of seedling stage in rice consequently affects the overall performance at reproductive stage due to stunted growth, deformed roots, delayed flowering, reduction in the number of effective tillers, the number of grains per panicle, and grain weight, and ultimately reducing the grain yield (Gregorio et al. 2002; Zhao et al. 2014). Salinity tolerance is crucial for crop establishment at the seedling stage leading to reproductive state, which is prerequisite for better grain yield (Krishnamurthy et al. 2020).
The growing population of Bangladesh is creating more pressure on food; hence, it has become necessary to explore the possibilities of increasing the potential use of saline areas. Although being high yielding, the existing modern rice varieties are not well adapted to the southern ecosystem due to the lack of salt tolerance (Huda et al. 2019). Therefore, developing salt-tolerant rice varieties has been considered as the most effective strategy to increase rice production in saline areas of Bangladesh. Landraces can play a vital role in the development of salt-tolerant rice genotypes due to their vast genetic diversity or variability (Rasel et al. 2018) which is an important criterion for crop improvement programs and useful to find the mean heredity effects of concerned genes and associated environmental effects. Accordingly, it is essential to partition the observed variability into heritable and non-heritable components measured as phenotypic coefficient of variation, genotypic coefficient of variation, broad sense heritability, genetic advance and genetic advance as percentage of mean (Mishra et al. 2015; Rasel et al. 2018). Screening of rice landraces at seedling stage under controlled conditions in a hydroponic system could reduce environmental effects and free from difficulties associated with other soil-related stress factors (Huda et al. 2019) and could be used as an ideal method of screening. Further, simple sequence repeats (SSR) markers are highly informative, co-dominant, multi-allele genetic markers, that are experimentally reproducible and transferable among related species and have been the most widely used for plant genotyping over the past twenty years (Ganie et al. 2016).
To date the commercially released rice varieties of Bangladesh can tolerate up to EC: 14 dS m−1 such as the newly released BRRI Dhan-97 and BRRI Dhan-99 along with the previously released and commercially cultivated Binadhan-10 (The Business Standard 2023). In this study, a higher salinity level has been introduced (EC: 15 dS m−1) to impose a selection pressure on the rice landraces to screen the salt tolerant genotypes. Therefore, the present study was undertaken to screen the local rice landraces for their salinity tolerance ability at the seedling stage, which is considered as the most sensitive stage of plant growth, to identify suitable salt-tolerant genetically diverse plant materials based on both morphological and molecular screening using SSR markers. The selected local germplasm can be used as potential resources of salinity tolerance for further rice breeding programs as well as the knowledge on molecular screening would provide valuable information on future marker development for use in salt tolerant rice variety development.
Materials and methods
Plant materials and experimental location
A total of fifty-seven rice landraces (Supplementary Table 1) along with check genotypes were collected from the different southern parts of Bangladesh as well as Bangladesh Agricultural University (BAU), Mymensingh-2202 and Bangladesh Institute of Nuclear Agriculture (BINA), BAU Campus, Mymensingh-2202, were used in the present study.
The experiment of salinity screening was conducted with hydroponic system at the growth room of the Department of Genetics and Plant Breeding, BAU, Mymensingh-2202 from March 2019 to March 2020. The molecular screening and analysis were performed based on SSR markers at the Molecular Plant Breeding Laboratory of BINA.
Seed sterilization and germination
Approximately one hundred and twenty rice seeds of each genotype were placed into brown bags and kept in the oven at 50 °C for 2 days to break the seed dormancy. Afterwards, seeds were sterilized by treating with 0.1% HgCl2 for about 2 min and washed three times with distilled water. Then the seeds were further sterilized with 70% ethanol for 3 min and washed three times with distilled water. The sterilized seeds were placed in Petridishes having moist filter paper and kept at room temperature (25 ± 2 °C) in a dark condition for 3–4 days for sprouting.
Experimental design and salt treatments
The experiment was laid out in completely randomized design (CRD) with five salt treatments, viz., control—nutrient solution without salt (EC: 0.0 dS m−1), EC: 08 dS m−1, 10 dS m−1, 13 dS m−1 and 15 dS m−1 with three replications in a well-established controlled plant growth room.
Hydroponic system
Rectangular plastic trays having dimensions of (40 cm × 30 cm × 35 cm) were used and filled up with 10 L of nutrient solution. Top of each trays, nylon net and rectangular-shaped Styrofoam having ten holes (each replication contained 10 seedlings) were placed. The 4-days old seedlings were transferred to the hydroponic medium containing normal water and placed into the holes keeping the roots down to the water in the pot and shoot up straight for 3 days. Afterwards Peter professional water-soluble fertilizer (Urea:TSP:MoP = 20:20:20) was used to supply nutrients for growth and development of plants. The nutrient solution was made with 1 g Peter professional and 0.1 g FeSO4 dissolved in one-liter water. The pH of the solution was adjusted to the range of 5.2–5.5 by adding the required volume of HCl and NaOH and the nutrient solution was replaced at seven-days intervals. The pH of the solution was monitored daily and maintained at 5.2 to 5.5. To ensure the continuous supply of the nutrients to the plants, the solution was stirred three times daily to keep the iron and some other nutrients dissolved as the nutrients could be precipitated within 7–8 h, and can be unavailable for the plants.
