Abstract
Mammals figure prominently in tropical ecology because of their role in seed dispersal, herbivore control, and nutrient cycling. Urbanization is a conservation concern for mammals not only because of lost habitat, but also other negative impacts such as illegal hunting and pollution. Some mammals are resilient to urbanization and can persist in degraded habitats; however, this has not been studied in many tropical sites. We used camera traps to study mammals in Peperpot Nature Park (PNP), a secondary forest located 5 km from the capital of Suriname, and compared it to the large Central Suriname Nature Reserve (CSNR). We found fewer species in PNP (20) compared to CSNR (30), however, PNP included apex predators and species of conservation concern, and the two sites had a similar relative abundance of large cats and most mesocarnivores. Smaller mammal species had higher relative abundance at PNP while larger herbivores were scarce or absent, likely from poaching, as PNP is still connected to interior forests. The detection models revealed that the park, the camera trap was set in, was the most common significant factor for most species, suggesting that broader landscape level effects are important. In occupancy models, the null model was the most common top model across species, but then followed by park. Despite fewer species being detected than in large protected areas, the presence of some species in PNP which are historically absent in developed areas shows promise for small urban tropical forests to support robust mammal communities, provided they maintain some connectivity to larger source habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protecting tropical forests is a priority for conservation. They are the most biodiverse ecosystems, harboring two-thirds of the world’s terrestrial species, and are reservoirs for carbon sequestration, yet are at imminent risk of largescale deforestation (Pimm and Raven 2000; Hansen et al. 2013). Historically, conservation efforts have focused on preserving primary tropical forests through protected areas, which are vital, but limited in their ability to conserve biodiversity alone (Rodrigues et al. 2004; Laurance et al. 2012). Urbanization, a major driver of deforestation in tropical forests, threatens biodiversity not only because of habitat loss for species, but associated anthropogenic impacts such as pollution and poaching that can penetrate into protected areas leading to defaunation and less effective parks (Laurance et al. 2012; Seto et al. 2012; Benítez-López et al. 2019).
Additionally, the amount of primary forest has declined in the tropics (Morales-Hidalgo et al. 2015), while degraded and/or fragmented forests now make up over 75% of all tropical forests (Lewis et al. 2015). Secondary succession has reclaimed some 15% of previously deforested areas and afforestation has been increasing in some middle income tropical countries (Wright 2005; Lewis et al. 2015; Sloan and Sayer 2015). Given that altered forests make up such a large percentage of all tropical forests, ecologists are now turning to these human-modified forests as important areas to conserve biodiversity in the tropics (Melo et al. 2013), even though questions remain as to how effective they are.
Previous studies show that secondary forests have overall lower biodiversity than primary forests (Barlow et al. 2007; Gibson et al. 2011; Sayer et al. 2017), but for some taxa, species diversity can be similar (Barlow et al. 2007; de Souza Laurindo et al. 2017). Some mammals appear to be notably resilient, and can even benefit from disturbance (Gibson et al. 2011; but see Ahumada et al. 2013). For example, mammal species richness in a fragmented agricultural landscape in southeastern Brazil was comparable to levels in protected areas (de Souza Laurindo et al. 2017). Secondary forests are especially important for large mammals, which require more space, and might use degraded habitat as corridors between higher quality habitats (Hogg et al. 2006; Mohd-Azlan 2006; McShea 2012). These species require protection in these landscapes as they are also more likely to be targeted for poaching. Maintaining intact mammal communities is important to tropical forest ecosystems because of the roles they play in seed dispersal, herbivore control, and nutrient cycling (Eisenberg and Thorington 1973; Sinclair 2003; Hirsch et al. 2012).
Urbanization adds an additional challenge for mammals persisting in degraded habitats, and has been found to particularly affect large carnivores because of their larger home range requirements, higher persecution rates, and the detrimental effect of a loss of prey (Woodroffe 2000; Cardillo et al. 2004). However, over the last few decades, some medium-to-large carnivores have been able to adapt to living in or near densely populated cities (Bateman and Fleming 2012), such as coyotes (Canis latrans) in Chicago (Gese et al. 2012), leopards (Panthera pardus) in Mumbai (Bhatia et al. 2013), and pumas (Puma concolor) near Los Angeles (Vickers et al. 2015). Large-scale studies in temperate areas have documented that urban mammal communities can be robust and even comparable to natural ones in abundance and species richness (Gallo et al. 2017; Parsons et al. 2018).
Few studies in the tropics have examined mammals specifically in the context of urbanization. Small mammal communities and bats show declining species richness with increasing urbanization (Hourigan et al. 2006; Wells et al. 2014). A meta-analysis including mammals of all sizes in tropical forests around the world found a peak in the probability of occurrence at intermediate human population densities (Newbold et al. 2014), however, most sites included were in primary forest and there were no truly urban sites with mammal data. Most studies on mammals in the tropics have been studied in the context of fragmentation and/or land use type, some of which include urbanization in the matrix (Umetsu et al. 2008; Galetti et al. 2009; Cove et al. 2013). In the Neotropics, most have taken place in the Atlantic Forest of South America, which has lost nearly 90% of its original forest and is a matrix of agriculture, mining, and settlements (Ribeiro et al. 2009; Melo et al. 2013; de Souza Laurindo et al. 2017). Typically, these studies show that smaller and more fragmented parks contain fewer mammal species. For example, in a study across 144 sites within the Atlantic forest, the average richness was 13 mammal species.
