Abstract
Significant progress has been made in unraveling the molecular biology of rice in the past two decades. Today, rice stands as a forerunner amongst the cereals in terms of details known on its genetics. Evidence show that salt tolerance in plants is a quantitative trait. Several traditional cultivars, landraces, and wild types of rice like Pokkali, CSR types, and Porteresia coarctata appear as promising materials for donation of requisite salt tolerance genes. A large number of quantitative trait loci (QTL) have been identified for salt tolerance in rice through generation of recombinant inbred lines and are being mapped using different types of DNA markers. Salt-tolerant transgenic rice plants have been produced using a host of different genes and transcript profiling by micro- and macroarray-based methods has opened the gates for the discovery of novel salt stress mechanisms in rice, and comparative genomics is turning out to be a critical input in this respect. In this paper, we present a comprehensive review of the genetic, molecular biology, and comparative genomics effort towards the generation of salt-tolerant rice. From the data on comprehensive transcript expression profiling of clones representing salt-stress-associated genes of rice, it is shown that transcriptional and translational machineries are important determinants in controlling salt stress response, and gene expression response in tolerant and susceptible rice plants differs mainly in quantitative terms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Salt stress in rice cultivation
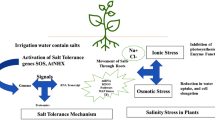
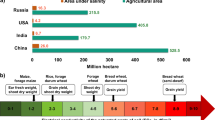
Although Na+ is a plant micronutrient, excess Na+ levels are toxic for plant growth. Salinity adversely affects quantity and quality of the crop produce (Gepstein et al. 2006; Blumwald and Grover 2006). About 20% of irrigated agricultural land is considered to be saline (Flowers and Yeo 1995). Salt-affected soils in arid and semi-arid regions of Asia, Africa, and South America cause considerable agronomic problems. In Asia alone, 21.5 million ha of land area is thought to be salt affected (of which 12 million ha is due to saline conditions and the remaining 9.5 million ha is due to alkaline/sodic conditions), with India having 8.6 million ha salt-affected area including 3.4 million ha sodic soils. It is further important to note that salt stress problems in field crops are getting accentuated with the increasing irrigation networks.
Rice is an important food crop for the entire world population. While active efforts are being made to increase rice productivity, a considerable amount of rice biomass for which genetic potential exists in the present-day cultivars is not harvested under field conditions, primarily because of the sensitivity of this crop to various stresses (Widawsky and O’Toole 1990; Shimamoto 1999; Minhas and Grover 1999). Rice is a salt-sensitive crop (Grover and Pental 2003).
Excess salts adversely affect all major metabolic activities in rice including cell wall damage, accumulation of electron-dense proteinaceous particles, plasmolysis, cytoplasmic lysis and damage to ER, accumulation of citrate, malate, and inositol in leaf blades within 1 d of salt treatment, increase in proline levels by 4- to 20-fold, decrease in Fv/Fm ratio, reduction in photosynthesis, and overall decline in germination and seedling growth (Yeo et al. 1985; Lutts et al. 1995; Garcia et al. 1997; Khan et al. 1997; Pareek et al. 1997; Sivakumar et al. 1998), leading ultimately to reduced growth and diminished grain yield. Relatively speaking, rice is considered as more sensitive to salts during early seedling than at reproductive stages (Flowers and Yeo 1981; Lutts et al. 1995). The parameters like high vigor, salt exclusion at the root level, compartmentalization of ions in structural and older tissues, high tissue tolerance, responsive stomata that close within minutes after exposure to salt stress but partially reopen after a period of acclimation, and up-regulation of antioxidant systems, particularly the ascorbate/glutathione pathway of oxidative stress tolerance, are among the major factors that govern the growth-stage-dependent response of rice to salt application.
A large body of evidence indicates that the response of plant cells to high salts is controlled by multiple genes (Bartels and Sunkar 2005; Chinnusamy et al. 2005; Sahi et al. 2006). To maximize the productivity of rice under saline soils, there is an urgent need to look for: (1) sources of genetic variation that can be used for developing new cultivars with greater yield potential and stability over seasons and ecogeographic locations, (2) identification of molecular markers associated with salt stress tolerance genes or QTL conferring tolerance to salt stress conditions for their use in marker-assisted breeding programs, and (3) discovery of genes imparting salt tolerance and their introduction in salt-sensitive rice cultivars (Blumwald and Grover 2006). For reasons such as the availability of well-saturated genetic maps (Harushima et al. 1998; Chen et al. 2002; McCouch et al. 2002), complete sequence information regarding the chloroplast, mitochondrial and nuclear genomes (Kanno and Hirai 1993; Notsu et al. 2002; IRGSP 2005), full-length cDNAs (Kikuchi et al. 2003), full-genome transcription profilings using high-density oligonucleotide tiling microarrays (Li et al. 2006), Tos17 insertion lines and enhancer trap lines (Miyao et al. 2003; Hirochika et al. 2004; Sallaud et al. 2004) and highly-efficient Agrobacterium-mediated genetic transformation (Minhas and Grover 1999; Roy et al. 2000), rice in recent years has emerged as a model species for understanding the cereal genetics (see special issue of Plant Molecular Biology, http://www.springerlink.com/openurl.asp?genre=issue&eissn=1573-5028&volume=59&issue=1).
To facilitate the comparison and the understanding of gene characteristics, genome organization, pathways, and phenotypes in cereals, there are attempts to develop a comparative genome mapping database for grasses using the rice genome as an anchor (http://www.gramene.org) (Jaiswal et al. 2006). We herewith discuss the progress made in understanding the details of the salt stress response using various tools like traditional plant breeding methods, marker-assisted selection-based approach, and transgenic rice production technique, and, finally, through the tools of comparative genomics.
Traditional breeding for augmenting salt tolerance in rice
The generation of salt-tolerant rice varieties through traditional breeding approach can only be realized if the genes for salt tolerance occur in the gene pool of rice species or in its closely related wild species. In a comparison of the relative tolerance of seven wild species and two cultivated species of rice (Oryza sativa and Oryza glaberrima), none of the wild species was found to be as tolerant as the most resistant of the cultivated lines of O. sativa tested (Akbar et al. 1987). According to Flowers et al. (1990), source(s) of salt tolerance is unavailable within the cultivated germplasm of O. sativa. Nonetheless, there are clear indications that some traditional cultivars and landraces of rice (e.g., Pokkali, Nona Bokra, Bura Rata, etc.) are more salt tolerant than many elite cultivars. In fact, Pokkali rice has been frequently used as a donor of salt tolerance genes in breeding programs. Extensive physiological and molecular studies have been carried out employing Pokkali rice for understanding the mechanisms of salt tolerance (Moons et al.1995; Kawasaki et al. 2001). The increased salt tolerance of Pokkali is usually attributed to both its capacity to maintain a low Na+/K+ratio in shoot tissues and its faster growth rate under saline conditions. According to Anil et al. (2005), Na+ is buffered in shoots of Pokkali by intracellular compartmentalization which is a basis of its robust salt stress response. A recombinant inbred population was developed at the International Rice Research Institute (IRRI), Philippines, using Pokkali and IR29 (IR29 is an improved indica cultivar often used as a salt-sensitive standard) (Bonilla et al. 2002). Several other salt-tolerant and salt-sensitive recombinant inbred lines (RILs) have also been identified in screening for salinity tolerance (Gregorio et al. 2002). Genetic studies have revealed that salt tolerance of these varieties is principally due to additive gene effects; however, the underlying molecular mechanism of salt tolerance has barely been experimentally investigated. At the Central Soil Salinity Research Institute (CSSRI), Karnal, India, CSR27 rice type has been bred through crossing Nona Bokra and IR 5657-33-2 rice types. CSR27 variety shows high tolerance to sodic (pH 9.6–9.9) and saline [electronic conductivity (EC) up to 9 dS m−1] soils. Another cultivar developed at CSSRI is CSR10 for sodic and inland saline soils. It is derived from the cross M40-431-24-114 and Jaya. CSR10 can withstand highly deteriorated alkaline (pH 9.8–10.2) and inland saline soil (EC 6–10 dS m−1) conditions under transplanted irrigated management system. Other promising rice types identified include Pobbeli (Indonesia), IR2151 (Sri Lanka), PSBRc 48, PSBRc 50, PSBRc 84, PSBRc 86, PSBRc 88, and NSIC 106 (IRRI, Indonesia). P. coarctata Tateoka, a native of salt marshes in Southeast Asia and considered a wild relative of O. sativa, is found to be highly salt tolerant (Flowers et al. 1990). It is suggested that P. coarctata can be used as a parent for evolving better salt-tolerant rice varieties. Majee et al. (2004) showed that l-myo-inositol-1-phosphate synthase (MIPS) enzyme (which catalyzes synthesis of inositol) from P. coarctata is more salt tolerant than MIPS from O. sativa under in vitro conditions of testing. It is further revealed that the structure of P. coarctata MIPS is relatively more stable towards high salt concentrations. An introgression of this gene rendered transgenic tobacco capable of growth in 200–300 mM NaCl with retention of ~40–80% of photosynthetic competence with concomitant increased inositol production compared with the unstressed control tobacco plants (Majee et al. 2004). MIPS protein isolated from transgenic tobacco plants also showed salt-tolerant properties in vitro. The same group is presently attempting to study how salt-sensitive rice type will respond if the MIPS protein from P. coarctatais transgressed in it, through transgenic methods (A. Lahiri-Majumdar, personal communication).
Finally, the fact remains that the landraces or the wild types discussed above are associated with a host of inherent problems of poor agronomic traits (such as tall plant stature, photo-sensitivity, poor grain quality and low yield). Therefore, the breeding for increased salt tolerance using such materials remains as a distant possibility.
Molecular markers and QTL linked to rice salt tolerance
Molecular/genetic rice maps have been constructed using F2 population or RILs derived from varieties that are genetically far apart, such as japonica and indica rice types as parents. Such a combination generates considerably more polymorphism than that between the same subspecies. Because of the ability to generate a large seed supply, RILs have been used to map several QTL in rice (Wang et al. 1994; Tsunematsu et al. 1996; Xiao et al. 1996; Nandi et al. 1997). Doubled haploid (DH) populations have also been used (Lorieux et al. 1996; Yadav et al. 1997); however, extensive use of DH plants is limited due to difficulty in generating sufficient number of plants through anther culture. Backcrosses and other types of crosses have not been widely used in rice gene mapping. McCouch et al. (1988) reported the first molecular genetic map of rice using RFLP technique; various fine maps have since been released using a variety of different markers including simple sequence repeat (SSR), amplified fragment length polymorphism, and random amplified polymorphic DNA (Causse et al. 1994; Kurata et al. 1994; Harushima et al. 1998). These as well as the genomic tools and methods that have become available [such as expressed sequence tags (ESTs) from salt-stressed libraries, complete genome sequence information, development of new bioinformatics tools, expression profiling by microarrays, random and targeted mutagenesis, and complementation and promoter-trapping strategies] provide opportunities to characterize the salt-tolerance-related gene networks in greater depths (Bohnert et al. 2001; Sahi et al. 2006). Prasad et al. (2000a,b) identified seven QTL for seedling traits associated with salt stress and mapped these to five different chromosomes. Using F2 population derived from a salt-tolerant japonica rice mutant, M-20, and the sensitive original variety 77-170A, Zhang et al. (1995) mapped a major gene for salt tolerance on chromosome 7. Koyama et al. (2001) reported the chromosomal location of ion transport and selectivity traits that are compatible with agronomic needs. This group showed that QTL for Na+ and K+transport are likely to act through the control of root development and structure and the regulation of membrane-localized transport compartmentalization, respectively. Gregorio et al. (2002) have mapped a major QTL designated Saltol on chromosome 1 (flanked by SSR markers RM23 and RM140) using a population generated from a cross between salt-sensitive IR29 and salt-tolerant Pokkali. This QTL accounted for more than 70% of the variation in salt uptake (Bonilla et al. 2002) and is now being mapped to within 1-cM distance using a large set of near isogenic lines (NILs). Pokkali was the source of positive alleles for this major QTL (Bonilla et al. 2002), which accounted for high K+ and low Na+ absorptions and low Na+-to-K+ ratio under salinity stress (Gregorio et al. 2002). Lin et al. (2004) have identified a major QTL for high shoot K+ under salinitystress in the same region of chromosome 1. Ren et al. (2005) have recently made a breakthrough in mapping SKC1, which maintains K+ homeostasis in the salt-tolerant variety Nona Bokra under salt stress. SKC1 was mapped to chromosome 1. Using finely mapped BC2F2 population, the SKC1 locus was cloned. This group further showed that the SKC1 locus was able to confer salt tolerance, when transformed into salt-sensitive variety. A detailed analysis showed that SKC1 protein is highly similar to HKT-type transporters. More details on analysis of salt-stress-associated QTL are provided in Table 1.
