Abstract
Bio-indicators are extremely useful for quickly assessing the true condition of a rapidly deteriorating freshwater environment at a low cost. Global research has shown that the spider (Arachnida: Araneae) is a well-known taxon with bio-indicator potential. Present study was performed to find out if any spider species could be used as a bio-indicator of undisturbed habitat for Gangetic riparian zone. For the study, total 27 sites were selected along the Ganga River’s banks, with an average distance of 75 kms between each site. Based on disturbance ratings, the sampling sites were divided into five groups: very low disturbed sites, low disturbed sites, moderately disturbed sites, highly disturbed sites and very highly disturbed sites. The non-parametric analysis of similarities (ANOSIM) test and non-parametric permutational multivariate analysis of variance (PERMANOVA) were used to understand similarities of species composition between these groups. The redundancy analysis (RDA) was performed to investigate the relationship between spider distribution and habitat disturbance. The indicator value (IndVal) method was used to find out significant bio-indicator spider species for habitat disturbance. The study found that the agriculture, human occupation, manmade embankment, and sand mining were the most effective combination of disturbance that affects spider assemblage and eight species can be used as indicator of very low disturbed sites and one species can be used as indicator of low disturbed sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bio-indicators are taxa or functional groups which reflect the state of the environment (Lencinas et al. 2015) as environmental indicators (by either acting as early warning indicators of any environmental change to the local environment) or as ecological indicators (by response to the specific ecosystem stress) or biodiversity indicators (by indicating the levels of taxonomic diversity at a site) (McGeogh 2007). The environmental and ecological bioindicators can be divided into detectors (naturally occurring indicators which are sensitive to environmental change and so decrease with added environmental stress), exploiters (increasing in abundance in response to environmental stress) and accumulators (organisms which take up chemicals such as heavy metals and can be used to measure toxin levels) (Jenkins 1971; Spellerberg 1991). Biomonitoring programs using resident, widespread and adapted species facilitate an efficient and early intervention, and represent a fundamental contribution to ecological risk assessment and environmental management (Conti et al. 2018). Bio-indicators are useful for conservation prioritisation by means of spatial comparisons of a site value, or monitoring of ecosystem recovery or response to management decisions (Peri et al. 2016; Schwerdt et al. 2018). Among the arthropods, the spiders have cosmopolitan distribution, they occupy different ecological niches, they have capacity of exploration and adaptation to various ecological conditions, they show rapid responses to disturbances, sensitive to changes in environmental conditions, and are relatively easy to sample. For having all these features, the spiders worldwide are considered to have potentials for bio-indicators (Pearce and Venier 2006; Mader et al. 2016; Schwerdt et al. 2018).
In recent years, the spiders are successfully used as bio-indicator for air pollution (Rachwał et al. 2018; Rutkowski et al. 2018), different agricultural practices, climatic conditions, or various cultivars grown (Radkova et al. 2019), disturbances of stream ecosystems (Reyes-Maldonado et al. 2018), effect of the canopy openness gradient on biodiversity conservation (Košulič et al. 2016), forest restoration (Borchard 2014), grazing effects (Kaltsas et al. 2019), ground of the central and peripheral area of olive agroecosystem (Benhadi‐Marín et al. 2019), health of natural grasslands (Schwerdt et al. 2018), heavy metal contamination (Yang et al. 2016; Conti et al. 2018; Hansson et al. 2019), low anthropogenic intervention (Rosa et al. 2019) and management strategies in fruit orchards (Mazzia et al. 2015).