Salt treatments were imposed on the seedlings at 2–3 leaves stage (about 10–12 days old). Crude salt was added to water at first and mixed it properly to make a saline solution. The saline solution was dissolved with nutrients to reach the desired salinity level. The salinity level was measured using an EC meter (TetraCon® 925—WTW, Germany) to maintain the desired salinity concentration. The control set of the genotypes were grown in only normal nutrient solution. All the collected genotypes (Supplementary Table 1) were exposed to salinity for seven days afterwards they were grown on the normal nutrient solution for another 7 days. Afterwards, data were collected to calculate their SES score. Based on the SES, the susceptible genotypes at EC: 08 dS m−1 were eliminated to minimize the population size and thirty genotypes were selected for second screening. These thirty genotypes (Table 1) were further screened under constant salinity stress for 14 days and data were recorded.
Data collection
The scoring and morphological data were collected after 14 days of salt treatment for thirty genotypes. Plants were harvested and data on morphological traits such as percentage of live leaves, shoot length (cm), root length (cm), root fresh weight (mg), root dry weight (mg), shoot fresh weight (mg) and shoot dry weight (mg) were recorded. The modified SES was used for scoring the visual symptoms of salt toxicity in the genotypes (Gregorio et al. 1997). The susceptible genotypes were distinguished from the tolerant and the moderately tolerant genotypes based on their SES.
Genotyping of rice germplasm
Genomic DNA was extracted using fresh leaves of 21-days-old rice seedlings through DNeasy Plant Mini Kit (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instruction. The quantity of DNA was determined using NanoDrop 2000c spectrophotometer (Thermo Scientific, USA). Two SSR markers (viz., RM10825 and RM7075) (https://archive.gramene.org/markers/) linked to QTL of salinity tolerance in rice and located on chromosome 1 were selected for the molecular screening of the selected rice genotypes (Ganie et al. 2016). The polymerase chain reaction (PCR) cocktail was prepared including 1 µl genomic DNA, 1 µl each of forward and reverse primers, 2.0 µl of nuclease-free water and 5 μl of PCR master mix (Thermo Scientific, USA). PCR (LabCycler, SensoQuest Biomedizinische Elektronik, Germany) program setup was maintained as initial denaturation at 94 °C for 4 min, followed by 35 cycles of denaturation at 94 °C for 45 s, annealing at 55 °C for 45 s and extension at 72 °C for 2 min with a final extension of 72 °C for 10 min and then incubated at 4 °C. PCR products were electrophoretically separated on an 8.0% polyacrylamide gel placed in a vertical electrophoresis tank (CBS Scientific, USA) containing 1.0X TBE buffer. The gel was soaked in SYBR green (0.5 µg ml−1) solution (Sigma-Aldrich) for 20 min and then de-stained with distilled water for a few minutes with gentle shaking. A gel documentation system was used to photograph the resolved bands (ProteinSimple, USA). The size of the amplified fragments of DNA was determined by comparing the migration distance of amplified fragments relative to the molecular weight of 100 bp DNA Ladder (Thermo Scientific, USA).
Statistical analysis
Morphological data were analyzed using RStudio with R (v 4.1.0) programs. The stress tolerance index (STI) values for morphological traits were also determined by the formula of Zeng et al. (2002). The amplified fragments of DNA banding pattern of each SSR marker was determined using AlphaEaseFC (v 4.0.0) software.
Results
Salt tolerance ability of rice genotypes based on SES
Rice seedlings grown under control treatment showed normal plant growth whereas under saline conditions the plants showed several symptoms of salt injury, such as leaf rolling and tip whitening, lower percentage of live leaves, reduction in root and shoot growth and dying of seedlings depending on their salt susceptibility (Fig. 1). Tolerant genotypes were least affected than susceptible genotypes under salt stress for different morphological traits. After 21 days of salinization, six genotypes i.e., Pokkali, Holdegotal-2, Vojon, Blockkhira, Rajashail and Hatibejor were identified as tolerant (SES of 3–4), whereas eleven genotypes were moderately tolerant, and rest of the genotypes were found as susceptible (Table 1). The maximum SES of 9 was observed in Ghunsi, Moulota, Rupeshwar-2, Jessoreswarna, Pothao, Nunia, Bashfulbalam, Swarnagota, Betichikon, Swarnajana, Mulagoboru, Khoiya, Ruhango, Machranga and Binadhan-17 at 15 dS m−1 (Table 1).
Morphological traits-based analysis of rice genotypes
The analysis of variance (ANOVA) revealed significant variations among different morphological parameters (Table 2). Live leaves (%) were significantly reduced in all genotypes when exposed to higher salinity levels. The highest live leaves were obtained in Holdegotal-2 (88.77% under control treatment and 47.69% at EC: 15 dS m−1) there were no live leaves observed in Moulota, Jessoreswarna, Pothao, Nunia, Swarnagota, Betichikon, Swarnajana. Mulagoboru, Khoiya and Binadhan-17 at EC: 13 dS m−1; and in Ghunsi, Rupeswar-2, Bashfulbalam, Ruhango and Machranga at EC: 15 dS m−1. The maximum shoot length was found in Holdegotal-2 (41.16 cm) in the control treatment and at EC: 15 dS m−1 (18.50 cm). Pothao (not survived at EC: 10 dS m−1), Moulota, Jessoreswarna, Nunia, Swarnagota, Betichikon, Swarnajana, Mulagoboru, Khoiya and Binadhan-17 were not survived both at EC: 13 dS m−1 and 15 dS m−1 followed by Ghunsi, Rupeshwar-2, Bashfulbalam, Ruhango, Machranga at EC: 15 dS m−1. Among the rice genotypes, Pokkali showed the highest of root fresh weight (61.14 mg) at EC: 15 dS m−1. Root dry weight was highest in Ranisalute (8.46 mg) followed by Rajashail (4.87 mg) at EC: 15 dS m−1. Further, the highest shoot fresh weight was found in Holdegotal-2 (120.16 mg) followed by Bohorimota (97.26 mg), Jotai (96.53 mg), Ranisalute (72.33 mg), Pokkali (71.46 mg) and Panbota (70.43 mg) at EC: 15 dS m−1. In addition, the highest shoot dry weight was found in Holdegotal-2 (19.13 mg) followed by Ranisalute (15.80 mg), Bohorimota (14.60 mg) and Rajashail (14.50 mg) at EC: 15 dS m−1. On the other hand, the lowest shoot fresh weight and dry weight were found in Ghunsi at EC: 15 dS m−1, Moulota at EC: 13 dS m−1 and 15 dS m−1, Rupeswar-2 at EC: 15 dS m−1, Jessoreswarna at EC: 13 dS m−1 and 15 dS m−1, Pothao at EC: 10 dS m−1, 13 dS m−1 and 15 dS m−1. The mean comparison of genotypes and treatments on different morphological traits at the seedling stage is shown in Supplementary Table 2.