Salt tolerance in rice through transgenics approach
Salinity imposes water deficit that results from the relatively high solute concentration in the soil, causes ion-specific stresses resulting from altered Na+/K+ ratios, and leads to buildup in Na+ and Cl− concentrations that are detrimental to growth and other life processes (Flowers 2004; Munns 2005; Vinocur and Altman 2005). Plants respond to salt stress by restricting the uptake of salt and adjust their osmotic pressure by the synthesis of compatible solutes (proline, glycinebetaine, sugars, etc.) (Greenway and Munns 1980) and sequestering salt into the cell vacuoles for the maintenance of low cytosolic Na+ levels (Glenn et al. 1999; Gepstein et al. 2006; Blumwald and Grover 2006). Transgenic rice plants tolerant to salt stress have been produced taking clues from the above account (Sahi et al. 2006). Xu et al. (1996) generated transgenic rice (cv Nipponbare) over-expressing barley hva1 gene (encoding a Late Embryogenesis Abundant, LEA) protein. This group exposed 3-week-old transformed and untransformed seedlings to two rounds of stress (200 mM NaCl for 10 d, followed by a recovery period of 10 d and a second round of 50 mM NaCl stress for 30 d) and noted that the transgenic plants maintained a higher growth rate initially and showed better recovery on removal of stress. Sakamoto et al. (1998) genetically engineered rice with the ability to synthesize glycinebetaine by introducing the codA gene (encoding for choline oxidase, obtained from the soil bacterium Arthrobacter globiformis). Levels of glycinebetaine were as high as 1 and 5 μmol per gram fresh weight of leaves in two types of transgenic plant in which choline oxidase was targeted to the chloroplasts (ChlCOD plants) and to the cytosol (CytCOD plants), respectively. Although treatment with 0.15 M NaCl inhibited the growth of both wild-type (WT) and transgenic plants, the transgenic plants recovered faster than the wild type plants and resumed normal growth after removal of the salt stress. It was indicated that ChlCOD plants are more tolerant than CytCOD plants to photoinhibition under salt stress (and low-temperature stress). Saijo et al. (2000) generated transgenic rice (cv Notohikari) plants over-expressing a Ca2+-dependent protein kinase (OsCDPK7). The youngest leaves wilted 3 days after salt stress of 200 mM NaCl in WT plants, while transgenic plants exhibited greater tolerance. Mohanty et al. (2002) generated transgenic lines of indica rice Pusa basmati 1 (PB 1) by Agrobacterium-mediated transformation with codA gene. In this work, more than 50% of the transgenic plants could survive salt stress and set seed whereas the WT plants failed to recover. Garg et al. (2002) generated transgenic indica rice PB 1, expressing the fusion gene comprising both trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase obtained from Escherichia coli. Compared to WT rice, transgenic lines exhibited sustained plant growth, less photo-oxidative damage, and more favorable mineral balance under salt (also drought and low temperature) stress conditions. Jang et al. (2003) reported the generation of trehalose-producing, transgenic japonica rice plants by the introduction of a gene encoding a bifunctional fusion (TPSP) of the trehalose-6-phosphate (T-6-P) synthase (TPS) and T-6-P phosphatase (TPP) of E. coli, under the control of the maize ubiquitin promoter. Increased trehalose accumulation in transgenic plants resulted in increased tolerance to drought, salt, and cold. Transgenic R1 rice overexpressing p5cs (encoding pyrroline carboxylate synthase, an enzyme that rate-limits proline biosynthesis) showed increased biomass than the control plants under salt stress and water stress conditions (Zhu et al. 1998). Considering that mechanisms that reduce cytosolic Na+ concentrations could provide a degree of tolerance to relatively low NaCl concentrations (Blumwald and Grover 2006), Ohta et al. (2002) engineered salt-sensitive rice cultivar Kinhuikari using the Na+/H+ antiporter gene from Atriplex gmelini (AgNHX1). The activity of the vacuolar-type Na+/H+ antiporter in the transgenic rice plants was eightfold higher than in WT rice plants. Salt tolerance assays followed by non-stress treatments showed that the transgenic plants over-expressing AgNHX1 could survive under conditions of 300 mM NaCl for 3 days while the WT plants could not. Zhao et al. (2006a) have recently generated transgenic rice plants with SsNHX1 from Suaeda salsa which showed markedly enhanced tolerance to salt stress (and to water deprivation) compared with non-transgenic controls grown under outdoor conditions. More details on rice transgenics produced thus far for increased salt tolerance are presented in Table 2.
Comparative genomics and proteomics
The salt stress response is a quantitative trait, involving activation of a large number of specific genes concomitant to the repression in activity of a large number of housekeeping genes (Sahi et al. 2006). Major alterations in transcriptional and post-transcriptional activities have been noted to accompany response of plants to salt stress. Micro- and macroarray-based transcriptional profiling provides quantitative information about the expression levels of a large number of genes simultaneously. In higher plants, different EST/cDNA collections have been employed for transcriptional profiling to identify genes whose expression levels change in response to salt (Seki et al. 2001; Kanesaki et al. 2002; Sahi et al. 2003; Kore-Eda et al. 2004; Marin et al. 2004; Sottosanto et al. 2004; Taji et al. 2004; Shiozaki et al. 2005). Transcriptional profiling data from Arabidopsis thaliana and O. sativa suggest that metabolic readjustment is one of the hallmarks of the salt stress response (Kawasaki et al. 2001; Kreps et al. 2002; Seki et al. 2002; Oono et al. 2003; Rabbani et al. 2003; Sottosanto et al. 2004). From the data obtained, it appears that overall tolerance to high salt levels is due to effectors that directly modulate stress etiology or attenuate stress effects and due to regulatory molecules that are involved in stress perception, signal transduction, and modulation of the effectors’ functions (Hasegawa et al. 2000; Sahi et al. 2003). Enzymes that catalyze rate-limiting steps in the biosynthesis of compatible osmolytes and proteins that protect membrane integrity, control water or ion homeostasis, and bring about scavenging of reactive oxygen species (ROS) are the examples of stress tolerance effectors. Regulatory determinants include transcription factors that interact with promoters of osmotic-stress-regulated genes (like rd22BP1, AtMYB2, DREBIA, and DREB2A) and intermediates that post-transcriptionally activate the effectors [SOS3 (Ca2+-binding protein), SOS2 (a Ca2+-dependant protein kinase), and SOS1 (putative plasma membrane Na+/H+ antiporter)] (Chinnusamy et al. 2005).
Further progress on the transcript changes in response to salt application has been made using the comparative genomics approach. Comparative stress genomics essentially means that various commonalities and differences in expression patterns of different genes relative to populations that differ in stress tolerance are scored. This approach appears highly valuable for unveiling the key genetic contributors to the complex physiological processes involved in salt tolerance trait (Bressan et al. 2001). The identification of salt-stress-specific changes in gene expression can be achieved by comparing gene expression in non-induced and stress-induced tissues or by comparing genetically different genotypes such as contrasting cultivars (Grover et al. 1993). Taji et al. (2004) noted that a fewer number of genes was induced by 250 mM NaCl stress in Thellungiella halophila (salt cress, a wild salt-tolerant relative of Arabidopsis), in contrast to Arabidopsis, indicating that the stress tolerance of salt cress may be due to the constitutive overexpression of many genes that function in stress tolerance and that are only stress inducible in Arabidopsis. A larger spectrum of gene expression changes noted between Atnhx1 knockout mutant (Apse et al. 2003) and wild type plants by Sottosanto et al. (2004) showed that salt-sensitive and salt-tolerant Arabidopsis phenotypes differ markedly in expression of their genetic machinery. Kawasaki et al. (2001) examined transcript regulation in response to high salinity in the salt-tolerant rice cultivar Pokkali and salt-sensitive rice cultivar IR29 using a microarray of 1,728 cDNAs from different libraries obtained from salt-stressed tissues. They suggested that the gene expression response to salt in contrasting rice plants is both qualitative as well as quantitative. Shiozaki et al. (2005) recently showed that transcript levels of a large number of genes (including those associated with detoxification, stress response, signal transduction, etc.) undergo pronounced changes upon subjecting seedlings of salt-tolerant rice (Dee-geo-woo-gen) to salt stress. Walia et al. (2005) investigated the response of salt stress in IR29 and salt-tolerant FL478 line (FL478 is RIL obtained from a cross of IR29 and Pokkali) using microarray. This group found that salinity stress induced a large number of genes involved in the flavanoid biosynthesis pathway in IR29 but not in FL478. Cell-wall-related genes were responsive in both genotypes, suggesting that cell wall restructuring is a general adaptive mechanism during salinity stress.