The freshwater ecosystems are facing various adversities all over the world such as flow modifications by construction of dams and reservoirs, overexploitation of biotic resources, unlimited extraction of water, invasion of exotic species, organic and inorganic pollution from agricultural, industrial and domestic sources and climate change (Dudgeon et al. 2005; Strayer and Dudgeon 2010; Vörösmarty et al. 2010; Collen et al. 2014). Some recent studies indicated that the fresh water ecosystem of the Ganga river is intimidated by overexploitation, pollution, riverbed mining, physical barriers, change of land use pattern and climate change (Siddiqui and Pandey 2019; Singh and Singh 2019; Kamboj and Kamboj 2019; Dey et al. 2019; Verma et al. 2019; Jain and Singh 2020; Paudel and Koprowski 2020; Santy et al. 2020). But, as these types of works are labour intensive, time consuming and costly, there is a great need for ecological indicators for the Ganga river to know the state of the ecosystem in a short time at low cost. However, no research has been done to find out any invertebrate taxa for bio-indicator of habitat disturbance of riparian zones of the Ganga river until now. With this background, the present work was carried out to find out significant bio-indicator spider taxa for undisturbed habitat for Gangetic riparian zones.
Materials and methods
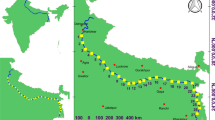
For the study, 27 sites were chosen on the banks of the Ganga river from Bijnour in Uttar Pradesh to Batanagar in West Bengal with average distance between two successive sites about 75 km (Fig. 1). For detail description of each study sites refer Ali et al. (2019). The fieldwork was performed during summer of 2018 (May and June) and 2019 (May and June) and winter of 2018 (November and December) and 2019 (November and December). Each site was visited once in each season in each year.
At each site, a 50 m (width) × 100 m (length) plot was chosen beside either left bank or right bank of the river, depending upon accessibility and within each plot 15 quadrates of 4 m × 4 m (3 across the width and 5 across the length) were employed for spider collection. Six different collection methods—pitfall trapping, vegetation beating, litter sampling, ground hand collection, aerial hand collection, and sweep netting (Coddington et al. 1996) were employed for spider collection. For pitfall trapping, at the centre of each quadrate, one plastic bottle of 10 cm diameter and 11 cm depth (Churchill and Arthur 1999) filled with preservative (69% water, 30% ethyl acetate, and 1% detergent) was placed for overnight. For the other approaches, 30 min in day time were spent collecting spiders in each quadrate for each technique. The collected spiders were preserved in 70% ethanol and identified the specimens up to at least genus level from published literatures.
At each site nine types of natural habitat disturbance namely—agricultural activities, boats, effluent discharge, garbage dump, ghats (series of steps descending into the water body), grazing, human settlement, manmade embankment and sand mining were recorded. According to Gezie et al. (2017), score of 1 for any or minimum disturbance (if disturbances were found in 0–5 quadrates), 2 for moderate (if disturbances were found in 5–10 quadrates), and 3 for high disturbance (if disturbances were found in 10–15 quadrates) were assigned. The overall disturbance of each site was calculated by adding individual values (a total of nine different factors). The overall disturbance score ranges from 9 to 22. Based on this disturbance scores, the sites were classified into five groups, namely very low disturbed sites (disturbance score up to 11), low disturbed sites (disturbance score between 12 and 14), moderately disturbed sites (disturbance score between 15 and 17), highly disturbed sites (disturbance score between 18 and 20) and very highly disturbed sites (disturbance score more than 20).