Genetic variability parameters
The presence of genetic variability in plant breeding materials is necessary for a successful breeding program. It was observed that the phenotypic variances were slightly higher than the genotypic variances. Similar findings were also found in the case of phenotypic coefficient of variation (PCV) and genotypic coefficient of variation (GCV) for all the traits under study (Table 3). Among all the traits root fresh weight exhibited high estimates of GCV and PCV of 82.01% and 82.04%, respectively; followed by shoot fresh weight (58.94% and 58.94%, respectively) shoot dry weight (57.84% and 57.85%, respectively). On the other hand, root dry weight showed a moderate value of 45.21% and 45.27%; shoot length 34.69% and 34.76% of GCV and PCV, respectively. Low GCV and PCV of 25.42% and 25.59% for root length and 19.03% and 19.07% for live leaves were also observed (Table 3).
The traits under the present experiment exhibited high heritability estimates ranging from 99 to 100% (Table 3). Among the traits, the highest heritability was recorded in shoot fresh weight (100%) followed by shoot dry weight (99.94%), root fresh weight (99.92%), root dry weight (99.75%), shoot length (99.60%), live leaves (99.54%) and root length (98.64%). Since the estimation of heritability is not enough to have a clear indication and screening of desirable genotypes, the genetic advance can provide a suitable indication of the progress that can be expected because of selecting on the pertinent genotypes. The highest genetic advance was found in shoot fresh weight (136.22) followed by root fresh weight (72.82) and the lowest genetic advance was found in root length (4.51) followed by root dry weight (6.59). The highest genetic advance as percentage of mean was found in root fresh weight (168.87%) followed by shoot fresh weight (121.41%) while the lowest was found for live leaves (39.11%) followed by root length (52.01%) (Table 3).
Estimation of correlation coefficients and path coefficient
Correlation analysis between the yield and yield contributing characters revealed that the genotypic correlation coefficients in most cases were higher than their phenotypic correlation coefficients. Significant positive correlations at both genotypic and phenotypic levels were recorded for all the traits where a significant negative correlation was observed with SES (Table 4).
Partitioning of genotypic and phenotypic correlation coefficients into direct and indirect effects on the studied traits of the rice genotypes by path analysis is shown in Table 5. At the genotypic level, live leaves percentage (− 0.677) had a direct negative effect on SES at genotypic correlation coefficients. Shoot length (-0.314), root length (-0.340), root fresh weight (-0.288) and shoot fresh weight (-0.177) showed a direct negative effect on SES whereas a direct positive effect was found for root dry weight (0.188) and shoot dry weight (0.751). At the phenotypic level, live leaves percentage (− 0.620) had a direct negative effect on SES. Shoot length (-0.341), root length (-0.321), root fresh weight (-0.265) and shoot fresh weight (-0.173) showed a direct negative effect on SES whereas a direct positive effect was found with root dry weight (0.189) and shoot dry weight (0.705). The indirect negative effect was found with live leaves (%), root length, root fresh weight and shoot fresh weight on SES whereas an indirect positive effect was found with root dry weight and shoot dry weight both at the genotypic and phenotypic levels (Table 5).
The residual effect of path analysis at genotypic and phenotypic levels were 0.241 and 0.299, respectively. It indicated that the effect of component traits on salt severity (SES) was 75.90% at the genotypic level and 70.10% at the phenotypic level with the rest of 24.10% and 29.90%, respectively contributed by other factors such as environmental factors or traits those were not studied.
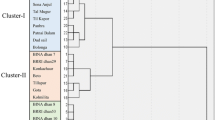
Grouping of rice genotypes based on cluster analysis
Depending on the range of diversity, thirty-two rice genotypes were grouped into four clusters based on Mahalanobis D2 results. The distribution pattern revealed that the maximum number of genotypes (34.37%) were in cluster I while cluster II included the lowest number of genotypes (15.63%). Cluster III and IV included eight genotypes in each (25.00%) (Table 6 and Fig. 2). In all cases, it was observed that the inter-cluster distances were greater than the intra-cluster distances (Supplementary Table 3). Maximum intra-cluster diversity was observed in cluster II (3.642) and minimum intra-cluster diversity was found in cluster III (2.656). The maximum inter-cluster distance was found between cluster II and Cluster III (10.857) followed by the distance between clusters II and IV (8.382), cluster I and III (7.019) and cluster I and II (6.682) where the minimum distance was observed between Cluster I and IV (4.333). The mean performance of different clusters for different morphological traits reflected that all the genotypes with the highest live leaves (%), shoot length, root length, root fresh weight, root dry weight, shoot fresh weight and shoot dry weight were grouped into cluster II whereas cluster III included all the genotypes with lowest values of above-mentioned traits. Genotypes having the highest SES were distributed in cluster III whereas genotypes having the lowest SES were found in cluster II (Supplementary Table 4).