Comparative profiling of salt-stress-response-associated transcripts in Pusa basmati 1, CSR27, and Pokkali rice types
To identify and isolate genes that are differentially regulated by salt stress in rice plants which are extensively grown in India, three indica cultivars, namely, Pusa basmati 1 (PB 1, salt sensitive), CSR 27 (salt tolerant), and Pokkali (salt tolerant), were employed by Sahi et al. (2003). These three rice varieties showed distinctive growth differences upon treating seedlings with NaCl stress (Sahi et al. 2003). All India evaluation studies have shown that CSR 27 is a highly salt-tolerant rice cultivar (Mishra and Singh 2000). Pokkali rice is a local selection in India noted for high salt tolerance. This rice type has in fact often been taken as a check in several national and international programs aimed at evolving genotypes combining high salt tolerance and high crop yield (Akbar et al. 1986a,b). PB 1 is a premier rice type from India combining high yield with a much sought-after “basmati” flavor. A sensitive and highly efficient polymerase chain reaction (PCR)-based subtraction method employed to construct four subtracted cDNA libraries (namely, T2D1, T4D1, T4D2, and T3D5; see Table 3 for selected details on these libraries) yielded 1,266 cDNA clones (Sahi et al. 2003). Expression profiling of 969 subtracted clones was carried out using macroarray analysis. Separate replicate membranes were probed with radiolabeled total cDNA corresponding to control and salt-stressed roots/shoots of different tester and driver tissues (details shown in Table 3). A representative blot corresponding to the analysis of T2D1 library is shown in Fig. 1. In this set, 234 clones were spotted on the nylon membrane (Fig. 1a). The details of the expression characteristics of specified clones are indicated in Fig. 1b. Salt-up regulated clones, salt-down regulated clones, and clones that are not significantly affected by salt stress are shown by dark blue, light blue, and white colored boxes, respectively, in this representation. Clones that are differentially expressed in the Pokkali and PB 1 are shown by grey colored boxes in Fig. 1b. Further details on expression of various ESTs from this and three other libraries based on macroarray data are shown in Table 5 (annotations for the selected clone numbers shown in Fig. 1b are presented in Table 4 as mentioned below). One hundred eighty-three cDNA clones representing both up- and down-regulation on the basis of macroarray profiles (chosen randomly) were sequenced either manually or by automated sequencing from 3′ ends. The sequences were annotated using different public databases. Most of the clones could be identified using the KOME database in particular, and their putative functions were assigned. In selected cases, our sequences did not match with any entry in KOME libraries but homologous counterparts were noted in EST databases. Based on the annotation analysis as presented in Table 4, a wide repertoire of genes belonging to different functionality classes (such as signaling molecules, transcriptional machinery, translational machinery, protein turnover and folding, ion transporters and metal binding proteins, and general metabolism) were seen associated with the response of different rice varieties to salt stress. The expression pattern of 77 ESTs (selected based on salt-regulated/cultivar-specific expression patterns as noted in macroarray blots) was further examined by Northern blotting (Fig. 2). Several ESTs were noted to be salt induced in this analysis. This category included putative Zn-finger protein (T4D1-144), subtilisin-chymotrypsin inhibitor (T4D2-165 and T4D2-50), cytosolic glyceraldehyde-3-phosphate dehydrogenase (T4D2-43), FAD-linked oxidoreductase (T2D1-173), NAD kinase (T4D2-114), vacuolar ATPase (T4D2-45), ribosomal protein genes (T4D2-66, T2D1-177 and T2D1-288), putative aldolase (T4D1-61), putative serine/threonine kinase (T2D1-99), ubiquitin ligase (T2D1-302), MD2 lipid recognition protein (T2D1-101), putative tumor protein (T3D5-302), salT(T4D2-96), ADP ribosylation factor (T3D5-13), etc. Genes showing homology to eukaryotic translation initiation factor (eIF2α) (T4D1-219) and an uncharacterized reteroelement (T4D2-130a) were also noted to be salt induced. Selected ESTs representing genes with unknown functions were found to be salt inducible. On the other hand, selected clones were noted to be down-regulated by salt treatment (such as T2D1-180, T4D1-209, and T4D2-231) or were expressed in comparable amounts in control and salt-treated tissues (such as T4D1-82 and T4D2-68). Clones showing up-regulation and down-regulation of the transcripts in response to salt treatment are shown by  and
and  arrows in Fig. 2, respectively. On the other hand, clones which did not show any marked change upon salt treatment are shown with
arrows in Fig. 2, respectively. On the other hand, clones which did not show any marked change upon salt treatment are shown with  symbol. The clones displaying differential response to salt application between the salt-sensitive PB 1 and salt-tolerant CSR 27 or Pokkali cultivars [such as ubiquitin ligase (T2D1-302)] are shown with bold font of the clone numbers in Fig. 2. On the other hand, clones showing differential transcript levels in response to salt application between the two salt-tolerant cultivars (CSR 27 and Pokkali) [such as putative amino acid transporter (T4D1-163)] are shown with underlining of the clone numbers in Fig. 2. The clones showing constitutive differential expression or salt-induced differential expression in the three rice varieties are summarized in Table 5. In the macroarray blots, homologous cDNA clones were detected using probes corresponding to both tester and driver rice varieties in a given subtraction set (Fig. 1). Thus, contrasting rice genotypes appear to have a similar genetic response to imposed stress in qualitative terms. This implies that the differences in the relative salt response in contrasting genotypes are controlled largely by the differential regulation of several stress-regulated genes. Kawasaki et al. (2001) noted that the stress-sensitive IR29 rice cultivar responded to stress slower than the salt-tolerant rice cultivar Pokkali. Several transcripts were induced within the first 15 min of the imposed stress in Pokkali, while the same took approximately 3 h in the salt-sensitive IR29 rice type (Kawasaki et al. 2001).
symbol. The clones displaying differential response to salt application between the salt-sensitive PB 1 and salt-tolerant CSR 27 or Pokkali cultivars [such as ubiquitin ligase (T2D1-302)] are shown with bold font of the clone numbers in Fig. 2. On the other hand, clones showing differential transcript levels in response to salt application between the two salt-tolerant cultivars (CSR 27 and Pokkali) [such as putative amino acid transporter (T4D1-163)] are shown with underlining of the clone numbers in Fig. 2. The clones showing constitutive differential expression or salt-induced differential expression in the three rice varieties are summarized in Table 5. In the macroarray blots, homologous cDNA clones were detected using probes corresponding to both tester and driver rice varieties in a given subtraction set (Fig. 1). Thus, contrasting rice genotypes appear to have a similar genetic response to imposed stress in qualitative terms. This implies that the differences in the relative salt response in contrasting genotypes are controlled largely by the differential regulation of several stress-regulated genes. Kawasaki et al. (2001) noted that the stress-sensitive IR29 rice cultivar responded to stress slower than the salt-tolerant rice cultivar Pokkali. Several transcripts were induced within the first 15 min of the imposed stress in Pokkali, while the same took approximately 3 h in the salt-sensitive IR29 rice type (Kawasaki et al. 2001).
Macroarray blot showing salt-stress-induced transcript alterations in Pokkali and Pusa basmati 1 roots corresponding to clones of the T2D1 library. Spotted on nylon membranes was 150 ng of clone plasmid DNA. The membranes were probed with radiolabeled total cDNA corresponding to control or salt-stressed (150 mM NaCl, 24 h) roots of PB 1 and control or salt-stressed (150 mM NaCl, 24 h) roots of Pokkali as shown in a. Representative up-regulated and down-regulated clones have been marked with dark blue and light blue boxes in b. Clone numbers of clones up-regulated in Pokkali but down-regulated in PB 1 are marked with grey boxes in b. A, B, C, D, E, and F represent controls from three other libraries constructed in our study (clones D5T3-13, D2T4-66, D2T4-50, D2T4-165, D2T4-216, and T4D1-227, respectively). Rice (O. sativa L.; cultivars Pusa basmati 1, CSR 27, and Pokkali) seeds were sown on wet cotton pads placed in plastic trays (Sahi et al. 2003). The seedlings were subjected to salt stress by placing these in beakers containing cotton pads soaked with 150 mM of NaCl solution instead of distilled water. The details on steps followed in the construction of subtractive cDNA libraries are shown in our earlier publication (Sahi et al. 2003). Replicate blots were probed with radiolabeled total cDNA prepared from control and salt-stressed roots/shoots of their respective rice types as shown in Results section. Northern blotting was carried out as per the standard protocol (Sambrook and Russell 2001). Radiolabeled probes were made either using “Megaprime Labeling Kit” (Amersham, UK) or “Random Primer Extension Labeling System” (NEN, USA). αP32-dCTP (BRIT, India) was used as the radiolabeling molecule. The nucleotide and protein sequences were analyzed using DNAStar software. Multiple alignments were done using MegAlign module of DNAStar. The nucleotide and the deduced amino acid sequences were searched for their homology with the previously existing sequences in the NCBI database using the BlastN and BlastP programs (Altschul et al. 1997). For protein domain analysis, sequences were searched against SMART (Simple Modular Architectural Research Tool) database (Letunic et al. 2004). The sequences were also searched on rice full-length cDNA consortium database at http://cdna01.dna.affrc.go.jp/cDNA/ (KOME database; Kikuchi et al. 2003)
Expression profiling of transcripts for selected clones by Northern blot analysis. PB 1, CSR 27, and Pokkali seedlings were salt stressed (150 mM NaCl, 24 h). An amount of 15 μg of total RNA isolated from root (in the case of analysis for the clones of T2D1, T4D1, and T4D2 libraries; see text and Table 3 for more details) or shoot tissues (in the case of analysis for the clones of T3D5 library; see text and Table 3 for more details) was resolved on 1% denaturing agarose gel. The blots were probed with PCR-amplified DNA fragment for the corresponding clones. C and S depict RNA from unstressed control and salt-stressed tissues, respectively. Methylene-blue-stained bands of rRNA depict equal RNA loading. , , , and D indicate clones showing increased transcript expression in tester and driver tissues, decreased transcript expression in tester and driver tissues, comparable transcript expression in tester and driver tissues, and differential transcript expression between tester and driver tissues, respectively. The clone numbers of the clones showing differential transcript expression between the salt sensitive PB 1 and salt tolerant cultivar (CSR27 or Pokkali) or within the two salt tolerant cultivars (CSR27 and Pokkali) are shown with bold font and with underlining, respectively. The annotations for the respective clone numbers are shown in Table 4
From the nucleotide sequence and annotation (Table 4; Sahi et al. 2003), macroarray profiling (Fig. 1), Northern blotting data (Fig. 2; Sahi et al. 2003), and the published literature, the following cellular processes appear relevant to the salt stress response of contrasting rice varieties:
Signaling processes
Different molecules have been shown to act as signals in the stress-associated signal transduction processes, and changes in cytosolic Ca2+ concentrations have been shown to act as a second messenger in salt stress (Chinnusamy et al. 2005). Kawasaki et al. (2001) observed that ESTs encoding Ca2+-dependent protein kinases were up-regulated during initial periods of salt stress in Pokkali rice. Salt-induced up-regulation of translationally controlled tumor protein has been reported. This protein is believed to play a role in salt stress signaling via Ca2+ binding (Gong et al. 2001). Isolation of cDNA clones encoding for EF-hand-containing proteins and Ca2+-binding proteins further substantiated the role(s) of Ca2+ in salt stress response. Clones like T3D5-13, T2D1-99, and T4D2-120 represent some of the signaling molecules identified (Table 4). It has been considered that most of the signaling components are induced and expressed within the initial time points of stress (Kawasaki et al. 2001). However, an EST corresponding to a putative serine/threonine protein kinase (T2D1-99) was noted at 24 h of salt exposure. A cDNA clone encoding a GDP inhibitor protein (T4D1-152) was also identified, suggesting that proteins binding GDP/GTP could play putative roles in salt-stress response in plants.
Transcriptional processes
RNA metabolism including varied transcriptional as well as post-transcriptional events appears as an important and well-conserved component of stress response in yeast, animal, and plant cells. The regulation of processes associated with RNA synthesis has been reported under diverse abiotic stresses (Sahi et al. 2003; Agarwal and Grover 2005). The activation of the transcription machinery to regulate salt-dependent gene expression requires the induction of specific transcription factors (TFs) as well as RNA polymerases (Estruch 2000). A microarray analysis in Arabidopsis showed that the levels of several TFs changed in response to environmental stresses (Chen et al. 2002).Several regulatory proteins have also been similarly identified. Clones showing homology to Zn-finger-containing TFs are found to be redundant in salt-stress-related gene expression profile studies. For instance, T4D1-144 (homologous to Zn-finger gene) was induced by salt stress (Fig. 2). Other clones obtained in similar kind of studies include ethylene response element binding protein (EREBP), basic helix loop helix (bHLH), and MADS (MCM1, agamous, deficiens, serum response factor) box containing transcription factors. bHLH and MYB transcription factors have been reported to function as transcriptional activators of ABA signaling in plants and were implicated in stress-regulated gene expression (Abe et al. 2003). Cooper et al. (2003) showed that MADS box TFs are important components of salt stress networking in plants. Pirin-like protein identified as an interacting molecule of nuclear factorI/CCAAT box TF (NFI/CTF1) stimulates RNA-polymerase-II-driven transcription in humans (Wendler et al. 1997). A representation of EST corresponding to pirin-like protein (T4D2-231) and DNA-dependent RNA polymerase II (T4D2-77 and T4D2-227) in salt-stressed libraries suggests that salt stress might regulate homologous events in the transcription machinery in plants.