For analysis, summed species matrix (i.e. pooled across all seasons) for each site was used. Prior to analysis the habitat disturbance score and species richness (mean = 0 and SD = 1) were normalized (Miyazono and Taylor 2013; Datry et al. 2016). All the analysis was performed in R (version 4.0.0) language and environment for statistical computing (R Core Team 2020). The non-parametric analysis of similarities (ANOSIM) test (Clarke 1993) and non-parametric permutational multivariate analysis of variance (PERMANOVA) (Anderson 2001) in R package ‘vegan’ (Oksanen et al. 2019) were used to understand similarities of species composition between the groups (of sites based on disturbance score). To facilitate interpretation of the results of ANOSIM and PERMANOVA, the non-metric multidimensional scaling (NMDS) based on spider composition was performed. The redundancy analysis (RDA) in R package ‘vegan’ (Oksanen et al. 2019) was performed to investigate the relationship between the spider distribution and habitat disturbance. To assess the significance of constraints, the permutation test (with 999 permutations) using both direct model (which permutes community data) and reduced model (which permutes residuals of the community data) was performed. The automatic backward stepwise model with 999 iteration steps was used to know which disturbance affected spider distribution in RDA ordination space. This method has the benefit of assessing the joint predictive potential of variables (Chowdhury and Turin 2020) as this process starts with all variables in the model and removes the least important variables early on, leaving only the most important variables in the model (Pham et al. 2020; Chowdhury and Turin 2020). The R package ‘MuMIn’ (Barton 2020) was used to find out best disturbance combination that affected the species richness of the spider community. Then the indicator value (IndVal: Dufrêne and Legendre 1997) was calculated for each species with ‘labdsv’ package (Roberts 2019). For the final list of indicator species, only those samples that were identified up to the species level were selected.
Results
It was found that the disturbance score ranged from 9 to 21 across different sites (Fig. 2). By non-parametric analysis of similarities (ANOSIM) test it was found that there was significant difference between the spider communities in different disturbed sites groups (ANOSIM statistic R = 0.3709, P = 0.001). By non-parametric permutational multivariate analysis of variance (PERMANOVA) test it was found that there was significant difference between the spider communities in different disturbed sites groups (ADONIS, F = 2.219, R2 = 0.287, P = 0.001). With the help of NMDS plot it was found that all the sites that had the similar species composition were clustered together and NMDS plot showed that different species composition were different in the very low disturbed sites and very highly disturbed sites (Fig. 3).
Comparative account of the disturbance score of 27 sites. In figure A, the size and shade of the bubbles (representing location of sampling sites) are proportional to the disturbance score. In figure B, the red horizontal line represents the mean value and the shaded area represents values within the mean ± standard deviation (color figure online)
The permutation test for redundancy analysis (RDA) under direct model (F = 1.421, P = 0.002) and reduced model (F = 1.421, P = 0.004) were found as significant. The variance of the RDA biplot (Fig. 4) of spider metrics and habitat disturbance based on the first two axes explained 55.10% of the variance. Axes 1 and 2 explained 40.94% and 14.16% of the variation in spider assemblages, respectively. This first axis was positively correlated with the entire habitat disturbances; the second axis was positively correlated with boats, manmade embankment, garbage dump, effluent discharge, ghats and human settlement but negatively correlated with grazing, agriculture and sand mining. The automatic backward stepwise model resulted in 8 steps and it was found that the human settlement and sand mining had significant (P < 0.05) affect on the spider distribution in RDA ordination space (Supplementary file 1). The study found that the agriculture, human settlement, manmade embankment and sand mining were the best disturbance combination (Table 1) that affected the species richness of the spider community.
Ordination diagram of the redundancy analysis (RDA) of the spider community. Arrows represent the direction of change of the habitat disturbance. The length of the arrow indicates the variable's importance in explaining the spider community. Angles between variables indicate the correlation between the habitat disturbances. Sites locations (black dots) and species locations (grey dots) relative to each other indicate their similarity in ordination space (color figure online)
The study found that eight species can be used as indicator of very low disturbed sites and only one species as bio-indicator for low disturbed sites (Table 2). For other sites, no spider species was found as significant bio-indicator.
Discussion
The ecological guild (following Cardoso et al. 2011) of the eight species of spiders found as indicator species for very low disturbed sites includes orb web builders (three species under families Araneidae and Uloboridae), sheet web builder (one species under family Pisauridae), ground hunter (one species under family Lycosidae) and other hunter (three species under family Salticidae) whereas the indicator species for low disturbed sites was space web builder (one species under family Theridiidae).
