Abstract
Assessment of habitat restoration often rely on single-taxa approach, plants being widely used. Arthropods might yet complement such evaluation, especially in hard, poorly-diversified environments such as maritime clifftops. In this study we compared the responses of spiders and plants (both at species and assemblage levels) to increasing time of heathland restoration. Sampling took place in different sites of Brittany (Western France), using a replicated design of both pitfall traps and phytosociological relevés. A total of 5056 spiders belonging to 160 species, and 103 plant species were found. No change in species richness between degradation states was found for spiders. Plant species richness was lower in highly degraded habitats of recently restored sites but was not in the oldest restored one. Species composition greatly changed through turnover mechanisms between all sites and degradation states, for both spider and vegetation. Heterogeneity was higher in references states, and increased over restoration time between sites. The number of indicator species decreased with restoration age for spiders, while no indicator species was found for plants. Restoration is still on-going after 15 years, with no recovery of reference assemblages for both plants and spiders, but there were some signals toward reference in the oldest restoration site. Plants and spiders were proved to be complementary bio-indicators of post disturbance restoration, as they bring different, scale-dependent information on restoration success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to strong environmental stresses (mainly wind and salt deposition: Malloch 1972; Sawtschuk 2010), maritime clifftops present particular conditions for flora and fauna, which lead to the establishment of maritime heathlands, a habitat protected by European legislation Natura 2000 (Sawtschuk et al. 2010), under the codes 4030 (European dry heaths) and 4040 (dry Atlantic coastal heaths with Erica vagans). Despite their high conservation values, maritime clifftops are often degraded along the French East-Atlantic coasts because of important touristic uses, leading to intensive human trampling and/or building constructions (especially since the 50s: Le Fur 2013; Sawtschuk 2010). These habitats have actively been restored since the 90s, first for landscape and then for biodiversity purposes (Le Fur 2013), locally resulting in sites with different state of degradation. In Brittany (Western France), most of restoration projects include passive restoration by precluding areas from human trampling or vehicle access. Some projects also use active restoration methods (e.g. biodegradable geotextile and fascines use) to initiate or accelerate the restoration of degraded sites. Some projects finally include the deconstruction of infrastructures such as building or car parks, together with public access restriction. Since the start of the restoration projects (between 2002 and 2012 for our study sites) vegetation monitoring plots are set up to assess the efficiency of restoration practises (Desdoigts 2000, Le Roy et al. 2019). This has proven that passive restoration, when performed in moderately or lightly degraded areas, is usually enough to create a good restoration dynamic (Sawtschuk et al. 2010). However, this assessment of ecological restoration success mainly relies on vegetation analysis. To the best of our knowledge, no study has been performed to assess the restoration of arthropod assemblages in degraded maritime heathlands yet.
Measurements of species diversity and abundance constitute the most widely used indicator of restoration success (Wortley et al. 2013), mainly using plants as bio-indicators (Morrison 1998; Babin-Fenske and Anand 2010; Kollmann et al. 2016). There are different reasons to explain this over-representation of plants, such as the facility to sample this group (Gatica-Saavedra et al. 2017) or the lesser impact of seasonality on plant assemblages compared to animal assemblages (Ruiz-Jaen and Aide 2005). Some authors also claimed that restoration of indigenous plants might naturally lead to the restoration of fauna (Young 2000), because of their structural and functional roles (Allen 1998). Fauna and more specifically arthropods are comparatively less used as bio-indicators (Ruiz-Jaen and Aide 2005; Wortley et al. 2013), while they represent more diversified taxonomic and functional groups (Longcore 2003). Using vegetation only as a proxy of global restoration success is thus increasingly criticised in literature (Morrison 1998; Andersen and Majer 2004). Arthropods are known to answer differently to restoration (Babin-Fenske and Anand 2010; Feest et al. 2011; Spake et al. 2016), and can actually complement assessments only based on plants (Pétillon et al. 2014). Arthropods are yet under-studied, probably because of their complexity and the specific skills required to identify some taxa (Gerlach et al. 2013). To encourage the use of arthropods in monitoring projects, some authors therefore recommended using bio-indicator taxa instead of the whole arthropod assemblage (Longcore 2003; Pearce and Venier 2006; Gerlach et al. 2013). Monitoring arthropod taxa is thus expected to complete conclusions based on vegetation studies, especially in habitats where specific abiotic conditions lead to specific arthropod assemblages (Leibold et al. 2004; Schirmel et al. 2012). Such taxa have to fulfil a number of criteria to be considered as “good” bio-indicators: nor too rare nor too common, to contain specialised taxa (e.g. by habitat, feeding regime, etc.: Caro and O’Doherty 1999), and easy to sample and to identify. Spiders, dominant arthropod predators in many terrestrial habitats, are one of these taxa described as good bio-indicators (Cristofoli et al. 2010; Gerlach et al. 2013; Ossamy et al. 2016), and also reported to react fast and differently from plants to local habitat disturbance (Lafage et al. 2019). In maritime grasslands and maritime heathlands, extreme conditions such as nutrient-limited and shallow soil (Bourlet 1980), high wind exposure and salt deposit (Malloch 1972; Sawtschuk 2010) lead to specific plant assemblages (Bioret & Géhu 2008). The same probably applies for arthropods that have to resist, and likely to be adapted, to these strong constraints.