Translational processes
Regulation of the translational machinery is considered an integral component in the cellular stress response (Wood and Oliver 1999). It has been indicated that ribosomal proteins are not only central to translational efficiency but have pleiotropic effects and have extra-ribosomal functions (Wool 1996). The polysomal retention of transcripts for ribosomal proteins RPS14, RPS16, and RPL23 has been correlated to desiccation response in Tortula ruralis (Wood et al. 2000). Ribosomal protein genes Gmrps6, Gmrps13, and Gmrpl37 were found responsive to low temperature stress in soybean (Kim et al. 2004). Arabidopsis plants mutated for ribosomal gene S27 displayed normal phenotype under control conditions but the same was noted to be indispensable for Arabidopsis plants under genotoxic conditions (Revenkova et al. 1999). Several different ribosomal genes are noted to be stress-regulated (like T2D1-81, T2D1-171, T2D1-288, and T4D2-66 in Figs. 1 and 2).
Apart from ribosomal proteins, clones associated with different other aspects of RNA metabolism were also found to be associated with salt stress (like T2D1-207, T2D1-217, T2D1-219, T2D1-313, T3D5-329, T3D5-362, T4D1-86, T4D1-157, T4D2-34, and T4D2-113 in Figs. 1 and 2). RNA binding proteins, mostly glycine-rich (GR) RNA binding proteins (RBPs) (GR–RBPs) are highly repetitive in stress related expression profiles (Sahi et al. 2006). Clones corresponding to small nuclear ribonucleoprotein (T3D5-70) and retrotransposon (T4D2-130a) were noted. EST encoding the retrotransposon was found to be salt inducible (Fig. 2). The active transcription of plant retrotransposons under diverse stress conditions would suggest their involvement in the stress response (Takeda et al. 2001). Phosphorylation of eIf2α by eIf2α kinase(s) is considered to be a key step in regulating stress-dependent translation in eukaryotes (Anderson and Kedersha 2002). cDNA encoding eIf2α (T4D1-219) was noted to be salt induced (Fig. 2). This protein has been reported to have a bearing on selected translation of stress-associated genes in several systems (Harding et al. 2002). GCN2 (eIf2α kinase) was also found to be stress-regulated (T2D1-36; Fig. 2). The latter protein has been shown to be implicated in salt toxicity in yeast (Goossens et al. 2001).
Protein turnover and folding
Protein degradation during stress is a highly conserved and regulated phenomenon in all the organisms reported so far (Vierstra and Callis 1999). Genes encoding proteins like ubiquitin ligase, polyubiquitin, proteasome subunit, and protease inhibitor are known to be expressed in the cDNA libraries of stressed tissues (Mahalingam et al. 2003). Increased transcript levels of a gene encoding a putative ubiquitin fusion protein (T2D1-10) were seen. It was observed that E3 ubiquitin ligases and the RING (Really Interesting New Gene) finger proteins are key components of the ubiquitin proteasome pathway (Freemont 2000; Taji et al. 2004), and RING finger proteins are thought to be involved in rapid degradation of regulatory proteins (Lee et al. 2001). Several polyubiquitin and related to ubiquitin (RUB) [encoding genes in our screen (such as T2D1-10, T3D5-25, and T4D2-224)] appear to be associated with salt stress response (Figs. 1 and 2). Ubiquitin has been noted to be involved with vegetative desiccation tolerance in plants (O’ Mahony and Oliver 1999).
It has been shown recently that salt stress induces wound-related genes, suggesting that salt and wounding stresses are closely linked (Dombrowski 2003). Bowman–Birk protease inhibitors, WRKY transcription factors, chitinase, EN242, and other PR proteins have been shown to be associated with salt stress (Dombrowski 2003; Sottosanto et al. 2004). At the transcription level, AtMYB102 has been identified as a key regulatory component, which integrates osmotic stress and wounding responses (Denekamp and Smeekens 2003). Clones coding for subtilisin-chymotrypsin protease inhibitors were amongst the abundant clones in salt-stressed root libraries (clones like T2D1-46, T2D1-133, T2D1-186, T2D1-223, T2D1-260, T4D1-15, T4D1-28, T4D2-50, and T4D2-106). Clones corresponding to the wheat WIN2 (T2D1-89 and T4D2-130), chitinase (T4D2-138), and EN242 gene (T4D1-77) were also obtained. As cDNA clones coding for proteins promoting proteolysis as well as inhibiting protein degradation were enriched in cDNA libraries, future work on the characterization of these proteins may reveal the intriguing complexity in co-coordination of protein metabolism under stress.
HSPs that help in proper protein folding or prevention of stress-induced folding problems (Grover 2002) have been reported to be expressed under salt stress (Asadulghani et al. 2004). Synechocystis cells mutated for a small molecular mass HSP were noted to be salt-sensitive and displayed severe protein folding aberrations under moderate salt stress (Asadulghani et al. 2004).EST clone corresponding to hsp90 (T4D2-178) was induced by salt stress in macroarray analysis. Hsp90 proteins have been shown to be expressed under salt stress in rice (Pareek et al. 1995).
Ion transporters and metal-binding proteins
Ion transporters have received a great deal of attention because of their crucial role in salt tolerance and potassium nutrition (Blumwald and Grover 2006). Ion homeostasis during salt stress is affected by sodium fluxes, transport, and compartmentalization. Clones homologous to vacuolar H+-ATPase (T4D1-227), vacuolar ATP synthase (T4D2-45 and T2D1-150), and various other uncharacterized proteins with putative transmembrane domains (T2D1-177 and T4D2-99) were notably present in salt stress cDNA libraries.
Metal-binding ESTs were also abundant in salt stress libraries (T2D1-20, T2D1-47, T4D1-99, etc.). Metallothionins were the most enriched among these (T2D1-20, T2D1-104, and T2D1-180). Other metal-binding proteins included copper binding proteins and copper chaperones (T2D1-47, T2D1-81, T2D1-221, T2D1-238, and T4D1-99). Metallothionins have been found in several other salt stress libraries (Kawasaki et al. 2001; Reddy et al. 2002) and have also been implicated in leaf senescence and cell death, and the induction of genes encoding copper binding proteins was reported in the response of Arabidopsis to salt (Sottosanto et al. 2004).
General metabolism
Stress leads to an oxidative stress response due to the accumulation of hydrogen peroxide, hydroxyl radicals, and superoxide anions, resulting in perturbance of the overall cellular metabolism (Mittler 2002). Salt stress cDNA libraries were rich in genes encoding peroxidases (T2D1-169 and T3D5-50), ascorbate peroxidase (T4D2-70), FAD-linked oxidoreductases (T2D1-172 and T2D1-173), and thioredoxins (T2D1-37, T2D1-39 and T2D1-157). Clones associated with senescence including cell cycle control and death such as cyclin H1 (T2D1-117), cytochrome c oxidase (T2D1-185), and tumor suppressor genes (T3D5-302) were also noted. The T3D5-302 and T2D1-276 clones were induced by salt treatment. EST clone homologous to 3-methyladenine DNA glycosylaseI gene (T4D2-131), which was reported to be involved with DNA repair pathway (Santerre and Britt 1994), was obtained.
Genes encoding proteins involved in amino acid metabolism (T2D1-145, T2D1-201, T2D1-215, T4D1-37, T4D1-38, T4D1-163, T4D1-213, and T4D2-68), mannosyltransferase (T2D1-259), glucosyltransferase (T4D2-88), fructose-2, 6,-bisphosphatase (T3D5-15), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (T4D2-43), NADP-dependent maleic enzyme (T4D2-55), and nucleoside diphospho kinase (NDPK1; T4D2-114 and T2D1-35) were noted to be abundant. The transcript corresponding to the NDPK gene was strongly up-regulated by salt stress in Pokkali rice (Kawasaki et al. 2001). Levels of T4D2-55 transcript in roots of both CSR27 and Pokkali were regulated by salt treatment. Upon salt treatment, this transcript was down-regulated in case of Pokkali but not in CSR27. Furthermore, clones related to photosynthesis (T2D1-37, T2D1-157 ,and T2D1-185), respiration (T4D1-161, T3D5-15, and T4D2-46), carbohydrate (T2D1-259, T4D2-88, and T4D2-122) and fat and lipid metabolism (T2D1-101 and T4D1-34) were obtained. According to Kawasaki et al. (2001), genes encoding proteins associated with cellular homeostasis such as those belonging to the TCA cycle, respiration, cellular biogenesis and DNA repair showed a distinct decline in the more sensitive IR29 rice but not in Pokkali rice type.
Clones like exostosin (T2D1-161), phragmoplastin, lipid-recognition protein (T2D1-101), cysteine synthase (T2D1-145), protein-containing domains like fascin (T2D1-142), TPR (tricopeptide repeat) (T4D2-191), and WD-40 (T4D2-120) were noted. SalT was detected in one of the subtracted libraries (T4D2-96). SalT is an organ-specific, 15-kDa glycine-rich cytosolic protein of unknown function regulated by salt, dehydration, ABA, and proline (Claes et al. 1990). cDNA encoding for putative membrane protein (T4D1-5) was detected to be salt inducible in PB 1 while it was constitutively expressed in CSR 27. Proteins with membrane spanning or lipid interacting domains could play significant roles in stress response (Urao et al. 1999).
One of the primary effects of salt stress is the dehydration effect, which is associated with the decrease in water potential in the extracellular solution (Munns 2005). This effect would increase the capacity of the cells to produce osmoregulants/compatible solutes (Munns 2005). An increased accumulation of proline and trehalose has been reported during salt stress in rice (Garcia et al. 1997). However, ESTs corresponding to synthesis of osmolytes were largely not represented in subtracted rice cDNA libraries, suggesting that osmolyte synthesis under salt stress may be a highly conserved salt stress response amongst different rice types.