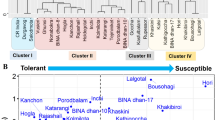
The heatmap based on salt tolerance indices of morphological parameters using Euclidean distance coefficient grouped all the rice genotypes into two main clusters (Fig. 3). The distribution pattern revealed that thirteen genotypes were found in Cluster I, viz., Jessoreswarna, Pothao, Binadhan-17, Nunia, Khoiya, Mulagoboru, Moulota, Swarnagota, Betichikon, Swarnajana, Morichshail, Ghunsi and Khoiramota. These genotypes were considered as salt-susceptible because these genotypes were clustered with susceptible check Binadhan-17, and the heatmap revealed that these genotypes had minimum stress tolerance indices (STIs) values. On the other hand, nineteen genotypes were clustered in Cluster II, viz., Bohorimota, Jotai, Benapole, Pokkali, Lalmatha, Machranga, Ruhango, Rupeshwar-2, Bashfulbalam, Binadhan-10, Blockkhira, Vojon, Gotalia, Holdegotal-2, Rajashail, Ranisalute, Mouthamota, Panbota and Hatibejor. These genotypes were considered as salt tolerant because these genotypes were clustered with tolerant check Binadhan-10 as well as tolerant landrace Pokkali, and the heatmap revealed that these genotypes had maximum STI values based on morphological parameters.
Salt tolerance indices of the rice genotypes based on their morphological traits RLSTI = Root length salt tolerance index, LLSTI = Live leaves (%) salt tolerance index, SLSTI = Shoot length salt tolerance index, RFWSTI = Root fresh weight salt tolerance index, RDWSTI = Root dry weight salt tolerance index, SFWSTI = Shoot fresh weight salt tolerance index, SDWSTI = Shoot dry weight salt tolerance index
Genetic similarity analysis
Molecular characterization of the rice genotypes was performed using two SSR markers, RM7075 and RM10825, to identify the rice genotypes, which possessed Saltol QTL. The DNA bands obtained from different genotypes compared with the DNA bands of phenotypically tolerant genotypes. In this study, considerable variability was found among different genotypes. In most cases, Binadhan-10 and other salt-tolerant cultivars exhibited similar banding patterns. Amplification profiles as revealed by RM10825 and RM7075 across the number of genotypes depicted in Fig. 4 and Table 7. Six genotypes viz., Benapole, Moulota, Mulagoboru, Mouthamota, Holdegotal-2 and Morichshail were found similar. As like as Swarnajana, Betichikon, Rajashail, Gotalia, Jessoreswarna, Khoiya, Ranisalute, Swarnagota, Vojon, Bashfulbalam, Nunia, Lalmatha and Ruhango were in same banding pattern, further, Pothao, Rupeshwar-2, Blockkhira, Bohorimota, Ghunsi, Hatibejor, Jotai, Khoiramota, Machranga and Panbota were genetically similar.
Discussion
After initial screening, thirty-two rice genotypes were selected and exposed to the five different levels of treatments. The genotypes were sorted out and selected as tolerant according to modified SES by studying their various stress symptoms such as yellowing and drying of leaves, leaf curling, reduced rate of growth and seedling dying which were also observed in several rice genotypes due to the effect of salt stress by Bonilla et al. (2002), Bhuiyan (2005) and Islam et al. (2007). However, the seedlings which were not subjected to saline treatments, i.e., grown under control treatment showed normal plant growth. The salt tolerant genotypes showed relatively better growth and fewer symptoms than the susceptible genotypes after salinization. Thirteen genotypes (Panbota, Pokkali, Holdegotal-2, Morichshail, Vojon, Mouthamota, Gotalia, Blockkhira, Lalmatha, Rajashail, Hatibejor, Bohorimota and Binadhan-10) were found highly tolerant and fifteen genotypes were found tolerant at EC: 08 dS m−1 (Table 1). After increasing salinity stress to EC: 10 dS m−1, the number of highly tolerant genotypes decreased to seven (Pokkali, Holdegotal-2, Vojon, Blockkhira, Lalmatha, Rajashail and Hatibejor). At EC: 13 dS m−1 and EC: 15 dS m−1, none of the studied genotypes showed high salt-tolerant ability. Only twelve genotypes (Panbota, Pokkali, Holdegotal-2, Morichshail, Vojon, Mouthamota, Gotalia, Blockkhira, Lalmatha, Rajashail, Hatibejor and Bohorimota) found tolerant at 13 dS m−1 compared with check varieties. Besides, six genotypes (Pokkali, Holdegotal-2, Vojon, Blockkhira, Rajashail and Hatibejor) were tolerant to salt stress at EC: 15 dS m−1 and the rest of them were either moderately tolerant or highly susceptible. It indicates that the tolerance level decreases with the increase of salinity level (Zeng and Shannon 2000).