In this study we assess the complementarity of plants and arthropods to monitor the restoration success of degraded maritime heathlands by comparing their patterns in taxonomic diversity (both alpha and beta, i.e. functional and rarity based metrics were not considered here, see e.g. Leroy et al. 2014). We first tested the hypothesis that reference habitats host few but constant specialist species, while degraded habitat host as many, but more context-dependent, generalist or opportunistic species and that their ratio is driven by the intensity of degradation (Fig. 1). We consequently expect (i) species richness to be similar between degradation states, (ii) species composition to differ between sites, with heterogeneity of assemblages (estimated by β-diversity) increasing with restoration process due to higher species turnover and a constant nestedness (i.e. species pool in degraded habitats is not a subset of species pool in reference habitat: see Baselga 2010) and (iii) a decreasing number of indicator species explained by a higher degradation. Although environmental filters act differently on plants and spiders (see e.g. Klejin et al. 2006), we do not expect significant differences in alpha- and beta-diversity patterns between these taxa (see e.g. Lafage et al. 2015). The number of indicators species in degraded states is expected to increase with time since restoration for both taxa (because pools of generalist species are larger and more site-dependent), while this number should remain constant in reference states. Lastly, we expect more indicator species for plants than for spiders, the latter being more mobile and thus more likely to be found in different adjacent degradation states.
Materials and methods
Study sites and sampling design
Fieldwork was done in three coastal sites of Brittany, Western France (Appendix Fig. 4). Sites were coded using the time (in years) since their restoration (at the time of fieldwork, i.e. 2017). L’Apothicairerie (S5) (47° 21′ 44.0″ N, 3° 15′ 34.9″ W) was heavily degraded by infrastructures (mainly car park and hotel) that were removed in 2012. La Pointe de l’Enfer (S11) (47° 37′ 18.3″ N 3° 27′ 46.9″ W) was degraded by human trampling and by frequent vehicle access. Both degradations sources were removed from the site in 2006. The last site La Pointe de Pen-Hir (S15), located on the mainland (48° 15′ 03″ N, 4° 37′ 25″ W), was mainly degraded by human trampling that was reduced in 2002. Therefore S5 is the youngest restoration site with 5 years of restoration while S15 has the longest restoration time. These sites were selected because of their increasing age of restoration, but also because they all present the same kind of reference vegetation (i.e. without degradation) which is a short and dry heathland dominated by Erica spp. and Ulex spp. (Appendix Fig. 4).
On top of the reference (further abbreviated RF) state, two other states were defined according to their morphology and selected at each site: heavily damaged (HD) and moderately damaged (MD). Two 400 m2 plots of homogeneous vegetation were designed for each degradation state, and four pitfall traps (80 mm in diameters and 100 mm deep) were set at each plot. Traps were half-filled of salted solution (250 g L−1) with a drop of odorless soap and settled 10 meters apart in order to avoid interference and local pseudoreplication (Topping and Sunderland 1992). This resulted in 71 traps (in one station, the sampling area was too restricted to set 4 traps spaced of 10 m apart, so 1 was removed) active between mid-March to mid-June 2017, and emptied every 2 weeks. Total plant cover was estimated in a 5 m radius circle around every trap, and all species were identified and their percentage cover estimated. Pitfall samples were sorted, arthropods transferred to ethanol 70°, and stored at the University of Rennes 1. Spiders were identified to species level using keys of Roberts (1985) and Nentwig et al. (2019). Data were pooled together by state of degradation and by site.
Data analysis
Spider and plant assemblages were consistently analyzed by (1) comparing alpha and beta-diversities between sites and between degradation states within sites, and (2) looking for indicator taxa of specific degradation states. Sampling coverage curves were computed (Appendix Fig. 5), and the above 90% values used to check the validity of the sampling protocol, and to assess the overall quality of spider and plant datasets. Site effect was tested through assemblage composition analysis (Anosim), which further determined whether sites were grouped or not in subsequent analyses.