Clones expressed differentially in contrasting rice varieties
Several transcripts that appeared to be expressed at higher levels in Pokkali but at lower levels in PB 1 were identified (such as FAD-linked oxidoreductase, MADS23 protein, subtilisin-chymotrypsin inhibitor, and thioredoxin-like protein; Table 5). Specific differences in transcriptional regulation were also apparent between Pokkali and CSR 27 rice varieties (such as nucleotide diphosphate kinase, subtilisin-chymotrypsin inhibitor, ubiquinol cytochrome c-reductase, and vacoular ATP synthase 22-kDa proteolipid subunit; Table 5). The transcript levels of selective clones were noted to be constitutively higher in Pokkali/CSR 27 compared to PB 1 (such as mannosyl transferase, EREBP-like protein, ribosomal protein R41, MADS23 protein, acetyl transferase, ubiquitin fusion protein, RING-finger-containing ubiquitin ligase, and subtilisin-chymotrypsin inhibitor; Table 5). Kawasaki et al. (2001) observed that a gene-encoding translation elongation factor-1α was up-regulated during initial periods of salt stress in Pokkali but was not induced at similar time points in the salt-sensitive IR29. This observation emphasized that tolerant cultivars are possibly more efficient in restructuring their translational machinery during salt stress and are able to respond faster to the imposed stress. A set of clones (T2D1-81, T2D1-157, T2D1-159, and T2D1-167) showed higher constitutive expression in Pokkali compared to PB 1 (Fig. 2). NaCl treatment notably caused up-regulation of these clones in PB 1 but down-regulation in Pokkali. Clone T2D1-288 encoding for R41 ribosomal protein was salt-up-regulated in Pokkali. The transcripts corresponding to this clone were not detected in PB 1. Three highly expressed clones isolated from Pokkali, namely, T2D1-273 (EREBP-like protein), T2D1-274 (ribosomal protein L10), and T2D1-276 (FAD-binding-domain-containing protein), showed not only high levels in CSR 27 but also differential expression in CSR 27 as compared to PB 1. Some of the clones that expressed differentially in these rice varieties (e.g., T4D2-220 and T4D2-144) have not yet been annotated.
The abundance of stress-regulated and transcriptional- and translational-associated genes in salt stress libraries (such as bHLH, EREBP, Zn-finger protein, GCN2, eIf2α etc.) indicate that cells undergo an adjustment to re-program their metabolism to survive under stress conditions. According to Sahi et al. (2006), there appears to be a coordinated action of trans-acting factors, ribosomal proteins, RNA binding proteins, translation initiation and elongation factors along with several accessory proteins for regulating stress-associated processes. Induction of specific ribosomal genes, translation initiation factors and elongation factors during salt stress in yeast, Arabidopsis and rice in recent studies (Sahi et al. 2006; this work) indicates that alterations in these cellular components represent a conserved stress response. From the above account, it is indicated that gene expression response in tolerant and susceptible plants differs mainly in quantitative terms. Further analysis of the salt stress-related cDNA clones in terms of transcript and protein expression may shed light on requisite promoters and interacting protein partners that are critical in controlling salt stress response.
Final comments
It is important to note that in spite of the high salt sensitivity, rice is the only cereal that is recommended as a desalinization crop because of its ability to grow well under flooded conditions, and because the standing water in rice fields can help leach the salts from the topsoil to a level low enough for subsequent crops (Bhumbla and Abrol 1978). This and also the fact that rice is an important food crop, increasing its salt tolerance has enormous implications. From the progress made thus far, several directions are emerging that may yield useful genes in breeding for rice salt tolerance. Strong evidence exist in showing that members of protein families involved in osmoregulation, ion transport and protection from oxidative damage are critical in governing high salt tolerance (Fig. 3). Recent work provides indications (through the abundance of stress-regulated and transcriptional- and translational-associated genes in salt stress libraries) that cells undergo an adjustment to re-program their metabolism to survive under stress conditions, and thus genes involved in this reprograming may as well turn out to be important players in controlling salt stress response. For discovery of novel salt-stress-tolerance-related genes, comparative genomics is clearly turning out to be an important tool. There are several attempts to unravel how Pokkali, Porteresia, and other related rice types are endowed with high salt stress tolerance using comparative genomics approach. Novel salt-stress-tolerant genes thus emerging from different directions of research need to be validated in future attempts. To undertake this, it is important that knockout mutants of rice for each and every individual gene are produced as has been done in the case of yeast and Arabidopsis. Such attempts are, in fact, in progress (http://www.iris.irri.org/IRFGC/). Gene knockout mutants may also help to validate the cellular functions of QTL identified by mapping. Furthermore, transgenic rice production has reached the stage where routine experiments with candidate genes are possible. With the requisite mutants and the optimized transformation protocols, validating the candidate genes for salt tolerance in rice appears a clear possibility. Finally, the above discussion has mainly emphasized transcript analysis. It is imperative that protein data is obtained in parallel. In recent years, two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) technique in conjunction with automated gel scanning and computer-assisted analysis has contributed greatly to the development of protein database (Dubey and Grover 2001; Komatsu et al. 2003). Abbasi and Komatsu (2004), using this approach to examine changes in protein expression in response to salt stress in rice seedlings, have reported that specific proteins expressed in defined regions of rice show a coordinated response to salt stress. Kong-ngern et al. (2005) analyzed the response of salt stress in leaf sheaths of rice seedlings. This group identified 13 different proteins which were induced and ten different proteins which were down-regulated by salt stress. The effect of salt stress on rice leaf lamina proteins has recently been analyzed by Parker et al. (2006). The highly optimized 2D-PAGE protocol used in this study resulted in separation of ~2,500 protein species of which 32 were observed to be significantly regulated by salinity. Tandem mass spectrometry analysis showed that rubisco activase and ferritin are increased predominantly during salt stress. However, information on salt stress-associated protein changes in various other tissues at varied growth stages and at the whole plant level is far from complete; this aspect therefore merits further research.
Schematic representation of mechanisms associated with salt tolerance phenotype in different cellular compartments of the plant cell. For validating the functionality of proteins shown in solid boxes in salt response, transgenic rice plants have been raised. Proteins shown in broken boxes represent examples where the transgenic host employed is other than rice. Evidence for the involvement of proteins not shown in solid or broken boxes in salt response has been obtained using varied other approaches (such as biochemical experiments, transcript/protein expression studies, mutant analysis, or yeast complementation assays). Involvement of SKC1 in salt tolerance was initially suggested on the basis of QTL analysis and, later on, it was functionally validated by producing transgenic rice plants. DREB, dehydration responsive element binding protein (Kasuga et al. 1999; Dubouzet et al. 2003); PDH45, pea DNA helicase 45 (Sanan-Mishra et al. 2005); Tsi1, tobacco stress-induced gene 1 (Park et al. 2001); BADH, betaine aldehyde dehydrogenase (Holmstrom et al. 2000; Jia et al. 2002; Yilmaz and Bulow 2002); CaN, calcineurin (Pardo et al. 1998); codA, choline oxidase (Lilius et al. 1996; Hayashi et al. 1997; Sakamoto et al. 1998; Alia et al. 1999; Prasad et al. 2000a, b); CDPK, calcium-dependent protein kinase (Saijo et al. 2000); ectA, ectB, and ectC, l-2,4-diaminobutyric acid acetyltransferase, l-2, 4-diaminobutyric acid transaminase, and l-ectoine synthase (Nakayama et al. 2000); eIF1A, eukaryotic translation initiation factor 1A (Rausell et al. 2003); eIF4A, eukaryotic translation initiation factor 4A (Montero-Lomeli et al. 2002); Gly I and II, glyoxylase I and II (Veena et al. 1999; Singla-Pareek et al. 2003); GST, glutathione-S-transferase (Roxas et al. 1997); GPX, glutathione peroxidase (Roxas et al. 1997); HspA (Asadulghani et al. 2004); Hsp17.6A (Sun et al. 2001); IMT1, myo-inositol O-methyl transferase (Vernon et al. 1993); P5CS, pyrroline-5-carboxylate synthetase (Kishore et al. 1995); ProDH, proline dehydrogenase (Nanjo et al. 1999); SOD2, sodium 2 (Gao et al. 2003); TPS, trehalose-6-phosphate synthase; TPSP, TPS phosphatase (Garg et al. 2002; Jang et al. 2003); TPX2, tomato peroxidase 2 (Amaya et al. 1999); AVP1, vacoular H+ pyrophosphatase (Gaxiola et al. 2001; Park et al. 2005); NHX1, Na+/H+ antiporter (Apse et al. 1999; Zhang and Blumwald 2001; Zhang et al. 2001; Ohta et al. 2002; Fukuda et al. 2004; Wang et al. 2004; Wu et al. 2004; Xue et al. 2004; Yin et al. 2004; He et al. 2005; Lu et al. 2005; Wu et al. 2005); SKC1, HKT type (Ren et al. 2005); SOS1, salt overly sensitive 1 (Shi et al. 2003); GS2, glutamine synthetase (Hoshida et al. 2000); MIPS, l-myo-inositol 1-phosphate synthase (Majee et al. 2004); MnSOD, Mn-superoxide dismutase (Tanaka et al. 1999)
While the above account of salt stress response has mainly been addressed on rice plant, a host of other plant species are being analyzed to unravel the salt stress biology. Among the crop species, salt stress response has been analyzed in appreciable details in barley, tomato, and wheat (Forster et al. 2000; Francki and Appels 2002; Colmer et al. 2005; Kawaura et al. 2006; Walia et al. 2006). Model plants like Arabidopsis and tobacco have particularly been exploited with the aim of testing the role(s) of candidate genes in governing salt stress tolerance using transgenic approach (Tarczynski et al. 1993; Apse et al. 1999; Zhang and Blumwald 2001; Zhang et al. 2001; Shi et al. 2003). Work on halophyte plants like salt cress (T. halophila) has been useful in comparative-genomics-based studies. Put together, it reflects that the understanding of the molecular events in plant salt stress response is making a fast progress (see March 2006 issue of Journal of Experimental Botany for more articles on plant salt stress biology, http://jbx.oxfordjournals.org/current.dtl#RESEARCH_PAPERS_PLANTS_AND_SALINITY).