Analysis of variance specified that the difference among the genotypes for all the traits studied was highly significant and indicated the importance of these traits with significant SES to determine the salt tolerance of the selected genotypes (Table 2) (Rahmanzadeh et al. 2008). Live leaves (%) of plants decreased significantly with the increased salinity levels and eventually caused total drying of leaves in some genotypes such as Pothao, Ruhango, Swarnajana, Betichikon at EC: 15 dS m−1 (Supplementary Table 2). It was observed due to the leaf rolling, drying of leaves, and brownish or whitish leaf tip under the saline condition, which are typical symptoms of salt stress causing a detrimental effect to plant survival (Islam et al. 2007). Shoot and root growth are important indices of salt tolerance (Tuna et al. 2008) which are restricted by salinity stress in rice cultivars. The results from this study showed a decreased root and shoot length in all the genotypes under salinity stress and the tolerant genotypes (Pokkali, Holdegotal-2, Vojon, Blockkhira, Rajashail and Hatibejor) showed higher root and shoot length compared to the susceptible genotypes. Similar observations of reduced root and shoot length under salinity stress were reported by Hussain et al. (2013) and Tahjib-Ul-Arif et al. (2018). In consequence, reduced shoot and root, fresh and dry weight were observed in the study that was also reported by Kapoor and Pande (2015). The reduction in root and shoot fresh and dry weight were lower in salt tolerant rice genotypes than in the sensitive ones (Supplementary Table 2), which were also observed in previous studies (Rasel et al. 2018; Huda et al. 2019). This could result in higher biomass production by the tolerant genotypes under salinity stress (Huda et al. 2019).
A wide range of variation was found in genotypes by treatments, which indicates the existence of huge genetic variation and diversity among the studied genotypes. The genetic analysis showed that the PCV was higher than the GCV for all the characters (Table 3) studied indicating the presence of environmental influence to some extent in the phenotypic expressions (Prajapati et al. 2011; Huda et al. 2019). The higher values of PCV and GCV would further facilitate the possibility of improvement of those genotypes through selection of desired characters. The highest PCV and GCV were found in root fresh weight and shoot fresh weight indicating a wide range of variability among these traits. Very low GCV was found in case of live leaves (%) indicating that lack of inherent variability and limited scope for improvement through selection for this trait among the genotypes (Huda et al. 2017; Rasel et al. 2018).
In plant breeding program, heritability is a crucial parameter that helps to select heritable plant traits (Rathi et al. 2010; Farshadfar et al. 2013; Roy and Shil 2020). The results from the present study reported that all the traits expressed high heritability (98.64–100%) (Table 3). High heritability values indicate that the traits under study are less influenced by the environment and have a greater possibility of genetic improvement through selection methods (Lipi et al. 2020; Roy and Shil 2020). These findings are in accordance with the minimum difference between phenotypic and genotypic variances. Such findings of high heritability coupled with less difference between phenotypic and genotypic variances among different morphological traits selected for the salinity screening of rice genotypes were previously reported by Huda et al. (2017), which allows selecting these traits for breeding purposes in the individual plant by adopting simple selection methods.
The traits studied such as root and shoot fresh weight showed high heritability with high genetic advances (Hosseini et al. 2012) that are controlled by additive gene action (Senguttuvel et al. 2016). It indicates that the accumulation of additive genes will be leading to further improvement in the performance of rice genotypes considering these traits. Furthermore, high heritability with moderate or low genetic advance was found in all other traits of the genotypes studied in this study, which could facilitate the possibility of improvement by inter-crossing with superior genotypes (Samadia 2005; Roy and Shil 2020).
Correlation coefficients of all morphological traits of thirty-two rice genotypes at genotypic and phenotypic levels revealed significant and negative relations with SES which implied the selection criteria of these traits in response to salt stress (Huda et al. 2019) (Table 4). The determination of direct and indirect effects of the traits on the selection criteria was very useful through path coefficient analysis. The path coefficient analysis showed that live leaves (%), shoot and root length, shoot and root fresh weight had a direct negative effect on SES whereas shoot dry weight and root dry weight had a direct positive effect on SES at both genotypic and phenotypic level (Table 5). This finding showed a comparatively higher effect of the shoot and root dry weight on salinity stress indices than the other traits studied (Osman et al. 2012; Rasel et al. 2018; Huda et al. 2019); suggesting that the selection based on these traits would be helpful for the screening and improvement of salt tolerance ability in rice.
Further, clustering of the genotypes based on their morphological performances under salinity stress would facilitate the evaluation of salt tolerance among the rice genotypes (Chunthaburee et al. 2016; Siddiqui et al. 2017). Depending upon the range of diversity, thirty-two rice genotypes were grouped into four clusters (Table 6 and Supplementary Table 3). When compared to intra-cluster distances, the inter-cluster distances were larger, indicating greater genotypic variation among the genotypes of the diverse groups (Pervin et al. 2017). The highest number of genotypes was found in cluster I whereas the lowest number was found in cluster II. The overall composition of the clustering pattern showed that local rice landraces were highly diverse and hence distributed in different clusters. These diverse genotypes could serve as potential germplasm for the improvement of both qualitative and quantitative traits (Rasel et al. 2020). Maximum intra-cluster diversity was observed in cluster II (3.642) along with the highest mean values of all the studied traits coupled with the lowest SES. Hence, mass selection followed by pure line selection within these clusters would be feasible to improve the cultivars of cluster II, in terms of the traits of interest or in general agronomic stability. A simple selection may be practiced based on the top priority for the desirable traits, which would be useful in crop improvement through inter-varietal hybridization (Joshi et al. 2008). On the contrary, the maximum inter-cluster distance was found between cluster II and cluster III (10.857) showing wider variation among the genotypes of these two groups would be beneficial for combining the agronomically superior features in inter-varietal hybridization among the genotypes (Subudhi et al. 2009). Hence, the genotypes from cluster II could be good materials for the development of salt tolerant cultivar (Table 6).