Here we report on a survey of mammals in Peperpot Nature Park (PNP), a former cacao and coffee plantation reclaimed by secondary forest located near the largest urban area in Suriname. We compared our results to those from a recent study in the Central Suriname Nature Reserve (CSNR), a large, protected area consisting of primary forest approximately 200 km away from PNP. Using camera traps, we calculated species richness, diversity, detection rate, and occupancy of terrestrial mammals to examine the extent to which an urban nature park can serve as habitat for medium-to-large mammals in the Neotropics.
Methods
Field sites and camera trap surveys
Peperpot Nature Park
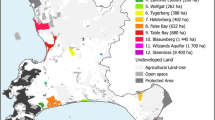
Peperpot Nature Park (PNP) is a wildlife reserve (est. 2009, 8 km2) comprising five abandoned cacao and coffee plantations in the Commewijne District of Suriname (Fig. 1). It is approximately five km from the center of Paramaribo, the capital of Suriname (pop. 241,000) and is separated from the city by the Suriname River. Commercial and residential development in the area surrounding PNP escalated significantly following the completion in 2000 of the Jules Wijdenbosch bridge, which provided the first land link between Paramaribo and the Commewijne District. The reserve is surrounded on three sides by intensive land use and ongoing construction projects; development is currently less advanced to the south, where natural vegetation remains intact. Tourists visit PNP year-round using an established network of trails for recreational hiking, biking, and motorized vehicles. PNP consists mostly of secondary coastal swamp forest with small patches of open habitat along old road corridors and areas mined for sand by local residents. Hunting is not permitted in PNP, but poaching of wildlife does occur.
We deployed twelve Reconyx PC 800 camera traps (Reconyx, Inc. Holmen, WI) across 20 sites from April 2015–November 2016 (Fig. 1). All cameras were secured on straight trees approximately 40 cm from the ground with box covers. Locations for camera traps were chosen in relation to accessibility within the trail network and were therefore all close to the perimeter of the park. Cameras were placed on and off trails. Cameras were equipped with an infrared flash and recorded five photographs per trigger, at a rate of 1 frame/s, re-triggering immediately if the animal was still in view. We grouped consecutive photos into sequences less than 60 s apart, which were used as independent records for data analysis (Kays et al. 2016). Cameras were left for 28 days, after which the memory cards were collected. Some cameras were redeployed at the same location, while others were moved. We uploaded photos and identified species using eMammal software (emammal.org). Images and data were permanently stored in a Smithsonian Data Repository (https://doi.org/10.25571/6q2m-rv23). As small mammals could not confidently be identified, we focused on species > 100 g.
Central Suriname Nature Reserve
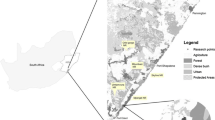
The Central Suriname Nature Reserve (CSNR) is located over 200 km southwest of PNP. Established in 1998, CSNR covers 15,920 km2 of primary tropical forest, swamp, and savanna habitats. Hunting is illegal in CSNR and tourism, although permitted, is limited due to a lack of road access.
A detailed description of camera trap protocols run by the Tropical Ecology Assessment & Monitoring (TEAM) Network are summarized in Ahumada et al. (2013). Briefly, researchers deployed 60 unbaited camera traps (model RM45, Reconyx Inc.) in a regular grid 120 km2 in area or larger, with one camera per 2 km2 for 30 days (Ahumada et al. 2011). Cameras were deployed at each site once per year between 2008 and 2015, but during different months across the years. Grid locations were close to animal trails and placed 30–40 cm above the ground.
Data analysis
We calculated the detection rate (DR) of each species captured on PNP and CSNR cameras to compare the relative abundance by dividing the count of detections for each species by the number of camera trap days for each site. For PNP, we included only camera locations that were > 200 m apart, greater than the recommended minimum distance of 25 m (Kays et al. 2011). Therefore we removed 5 sites for a total of 15 unique sites from the original 20. Cameras were set without bait, making relative abundance comparable across sites (Rowcliffe et al. 2008, 2013; Parsons et al. 2017). We compared the mean DR for species within PNP for camera traps set on and off trails. Where possible, we identified individual jaguars (Panthera onca) from unique pelage spot patterns.