References
Abbasi FM, Komatsu S (2004) A proteomic approach to analyze salt-responsive proteins in rice leaf sheath. Proteomics 4:2072–2081
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15:63–78
Agarwal S, Grover A (2005) Isolation and transcription profiling of anaerobic stress associated cDNA clones from flooding stress tolerant FR13A rice genotype. Ann Bot 96:831–844
Akbar M, Gunawardena IE, Ponnamperuma FN (1986a) Breeding for soil stresses. In: International Rice Research Institute (ed) Progress in rainfed lowland rice. International Rice Research Institute, Manila, Philippines, pp 263–272
Akbar M, Khush GS, Hillerislambers D (1986b) Genetics of salt tolerance in rice. In: International Rice Research Institute (ed) Progress in rainfed lowland rice. International Rice Research Institute, Manila, Philippines, pp 399–409
Akbar M, Jena KK, Seshu DV (1987) Salt tolerance in wild rices. Int Rice Res Newsl 12:15
Alia, KondoY, Sakamoto A, Nonaka H, Hayashi H, Saradhi PP, Chen THH, Murata N (1999) Enhanced tolerance to light stress of transgenic Arabidopsis plants that express the codA gene for a bacterial choline oxidase. Plant Mol Biol 40:279–288
Altschul SF, Madden TL, Scheffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Amaya I, Botella MA, De La Calle M, Medina MI, Heredia A, Bressan RA, Hasegawa PM, Quesada MA, Valpuesta V (1999) Improved germination under osmotic stress of tobacco plants overexpressing a cell wall peroxidase. FEBS Lett 457:80–84
Anderson P, Kedersha N (2002) Stressful initiations. J Cell Sci 115:3227–3234
Anil VS, Krishnamurthy P, Kuruvilla S, Sucharitha K, Thomas G, Mathew MK (2005) Regulation of the uptake and distribution of Na+ in shoots of rice (Oryza sativa) variety Pokkali: role of Ca2+ in salt tolerance response. Physiol Plant 124:451–464
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285:1256–1258
Apse MP, Sottosanto JB, Blumwald E (2003) Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J 36:229–239
Asadulghani, Nitta K, Kaneko Y, Kojima K, Fukuzawa H, Kosaka H, Nakamoto H (2004) Comparative analysis of the hspA mutant and wild-type Synechocystissp. Strain PCC 6803 under salt stress: evaluation of the role of hspA in salt-stress management. Arch Microbiol 182:487–497
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24:23–58
Bhumbla D, Abrol I (1978) Saline and sodic soils. In: Soils and rice. International Rice Research Institute, Manila, Philippines, pp 719–738
Blumwald E, Grover A (2006) Salt tolerance. In: Halford NG (ed) Plant biotechnology: current and future uses of genetically modified crops. John Wiley and Sons Ltd., UK, pp 206–224
Bohnert HJ, Ayoubi P, Borchert C, Bressan RA, Burnap RL, Cushman JC, Cushman, MA, Deyholos M, Fischer R, Galbraith DW, Hasegawa PM, Jenks M, Kawasaki S, Koiwa H, Kore-Eda S, Lee B-H, Michalowski CB, Misawa E, Nomura M, Ozturk N, Postier B, Prade R, Song CP, Tanaka Y, Wang H, Zhu JK (2001) A genomics approach toward salt stress tolerance. Plant Physiol Biochem 39:295–311
Bonilla P, Dvorak J, Mackill D, Deal K, Gregorio G (2002) RFLP and SSLP mapping of salinity tolerance genes in chromosome 1 of rice (Oryza sativa L.) using recombinant inbred lines. Philipp Agric Sci 85:68–76
Bressan RA, Zhang C, Zhang H, Hasegawa PM, Bohnert HJ, Zhu JK (2001) Learning from the Arabidopsis experience. The next gene search paradigm. Plant Physiol 127:1354–1360
Causse M, Fulton T, Cho Y, Ahn S, Chunwongse J, Wu K, Xiao J, Yu Z, Ronald P, Harrington S, Second G, McCouch S, Tanksley S (1994) Saturated molecular map of the rice genome based on an interspecific backcross population. Genetics 138:1251–1274
Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, Mauch F, Luan S, Zou G, Whitham SA, Budworth PR, Tao Y, Xie Z, Chen X, Lam S, Kreps JA, Harper JF, Si-Ammour A, Mauch-Mani B, Heinlein M, Kobayashi K, Hohn T, Dangl JL, Wang X, Zhu T (2002) Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 3:559–574
Chinnusamy V, Jagendorf A, Zhu JK (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448
Claes B, Dekeyser R, Villarroel R, van den Bulcke M, Bauw G, Van Montagu M (1990) Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell 2:19–27
Colmer TD, Munns R, Flowers TJ (2005) Improving salt tolerance of wheat and barley: future prospects. Aust J Exp Agric 45:1425–1443
Cooper B, Clarke J D, Budworth P, Kreps J, Hutchison D, Park S, Guimil S, Dunn M, Luginbühl P, Ellero C, Goff SA, Glazebrook J (2003) A network of rice genes associated with stress response and seed development. Proc Natl Acad Sci USA 100:4945–4950
Denekamp M, Smeekens SC (2003) Integration of wounding and osmotic stress signals determines the expression of the AtMYB102 transcription factor gene. Plant Physiol 132:1415–1423
Dombrowski JE (2003) Salt stress activation of wound-related genes in tomato plants. Plant Physiol 132:2098–2107
Dubey H, Grover A (2001) Current initiatives in proteomics research: the plant perspective. Curr Sci 80:262–269
Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J 33:751–763
Estruch F (2000) Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol Rev 25:469–486
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55:307–319
Flowers TJ, Yeo AR (1981) Variability in the resistance of sodium chloride salinity within rice (Oryza sativa L.) varieties. New Phytol 88:363–373
Flowers TJ, Yeo AR (1995) Breeding for salinity tolerance in crop plants. Aust J Plant Physiol 22:875–884
Flowers TJ, Flowers SA, Hajibagheri MA, Yeo AR (1990) Salt tolerance in the halophytic wild rice Porteresia coarctata Tateoka. New Phytol 114:675–684
Flowers TJ, Koyama ML, Flowers SA, Sudhakar C, Singh KP, Yeo AR (2000) QTL: their place in engineering tolerance of rice to salinity. J Exp Bot 51:99–106
Forster BP, Ellis RP, Thomas WTb, Newton AC, Tuberosa R, This D, El-Enein RA, Bahri MH, Salem MB (2000) The development and application of molecular markers for abiotic stress tolerance in barley. J Exp Bot 51:19–27
Francki M, Appels R (2002) Wheat functional genomics and engineering crop improvement. Genome Biol 3:1013.1–1013.5
Freemont PS (2000) Ubiquitination: RING for destruction? Curr Biol 10:84–87
Fukuda A, Nakamura A, Tagiri A, Tanaka H, Miyao A, Hirochika H, Tanaka Y (2004) Function, intracellular localization and the importance in salt tolerance of a vacuolar Na(+)/H(+) antiporter from rice. Plant Cell Physiol 45:146–159
Gao X, Ren Z, Zhao Y, Zhang H (2003) Overexpression of SOD2 increases salt tolerance of Arabidopsis. Plant Physiol133:1873–1881
Garcia AB, de Almeida JE, Iyer S, Gerats T, Van Montagu M, Caplan AB (1997) Effects of osmoprotectants upon NaCl stress in rice. Plant Physiol 115:159–169
Garg AK, Kim JK, Owens TG, Ranwala AP, Choi YD, Kochian LV, Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 99:15898–15903
Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, Fink GR (2001) Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci USA 98:11444–11449
Gepstein S, Grover A, Blumwald E (2006) Producing biopharmaceuticals in the desert: building an abiotic stress tolerance in plants for salt, heat and drought. In: Knablein J, Muller RH (eds) Modern biopharmaceuticals. Wiley-VCH Verlag GmbH, Weinhaum, pp 967–994
Glenn EP, Brown JJ, Blumwald E (1999) Salt tolerance and crop potential of halophytes. Crit Rev Plant Sci 18:227–255
Gong Z, Koiwa H, Cushman MA, Ray A, Bufford D, Kore-eda S, Matsumota TK, Zhu J, Cushman JC, Bressan RA, Hasegawa PM (2001) Genes that are uniquely stress regulated in salt overly sensitive (sos) mutants. Plant Physiol 126:363–375
Goossens A, Dever TE, Pascual-Ahuir A, Serrano R (2001) The protein kinase Gcn2p mediates sodium toxicity in yeast. J Biol Chem 276:30753–30760
Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol 31:149–190
Gregorio GB, Senadhira D, Mendoza RD, Manigbas NL, Roxas JP, Guerta CQ (2002) Progress in breeding for salinity tolerance and associated abiotic stresses in rice. Field Crops Res 76:91–101
Grover A (2002) Molecular biology of stress responses. Cell Stress Chaperones 7:1–15
Grover A, Pental D (2003) Breeding objectives and requirements for producing transgenic for the major field crops of India. Curr Sci 84:310–320
Grover A, Pareek A, Maheshwari SC (1993) Molecular approaches for genetically engineering plants tolerant to salt stress. Proc Indian Natl Sci Acad B 59:113–127
Harding HP, Calfon M, Urano F, Novoa I, Ron D (2002) Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Cell Dev Biol 18:575–599
Harushima Y, Yano M, Shomura A, Sato M, Shimano T, Kuboki Y, Yamamoto T, Lin SY, Antonio BA, Parco A, Kajiya H, Huang N, Yamamoto K, Nagamura Y, Kurata N, Khush GS, Sasaki T (1998) A high-density rice genetic linkage map with 2275 markers using a single F2 population. Genetics 148:479–494
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Hayashi H, Alia Mustardy L, Deshnium PG, Ida M, Murata N (1997) Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycinebetaine and enhanced tolerance to salt and cold stress.Plant J 12:133–142
He C, Yan J, Shen G, Fu L, Holaday AS, Auld D, Blumwald E, Zhang H (2005) Expression of an Arabidopsis vacuolar sodium/proton antiporter gene in cotton improves photosynthetic performance under salt conditions and increases fibre yield in the field. Plant Cell Physiol 46:1848–1854
Hirochika H, Guiderdoni E, AnG, Hsing Y, Eun MY, Han C, Upadhyaya N, Ramachandran R, Zhang Q, Pereira A, Sundaresan V, Leung H (2004) Rice mutant resources for gene discovery. Plant Mol Biol 54:325–334
Holmstrom KO, Somersalo S, Manda A, Palva TE, Welin B (2000) Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J Exp Bot 51:177–185
Hoshida H, Tanaka Y, Hibino T, Hayashi Y, Tanaka A, Takabe T, Takabe T (2000) Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Mol Biol 43:103–111
Huang J, Hirji R, Adam L, Rozwadowski KL, Hammerlindl JK, Keller WA, Selvaraj G (2000) Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: metabolic limitations. Plant Physiol 122:747–756
International Rice Genome Sequencing Project (2005) The map based sequence of the rice genome. Nature 436:793–800
Jaiswal P, Ni J, Yap I, Ware D, Spooner W, Youens-Clark K, Ren L, Liang C, Zhao W, Ratnapu K, Faga B, Canaran P, Fogleman M, Hebbard C, Avraham S, Schmidt S, Casstevens TM, Buckler ES, Stein L, McCouch S (2006) Gramene: a bird’s eye view of cereal genomes. Nucleic Acids Res 34:D717–D723
Jang IC, Oh SJ, Seo JS, Choi WB, Song SI, Kim CH, Kim YS, Seo HS, Choi YD, Nahm BH, Kim JK (2003) Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiol 131:516–524
Jia GX, Zhu ZQ, Chang FQ, Li YX (2002) Transformation of tomato with the BADH gene from Atriplex improves salt tolerance. Plant Cell Rep 21:141–146