The SSR marker-based screening was reported as the most used and applicable method in rice for salinity tolerance (Kordrostami et al. 2017; Rasel et al. 2018; Huda et al. 2019). In this study, clear bands were observed in almost all genotypes using two SSR markers; RM10825 and RM7075 (Fig. 4) which indicated the presence of Saltol QTL in those genotypes. However, this finding was not absolute to determine the tolerance level of all genotypes although they have Saltol QTL. Using only two markers would not be able to explore the genomic regions of Saltol QTL to find the salt-tolerant germplasm (Singh et al. 2018). Further research in this regard or based on allele-specific markers would be rational to consider. The phenotypic study showed that six genotypes (Pokkali, Holdegotal-2, Vojon, Blockkhira, Rajashail and Hatibejor) were tolerant and ten genotypes were moderately tolerant at EC: 15 dS m−1 (Table 1) which produced DNA bands at molecular experiment by using two SSR markers. Kumari et al. (2018) conducted a similar study to identify salt-tolerant rice lines where Pokkali was identified as a salt-tolerant genotype by using SSR markers. Ghunsi, Holdegotal and Rajashail were also reported as salt-tolerant genotypes likewise salt-tolerant check Binadhan-10 (Rasel et al. 2020). Therefore, the selected rice genotypes through morphological (Table 1) and molecular (Fig. 4 and Table 7) screening could be the potential sources for the use in salinity-tolerant rice breeding as well as molecular marker development.
Conclusions
Rice production is drastically threatened over the southern regions of Bangladesh due to one of the major abiotic stresses, i.e., soil salinity. There are a number of local varieties available and cultivated within this region without prior knowledge of their salt tolerance ability. The present study reported and conserved fifty-seven local rice landraces that could be a milestone for the researchers in studying important traits of rice including yield and salinity tolerance. Moreover, morpho-molecular screening of all these rice genotypes at the most sensitive plant growth stage to salinity i.e., seedling stage revealed six genotypes such as Pokkali, Holdegotal-2, Vojon, Blockkhira, Rajashail and Hatibejor as the best performer in the highest level of salinity treatment i.e., EC: 15 dS m−1. The salt tolerance ability of these local rice genotypes showed better performance than that of the tolerant check, Binadhan-10. Therefore, these genotypes could be used as potential donors of Saltol QTL to develop salt tolerant commercial rice varieties through plant breeding.
References
Ali MN, Yeasmin L, Gantait S, Goswami R, Chakraborty S (2014) Screening of rice landraces for salinity tolerance at seedling stage through morphological and molecular markers. Physiol Mol Biol Plants 20:411–423. https://doi.org/10.1007/s12298-014-0250-6
BBS (2020) The Year Book of Agricultural Statistics of Bangladesh. Bangladesh Bureau of Statistics. Statistics and Informatics Division. Ministry of Planning, Government of the People’s Republic of Bangladesh. Dhaka
Bhuiyan MAR (2005) Efficiency in evaluating salt tolerance in rice using phenotypic and marker assisted selection. MS Thesis, Department of Genetics and Plant Breeding, Bangladesh Agricultural University, Mymensingh, Bangladesh
Bonilla P, Dvorak J, Mackill D, Deal K, Gregorio G (2002) RFLP and SSLP mapping of salinity tolerance genes in chromosome 1 of rice (Oryza sativa L.) using recombinant inbred lines. Philipp Agric Sci 85:68–76
Brindha K, Schneider M (2019) Impact of urbanization on groundwater quality. In Venkatramanan M, Prasanna MV, Chung SY (Editors), GIS and geostatistical techniques for groundwater science. Elsevier, pp 179–196. https://doi.org/10.1016/C2017-0-02667-8
Chowdhury AD, Haritha G, Sunitha T, Krishnamurthy SL, Divya B, Padmavathi G, Ram T, Sarla N (2016) Haplotyping of rice genotypes using simple sequence repeat markers associated with salt tolerance. Rice Sci 23:317–325. https://doi.org/10.1016/j.rsci.2016.05.003
Chunthaburee S, Dongsansuk A, Sanitchon J, Pattanagul W, Theerakulpisut P (2016) Physiological and biochemical parameters for evaluation and clustering of rice cultivars differing in salt tolerance at seedling stage. Saudi J Biol Sci 23:467–477. https://doi.org/10.1016/j.sjbs.2015.05.013
Climate Change Cell (2007) Climate change and Bangladesh. Department of Environment, Government of the People’s Republic of Bangladesh, Dhaka
Dasgupta S, Hossain MM, Huq M, Wheeler D (2015) Climate change and soil salinity: the case of coastal Bangladesh. Ambio 44:815–826. https://doi.org/10.1007/s13280-015-0681-5
FAOSTAT (2015) Worlds’ crop production. Food and Agriculture Organization of the United Nations. Rome, Italy
FAOSTAT (2017) Statistical database. Food and Agriculture Organization of the United Nations, Rome, Italy
Farshadfar E, Romena H, Shabani A (2013) Evaluation of genetic parameters in agro-physiological traits of wheat (Triticum aestivum L.) under irrigated condition. Int J Adv Biol Biom Res 1:331–340