We compared alpha-diversity (species richness) between states using estimated richness based on the methods developed by Chao (1984, 1987). The “iNEXT” function from iNEXT package (Chao et al. 2014). This method was selected to account for possible influence of sampling coverage. The test was ran with 40 knots and 200 bootstrap replication. Significant differences were assessed through the absence of overlapping confidence intervals on iNEXT curves (Chao et al. 2014).
Assemblages were compared between sites using NMDS with a Sørensen dissimilarity matrix that is not affected by joint absence (Borcard et al. 2011). Data was transformed to presence/absence data to avoid abundance bias from difference in species activity rate and/or in sensitivity to environment structures (Lang 2000). If ANOSIMs were significant, subsequent analyses on beta-diversity and indicator species were performed site by site, if not sites were pooled together for comparing degradation states.
Beta-diversities were compared using NMDS and ANOSIM on dissimilarity matrices to identify significant differences between assemblages. NMDS and ANOSIM tests were performed using “MetaMDS” and “anosim” functions of the “vegan” package (Oksanen et al. 2019) respectively. When ANOSIMs were performed on pairs of states, level of significance was adjusted using correction (here α = 0.016). Dissimilarity matrices were calculated with the Sørensen dissimilarity on presence/absence data with the “beta.pair” function (“betapart” package, Baselga et al. 2018). This method assesses for broad dissimilarity and its partition following Baselga (2012). Between-site dissimilarity was calculated to assess beta diversity patterns (Baselga 2010) within degradation states using the “beta.multi” function (“betapart” package, Baselga et al. 2018).
IndVal were calculated using presence absence data (Dufrêne and Legendre 1997) in order to identify the species representative of each state in terms of fidelity and exclusivity. Significance of IndVal was tested using a random reallocation procedure with 1000 permutations using the “indval” function from “labdsv” package (Roberts 2016). Only taxa both with an IndVal > 0.5 and with a significant p-value were considered accurate indicators of their respective degradation state (Pétillon et al. 2010).
All analyses were carried out using R software (version 3.2.3 2015/12/10).
Results
5056 adult spiders belonging to 160 species were identified (Appendix Table 3). The most common species were Pardosa monticola (1458 individuals) and Pardosa nigriceps (599 individuals). A total of 133 plant species were identified (Appendix Table 4). The most common species were Leontodon saxatilis ssp. saxatilis and Plantago coronopus, both found in more than 60% of sampled plots. Festuca ovina, Calluna vulgaris and Agrostis stolonifera were also found in the three sites, and were found in more than 10% of plots.
Species richness
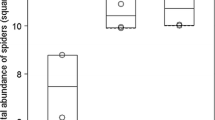
Spider estimated richness showed no significant pattern between degradation states (Fig. 2). No statistical differences were found in estimated species richness of spiders, with all confidence intervals overlapping. Plant assemblages showed a lower richness in the most degraded state for both S5 and S11 sites. The oldest restoration site (S15) showed no significant differences in plant species richness between the three states.
Estimated species richness for spiders and plants in each degradation state of the three sites (highly degraded: red curve; mildly degraded: blue curve; reference: green curve). The coloured area around the curves represents the 95% confidence interval based on the bootstrap method. Figure made with R and iNext package (Chao et al. 2014)
Assemblage composition
Both taxa significantly differed in their assemblage composition between sites (ANOSIM on presence/absence data, spider: R = .76, P = 0.001; plants: R = .69, P = 0.001, Fig. 3), which can be explained by important differences in species occurrence. S5 was the spider richer site with 112 different species, half were found only in this site (Fig. 3). Out of the 81 species identified in S11, a third was unique to this site. S15 was the less spider diversified site (57 species), and a fifth of these was found only there. The same pattern was found for plant assemblages (Fig. 3). Site S5 was indeed the most diversified site (63 species), followed by S11 (48 species) and S15 (39 species), with only 15 species common to the three sites. Half of S5 and S11 plant species were found only on these sites, whereas a quarter of S15 species was unique to this site. Sites were finally ordered by geographic proximity, for both spider and plant assemblages, and total species richness of sites increased from North to South. Because of such strong differences between assemblages of the three studied sites, further analyses on beta-diversities and indicator species were done site by site.
NMDS based on Sørensen dissimilarity matrix for spider and plant assemblages with the envelopes grouping sites. Venn diagrams with the number of species unique or shared by site for each taxa. Figure made with R with ggplot2 package (Wickham 2016)
RF states had the highest beta-diversities, while degraded states had similar, lower, beta-diversities (Table 1). Patterns in beta-diversities were driven by changes in turn-over, while nestedness was negligible. NMDS displayed results consistent with beta-diversity analyses (Appendix Fig. 6), i.e. higher heterogeneity in RF sites. An overlap in species assemblages in the oldest restoration site was finally observed for both plants and spiders (although while spider assemblages between MD and HD in S15 seem closer, they still differ significantly: ANOSIM, R = 0.17, P = 0.04).