Kanesaki Y, Suzuki I, Allakhverdiev SI, Mikami K, Murata N (2002) Salt stress and hyperosmotic stress regulate the expression of different sets of genes in Synechocystis sp. PCC 6803. Biochem Biophys Res Commun 290:339–348
Kanno A, Hirai A (1993) A transcription map of the chloroplast genome from rice (Oryza sativa). Curr Genet 23:166–174
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcriptional factor. Nat Biotechnol 17:287–291
Kawasaki S, Borchert C, Deyholos M, Wang H, Brazille S, Kawai K, Galbraith D, Bohnert HJ (2001) Gene expression profiles during the initial phase of salt stress in rice. Plant Cell 13:889–905
Kawaura K, Mochida K, Yamazaki Y, Ogihara Y (2006) Transcriptome analysis of salinity stress responses in common wheat using a 22k oligo-DNA microarray. Funct Integr Genomics 6:132–142
Khan MSA, Hamid A, Karim MA (1997) Effect of sodium chloride on germination and seedling characters of different types of rice (Oryza sativa L.). J Agron Crop Sci 179:163–169
Kikuchi S, Satoh K, Nagata T, Kawagashira N, Doi K, Kishimoto N, Yazaki J, Ishikawa M, Yamada H, Ooka H, Hotta I, Kojima K, Namiki T, Ohneda E, Yahagi W, Suzuki K, Li CJ, Ohtsuki K, Shishiki T, Otomo Y, Murakami K, Iida Y, Sugano S, Fujimura T, Suzuki Y, Tsunoda Y, Kurosaki T, Kodama T, Masuda H, Kobayashi M, Xie Q, Lu M, Narikawa R, Sugiyama A, Mizuno K, Yokomizo S, Niikura J, Ikeda R, Ishibiki J, Kawamata M, Yoshimura A, Miura J, Kusumegi T, Oka M, Ryu R, Ueda M, Matsubara K, Kawai J, Carninci P, Adachi J, Aizawa K, Arakawa T, Fukuda S, Hara A, Hashizume W, Hayatsu N, Imotani K, Ishii Y, Itoh M, Kagawa I, Kondo S, Konno H, Miyazaki A, Osato N, Ota Y, Saito R, Sasaki D, Sato K, Shibata K, Shinagawa A, Shiraki T, Yoshino M, Hayashizaki Y, Yasunishi A (2003) Collection, mapping, and annotation of over 28,000 cDNA clones from japonica rice. Science 301:376–379
Kim KY, Park SW, Chung YS, Chung CH, Kim JI, Lee JH (2004) Molecular cloning of low-temperature-inducible ribosomal proteins from soybean. J Exp Bot 55:1153–1155
Kishore PBK, Hong Z, Miao GH, Hu CAA, Verma DPS (1995) Overexpression of Δ-1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108:1387–1394
Komatsu S, Konishi H, Shen S, Yang G (2003) Rice proteomics. Mol Cell Proteomics 2.1:2–10
Kong-ngern K, Daduang S, Wongkham C, Bunnag S, Kosittrakun M, Theerakulpisut (2005) Protein profiles in response to salt stress in leaf sheaths of rice seedlings. Sci Asia 31:403–408
Kore-Eda S, Cushman MA, Akselrod I, Bufford D, Fredrickson M, Clark E, Cushman JC (2004) Transcript profiling of salinity stress responses by large-scale expressed sequence tag analysis in Mesembryanthemum crystallinum. Gene 341:83–92
Koyama ML, Levesley A, Koebner RM, Flowers TJ, Yeo AR (2001) Quantitative trait loci for component physiological traits determining salt tolerance in rice. Plant Physiol 125:406–422
Kreps JA, Wu Y, Chang HS, Zhu T, Wang X, Harper JF (2002) Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol 130:2129–2141
Kurata N, Nagamura Y, Yamamoto K, Harushima Y, Sue N, Wu J, Antonio BA, Shomura A, Shimizu T, Lin SY, Inoue T, Fukuda A, Shimano T, Kuboki Y, Toyama T, Miyamoto Y, Kirihara T, Hayasaka K, Miyao A, Monna L, Zhong HS, Tamura Y, Wang ZX, Momma T, Umehara Y, Yano M, Sasaki T, Minobe Y (1994) A 300 kilobase interval genetic map of rice including 883 expressed sequences. Nat Genet 8:365–372
Lee H, Xiong L, Gong Z, Ishitani M, Stevenson B, Zhu JK (2001) The Arabidopsis HOS1 gene negatively regulates cold signal transduction and encodes a RING finger protein that displays cold-regulated nucleo-cytoplasmic partitioning. Genes Dev 15:912–924
Letunic I, Copley RR, Schmidt S, Ciccarelli FD, Doerks T, Schultz J, Ponting CP, Bork P (2004) SMART 4.0: towards genomic data integration. Nucleic Acids Res 32:D142–D144
Li L, Wang X, Stolc V, Li X, Zhang D, Su N, Tongprasit W, Li S, Cheng Z, Wang J, Deng XW (2006) Genome-wide transcription analyses in rice using tiling microarrays. Nat Genet 38:124–129
Lilius G, Holmberg N, Bulow L (1996) Enhanced NaCl stress tolerance in transgenic tobacco expressing bacterial choline dehydrogenase. Biotechnology 14:177–180
Lin HX, Zhu MZ, Yano M, Gao JP, Liang ZW, Su WA, Hu XH, Ren ZH, Chao DY (2004) QTLs for Na+ and K+ uptake of the shoots and roots controlling rice salt tolerance. Theor Appl Genet 108:253–260
Lorieux M, Petror M, Huang N, Guiderdoni E, Ghesquier A (1996) Aroma in rice: genetic analysis of a quantitative trait. Theor Appl Genet 93:1145–1151
Lu SY, Jing YX, Shen SH, Zhao HY, Ma LQ, Zhou XJ, Ren Q, Li YF (2005) Antiporter gene from Hordum brevisubulatum (Trin.) Link and its overexpression in transgenic tobaccos. J Integr Plant Biol 47:343–349
Lutts S, Kinet JM, Bouharmont J (1995) Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J Exp Bot 46:1843–1852
Mahalingam R, Gomez-Buitrago A, Eckardt N, Shah N, Guevara-Garcia A, Day P, Raina R, Fedoroff NV (2003) Characterizing the stress/defense transcriptome of Arabidopsis. Genome Biol 4:R20
Majee M, Maitra S, Dastidar KG, Pattnaik S, Chatterjee A, Hait NC, Das KP, Majumder AL (2004) A novel salt-tolerant l-myo-inositol-1-phosphate synthase from Porteresia coarctata (Roxb.) Tateoka, a halophytic wild rice: molecular cloning, bacterial overexpression, characterization, and functional introgression into tobacco-conferring salt tolerance phenotype. J Biol Chem 279:28539–28552
McCouch, SR, Kochert G, Yu ZH, Wang ZY, Khush GS, Coffiman WR, Tanksley SD (1988) Molecular mapping of rice chromosomes. Theor Appl Genet 76:815–829
McCouch S, Teytelman L, Xu Y, Lobos K, Clare K, Walton M, Fu B, Maghirang R, Li Z, Xing Y, Zhang Q, Kono I, Yano M, Fjellstrom R, DeClerck G, Schneider D, Cartinhour S, Ware D, Stein L (2002) Development of 2,240 new SSR markers for rice (Oryza sativa L.) DNA Res 9:199–207
Marin K, Kanesaki Y, Los DA, Murata N, Suzuki I, Hagemann M (2004) Gene expression profiling reflects physiological processes in salt acclimation of Synechocystis sp. strain PCC 6803. Plant Physiol 136:3290–3300
Minhas D, Grover A (1999) Transcript levels of genes encoding various glycolytic and fermentation enzymes change in response to abiotic stress. Plant Sci 146:41–51
Mishra B, Singh RK (2000) CSR27, a fine grain salt tolerant variety of rice released. Salinity News 11:3
Mittler R (2002) Oxidative stress, antioxidants, and stress tolerance. Trends Plant Sci 7:405–410
Miyao A, Tanaka K, Murata K, Sawaki H, Takeda S, Abe K, Shinozuka Y, Onosato K, Hirochika H (2003) Target site specificity of the Tos17 retrotransposon shows a preference for insertion within genes and against insertion in retrotransposon-rich regions of the genome. Plant Cell 15:1771–1780
Mohanty A, Kathuria H, Ferjani A, Sakamoto A, Mohanty P, Murata N, Tyagi AK (2002) Transgenics of an elite indica rice variety Pusa Basmati 1 harbouring the codA gene are highly tolerant to salt stress. Theor Appl Genet 106:51–57
Montero-Lomeli M, Morais BLB, Figueiredo DL, Neto DCS, Martins JRP, Masuda CA (2002) The initiation factor eIF4A is involved in the response to lithium stress in Saccharomyces cerevisiae. J Biol Chem 277:21542–21548
Moons A, Bauw G, Prinsen E, Van-Montagu M, Straeten VDD (1995) Molecular and physiological responses to abscisic acid and salt in roots in salt-sensitive and salt tolerant indica rice varieties. Plant Physiol 107:177–186
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Nakayama H, Yoshida, K, Ono H, Murooka Y, Shinmyo A (2000) Ectoine, the compatible solute of Halomonas elongata, confers hyperosmotic tolerance in cultured tobacco cells. Plant Physiol 122:1239–1247
Nandi S, Subudhi PK, Senadhira D, Maigbas NL, Sen-Mandi S, Huang N (1997) Mapping QTLs for submergence tolerance in rice by AFLP analysis and selective genotyping. Mol Gen Genet 255:1–8
Nanjo T, Kobayashi M, Yoshiba Y, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (1999) Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana.FEBS Lett 461:205–210
Notsu Y, Masood S, Nishikawa T, Kubo N, Akiduki G, Nakazono M, Hirai A, Kadowaki K (2002) The complete sequence of the rice (Oryza sativa L.) mitochondrial genome: frequent DNA sequence acquisition and loss during the evolution of flowering plants. Mol Gen Genet 268:434–445
O’ Mahony PJ, Oliver MJ (1999) The involvement of ubiquitin in vegetative desiccation tolerance. Plant Mol Biol 41:657–667
Ohta M, Hayashi Y, Nakashima A, Hamada A, Tanaka A, Nakamura T, Hayakawa T (2002) Introduction of a Na+/H+ antiporter gene from Atriplex gmelini confers salt tolerance to rice. FEBS Lett 532:279–282
Oono Y, Seki M, Nanjo T, Narusaka M, Fujita M, Satoh R, Satou M, Sakurai T, Ishida J, Akiyama K, Iida K, Maruyama K, Satoh S, Yamaguchi-Shinozaki K, Shinozaki K (2003) Monitoring expression profiles of Arabidopsis gene expression during rehydration process after dehydration using a 7000 full-length cDNA microarray. Plant J 34:868–887
Pardo JM, Reddy MP, Yang S, Maggio A, Huh GH, Matsumoto T, Coca MA, Paino-D’Urzo M, Koiwa H, Yun DJ, Watad AA, Bressan RA, Hasegawa PM (1998) Stress signaling through Ca2+/calmodulin-dependent protein phosphatase calcineurin mediates salt adaptation in plants. Proc Natl Acad Sci USA 95:9681–9686
Pareek A, Singla SL, Grover A (1995) Immunological evidence for accumulation of two high-molecular-weight (104 and 90 kDa) HSPs in response to different stresses in rice and in response to high temperature stress in different plant genera. Plant Mol Biol 29:293–301
Pareek A, Singla SL, Grover A (1997) Salt responsive proteins/genes in crop plants. In: Jaiwal PK, Singh RP, Gulati A (eds) Strategies for improving salt tolerance in higher plants. Science Publishers, USA, pp 365–382
Park JM, Park CJ, Lee SB, Ham BK, Shin R, Paek KH (2001) Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell 13:1035–1046
Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, Morris J, Hirschi KD, Gaxiola RA (2005) Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci USA 102:18830–18835
Parker R, Flowers TJ, Moore AL, Harpham NVJ (2006) An accurate and reproducible method for proteome profiling of the effects of salt stress in the rice leaf lamina. J Exp Bot 57:1109–1118
Prasad SR, Bagali PG, Hittalmani S, Shashidhar HE (2000a) Molecular mapping of quantitative trait loci associated with seedling tolerance to salt (Oryza sativa L.). Curr Sci 78:162–164
Prasad KVSK, Sharmila P, Kumar PA, Saradhi PP (2000b) Transformation of Brassica juncea (L.) Czern with bacterial codA gene enhances its tolerance to salt stress. Mol Breed 6:489–499
Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, Yoshiwara K, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Monitoring expression profiles of rice genes under cold, drought, and high- salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol 133:1755–1767