Ganie SA, Borgohain MJ, Kritika K, Talukdar A, Pani DR, Mondal TK (2016) Assessment of genetic diversity of Saltol QTL among the rice (Oryza sativa L.) genotypes. Physiol Mol Biol Plants 22:107–114. https://doi.org/10.1007/s12298-016-0342-6
Gregorio GB, Senadhira D, Mendoza RD, Manigbas NL, Roxas JP, Guerta CQ (2002) Progress in breeding for salinity tolerance and associated abiotic stresses in rice. Field Crops Res 76:91–101. https://doi.org/10.1016/S0378-4290(02)00031-X
Gregorio GB, Senadhira D, Mendoza RD (1997) Screening rice for salinity tolerance. Plant Breeding, Genetics and Biochemistry Division, International Rice Research Institute, The Philippines
Hosseini SJ, Sarvestani ZT, Pirdashti H, Afkhami A, Hazrati S (2012) Estimation of heritability and genetic advance for screening some rice genotypes at salt stress conditions. Int J Agron Plant Prod 3:475–482
Huda MM, Ferdausi A, Hassan L, Hossain MA (2017) Evaluation of the genetic variability and genetic advance for salt tolerance in rice genotypes. Prog Agric 28:262–270
Huda MM, Nuruzzaman M, Prodhan MA, Ferdausi A, Hossain A (2019) Characterization of salt tolerance in rice landraces (Oryza sativa L.) at seedling stage. Indian J Nat Sci 10:17613–17629
Hussain M, Park HW, Farooq M, Jabran K, Lee DJ (2013) Morphological and physiological basis of salt resistance in different rice genotypes. Int J Agric Biol 15:113–118
Islam MZ, Baset Mia MA, Islam MR, Akter A (2007) Effect of different saline levels on growth and yield attributes of mutant rice. J Soil Nat 1:18–22
Joshi MA, Singh P, Sarao NK, Sharma RC, Bharaj TS (2008) Genetic diversity among the rice varieties cultivated in Punjab. Oryza 45:277–279
Kapoor N, Pande V (2015) Effect of salt stress on growth parameters, moisture content, relative water content and photosynthetic pigments of fenugreek variety RMt-1. J Plant Sci 10:210–221. https://doi.org/10.3923/jps.2015.210.221
Kordrostami M, Rabiei B, Kumleh HH (2017) Biochemical, physiological and molecular evaluation of rice cultivars differing in salt tolerance at the seedling stage. Physiol Mol Biol Plants 23:529–544. https://doi.org/10.1007/s12298-017-0440-0
Krishnamurthy SL, Sharma PC, Sharma SK, Batra V, Kumar V, Rao LVS (2016) Effect of salinity and use of stress indices of morphological and physiological traits at the seedling stage in rice. Indian J Exp Biol 54:843–850
Krishnamurthy SL, Pundir P, Warraich AS, Rathor S, Lokeshkumar BM, Singh NK, Sharma PC (2020) Introgressed Saltol QTL lines improves the salinity tolerance in rice at seedling stage. Front Plant Sci 11:1–13. https://doi.org/10.3389/fpls.2020.00833
Kumari R, Kumar P, Sharma VK, Kumar H (2018) Seedling stage salt stress response specific characterization of genetic polymorphism and validation of SSR markers in rice. Physiol Mol Biol Plants 25:407–419. https://doi.org/10.1007/s12298-018-0623-3
Lipi LF, Hasan MJ, Akter A, Quddus MR, Biswas PL, Ansari A, Akter S (2020) Genetic variation, heritability and genetic advance in some promising rice hybrids. SAARC J Agric 18:39–49. https://doi.org/10.3329/sja.v18i2.51107
Mishra PK, Ram RB, Kumar N (2015) Genetic variability, heritability, and genetic advance in strawberry (Fragaria × ananassa Duch.). Turk J Agric for 39:451–458. https://doi.org/10.3906/tar-1408-99
Osman KA, Mustafa AM, Ali F, Yonglain Z, Fazhan Q (2012) Genetic variability for yield and related attributes of upland rice genotypes in semi arid zone (Sudan). Afr J Agric Res 7:4613–4619. https://doi.org/10.5897/AJAR12.529
Pervin MS, Halder T, Khalequzzaman M, Kader MA, Aditya TL, Yasmeen R (2017) Genetic diversity and screening of rice (Oryza sativa L.) genotypes for drought tolerance at reproductive phase. Bangladesh Rice J 21:27–34. https://doi.org/10.3329/brj.v21i1.37375
Prajapati MK, Singh CM, Babu GS, Lavanya GR, Jadhav P (2011) Genetic parameters for grain yield and its component characters in rice. Electron J Plant Breed 2:235–238
Rahmanzadeh S, Kazemitabar K, Yazdifar S, Jafroudi AT (2008) Evaluation of rice (Oryza sativa L.) cultivars response to salinity stress through greenhouse experiment and tissue culture technique. Asian J Plant Sci 7:207–212. https://doi.org/10.3923/ajps.2008.207.212
Rasel M, Hassan L, Injamum-Ul-Hoque M, Saha SR (2018) Estimation of genetic variability, correlation and path coefficient analysis in local landraces of rice (Oryza sativa L.) for the improvement of salinity tolerance. J Bangladesh Agric Univ 16:41–46
Rasel M, Tahjib-Ul-Arif M, Hossain MA, Sayed MA, Hassan L (2020) Discerning of rice landraces (Oryza sativa L.) for morpho-physiological, antioxidant enzyme activity, and molecular markers’ responses to induced salt stress at the seedling stage. J Plant Growth Regul 39:41–59. https://doi.org/10.1007/s00344-019-09962-5
Rathi S, Yadav RNS, Sarma RN (2010) Variability in grain quality characters of upland rice of Assam, India. Rice Sci 17:330–333. https://doi.org/10.1016/S1672-6308(09)60035-4