To evaluate the community composition of mammals in PNP and CSNR, we compared the mean detection rates of mammals in different body mass categories. For each species, we generated a single body mass value calculated as a mean of the range of masses reported in Emmons (1990). To account for differences in detections associated with body mass (i.e. smaller mammals have a smaller detection range), we calculated the detection range using the formula: area = 1.65 × mass0.33 (Rowcliffe et al. 2011). We grouped mammals into four categories based on body mass (< 1 kg, 1–5 kg, 6–10 kg, and 10.1 + kg) and calculated a cumulative size-adjusted relative abundance for each category by dividing the total number of detections of each species within the group by the total number of days camera traps were active, and the area generated for that group’s body mass: size-adjusted relative abundance = detections/days/area. To compare overall biomass differences between the parks, we multiplied the size-adjusted relative abundance for each species with their mean body mass and summed the results.
Detection rate models
We used generalized linear models for each species separately to evaluate which covariates best explained detection rates (count/day), or the relative degree of use, of areas between the two parks. We included covariates representing human detections at the site (count/day), vehicle detections at the site (count/day), whether the site was set on or off of a trail (1/0), and domestic dog detection rate (count/day). Cars are not permitted in either park, but motorbikes are used by staff at PNP. Before running models, we tested for correlations between covariates and removed any that were greater than 50%. We therefore removed dog detections as a covariate, which was correlated with humans at 0.51.
We assumed the number of detections of a given species obtained at camera trap site i was a Poisson random variable and we modeled the expected number of photographs at site i as a log-linear model with covariates, slope parameters and an offset equal to the log of the number of days camera trap i operated. We added an additional term for extra-Poisson variation to capture additional variation in the number of detections. We assumed independent normal prior distributions for the slope parameters and assumed a uniform prior distribution for the random error standard deviation parameter. We assessed model fit with posterior predictive checks (PPC, Gelman et al. 2014, Kery and Schaub 2012). We calculated the sum of squared Pearson residuals from observed data (T(y)) and from data simulated assuming model (1) was the data-generating model (T(ysim)). We calculated a Bayesian p value as pB = Pr(T(ysim) > T(y)) from posterior simulations and assumed adequate fit if 0.1 < pB < 0.9.
We fit detection rate models in JAGS (Plummer 2003) via package rjags (Plummer 2016) in Program R (R Core Development Team 2008) via RStudio (RStudio 2015). The inference was based on posterior samples generated from three markov chains. All models achieved adequate convergence (R1.1, Gelman et al. 2014) by running for 50,000 iterations following a burn-in phase of 1000 iterations, thinning every 10 iterations. The significance was based on whether parameter 95% credible intervals overlapped zero.
Occupancy analysis
To compare the percentages of sites used by each species and account for imperfect detection, we also estimated occupancy probabilities using a single season single species occupancy modeling framework (Mackenzie 2006). To simplify analyses, we used one camera deployment per site as sites were sampled multiple times and only included deployments with at least seven trap nights. For sites that were sampled multiple times, we chose the deployment with the most camera trap nights and collapsed detection/non-detection of species into 5-day periods not exceeding 55 days to meet assumptions of closure. We excluded primates as they are largely arboreal and only included species with more than ten detections.
For each species detected in PNP, we used the following covariates in our occupancy models: Park (CSNR or PNP), Start Month (the month the deployment started, to account for seasonality), and Human and Vehicle detection rate. We did not include covariates for detection probability because we did not have any site-specific data associated with the ability of a camera to detect an animal. To improve maximum likelihood convergence with covariates and ensure covariates were on the same scale, we Z-scored covariates with continuous data. To ensure model convergence, we used Goodness-of-Fit tests to estimate c-hat. We removed models with a c-hat value of > 3.0, as this was considered overdispersion. We ranked our models based on Akaike’s Information Criterion (AIC, Burnham and Anderson 2002) ranking and models with a ∆AIC of ≥ 2.0 were considered competing. For each species, we reported all competing model outputs as well as the estimates of occupancy and detection estimates with standard error. We used occupancy models and Goodness-of-Fit tests in the program PRESENCE (version 12.24, Hines 2006) and all other analyses in R (Version 1.1.463, R Development Core Team 2008).
Results
We deployed camera traps over 2449 trap-nights in PNP yielding 2112 detections of 20 native medium-to-large mammals (Fig. 2). Cameras at PNP sites were run on average 163.7 (± 92.2) trap nights and the detection rate for all species was 0.862. The species with the largest number of detections were red-rumped agouti (Dasyprocta leporina, n = 1402), black-eared opossum (Didelphis marsupialis, n = 286) and lowland paca (Cuniculus paca, n = 127). The species with the fewest number of detections in PNP included the brown four-eyed opossum (Metachirus nudicaudatus) with one detection, and the Southern tamandua (Tamandua tetradactyla), jaguarundi (Herpailurus yagouaroundi), and red brocket deer (Mazama americana) with three detections each. Species on the IUCN’s Red List of Threatened Species detected on PNP camera traps included the giant anteater (Myrmecophaga tridactyla, n = 39, IUCN Vulnerable), lowland tapir (Tapirus terrestris, n = 6, IUCN Vulnerable), and the jaguar (n = 12, IUCN Near Threatened). From 12 detections of jaguars over 8 months, we identified at least three unique individuals. Camera traps also photographed domestic dogs (n = 76, DR = 0.032 ± 0.042 SD) and, on trails, humans (n = 2981, DR = 1.66 ± 3.317 SD) and vehicles (DR = 0.073 ± 0.156 SD).