Indicator taxa
Several significant indicator taxa above the 0.5 IndVal value threshold were found for spiders (Table 2). They were no spider indicator of reference state in S5, 9 in S11 and 1 in S15. Mildly degraded states had 14 indicator spider species in S5, 2 in S11 and 0 in S15. Highly degraded had 4 indicator spider species in S5, 1 in S11 and 0 in S15 (see Appendix Table 3 for species list). There was no significant indicator plant species, i.e. having an IndVal above the 0.5 threshold.
Discussion
Species richness
Contrary to other studies reporting that species richness follows perturbation or degradation patterns (Varet et al. 2013; Bargmann et al. 2015), species richness remained stable for spider assemblages. This is overall consistent with Bell et al. (1998) who described species richness a quite unreliable metrics for monitoring management effects. Plants responded differently, with a lower richness in highly degraded habitats for the two most recent restored sites and no significant differences for the oldest one, which indicates a success of restoration on this metric over time. Species richness can hardly be used alone as restoration proxy in a maritime clifftop context, especially for spiders. This result has already been found by other authors (Matthews et al. 2009; Déri et al. 2011), but can hardly be generalized to all ecosystems. Some studies indeed proved the effectiveness of this metric for the same taxa, like Pétillon and Garbutt (2008) for spiders of disturbed salt marshes and Borchard et al. (2014) for plants of degraded mountain heathlands. In a grassland context, spider and plant species richness can also be higher in reference states (Perner and Malt 2003; Borchard et al. 2014; DiCarlo and DeBano 2018).
Species composition
Assemblage compositions of the two taxa (spiders and plants) were very different between the three studied sites. Only a few plant and spider species were common to all sites. Differences between plant assemblages are not surprising, maritime heathland being well differentiated along Brittany’s coast (Bioret 1989; Demartini 2016). Differences in spider assemblages between sites is an interesting result that can be partly explained by their great sensitivity to changes in environmental, including climatic, conditions (Pik et al. 2002; Andersen and Majer 2004; Pearce and Venier 2006) and by their high diversity (Roberts 1985).
Almost all assemblages from the different degradation states within a given site significantly differed between each other. As expected, most of these differences were driven by changes in turnover, nestedness being negligible in most cases. Such a pattern due to turnover is consistent with other studies on spider and plant beta diversity (Schirmel and Buchholz 2011; Rickert et al. 2012; Lafage et al. 2015; Coccia and Fariña 2019; see also Almeida-neto et al. 2011).
The observed patterns validate our hypothesis of a higher heterogeneity in reference states as compared to references states that also increases over time since restoration. Both spiders and plants displayed this pattern, which is consistent with several previous studies that showed similar responses of these taxa (Perner and Malt 2003; Ilg et al. 2008; Rickert et al. 2012; Lafage et al. 2015).
Therefore heterogeneity, as revealed by analyses of beta-diversity, can be used as a restoration criteria of coastal heathland restoration for both spider and plant assemblages. Spiders appear to increase in heterogeneity over time, but without converging toward the same referential state. Differences in sites could explain such a result. It has indeed been shown that spider beta diversity is strongly impacted by landscape-level factors (Carvalho et al. 2011; Lafage et al. 2015). The three sampling sites are split between continental (S15) and islands (S5 and S11), and also show a temperature gradient from North to South, which are some example on how site specificity may induce differences in landscape composition. Spider assemblages in all three sites became more and more variable with an increasing restoration level but differently for each site, which is probably driven by site-specific ecological variables. Spider assemblages are known to be shaped by the interplay of environmental structures (Uetz 1991), and to rapidly colonize empty niches (Pearce and Venier 2006; Cristofoli et al. 2010; Borchard et al. 2014). As the environment tends to be more complex along the restoration process, spiders that are known for their high dispersion abilities (e.g. Foelix 2011) colonize habitats from the surrounding landscapes.
Contrary to spiders, plant beta diversity is known to be mostly driven by local factors (Lafage et al. 2015). Coastal heathlands are constraining habitats (Bioret and Géhu 2008), which can induce a stable reference state for plants toward which degraded states are converging. The remaining differences with assemblages of reference state could come from the restoration dynamic that stabilized into an alternative stable state (Le Roy 2019).