Razzaque S, Haque T, Elias SM, Rahman MS, Biswas S, Schwartz S, Ismail AM, Walia H, Juenger TE, Seraj ZI (2017) Reproductive stage physiological and transcriptional responses to salinity stress in reciprocal populations derived from tolerant (Horkuch) and susceptible (IR29) rice. Sci Rep 7:1–16. https://doi.org/10.1038/srep46138
Roy SC, Shil P (2020) Assessment of genetic heritability in rice breeding lines based on morphological traits and caryopsis ultrastructure. Sci Rep 10:1–17. https://doi.org/10.1038/s41598-020-63976-8
Sahi C, Singh A, Kumar K, Blumwald E, Grover A (2006) Salt stress response in rice: genetics, molecular biology, and comparative genomics. Funct Integr Genom 6:263–284. https://doi.org/10.1007/s10142-006-0032-5
Samadia DK (2005) Genetic variability studies in Lasora (Cordia myxa Roxb.). Indian J Plant Genet Resour 18:236–240
Schneider P, Asch F (2020) Rice production and food security in Asian Mega deltas—a review on characteristics, vulnerabilities and agricultural adaptation options to cope with climate change. J Agron Crop Sci 206:491–503. https://doi.org/10.1111/jac.12415
Senguttuvel P, Sravan Raju N, Padmavathi G, Sundaram RM, Madhav S, Hariprasad AS, Kota S, Bhadana VP, Subrahmanyam D, Subba Rao LV, Brajendra RV (2016) Identification and quantification of salinity tolerance through salt stress indices and variability studies in rice (Oryza sativa L.). SABRAO J Breed Genet 48:172–179
Siddiqui MN, Mostofa MG, Akter MM, Srivastava AK, Sayed MA, Hasan MS, Tran LSP (2017) Impact of salt-induced toxicity on growth and yield potential of local wheat cultivars: oxidative stress and ion toxicity are among the major determinants of salt-tolerant capacity. Chemosphere 187:385–394. https://doi.org/10.1016/j.chemosphere.2017.08.078
Singh VK, Singh BD, Kumar A, Maurya S, Krishnan SG, Vinod KK, Singh MP, Ellur RK, Bhowmick PK, Singh AK (2018) Marker-assisted introgression of Saltol QTL enhances seedling stage tolerance in the rice variety “Pusa Basmati 1.” Int J Genomics 2018:1–12. https://doi.org/10.1155/2018/8319879
SRDI (2010) Saline soils of Bangladesh. SRMAF Project, Ministry of Agriculture, Banglades, pp 1–60
Subudhi HN, Meher J, Bose LK, Das S (2009) Genetic diversity studies of promising rice varieties of eastern India based on quality characters. Oryza 46:271–274
Tahjib-Ul-Arif M, Sayed MA, Islam MM, Siddiqui MN, Begum SN, Hossain MA (2018) Screening of rice landraces (Oryza sativa L.) for seedling stage salinity tolerance using morpho-physiological and molecular markers. Acta Physiol Plant 40:1–12. https://doi.org/10.1007/s11738-018-2645-4
The Business Standard, 2023. https://www.tbsnews.net/bangladesh/salt-tolerant-rice-varieties-bring-hope-bangladesh-farmers-410202
Tuna AL, Kaya C, Higgs DM, D, (2008) The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ Exp Bot 62:1–9. https://doi.org/10.1016/j.envexpbot.2007.06.007
Wankhade SD, Cornejo MJ, Mateu-Andrés I, Sanz A (2013) Morpho-physiological variations in response to NaCl stress during vegetative and reproductive development of rice. Acta Physiol Plant 35:323–333. https://doi.org/10.1007/s11738-012-1075-y
Zeng L, Shannon MC (2000) Salinity effects on seedling growth and yield components of rice. Crop Sci 40:996–1003. https://doi.org/10.2135/cropsci2000.404996x
Zeng L, Shannon MC, Grieve CM (2002) Evaluation of salt tolerance in rice genotypes by multiple agronomic parameters. Euphytica 127:235–245. https://doi.org/10.1023/A:1020262932277
Zhao X, Wang W, Zhang F, Deng J, Li Z, Fu B (2014) Comparative metabolite profiling of two rice genotypes with contrasting salt stress tolerance at the seedling stage. PLoS ONE 9:1–7. https://doi.org/10.1371/journal.pone.0108020
Acknowledgements
The financial support provided by the granted project (Code No.: 2018/623/BAU) from the Bangladesh Agricultural University Research System (BAURES), Bangladesh Agricultural University, Mymensingh-2202. The authors are grateful to the Bangladesh Institute of Nuclear Agriculture (BINA), Mymensingh-2202, Bangladesh for providing rice seeds and also grateful to the Shamsun Nahar Begum, Chief Scientific Officer and Head, Plant Breeding Division, BINA, for providing facilities in molecular analyses at the molecular plant breeding laboratory, BINA.
Author information
Authors and Affiliations
Contributions
Md. Abdus Sogir performed the morphological and molecular experiments, collected and analyzed the data and drafted the manuscript. Anjan Chandra Sharma performed the molecular experiments, curated and analyzed the data and wrote the final version of the manuscript. Snigdha Sharmin Mithila performed the experiments and collected the data. Md. Rasel involved in experimental planning and supervision. Aleya Ferdausi planned the project, acquired fund, supervised the project and reviewed and edited the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sogir, M.A., Sharma, A.C., Mithila, S.S. et al. Morpho-molecular assessment of local rice (Oryza sativa L.) genotypes at seedling stage for salinity tolerance. CEREAL RESEARCH COMMUNICATIONS 52, 423–437 (2024). https://doi.org/10.1007/s42976-023-00438-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-023-00438-7