Mean detection rates (± standard error) for all species > 100 g detected in either PNP or CSNR. 0.01 was added to all values except for species that were not detected within a park to visualize smaller values. *Statistical significance as interpreted from non-overlapping standard error values for species that were found at both parks
Camera traps in CSNR were run for much longer (16, 939 trap-nights), and at 60 sites. Camera traps yielded 17,282 detections of 30 native mammal species and a higher overall detection rate than PNP (1.02). The most commonly detected species in CSNR was the collared peccary (Pecari tajacu, n = 4983), while the least photographed species was the Southern naked tailed armadillo (Cabassous unicinctus) with a single detection. CSNR camera traps photographed 12 species not detected in PNP whereas PNP camera traps only captured two not photographed on CSNR cameras (Fig. 2).
When detections were grouped by body mass, the highest average detection rates of species in PNP were mammals of 5 kg or less, which were more than twice those of the same category in CSNR (Fig. 3). PNP and CSNR had more similar values for the < 1 kg and 6–10 kg categories, but in the 10 + kg category, the detection rate at CSNR was ten times that of PNP (Fig. 3). The accumulative daily detection rate adjusted for area in PNP was 1.59 kg/day/area, while in CSNR it was 5.25 kg/day/area.
Cameras at PNP were placed almost equally on and off trail (n = 7, n = 6, respectively). All species were found both on and off trail in PNP except for the red brocket deer (off-trail only) and the brown four-eyed opossum (on-trail only, Fig. 4). Species that were detected on-trail significantly more than off-trail as inferred from non-overlapping standard error bars included the black-eared opossum and ocelot. The lowland paca and the nine-banded armadillo were detected significantly more off-trail than on-trail.
Detection rate models
When comparing the relative abundance of each species through models of detection rates, we found Park to be the most widespread significant covariate. Eight species—collared peccary, brown brocket deer (Mazama gouazoubira), greater long-nosed armadillo (Dasypus kappleri), Brown four-eyed opossum (Metachirus nudicaudatus), margay (Leopardus wiedii), red brocket deer, white-lipped peccary (Tayassu pecari), and red acouchi (Myoprocta acouchy)—were significantly associated with CSNR, whereas the giant anteater, crab-eating raccoon (Procyon cancrivorus), Guianan capuchin (Sapajus apella), and South American squirrel monkey (Saimiri sciureus) were more significantly associated with PNP (Table 1, Supplementary Table 1). The other covariates, Human and Vehicle were significant for five and four species, respectively. The black-eared opossum, ocelot, and capybara (Hydrochoerus hydrochaeris) were all positively associated with humans or vehicles, while the grey four-eyed opossum, red brocket deer, Guianan capuchin, and lowland tapir were all negatively associated with humans or vehicles (Table 1, Supplementary Table 1).
Occupancy models
The null model was the most common top model for occupancy, being in five of the species’ top models, followed by Park (n = 4, including red brocket deer which had an additive effect), Human (n = 3), Month (n = 2), and Vehicle (n = 2). Only two models were significant; the occupancy in the lowland paca significantly decreased in PNP, while the lowland tapir was negatively associated with Month, indicating occupancy decreased with time. The red-rumped agouti also decreased in occupancy over time, while the black-eared opossum, grey four-eyed opossum, and coati increased in occupancy with human detections. All species with Park included in the top model had higher occupancy in CSNR except for the tayra (Eira barbara), which had higher occupancy in PNP. Vehicle had opposing effects for the ocelot and the crab-eating raccoon; ocelot decreased in occupancy, whereas the crab-eating raccoon increased (Table 2).
Discussion
Our results indicated that a small, protected area of secondary forest within the most urbanized region of Suriname contained fewer mammal species than a large protected area in the relatively undisturbed primary forest of the country’s interior. However, in PNP we were surprised to find 20 mammal species, of which three were of conservation concern, and both apex predators (puma and jaguar). The presence of some species known to be sensitive to human activity shows promise for forest reserves near urban areas in the Neotropics to support diverse mammal communities.
Prior to urbanization, PNP and CSNR would have had similar mammal communities of the species studied (Husson 1978). The greatest difference between the two sites was that large herbivores were less common in, or completely absent from PNP. White-lipped peccary, collared peccary, and brown brocket deer were not detected at all, and red brocket deer and tapir had lower detection rates and occupancy in PNP than in CSNR. Our model results revealed that the park, the animal was detected in, had the most influence for most species, suggesting that large-scale landscape level factors that were not measured in this study are important in predicting species’ relative abundance. Previous studies have shown that non-consumptive human recreation alone can have negative impacts on wildlife (Boyle and Samson 1985; Green and Giese 2004, but see Kays et al. 2016), which could lead one to believe that perhaps tourism in PNP was the main reason for the differences observed. However, we failed to detect strong impacts of people or vehicles, although species most sensitive to recreation have likely already been extirpated in PNP. A study in Brownsberg Nature Park, Suriname, where tourism levels are similar to PNP, had higher species richness and large herbivore detection rates more like CSNR (Ouboter and Kadosoe 2016), but this park is also not nearly as disturbed and fragmented as PNP. Tourism does not seem to have a substantial impact on mammals in Suriname, however tourist volume is low compared to many other countries.