Indicator taxa
Ground-dwelling spiders were both abundant and diverse in pitfall traps (they were actually the dominant predators here: Hacala et al. unpublished data), which confirms their value as potential bio-indicators. The low and inconsistent number of reference indicator species concurs with the hypothesis of a heterogeneous reference habitat driven by site specificity. That was especially true for the site S5, where no indicator species of the reference state was found. This might be due to the fact that the dominant plant species of this habitat was Erica vagans that creates more patchy vegetation, hereby inducing more spatial heterogeneity in spider assemblages than in the two other sites and preventing the highlight of indicator species. Since habitat structure is known to be a strong driver of spider assemblages (e.g. Uetz 1991), the heterogeneity in reference state of site S5 may induce variations that prevent indicator species to be detected. The number of indicator species in degraded states decreased with increasing restoration from S5 to S15. This expected shift in assemblage composition with more specialist species in preserved habitats is consistent with other previous studies (Bonte et al. 2006; Cristofoli et al. 2010). The higher number of indicator species in MD than in HD state is probably linked to the greater number of ecological niches in MD, whereas HD state is mostly characterized by high levels of bare grounds. Several indicator species found in this study match well with their known ecology. Degraded habitats were associated with open habitat species like Linyphiidae (e.g. Erigone dentipalpis, Diplostyla concolor etc.) and reference states were more characterized by plant-dwelling species (e.g. Pardosa nigriceps, Zora spinimana etc).
Finally, the absence of plant indicator species strongly invalidates our hypothesis about the ratio of indicator species between spiders and plants. This result could be due to the relatively low spatial scale of sites together with the high dispersal ability of plants (Dufrêne and Legendre 1997).
Concluding remarks
Recovery is still on-going after 15 years after initiating restoration, with no recovery of reference assemblages for both plants and spiders. There were some signals that in the oldest restoration site, HD resembles MD in terms of spider and plant species composition (although the latter was significant different between degradation states), and plant species richness. This study allows us to confirm what has been shown in other ecosystems (Babin-Fenske and Anand 2010; Borchard et al. 2014; McCary et al. 2015; Cole et al. 2016), i.e. arthropods strongly responds to disturbance through changes in assemblage composition and species occurrence. Monitoring both these taxa should provide a better understanding of the restoration dynamic (Harry et al. 2019). The use of multiple bio-indicators, as the ones shown in this study, seems to be the best way of assessing restoration operation (Lambeck 1997; Sattler et al. 2013; Fournier et al. 2015; Harry et al. 2019). This was illustrated here by the complementary responses of spiders and plants. Our study is consistent with previous studies that showed arthropod assemblages respond differently to disturbances compared to vegetation (Babin-Fenske and Anand 2010; Feest et al. 2011; Spake et al. 2016, Harry et al. 2019), because of their greater diversity (Longcore 2003) and sensitivity to changes in microhabitat (Pearce and Venier 2006). Thus a habitat where vegetation has been restored does not mean that arthropods are restored as well (Pétillon et al. 2014), and the other way around may probably apply.
These results stress the need to use multiple taxa in restoration studies as one cannot necessarily be the proxy of others (Coelho et al. 2009; Gerlach et al. 2013). Taxa from various trophic guilds, such as detrivorous, phytophagous or polyphagous arthropods (e.g. weevils, ground beetles or ants respectively), will be studied in the future since they are also likely to display different and complementary information (Lafage et al. 2015; Coccia and Fariña 2019). Such comparisons groups should be done using functional or phylogenetical (Pavoine and Ricotta 2014; Cardoso et al. 2015) distances aside from taxonomic one to understand the dynamics of arthropod assemblages, and therefore the ongoing, complex, processes that drive them.