No indicator species were found for moderately disturbed sites, highly disturbed sites and very highly disturbed sites, but we did find indicator species for very low disturbed sites and low disturbed sites, which were also present in other sites. This interesting composition of spider community indicates that the community was likely to be nested because in nestedness, small communities form proper subsets of large communities which a typical bio-geographic pattern (Ulrich and Gotelli 2007).
Geographically any river is an ever-changing entity because the rainfall, temperature, amount of forest and the nature of the land use affect the river hydrology and in many cases it is seasonal and this change is accompanied by changes in the river-dependent biota. Prolonged bio-monitoring on a long river like the Ganga is a daunting task for any scientific body. For this, researchers have to rely on the people living on the banks of the river and depend on the river for their livelihood. Such animals and plants should be identified as bio-indicators which are readily available and known to them, so that they can present any visible change in an area to the scientists. Spiders are extremely useful for this task as they are a well-known animal to human. As the habitats of the riparian zones are altered by anthropogenic activities the riparian spider communities respond strongly to urbanization and anthropogenic activities (de Jesús-Crespo and Ramírez 2011; Ramírez et al. 2011; Reyes-Maldonado et al. 2018). As anthropogenic stressors likely make disturbance-tolerant species inhabiting disturbed habitats and disturbance-sensitive species vulnerable to extinction, there is an urgent need to develop methodologies for evaluating riparian ecological health from a multiple and integrated perspective (Zina et al. 2021). Small-scale monitoring, such as protocols and bio-indicators, should be utilised when specific parts of the watershed that are substantially degraded are recognised (Zaimes and Iakovoglou 2020). A vital step in developing such monitoring programmes is selecting cost-effective bio-indicators (Valente-Neto et al. 2021).
This is an initial work on the bio-indicator spiders for the Ganga river and the work should be iterated with the help of new information because scientific community has limited but always evolving knowledge about pattern of diversity and distribution of plants and animals, especially for invertebrates, the change in conservation strategy will be taken place with the increase of information and knowledge about ecology of any species (Abellán et al. 2005).
Conclusion
Through the present work it can be concluded that spiders can be used as bio-indicator of habitat disturbance in the riparian zone of river ecosystem. But as a single taxon cannot indicate overall biodiversity or environmental conditions (Lehmitz et al. 2020), it is expected that researchers will study the bio-indicator capacity of plants and animals living in the water and on the banks of the Ganga river and present new information for effective conservation, restoration and management of this freshwater ecosystem.
References
Abellán P, Sánchez-Fernández D, Velasco J, Millán A (2005) Assessing conservation priorities for insects: status of water beetles in southeast Spain. Biol Cons 121:79–90. https://doi.org/10.1016/j.biocon.2004.04.011
Ali ZSK, Johnson JA, Hussain SA et al (2019) Study area and sampling strategy. In: Johnson JA, Hussain SA, Badola R (eds) Biodiversity profile of the Ganga river. Wildlife Institute of India, Dehradun, pp 24–61
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Barton K (2020) MuMIn: multi model inference. R Package Version 1(43):17
Benhadi-Marín J, Pereira JA, Sousa JP, Santos SAP (2019) Distribution of the spider community in the olive grove agroecosystem (Portugal): potential bioindicators. Agr Forest Entomol 22:10–19. https://doi.org/10.1111/afe.12352