We hypothesize hunting, park size, and development pressures outside of park boundaries, rather than the number of people in the park, explain the lack of large herbivores in PNP. Large herbivores can thrive in secondary forests if they are not overhunted (Redford 1992; Wright et al. 2000; Fa and Peres 2001), and hunting pressure is an important predictor of mammal abundance (Galetti et al. 2009). Given the accessibility of PNP and the growing human population around it, poaching is likely to influence the mammal community. Shotgun shells have frequently been found in the park and at times, gunshots are even heard. Camera trap locations may also partly explain the lower detection rates and absence of some species at PNP. Sites were chosen based on trail access, which were mostly on the perimeter. Larger herbivores may have learned to avoid trails due to a higher poaching risk. Some of these species may have been detected if cameras were placed in the interior. Finally, the small sample size of PNP, which only had 15 sites compared to CSNR’s 60, makes it more difficult to estimate detection rate and occupancy with good precision, especially for species with fewer detections overall, potentially affecting results.
While PNP lacked larger herbivores, smaller herbivores were much more common. The red-rumped agouti was detected almost three times as frequently in PNP as in CSNR. We suspect this is the result of competitive release, with smaller predators benefiting from more food resources given the absence of competition from larger animals (Moreno et al. 2006; Samudio et al. 2006). The abundance of fruit trees in the park, including species planted by people that are not found in interior forests, may also be a contributing factor.
Surprisingly, we detected jaguars in PNP. This apex predator typically declines with an increasing human footprint due to widespread poaching and retaliation for presumed and/or actual depredation on livestock (Cavalcanti et al. 2010; De Angelo et al. 2013). The detection rate of jaguars in PNP was comparable to that at CSNR, however results may be impacted from the smaller sample size of PNP. From spot patterns we identified at least three individuals in the park over 8 months. The presence of jaguars was also surprising given the small (8 km2) size of PNP; home ranges vary from 22 to 690 km2 (Gonzalez-Borrajo et al. 2017) with densities as high as 8.8 per 100 km2 (Silver et al. 2004). The high connectivity of natural habitats surrounding PNP likely allows for jaguars to make PNP a portion of their home ranges. With few large ungulates in PNP, jaguars must shift their prey source to smaller species. In an overhunted forest in Brazil, jaguars most frequently consumed species < 5 kg, primarily yellow armadillo (Euphractus sexcinctus) and the Southern tamandua. Finally, jaguars may also be attracted to PNP due to the high number of dogs. In the Guianas, jaguars are known predators on domestic dogs (Berzins et al. in review).
Within PNP, we did not find strong differences between the presence of species or their detection rates according to trail use except for ocelots and black-eared opossums, both of which were common and detected significantly more on trails. In previous studies, some species have demonstrated preference for, or avoidance of trails. Cavalcanti et al. (2010) found prey to prefer trails, especially those with more humans to avoid predators, whereas Kays et al. (2016) and Harmsen et al. (2010) detected many predators on trails.
While PNP had lower overall species richness than CSNR and other protected areas in Suriname (Gajapersad et al. 2011; Ouboter and Kadosoe 2016), the 20 species detected in this study indicates comparable or greater diversity than has been reported from other degraded landscapes and secondary forest sites in the Neotropics (Espartosa et al. 2011; Cove et al. 2013; Meyer et al. 2015; de Souza Laurindo et al. 2017; Lima et al. 2017). For instance, in the Brazilian Atlantic Forest, 144 sites averaged had 13 mammal species (Lima et al. 2017), and in a study of forest fragments in Panama, the average was 15 species, with few or no apex predators or large terrestrial frugivores (Meyer et al. 2015). Suriname’s overall low human population density and the continued existence of corridors connecting PNP to extensive interior forests are likely the primary reasons for higher species richness in the park.
Conclusion
Our survey results demonstrate the potential for small reserves, even those close to urban areas, to preserve biodiversity in the Neotropics. PNP held some important species of conservation concern, notably the jaguar, giant anteater, and lowland tapir, however due to our small sample size, similar studies should be conducted to determine the resilience of these species. With stronger anti-poaching protections and efforts, PNP may even be able to support populations of large herbivores found in mammal communities of larger protected areas, as much habitat outside of PNP remains intact. Success will also depend on maintaining the connectivity of the landscape surrounding the small park. With connectivity becoming more limited amid intensifying land use, ensuring the survival of many species may become increasingly difficult.