References
Allen EB (1998) Response. Restor Ecol 6:134
Almeida-Neto M, Frensel DM, Ulrich W (2011) Rethinking the relationship between nestedness and beta diversity: a comment on Baselga (2010). Glob Ecol Biogeogr 21(7):772–777
Andersen AN, Majer JD (2004) Ants show the way down under: invertebrates as bioindicators in land management. Front Ecol Environ 2:291–298
Babin-Fenske J, Anand M (2010) Terrestrial insect communities and the restoration of an industrially perturbed landscape: assessing success and surrogacy. Restor Ecol 18:73–84
Bargmann T, Hatteland BA, Grytnes JA (2015) Effects of prescribed burning on carabid beetle diversity in coastal anthropogenic heathlands. Biodivers Conserv 24(10):2565–2581
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19:134–143
Baselga A (2012) The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob Ecol Biogeogr 21:1223–1232
Baselga A, Orme D, Villeger S, De Bortoli J, Leprieur F (2018). betapart: Partitioning Beta Diversity into Turnover and Nestedness Components. R package version 1.5.1. https://CRAN.R-project.org/package=betapart
Bell JR, Rod Cullen W, Wheater P (1998) The structure of spider communities in limestone quarry environments. In Proceedings of the 17th European Colloquium of Arachnology. British Arachnological Society, Burnham Beeches, Bucks, pp. 253–259
Bioret F (1989) Contribution à l’étude de la flore et de la végétation de quelques îles et archipels ouest et sud armoricains. Ph.D. thesis, Université de Nantes, Nantes, France
Bioret F, Géhu J-M (2008) Révision phytosociologique des végétations halophiles des falaises littorales atlantiques françaises. Fitosociologia 45:75–116
Bonte D, Lens L, Maelfait JP (2006) Sand dynamics in coastal dune landscapes constrain diversity and life-history characteristics of spiders. J Appl Ecol 43:735–747
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer, New York
Borchard F, Buchholz S, Helbing F, Fartmann T (2014) Carabid beetles and spiders as bioindicators for the evaluation of montane heathland restoration on former spruce forests. Biol Conserv 178:185–192
Bourlet Y (1980) Les landes de Bretagne septentrionale. Etudes de biogéographie végétale. Norois 107:417–432
Cardoso P, Rigal F, Carvalho JC (2015) BAT—Biodiversity Assessment Tools, an R package for the measurement and estimation of alpha and beta taxon, phylogenetic and functional diversity. Methods Ecol Evol 6(2):232–236. https://doi.org/10.1111/2041-210x.12310
Caro TM, O’Doherty G (1999) On the use of surrogate species in conservation biology. Conserv Biol 13:805–814
Carvalho JC, Cardoso P, Crespo LC, Henriques S, Carvalho R, Gomes P (2011) Determinants of beta diversity of spiders in coastal dunes along a gradient of mediterraneity. Divers Distrib 17(2):225–234
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Chao A (1987) Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43:783–791
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67
Coccia C, Fariña JM (2019) Partitioning the effects of regional, spatial, and local variables on beta diversity of salt marsh arthropods in Chile. Ecol Evol 9(5):2575–2587
Coelho MS, Quintino AV, Fernandes GW, Santos JC, Delabie JHC (2009) Ants (Hymenoptera: Formicidae) as bioindicators of land restoration in a Brazilian Atlantic forest fragment. Sociobiology 54(1):51–63
Cole RJ, Holl KD, Zahawi RA, Wickey P, Townsend AR (2016) Leaf litter arthropod responses to tropical forest restoration. Ecol Evol 6:5158–5168
Cristofoli S, Mahy G, Kekenbosch R, Lambeets K (2010) Spider communities as evaluation tools for wet heathland restoration. Ecol Ind 10:773–780
Demartini C (2016) Les végétations des côtes Manche-Atlantique françaises: essai de typologie et de cartographie dynamico-caténales. Ph.D. thesis, Université de Bretagne occidentale - Brest
Déri E, Magura T, Horváth R, Kisfali M, Ruff G, Lengyel S, Tóthmérész B (2011) Measuring the short-term success of grassland restoration: The use of habitat affinity indices in ecological restoration. Restor Ecol 19:520–528
Desdoigts J-Y (2000) L’extrémité du Cap Sizun : restauration de la nature et tourisme. L’opération grand site de la pointe du Raz, de la pointe du Van et de la baie des Trépassés. Norois 186:283–293
DiCarlo LAS, DeBano SJ (2018) Spider community responses to grassland restoration: balancing trade-offs between abundance and diversity. Restor Ecol 27(1):210–219
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Feest A, Merrill I, Aukett P (2011) Does botanical diversity in sewage treatment reed-bed sites enhance invertebrate biodiversity? Int J Ecol 1:1–9
Foelix R (2011) Biology of spiders. OUP USA
Fournier B, Gillet F, Le Bayon RC, Mitchell EA, Moretti M (2015) Functional responses of multitaxa communities to disturbance and stress gradients in a restored floodplain. J Appl Ecol 52:1364–1373