Borchard F, Buchholz S, Helbing F, Fartmann T (2014) Carabid beetles and spiders as bioindicators for the evaluation of montane heathland restoration on former spruce forests. Biol Cons 178:185–192. https://doi.org/10.1016/j.biocon.2014.08.006
Cardoso P, Pekár S, Jocqué R, Coddington JA (2011) Global patterns of guild composition and functional diversity of spiders. PLoS One 6:e21710. https://doi.org/10.1371/journal.pone.0021710
Chowdhury MZI, Turin TC (2020) Variable selection strategies and its importance in clinical prediction modelling. Fam Med Com Health 8:e000262. https://doi.org/10.1136/fmch-2019-000262
Churchill TB, Arthur JM (1999) Measuring spider richness: effects of different sampling methods and spatial and temporal scales. J Insect Conserv 3:287–295. https://doi.org/10.1023/a:1009638706789
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Austral Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Coddington JA, Young LH, Coyle FA (1996) Estimating spider species richness in a southern Appalachian cove hardwood forest. J Arachnol 24:111–128
Collen B, Whitton F, Dyer EE et al (2014) Global patterns of freshwater species diversity, threat and endemism. Glob Ecol Biogeogr 23:40–51. https://doi.org/10.1111/geb.12096
Conti E, Costa G, Liberatori G et al (2018) Ariadna spiders as bioindicator of heavy elements contamination in the Central Namib Desert. Ecol Ind 95:663–672. https://doi.org/10.1016/j.ecolind.2018.08.014
da Rosa MG, Brescovit AD, Baretta CRDM et al (2019) Diversity of soil spiders in land use and management systems in Santa Catarina. Brazil Biota Neotrop. https://doi.org/10.1590/1676-0611-bn-2018-0619
Datry T, Melo AS, Moya N et al (2016) Metacommunity patterns across three neotropical catchments with varying environmental harshness. Freshw Biol 61:277–292. https://doi.org/10.1111/fwb.12702
de Jesús-Crespo, Ramírez A (2011) Effects of urbanization on stream physicochemistry and macroinvertebrate assemblages in a tropical urban watershed in Puerto Rico. J N Am Benthol Soc 30:739–750. https://doi.org/10.1899/10-081.1
Dey S, Choudhary SK, Dey S et al (2019) Identifying potential causes of fish declines through local ecological knowledge of fishers in the Ganga river, eastern Bihar, India. Fish Manag Ecol 27:140–154. https://doi.org/10.1111/fme.12390
Dudgeon D, Arthington AH, Gessner MO et al (2005) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163. https://doi.org/10.1017/s1464793105006950
Dufrêne M, Legendre P (1997) Species assemblages and indicator species:the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. https://doi.org/10.1890/0012-9615(1997)067[0345:saaist]2.0.co;2
Gezie A, Anteneh W, Dejen E, Mereta ST (2017) Effects of human-induced environmental changes on benthic macroinvertebrate assemblages of wetlands in Lake Tana Watershed Northwest Ethiopia. Environ Monit Assess 189(4):152. https://doi.org/10.1007/s10661-017-5853-2
Hansson SV, Høye TT, Bach L et al (2019) Spiders as biomonitors of metal pollution at Arctic mine sites: the case of the Black Angel Pb-Zn-mine, Maarmorilik West Greenland. Ecol Ind 106:105489. https://doi.org/10.1016/j.ecolind.2019.105489
Jain CK, Singh S (2020) Impact of climate change on the hydrological dynamics of River Ganga, India. J Water Clim Change 11:274–290. https://doi.org/10.2166/wcc.2018.029
Jenkins DW (1971) Global biological monitoring. In: Matthews WH, Smith FE, Goldberg ED (eds) Man’s impact on terrestrial and oceanic ecosystems. The Colonial Press, New York, pp 351–370
Kaltsas D, Panayiotou E, Kougioumoutzis K, Chatzaki M (2019) Overgrazed shrublands support high taxonomic, functional and temporal diversity of Mediterranean ground spider assemblages. Ecol Ind 103:599–609. https://doi.org/10.1016/j.ecolind.2019.04.024