References
Ahumada J, Silva Carlos EF, Gajapersad K et al (2011) Community structure and diversity of tropical forest mammals: data from a global camera trap network. Philos Trans R Soc B Biol Sci 366:2703–2711. https://doi.org/10.1098/rstb.2011.0115
Ahumada JA, Hurtado J, Lizcano D (2013) Monitoring the status and trends of tropical forest terrestrial vertebrate communities from camera trap data: a tool for conservation. PLoS One 8:e73707. https://doi.org/10.1371/journal.pone.0073707
Barlow J, Gardner TA, Araujo IS et al (2007) Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc Natl Acad Sci 104:18555. https://doi.org/10.1073/pnas.0703333104
Bateman PW, Fleming PA (2012) Big city life: carnivores in urban environments. J Zool 287:1–23. https://doi.org/10.1111/j.1469-7998.2011.00887.x
Benítez-López A, Santini L, Schipper AM et al (2019) Intact but empty forests? Patterns of hunting-induced mammal defaunation in the tropics. PLoS Biol 17:e3000247. https://doi.org/10.1371/journal.pbio.3000247
Berzins R, Paemelaere EAD, Hallett MT, Ouboter P, Jędrzejewski W, Ramalho E, Kadosoe V, Morato R, Cromwell L (in review) Distribution and stauts of the jaguar in the Guiana Shield. Cat News
Bhatia S, Athreya V, Grenyer R, Macdonald DW (2013) Understanding the role of representations of human–leopard conflict in Mumbai through media-content analysis. Conserv Biol 27:588–594. https://doi.org/10.1111/cobi.12037
Boyle SA, Samson FB (1985) Effects of nonconsumptive recreation on wildlife: a review. Wildl Soc Bull 1973–2006(13):110–116
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Cardillo M, Purvis A, Sechrest W et al (2004) Human population density and extinction risk in the world’s carnivores. PLoS Biol 2:e197. https://doi.org/10.1371/journal.pbio.0020197
Cavalcanti SMC, Marchini S, Zirnmermann A et al (2010) Jaguars, livestock, and people in Brazil: realities and perceptions behind the conflict. In: Macdonald DW, Loveridge A (eds) The biology and conservation of wild felids. Oxford University Press, Oxford, pp 383–402
Cove MV, Spínola RM, Jackson VL et al (2013) Integrating occupancy modeling and camera-trap data to estimate medium and large mammal detection and richness in a Central American biological corridor. Trop Conserv Sci 6:781–795. https://doi.org/10.1177/194008291300600606
De Angelo C, Paviolo A, Wiegand T et al (2013) Understanding species persistence for defining conservation actions: a management landscape for jaguars in the Atlantic Forest. Biol Conserv 159:422–433. https://doi.org/10.1016/j.biocon.2012.12.021
de Souza LR, Novaes RLM, de França SR et al (2017) Mammals in forest remnants of an ecotonal Atlantic Forest-Cerrado area from southeastern Brazil. Neotrop Biol Conserv 12:19–29
Eisenberg JF, Thorington RW (1973) A preliminary analysis of a neotropical mammal fauna. Biotropica 5:150–161. https://doi.org/10.2307/2989807
Emmons L (1990) Neotropical rainforest mammals: a field guide. The University of Chicago Press, Chicago
Espartosa KD, Pinotti BT, Pardini R (2011) Performance of camera trapping and track counts for surveying large mammals in rainforest remnants. Biodivers Conserv 20:2815. https://doi.org/10.1007/s10531-011-0110-4
Fa JE, Peres CA (2001) Game vertebrate extraction in African and Neotropical forests: an intercontinental comparison. In: Reynolds J, Mace G, Robinson JG, Redford K (eds) Conservation of exploited species. Cambridge University Press, Cambridge
Gajapersad K, Mackintosh A, Benitez A, Payán E (2011) A survey of the large mammal fauna of the Kwamalasamutu region, Suriname. In: O’Shea BJ, Alonso LE, Larsen TH (eds) A rapid biological assessment of the Kwamalasamutu region, Southwestern Suriname. RAP Bulletin of Biological Assessment 63. Springer, Arlington, pp 150–156
Galetti M, Giacomini HC, Bueno RS et al (2009) Priority areas for the conservation of Atlantic forest large mammals. Biol Conserv 142:1229–1241. https://doi.org/10.1016/j.biocon.2009.01.023
Gallo T, Fidino M, Lehrer EW, Magle SB (2017) Mammal diversity and metacommunity dynamics in urban green spaces: implications for urban wildlife conservation. Ecol Appl 27:2330–2341. https://doi.org/10.1002/eap.1611
Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB (2014) Bayesian data analysis. CRC Press, Boca Raton, FL, USA
Gese EM, Morey PS, Gehrt SD (2012) Influence of the urban matrix on space use of coyotes in the Chicago metropolitan area. J Ethol 30:413–425. https://doi.org/10.1007/s10164-012-0339-8