Rausell A, Kanhonou R, Yenush L, Serrano R, Ros R (2003) The translation initiation factor eIF1A is an important determinant in the tolerance to NaCl stress in yeast and plants. Plant J 34:257–267
Reddy AR, Ramakrishna W, Chandra Shekhar A, Ithal N, Babu PR, Bonaldo FM, Soares B, Bennetzen JL (2002) Novel genes are enriched in normalized cDNA libraries from drought stressed seedlings of indica rice (Oryza sativa L. subsp. indica cv. Nagina 22). Genome 45:204–211
Ren ZH, Gao JP, Li LG, Cai XL, Huang W, Chao DY, Zhu MZ, Wang ZY, Luan S, Lin HX (2005) A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet 37:1141–1146
Revenkova E, Masson J, Koncz C, Afsar K, Jakovleva L, Paszkowski J (1999) Involvement of Arabidopsis thaliana ribosomal protein S27 in mRNA degradation triggered by genotoxic stress. EMBO J 18:490–499
Roxas VP, Smith RK, Allen ER, Allen RD (1997) Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat Biotechnol 15:988–991
Roy M, Jain RK, Rohila JS, Wu R (2000) Production of agronomically superior transgenic rice plants using Agrobacterium transformation methods: present status and future perspectives. Curr Sci 79:954–960
Sahi C, Agarwal M, Reddy MK, Sopory SK, Grover A (2003) Isolation and expression analysis of salt stress-associated ESTs from contrasting rice cultivars using a PCR-based subtraction method. Theor Appl Genet 106:620–628
Sahi C, Singh A, Blumwald E, Grover A (2006) Beyond osmolytes and transporters: novel plant salt stress tolerance-related genes from transcriptional profiling data. Physiol Plant 127:1–9
Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K (2000) Over-expression of a single Ca2+dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J 23:319–327
Sakamoto A, Alia, Murata N, Murata A (1998) Metabolic engineering of rice leading to biosynthesis of glycinebetaine and tolerance to salt and cold. Plant Mol Biol 38:1011–1019
Sallaud C, Gay C, Larmande P, Bes M, Piffanelli P, Piegu B, Droc G, Regad F, Bourgeois E, Meynard D, Perin C, Sabau X, Ghesquiere A, Glaszmann JC, Delseny M, Guiderdoni E (2004) High throughput T-DNA insertion mutagenesis in rice: a first step towards in silico reverse genetics. Plant J 39:450–464
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, New York
Sanan-Mishra N, Pham XH, Sopory SK, Tuteja N (2005) Pea DNA helicase 45 overexpression in tobacco confers high salinity tolerance without affecting yield. Proc Natl Acad Sci USA 102:509–514
Santerre A, Britt AB (1994) Cloning of a 3-methyladenine-DNA glycosylase from Arabidopsis thaliana. Proc Natl Acad Sci USA 91:2240–2244
Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninici P, Hayashizaki Y, Shinozaki K (2001) Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray.Plant Cell 13:61–72
Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nokajima M, Enju A, Sakurai T, Satou M, Akiyama K, Taji T, Yamaguchi-Shinozaki K, Carninci P, Kawai J, Hayashizaki Y, Shinozaki K (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J 31:279–292
Shi H, Lee BH, Wu SJ, Zhu JK (2003) Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol 21:81–85
Shimamoto K (1999) Molecular biology of rice, Springer-Verlag, Tokyo
Shiozaki N, Yamada M, Yoshiba Y (2005) Analysis of salt-stress-inducible ESTs isolated by PCR-subtraction in salt-tolerant rice. Theor Appl Genet 110:1177–1186
Singla-Pareek SL, Reddy MK, Sopory SK (2003) Genetic engineering of the glyoxylase pathway in tobacco leads to enhanced salinity tolerance. Proc Natl Acad Sci USA 100:14672–14677
Sivakumar P, Sharmila P, Saradhi PP (1998) Proline suppresses Rubisco activity in higher plants. Biochem Biophys Res Commun 252:428–432
Sottosanto JB, Gelli A, Blumwald E (2004) DNA array analyses of Arabidopsis thaliana lacking a vacuolar Na/H antiporter: impact of AtNHX1 on gene expression. Plant J 40:752–771
Sun W, Bernard C, van de Cotte B, Van Montagu M, Verbruggen N (2001) At-HSP17.6A, encoding a small heat-shock protein in Arabidopsis can enhance osmotolerance upon overexpression. Plant J 27:407–415
Taji T, Seki M, Satou M, Sakurai T, Kobayashi M, Ishiyama K, Narusaka Y, Narusaka M, Zhu JK, Shinozaki K (2004) Comparative genomics in salt tolerance between Arabidopsis and Arabidopsis-related halophyte salt cress using Arabidopsis microarray. Plant Physiol 135:1697–1709
Takeda S, Sugimoto K, Kakutani T, Hirochika H (2001) Linear DNA intermediates of the Tto1 retrotransposon in Gag particles accumulated in stressed tobacco and Arabidopsis thaliana. Plant J 28:307–317
Takehisa H, Shimodate T, Fukuta Y, Ueda T, Yano M, Yamayad T, Kameya T, Sato T (2004) Identification of quantitative trait loci for plant growth of rice in paddy field flooded with salt water. Field Crops Res 89:85–95
Tanaka Y, Hibin T, Hayashi Y, Tanaka A, Kishitani S. Takabe T, Yokota S, Takabe T (1999) Salt tolerance of transgenic rice overexpressing yeast mitochondrial Mn-SOD in chloroplasts. Plant Sci 148:131–138
Tarczynski MC, Jensen RG, Bohnert H (1993) Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 259:508–510
Tsunematsu H, Yoshimura A, Horushima Y, Nagamura Y, Kurata N, Yano M, Sasaki T, Iwata N (1996) RFLP framework map using recombinant inbred lines in rice. Breed Sci 46:279–284
Urao T, Yakubov B, Satoh R, Yamaguchi-Shinozaki K, Seki B, Hirayama T, Shinozaki K (1999) A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell 11:1743–1754
Veena J, Reddy VS, Sopory SK (1999) Glyoxalase I from Brassica juncea: molecular cloning, regulation and its over-expression confer tolerance in transgenic tobacco under stress. Plant J 17:385–395
Vernon DM, Tarczynski, MC, Jensen RG, Bohnert HJ (1993) Cyclitol production in transgenic tobacco. Plant J 4:199–205
Vierstra RD, Callis J (1999) Polypeptide tags, ubiquitous modifiers for plant protein regulation. Plant Mol Biol 41:435–442
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol 16:123–132
Walia H, Wilson C, Condamine P, Liu X, Ismail AM, Zeng L, Wanamaker SI, Mandal J, Xu J, Cui X, Close TJ (2005) Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiol 139:822–835
Walia H, Wilson C, Wahi A, Condamine P, Cui X, Close TJ (2006) Expression analysis of barley (Hordeum vulgare L.) during salinity stress. Funct Integr Genomics 6:143–156
Wang GL, Mackill DJ, Bonman JM, McCouch SR, Champoux MC, Nelson RJ (1994) RFLP mapping of genes conferring complete and partial resistance to blast in a durably resistant rice cultivar. Genetics 136:1421–1434
Wang J, Zuo K, Wu W, Song J, Sun X, Lin J, Li X, Tang K (2004) Expression of a novel antiporter gene from Brassica napus resulted in enhanced salt tolerance in transgenic tobacco plants. Biol Plant 48:509–515
Wendler WM, Kremmer E, Forster R, Winnacker EL (1997) Identification of pirin, a novel highly conserved nuclear protein. J Biol Chem 272:8482–8489
Widawsky DA, O’Toole JC (1990) Prioritizing rice biotechnology research agenda for Eastern India. The Rockefeller Foundation, New York, USA
Wood AJ, Oliver MJ (1999) Translational control in plant stress: formation of messenger ribonucleoprotein complexes (mRNPs) in Tortula ruralis in response to desiccation.Plant J 18:359–370
Wood AJ, Duff RJ, Oliver MJ (2000) The translational apparatus of Tortula ruralis: polysomal retention of transcripts encoding the ribosomal proteins RPS14, RPS16 and RPL23 in desiccated and rehydrated gametophytes. J Exp Bot 51:1655–1662
Wool IG (1996) Extraribosomal functions of ribosomal proteins. Trends Biochem Sci 21:164–165
Wu CA, Yang GD, Meng QW, Zheng CC (2004) The cotton GhNHX1 gene encoding a novel putative tonoplast Na+/K+ antiporter plays an important role in salt stress. Plant Cell Physiol 45:600–607
Wu L, Fan Z, Guo L, Li Y, Chen ZL, Qu LJ (2005) Overexpression of the bacterial nhaA gene in rice enhances salt and drought tolerance. Plant Sci. 168:297–302
Xiao J, Li J, Yuan L, Tanksley SD (1996) Identification of QTLs affecting traits of agronomic importance in a recombinant inbred population derived from a subspecific rice cross. Theor Appl Genet 92:230–244
Xu D, Duan X, Wang B, Hong B, Ho THD, Wu R (1996) Expression of a late embryogenesis abundant (LEA) protein gene, HVA1, from barley confers tolerance to drought and salinity in transgenic rice. Plant Physiol 110:249–257
Xue ZY, Zhi DY, Xue GP, Zhang H, Zhao YX, Xia GM (2004) Enhanced salt tolerance of transgenic wheat (Tritivum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Sci 167:849–859
Yadav R, Courtois B, Huang N, McLaren G (1997) Mapping genes controlling root morphology and root distribution in a doubled-haploid population of rice. Theor Appl Genet 94:619–632
Yeo AR, Caporn SJM, Flowers TJ (1985) The effect of salinity upon photosynthesis in rice (Oryza sativa L.): gas exchange by individual leaves in relation to their salt content. J Exp Bot 36:1240–1248
Yilmaz JL, Bulow L (2002) Enhanced stress tolerance in Escherichia coli and Nicotiana tabacum expressing a betaine aldehyde dehydrogenase/choline dehydrogenase fusion protein. Biotechnol Prog 18:1176–1182
Yin XY, Yang AF, Zhang KW, Zhang JR (2004) Production and analysis of transgenic maize with improved salt tolerance by the introduction of AtNHX1gene. Acta Bot Sin 46:854–861
Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768
Zhang G-Y, Guo Y, Chen S-L, Chen S-Y (1995) RFLP tagging of a salt tolerant gene in rice. Plant Sci 110:227–224
Zhang HX, Hodson JN, Williams JP, Blumwald E (2001) Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc Natl Acad Sci USA 98:6896–6901
Zhao F, Guo S, Zhang H, Zhao Y (2006a) Expression of yeast SOD2 in transgenic rice results in increased salt tolerance. Plant Sci 170:216–224
Zhao F, Wang Z, Zhang Q, Zhao Y, Zhang H (2006b) Analysis of the physiological mechanism of salt-tolerant transgenic rice carrying a vacuolar Na+/H+ antiporter gene from Suaeda salsa. J Plant Res 119:95–104
Zhu B, Su J, Chang MC, Verma DPS, Fan YL, Wu R (1998) Overexpression of a pyrroline-5-carboxylate synthetase gene and analysis of tolerance to water and salt stress in transgenic rice. Plant Sci 139:41–48
Acknowledgements
AG is thankful to the Department of Biotechnology (DBT), Government of India and National Agriculture Technology Project (NATP), Indian Council of Agricultural Research (ICAR), Government of India, for the financial support. CS and AS acknowledge the Council of Scientific and Industrial Research (CSIR), New Delhi for the fellowship grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahi, C., Singh, A., Kumar, K. et al. Salt stress response in rice: genetics, molecular biology, and comparative genomics. Funct Integr Genomics 6, 263–284 (2006). https://doi.org/10.1007/s10142-006-0032-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-006-0032-5