Kamboj N, Kamboj V (2019) Riverbed mining as a threat to in-stream agricultural floodplain and biodiversity of Ganges River, India. In: Kumar V, Kumar R, Singh J et al (eds) Contaminants in agriculture and environment: health risks and remediation, vol 1. Agro Environ Media, Haridwar, pp 250–263
Košulič O, Michalko R, Hula V (2016) Impact of canopy openness on spider communities: implications for conservation management of formerly coppiced oak forests. PLoS One 11:e0148585. https://doi.org/10.1371/journal.pone.0148585
Lehmitz R, Haase H, Otte V, Russell D (2020) Bioindication in peatlands by means of multi-taxa indicators (Oribatida, Araneae, Carabidae, Vegetation). Ecol Ind 109:105837. https://doi.org/10.1016/j.ecolind.2019.105837
Lencinas MV, Kreps G, Soler R et al (2015) Neochelanops michaelseni (Pseudoscorpiones: Chernetidae) as a potential bioindicator in managed and unmanaged Nothofagus forests of Tierra del Fuego. J Arachnol 43:406–412. https://doi.org/10.1636/0161-8202-43.3.406
Mader V, Birkhofer K, Fiedler D et al (2016) Land use at different spatial scales alters the functional role of web-building spiders in arthropod food webs. Agric Ecosyst Environ 219:152–162. https://doi.org/10.1016/j.agee.2015.12.017
Mazzia C, Pasquet A, Caro G et al (2015) The impact of management strategies in apple orchards on the structural and functional diversity of epigeal spiders. Ecotoxicology 24:616–625. https://doi.org/10.1007/s10646-014-1409-1
McGeogh MA (2007) The selection, testing and application of terrestrial insects as bioindicators. Biol Rev 73:181–201. https://doi.org/10.1111/j.1469-185x.1997.tb00029.x
Miyazono S, Taylor CM (2013) Effects of habitat size and isolation on species immigration-extinction dynamics and community nestedness in a desert river system. Freshw Biol 58:1303–1312. https://doi.org/10.1111/fwb.12127
Oksanen J, Guillaume Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs H, Wagner H (2019) Vegan: community ecology package. R package version 2.5-6.
Paudel S, Koprowski JL (2020) Factors affecting the persistence of endangered Ganges River dolphins (Platanista gangetica gangetica). Ecol Evol 10:3138–3148. https://doi.org/10.1002/ece3.6102
Pearce JL, Venier LA (2006) The use of ground beetles (Coleoptera: Carabidae) and spiders (Araneae) as bioindicators of sustainable forest management: a review. Ecol Ind 6:780–793. https://doi.org/10.1016/j.ecolind.2005.03.005
Peri PL, Bahamonde HA, Lencinas MV et al (2016) A review of silvopastoral systems in native forests of Nothofagus antarctica in southern Patagonia, Argentina. Agrofor Syst 90:933–960. https://doi.org/10.1007/s10457-016-9890-6
Pham BT, Nguyen-Thoi T, Ly H-B et al (2020) Extreme learning machine based prediction of soil shear strength: a sensitivity analysis using monte carlo simulations and feature backward elimination. Sustainability 12:2339. https://doi.org/10.3390/su12062339
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rachwał M, Rybak J, Rogula-Kozłowska W (2018) Magnetic susceptibility of spider webs as a proxy of airborne metal pollution. Environ Pollut 234:543–551. https://doi.org/10.1016/j.envpol.2017.11.088
Radkova M, Naumova M, Stoichev S et al (2019) Biodiversity of epigeic spiders in conventional potato fields in South-western Bulgaria (Ten Years Later). Cr Acad Bulg Sci 72:1052–1058. https://doi.org/10.7546/CRABS.2019.08.07
Ramírez A, Engman A, Rosas KG et al (2011) Urban impacts on tropical island streams: some key aspects influencing ecosystem response. Urban Ecosyst 15:315–325. https://doi.org/10.1007/s11252-011-0214-3
Reyes-Maldonado R, Sánchez-Ruiz JA et al (2018) Comunidades de arañas ribereñas como indicadores de la condición de los ecosistemas fluviales en la cuenca del Río Piedras de Puerto Rico. Actual Biol 39:1–23
Roberts DW (2019) labdsv: Ordination and multivariate analysis for ecology. R package version 2.0-1.