Gibson L, Lee TM, Koh LP et al (2011) Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478:378. https://doi.org/10.1038/nature10425
Gonzalez-Borrajo N, López-Bao JV, Palomares F (2017) Spatial ecology of jaguars, pumas, and ocelots: a review of the state of knowledge. Mammal Rev 47:62–75. https://doi.org/10.1111/mam.12081
Green R, Giese M (2004) Negative effects of wildlife tourism on wildlife. In: Higginbottom K (ed) Wildlife tourism: impacts, planning and management. Victoria, Australia, pp 81–93
Hansen MC, Potapov PV, Moore R et al (2013) High-resolution global maps of 21st-century forest cover change. Science 342:850–853
Harmsen BJ, Foster RJ, Silver S et al (2010) Differential use of trails by forest mammals and the implications for camera-trap studies: a case study from Belize. Biotropica 42:126–133. https://doi.org/10.1111/j.1744-7429.2009.00544.x
Hines JE (2006) PRESENCE – Software to estimate patch occupancy and related parameters. https://www.mbr-pwrc.usgs.gov/software/presence.html
Hirsch BT, Kays R, Pereira VE, Jansen PA (2012) Directed seed dispersal towards areas with low conspecific tree density by a scatter-hoarding rodent. Ecol Lett 15:1423–1429. https://doi.org/10.1111/ele.12000
Hogg JT, Forbes SH, Steele BM, Luikart G (2006) Genetic rescue of an insular population of large mammals. Proc Biol Sci 273:1491–1499. https://doi.org/10.1098/rspb.2006.3477
Hourigan CL, Johnson C, Robson SKA (2006) The structure of a micro-bat community in relation to gradients of environmental variation in a tropical urban area. Urban Ecosyst 9:67–82. https://doi.org/10.1007/s11252-006-7902-4
Husson AM (1978) The mammals of Surinam. Zoölogische Monographieën van het Rijksmuseum van Natuurlijke Historie. Brill, Leiden
Kays R, Tilak S, Kranstauber B et al (2011) Camera traps as sensor networks for monitoring animal communities. Int J Res Rev Wirel Sens Netw 1:19–29
Kays R, Parsons AW, Baker MC et al (2016) Does hunting or hiking affect wildlife communities in protected areas? J Appl Ecol 54:242–252. https://doi.org/10.1111/1365-2664.12700
Kery M, Schaub M (2012) Bayesian population analysis using Win-BUGS: a hierarchical perspective. Academic Press, Waltham, MA, USA
Laurance WF, Carolina Useche D, Rendeiro J et al (2012) Averting biodiversity collapse in tropical forest protected areas. Nature 489:290. https://doi.org/10.1038/nature11318
Lewis SL, Edwards DP, Galbraith D (2015) Increasing human dominance of tropical forests. Science 349:827. https://doi.org/10.1126/science.aaa9932
Lima F, Beca G, Muylaert RL et al (2017) ATLANTIC-CAMTRAPS: a dataset of medium and large terrestrial mammal communities in the Atlantic Forest of South America. Ecology 98:2979–2979. https://doi.org/10.1002/ecy.1998
Mackenzie DI (2006) Modeling the probability of resource use: the effect of, and dealing with, detecting a species imperfectly. J Wildl Manag 70:367–374. https://doi.org/10.2193/0022-541X(2006)70[367:MTPORU]2.0.CO;2
McShea WJ (2012) Ecology and management of white-tailed deer in a changing world. Ann N Y Acad Sci 1249:45–56. https://doi.org/10.1111/j.1749-6632.2011.06376.x
Melo FPL, Arroyo-Rodríguez V, Fahrig L et al (2013) On the hope for biodiversity-friendly tropical landscapes. Trends Ecol Evol 28:462–468. https://doi.org/10.1016/j.tree.2013.01.001
Meyer NFV, Esser HJ, Moreno R et al (2015) An assessment of the terrestrial mammal communities in forests of Central Panama, using camera-trap surveys. J Nat Conserv 26:28–35. https://doi.org/10.1016/j.jnc.2015.04.003
Mohd-Azlan J (2006) Mammal diversity and conservation in a secondary forest in peninsular Malaysia. Biodivers Conserv 15:1013–1025. https://doi.org/10.1007/s10531-004-3953-0
Morales-Hidalgo D, Oswalt SN, Somanathan E (2015) Status and trends in global primary forest, protected areas, and areas designated for conservation of biodiversity from the Global Forest Resources Assessment 2015. For Ecol Manag 352:68–77. https://doi.org/10.1016/j.foreco.2015.06.011
Moreno RS, Kays RW, Samudio R (2006) Competitive release in diets of ocelot (Leopardus pardalis) and puma (Puma concolor) after jaguar (Panthera onca) decline. J Mammal 87:808–816. https://doi.org/10.1644/05-MAMM-A-360R2.1
Newbold T, Hudson LN, Phillips HRP et al (2014) A global model of the response of tropical and sub-tropical forest biodiversity to anthropogenic pressures. Proc R Soc B Biol Sci 281:20
Ouboter P, Kadosoe VS (2016) Three years of continuous monitoring of the large terrestrial mammals of Brownsberg Nature Park, Suriname. Acad J Suriname 7:643–660