Gatica-Saavedra P, Echeverría C, Nelson CR (2017) Ecological indicators for assessing ecological success of forest restoration: a world review. Restor Ecol 25:850–857
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. J Insect Conserv 17:831–850
Harry I, Höfer H, Schielzeth H, Assman T (2019) Protected habitats of Natura 2000 do not coincide with important diversity hotspots of arthropods in mountain grasslands. Insect Conserv Divers 12(4):329–338
Ilg C, Dziock F, Foeckler F, Follner K, Gerisch M, Glaeser J, Rink A, Schanowski A, Scholz M, Deichner O, Henle K (2008) Long-term reactions of plants and macroinvertebrates to extreme floods in floodplain grasslands. Ecology 98(9):2392–2398
Klejin D, Baquero RA, Clough Y, Días M, De Esteban J, Fernández F, Gabriel D, Herzog F, Holzschuh A, Jöhl R, Knop E, Kruess A, Marshall EJP, Steffan-Dewenter I, Tscharntke T, Verhulst J, West TM, Yela JL (2006) Mixed biodiversity benefits of agri-environment schemes in five European countries. Ecol Lett 9:243–254
Kollmann J, Meyer ST, Bateman R, Conradi T, Gossner MM, de Souza Mendoza M Jr., Fernandes GW, Hermann J-M, Koch C, Müller SC, Oki Y, Overbeck GE, Paterno GB, Rosenfield MF, Toma TSP, Weisser WW (2016) Integrating ecosystem functions into restoration ecology—recent advances and future directions. Restor Ecol 24:722–730
Lafage D, Maugenest S, Bouzillé JB, Pétillon J (2015) Disentangling the influence of local and landscape factors on alpha and beta diversities: opposite response of plants and ground-dwelling arthropods in wet meadows. Ecol Res 30(6):1025–1035
Lafage D, Djoudi EA, Perrin G, Gallet S, Pétillon J (2019) Responses of ground-dwelling spider assemblages to changes in vegetation from wet oligotrophic habitats of Western France. Arthropod Plant Interact. https://doi.org/10.1007/s11829-019-09685-0
Lambeck RJ (1997) Focal species: a multi-species umbrella for nature conservation. Conserv Biol 11:849–856
Lang A (2000) The pitfalls of pitfalls: a comparison of pitfalls trap catches and absolute density estimates of epigeal invertebrate predators in arable land. J Pest Sci 73(4):99–106
Le Fur Y (2013) La patrimonialisation des grands sites : évolution des doctrines et transformation des espaces : exemple des promontoires littoraux emblématiques bretons. Ph.D. thesis, Université de Bretagne Occidentale, Brest, France
Le Roy M (2019) Contribution à la connaissance socio-écologique des opérations de restoration des hauts de falaises littorales de Bretagne. Ph.D. thesis, Université de Bretagne Occidentale, Brest, France
Le Roy M, Sawtschuk J, Bioret F, Gallet S (2019) Toward a social-ecological approach to ecological restoration: a look back at three decades of maritime clifftop restoration. Restor Ecol 27(1):228–238
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzales A (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Leroy B, Le Viol I, Pétillon J (2014) Complementarity of rarity, specialisation and functional diversity metrics to assess community responses to environmental changes, using an example of spider communities in salt marshes. Ecol Ind 46:351–357
Longcore T (2003) Terrestrial arthropods as indicators of ecological restoration success in coastal sage scrub (California, U.S.A.). Restor Ecol 11:397–409
Malloch AJC (1972) Salt-spray deposition on the maritime cliffs of the lizard peninsula. J Ecol 60:103–112
Matthews JW, Spyreas G, Endress AG (2009) Trajectories of vegetation-based indicators used to assess wetland restoration progress. Ecol Appl 19:2093–2107
McCary MA, Martinez J-C, Umek L, Heneghan L, Wise DH (2015) Effects of woodland restoration and management on the community of surface-active arthropods in the metropolitan Chicago region. Biol Conserv 190:154–166
Minchin P R, O’Hara R B, Simpson G L, Solymos P, Henry M, Stevens H, Szoecs E, Wagner H (2019). vegan: Community ecology package. R package version 2.5-4. https://CRAN.R-project.org/package=vegan
Morrison ML (1998) Letter to the editor. Restor Ecol 6:133
Nentwig W, Blick T, Gloor D, Hänggi A, Kropf C (2019) Version 06.2019. Online at https://www.araneae.nmbe.ch. Accessed 19 June 2019. doi: 10.24436/1
Oksanen J, Blanchet F G, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin P R, O’Hara R B, Simpson G L, Solymos P, Stevens H M H, Szoecs E, Wagner H (2019). vegan: Community ecology package. R package version 2.5-4. https://CRAN.R-project.org/package=vegan
Ossamy S, Elbanna SM, Orabi GM, Semida FM (2016) Assessing the potential role of spiders as bioindicators in Ashtoum el Gamil natural protected area, Port Said, Egypt. Indian J. Arachnol 5:101
Pavoine S, Ricotta C (2014) Functional and phylogenetic similarity among communities. Methods Ecol Evol 5(7):666–675
Pearce JL, Venier LA (2006) The use of ground beetles (Coleoptera: Carabidae) and spiders (Araneae) as bioindicators of sustainable forest management: a review. Ecol Ind 6:780–793