Rutkowski R, Rybak J, Mach T, Rogula-Kozłowska W (2018) Spider webs in monitoring of air pollution. SHS Web Conf 57:02011. https://doi.org/10.1051/shsconf/20185702011
Santy S, Mujumdar P, Bala G (2020) Potential impacts of climate and land use change on the water quality of Ganga river around the industrialized Kanpur Region. Sci Rep 10:9107. https://doi.org/10.1038/s41598-020-66171-x
Schwerdt L, Elena de Villalobos A, Miles FP (2018) Spiders as potential bioindicators of mountain grasslands health: the Argentine tarantula Grammostola vachoni (Araneae, Theraphosidae). Wildl Res 45:64. https://doi.org/10.1071/wr17071
Siddiqui E, Pandey J (2019) Assessment of heavy metal pollution in water and surface sediment and evaluation of ecological risks associated with sediment contamination in the Ganga river: a basin-scale study. Environ Sci Pollut Res 26:10926–10940. https://doi.org/10.1007/s11356-019-04495-6
Singh R, Singh GS (2019) Climate change impacts on the Ganga river ecosystem services: challenges for the well-being of millions. Clim Change Environ Sustain 7:108. https://doi.org/10.5958/2320-642x.2019.00014.0
Spellerberg IF (1991) Monitoring ecological change. Cambridge University Press, Cambridge
Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation: recent progress and future challenges. J N Am Benthol Soc 29:344–358. https://doi.org/10.1899/08-171.1
Ulrich W, Gotelli NJ (2007) Null model analysis of species nestedness patterns. Ecology 88:1824–1831. https://doi.org/10.1890/06-1208.1
Valente-Neto F, Martínez BT, Hughes RM et al (2021) Incorporating costs, thresholds and spatial extents for selecting stream bioindicators in an ecotone between two Brazilian biodiversity hotspots. Ecol Ind 127:107761. https://doi.org/10.1016/j.ecolind.2021.107761
Verma K, Pandey J, Siddiqui E (2019) Heavy metal pollution in the ganga river enhances carbon storage relative to flux. Bull Environ Contam Toxicol 104:41–48. https://doi.org/10.1007/s00128-019-02761-4
Vörösmarty CJ, McIntyre PB, Gessner MO et al (2010) Global threats to human water security and river biodiversity. Nature 467:555–561. https://doi.org/10.1038/nature09440
Yang H, Peng Y, Tian J et al (2016) Spiders as excellent experimental models for investigation of heavy metal impacts on the environment: a review. Environ Earth Sci 75:1059. https://doi.org/10.1007/s12665-016-5828-6
Zaimes GN, Iakovoglou V (2020) Assessing riparian areas of greece—an overview. Sustainability 13:309. https://doi.org/10.3390/su13010309
Zina V, Ordeix M, Franco JC et al (2021) Ants as bioindicators of riparian ecological health in Catalonian rivers. Forests 12:625. https://doi.org/10.3390/f12050625
Acknowledgements
Authors are thankful to the National Mission for Clean Ganga, Ministry of Jal Shakti, Department of Water Resources, River development and Ganga Rejuvenation, Government of India for sponsoring the work under the project “Biodiversity conservation and Ganga Rejuvenation” (Grant No. B-02/2015-16/1259/NMCG-WIIPROPOSAL). Authors express gratitude to the Director and Dean, Wildlife Institute of India for their administrative support for the study. Authors are thankful the Environment, Forest and Climate Change Department, Government of Uttar Pradesh; the Department of Environment, Forest and Climate Change, Government of Bihar; the Environment, Forest and Climate Change Department, Government of Jharkhand and the Department of Forests, Government of West Bengal for providing support and necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De, K., Siliwal, M., Uniyal, V.P. et al. Spiders as bio-indicators of habitat disturbance in the riparian zone of the Ganga river: a preliminary study. Trop Ecol 63, 209–215 (2022). https://doi.org/10.1007/s42965-021-00192-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42965-021-00192-z