Parsons AW, Forrester T, McShea WJ et al (2017) Do occupancy or detection rates from camera traps reflect deer density? J Mammal 98:1547–1557. https://doi.org/10.1093/jmammal/gyx128
Parsons AW, Forrester T, Baker-Whatton MC et al (2018) Mammal communities are larger and more diverse in moderately developed areas. eLife 7:e38012. https://doi.org/10.7554/eLife.38012
Pimm SL, Raven P (2000) Extinction by numbers. Nature 403:843. https://doi.org/10.1038/35002708
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Redford KH (1992) The empty forest. Bioscience 42:412–422. https://doi.org/10.2307/1311860
Ribeiro MC, Metzger JP, Martensen AC et al (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153. https://doi.org/10.1016/j.biocon.2009.02.021
Rodrigues ASL, Andelman SJ, Bakarr MI et al (2004) Effectiveness of the global protected area network in representing species diversity. Nature 428:640. https://doi.org/10.1038/nature02422
Rowcliffe JM, Field J, Turvey ST, Carbone C (2008) Estimating animal density using camera traps without the need for individual recognition. J Appl Ecol 45:1228–1236. https://doi.org/10.1111/j.1365-2664.2008.01473.x
Rowcliffe JM, Carbone C, Jansen PA et al (2011) Quantifying the sensitivity of camera traps: an adapted distance sampling approach. Methods Ecol Evol 2:464–476. https://doi.org/10.1111/j.2041-210X.2011.00094.x
Rowcliffe JM, Kays R, Carbone C, Jansen PA (2013) Clarifying assumptions behind the estimation of animal density from camera trap rates. J Wildl Manag 77:876–876. https://doi.org/10.1002/jwmg.533
Samudio R, Moreno RS, Kays RW (2006) Competitive release in diets of ocelot (Leopardus pardalis) and puma (Puma concolor) after jaguar (Panthera onca) decline. J Mammal 87:808–816. https://doi.org/10.1644/05-MAMM-A-360R2.1
Sayer CA, Bullock JM, Martin PA (2017) Dynamics of avian species and functional diversity in secondary tropical forests. Biol Conserv 211:1–9. https://doi.org/10.1016/j.biocon.2017.05.004
Seto KC, Güneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci 109:16083
Silver SC, Ostro LET, Marsh LK et al (2004) The use of camera traps for estimating jaguar Panthera onca abundance and density using capture/recapture analysis. Oryx 38:148–154. https://doi.org/10.1017/S0030605304000286
Sinclair ARE (2003) The role of mammals as ecosystem landscapers. Alces 39:161–176
Sloan S, Sayer JA (2015) Forest Resources Assessment of 2015 shows positive global trends but forest loss and degradation persist in poor tropical countries. For Ecol Manag 352:134–145. https://doi.org/10.1016/j.foreco.2015.06.013
Umetsu F, Paul Metzger J, Pardini R (2008) Importance of estimating matrix quality for modeling species distribution in complex tropical landscapes: a test with Atlantic forest small mammals. Ecography 31:359–370. https://doi.org/10.1111/j.0906-7590.2008.05302.x
Vickers TW, Sanchez JN, Johnson CK et al (2015) Survival and mortality of pumas (Puma concolor) in a fragmented, urbanizing landscape. PLoS One 10:e0131490. https://doi.org/10.1371/journal.pone.0131490
Wells K, Lakim MB, O’Hara RB (2014) Shifts from native to invasive small mammals across gradients from tropical forest to urban habitat in Borneo. Biodivers Conserv 23:2289–2303. https://doi.org/10.1007/s10531-014-0723-5
Woodroffe R (2000) Predators and people: using human densities to interpret declines of large carnivores. Anim Conserv 3:165–173
Wright SJ (2005) Tropical forests in a changing environment. Trends Ecol Evol 20:553–560. https://doi.org/10.1016/j.tree.2005.07.009
Wright SJ, Zeballos H, Domínguez I et al (2000) Poachers alter mammal abundance, seed dispersal, and seed predation in a neotropical forest. Conserv Biol 14:227–239. https://doi.org/10.1046/j.1523-1739.2000.98333.x
Acknowledgements
We thank Sara Fernandes, Michael Fede, Anne Premchand, Fabian Lingaard, Fabriella Nain, Rachel Tsie Foen, Natasha Fernandes, Johnny Fernandes, and Emiliano Mohabir for deploying camera traps, Rebecca Sears for photo uploads, and Arielle Parsons and Michael Cove for input on data analyses. This project was generously supported by the Peperpot Foundation and WWF-Guianas.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schuttler, S., Ramcharan, S., Boone, H. et al. Can mammals thrive near urban areas in the Neotropics? Characterizing the community of a reclaimed tropical forest. Trop Ecol 62, 174–185 (2021). https://doi.org/10.1007/s42965-020-00134-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42965-020-00134-1