Perner J, Malt S (2003) Assessment of changing agricultural land use: response of vegetation, ground-dwelling spiders and beetles to the conversion of arable land into grassland. Agric Ecosyst Environ 98:169–181
Pétillon J, Garbutt A (2008) Success of managed realignment for the restoration of salt-marsh biodiversity: preliminary results on ground-active spiders. J Arachnol 36:388–393
Pétillon J, Lasne E, Lambeets K, Canard A, Vernon P, Ysnel F (2010) How do alterations in habitat structure by an invasive grass affect salt-marsh resident spiders? Ann Zool Fenn 47:79–89
Pétillon J, Potier S, Carpentier A, Garbutt A (2014) Evaluating the success of managed realignment for the restoration of salt marshes: lessons from invertebrate communities. Ecol Eng 69:70–75
Pik AJ, Dangerfield JM, Bramble RA, Angus C, Nipperess DA (2002) The use of invertebrates to detect small-scale habitat heterogeneity and its application to restoration practices. Environ Monit Assess 75:179–199
Rickert C, Fichtner A, Van Klink R, Bakker JP (2012) α- and β- diversity in moth communities in salt marshes is driven by grazing management. Biol Conserv 146:24–31
Roberts MJ (1985) The spiders of Great Britain and Ireland. Brill Archive
Roberts D W (2016). labdsv: Ordination and multivariate analysis for ecology. R package version 1.8-0. https://CRAN.R-project.org/package=labdsv
Ruiz-Jaen MC, Aide TM (2005) Restoration success: how is it being measured? Restor Ecol 13:569–577
Sattler T, Pezzatti GB, Nobis MP, Obrist MK, Roth T, Moretti M (2013) Selection of multiple umbrella species for functional and taxonomic diversity to represent urban biodiversity. Conserv Biol 00:1–13
Sawtschuk J (2010) Restauration écologique des pelouses et des landes des falaises littorales atlantiques : analyse des trajectoires successionnelles en environnement contraint. Ph.D. thesis, Université de Bretagne Occidentale, Brest, France
Sawtschuk J, Bioret F, Gallet S (2010) Spontaneous succession as a restoration tool for maritime cliff-top vegetation in Brittany, France. Restor Ecol 18:273–283
Schirmel J, Buchholz S (2011) Response of carabid beetles (Coleoptera: Carabidae) and spiders (Araneae) to coastal heathland succession. Biodivers Conserv 20(7):1469
Schirmel J, Blindow I, Buchholz S (2012) Life-history trait and functional diversity patterns of ground beetles and spiders along a coastal heathland successional gradient. Basic Appl Ecol 13:606–614
Spake R, Barsoum N, Newton AC, Doncaster CP (2016) Drivers of the composition and diversity of carabid functional traits in UK coniferous plantations. For Ecol Manag 359:300–308
Topping CJ, Sunderland KD (1992) Limitations to the use of pitfall traps in ecological studies exemplified by a study of spiders in a field of winter wheat. J Appl Ecol 29(2):485–491
Uetz GW (1991) Habitat structure and spider foraging. Habitat structure. Springer, Dordrecht, pp 325–348
Varet M, Burel F, Lafage D, Pétillon J (2013) Age-dependent colonization of urban habitats: a diachronic approach using carabid beetles and spiders. Anim Biol 63:257–269
Wickham H (2016) ggplot2: Elegant graphics for data analysis. Springer, New York
Wortley L, Hero J-M, Howes M (2013) Evaluating ecological restoration success: a review of the literature. Restor Ecol 21:537–543
Partitioning Beta Diversity into Turnover and Nestedness Components. R package version 1.5.1. https://CRAN.R-project.org/package=betapart
Young TP (2000) Restoration ecology and conservation biology. Biol Conserv 92:73–83
Acknowledgements
We thank Denis Lafage and Kaïna Privet for fruitful discussions, Aurélien Ridel and Timothée Scherer for their help in identifying spiders and sorting samples respectively, and “Bretagne Vivante” and “Communauté de communes de Belle-île-en-Mer” for continuous support during fieldwork. Two anonymous reviewers and Marie Handock provided constructive comments on a first draft.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David L Hawksworth.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Figs. 4, 5, 6, and Tables 3, 4.
Accumulation curves for a spider and b plant sampling coverage. Curve colours correspond to different sampling sites (Blue: S5; Red: S11; Green: S15). The coloured area around the curves is the 95% confidence interval based on bootstrapping (see “Methods” section for more details). Figure made with R and iNext package (Chao et al. 2014)
NMDS per site for Spiders and Vegetation. Coloured area corresponding to degradation state. Light grey: reference; grey: mildly degraded; dark grey: highly degraded. Figure made with R and ggplot2 package (Wickham 2016)
Rights and permissions
About this article
Cite this article
Hacala, A., Le Roy, M., Sawtschuk, J. et al. Comparative responses of spiders and plants to maritime heathland restoration. Biodivers Conserv 29, 229–249 (2020). https://doi.org/10.1007/s10531-019-01880-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-019-01880-y