Abstract
At the end of 2019, the world witnessed the beginning of the COVID-19 pandemic. As an aggressive viral infection, the entire world remained attentive to new discoveries about the SARS-CoV-2 virus and its effects in the human body. The search for new antivirals capable of preventing and/or controlling the infection became one of the main goals of research during this time. New biocompounds from marine sources, especially microalgae and cyanobacteria, with pharmacological benefits, such as anticoagulant, anti-inflammatory and antiviral attracted particular interest. Polysaccharides (PS) and extracellular polymeric substances (EPS), especially those containing sulfated groups in their structure, have potential antiviral activity against several types of viruses including HIV-1, herpes simplex virus type 1, and SARS-CoV-2. We review the main characteristics of PS and EPS with antiviral activity, the mechanisms of action, and the different extraction methodologies from microalgae and cyanobacteria biomass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Viral infections are a significant threat to public health, particularly seasonal epidemics and occasional pandemics. The most effective way to prevent infections is through vaccination [1]. However, for some viruses, such as human immunodeficiency virus (HIV), Zaire ebola virus, and dengue virus, vaccines are still not available [2]. The COVID-19 outbreak and emerging variants of the SARS-CoV-2 virus also highlighted the high probability of the emergence or re-emergence of viral infections. A way of circumventing this problem is through the use of effective antiviral agents from natural sources. The sulfated polysaccharides (PS) from microalgae and cyanobacteria, for example, have been demonstrated as a promising option for the treatment of viral infections [3].
Certain organisms that inhabit aquatic environments have received increasing attention in the search for sources of natural products with biological activities. Microalgae and cyanobacteria are photosynthetic microorganisms that accumulate PS and extracellular polymeric substances (EPS) under different conditions. The structures of PS and EPS will typically depend on the species [4]; however, sulfated PS and EPS have gained particular prominence owing to their demonstrated antiviral activities. Since the 90s, when the first report of a sulfated PS produced by the planktonic filamentous cyanobacterium Arthrospira platensis was shown to exhibit anti-HIV activity, many studies have focused on discovering new producers of these substances. Some studies have reported that PS and EPS could be used against enveloped RNA viruses, such HIV [5] and influenza [6], as well as the new coronavirus SARS-CoV-2, which belongs to this group [7].
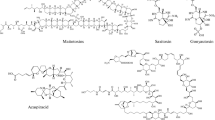
Most studies with sulfated PS are derived from marine macroalgae (seaweeds), such as ulvans from green macroalgae [8], carrageenans and agar from red macroalgae [9], and fucoidans and laminarians from brown macroalgae [10] (Fig. 1). PS and EPS from microalgae and cyanobacteria are still poorly studied and considerably misunderstood. As previous studies have shown that PS and EPS have biological activities against retroviruses without toxic effects on host cells, they are considered to be a “new generation antiretroviral drugs” [11].
Polysaccharide structures produced by marine macroalgae. a Ulvan; b Carragenan; c Fucoidan; d Laminarian. Reused with permission [12]
The aim of this review is to provide an overview of PS and EPS structures, their potentialities as antiviral compounds against the novel coronavirus SARS-CoV-2 and other viruses, and the extraction techniques for their recovery from biomass.
Polysaccharides (PS)
PS are composed of monomers of sugars and sugar derivatives, such as uronic acids and amino sugars [13]. According to Donot et al. [14], PS can be divided in two categories: (1) cytosolic PS, which provide a carbon and energy source for the cell, and (2) cell wall PS including peptidoglycans, teichoic acids, and lipopolysaccharides. Therefore, carbohydrate production serves two main purposes: to provide energy and nutrients, and to maintain the structure of the cell walls [15, 16].
Some cyanobacteria and microalgae PS contain sulfate esters. These sulfated PS have been extensively studied in their respective literature, with focus on their pharmaceutical properties, such as antiviral [17], anticoagulant [18], and antioxidant activities [19]. Sulfated PS are the most studied class of antiviral PS. Among these sulfated PS, spirulan [18, 20] and nostoflan [20] can be highlighted, which are derived from the cyanobacteria A. platensis and Nostoc flagelliforme, respectively. The composition of these PS varies according to species (Table 1). A. platensis was found to produce a water-soluble sulfated PS with xylose as the main component, followed by glucose, rhamnose, fucose, mannose, and galactose [19]. The red microalgae Dixoniella grisea and Porphyridium aerugineum were shown to produce sulfated PS composed of 6–8 monosaccharides, predominantly xylose, and had a sulfur content of 1.6% and 0.8% respectively [21].
Although sulfated PS are found in nature, they can be also obtained by chemical modification with sulfonating agents, such as chlorosulfonic acid-pyridine, concentrated sulfuric acid, and sulfur trioxide pyridine [31]. The sulfation modification has been demonstrated to enhance the antiviral activity. The sulfate may improve the water solubility of the PS and alter the chain conformation resulting in an alteration of biological activities [32, 33].
Some studies in literature have focused on the optimization of the culture conditions of microalgae and cyanobacteria to induce the production of sulfated PS. Sulfated PS can be accumulated in response to nutrient and physical stress, which cause an induction of metabolic pathways that produce the sulfated PS [34]. A study carried out with Arthospira platensis showed induction of sulfated PS as a response to nutrient stress, such as excess nitrogen and high salinity, and physical stress conditions, such as UV radiation and aging. A yield of PS with the highest degree of sulfation (14.63%) [35] was observed by the authors, as a result of cells subjected to UV radiation treatment. The sulfation of PS produced by Porphyridium sp. was studied by incorporating radiosulfate (Na235SO4) or [35S]cysteine. The authors suggested that under conditions of sulfate starvation, the microorganism can assimilate the sulfur-containing amino acid cysteine while incorporating sulfur from [35S]cysteine into the cell wall PS [36]. A study carried out with Porphyridium cruentum demonstrated that sulfate (MgSO4) supplementation of the culture medium enhanced antiviral properties of PS against vesicular stomatitis virus (VSV) and herpes simplex virus type 1 (HSV-1) and suggested that it could be related to the higher degree of sulfation [37].
Extracellular polymeric substances (EPS)
EPS are secondary metabolic products that accumulate on the cell surface, providing protection against external stimuli. EPS are complex blends of carbohydrates, proteins, lipids, nucleic acids, and humic substances [38]. These complex polymers have a molecular weight between 10 KDa and 2 MDa. They are formed by branching amongst the monomers, as observed frequently with other compounds [39].
EPS can be subdivided into bound EPS and soluble EPS. Bound EPS are closely with cells, being sheaths and capsular polymers. Soluble EPS are weakly bound with cells or are dissolved into the cytoplasm and secreted into the extracellular medium [40]. EPS can surround the cell by forming a highly hydrated exopolysaccharide layer, which acts as a barrier against desiccation [41]. The function of EPS is related to protection against nutrient stress and predation; however, other functions have also been described in the literature. These functions include antibiotic resistance, exo-enzymatic degradation, and mechanical strength [28]. In scenarios of nutrient scarcity, the abundance of organic carbon in EPS establishes their value as a rich source. According to Flemming & Wingender [42], EPS present the following functions: adhesion (initial steps in the colonization of abiotic and biotic surfaces), protective barrier (resistance and tolerance to antimicrobial agents), sorptive agent (sorption of xenobiotics and heavy metals), retention of water (important in the tolerance of desiccation), redox-active (electron donor or acceptor), and nutritive source (source of carbon, nitrogen, and phosphorus).
EPS from cyanobacteria often possess five or six sugar monosaccharides, while the EPS of other prokaryotes usually have less than four monosaccharides [43]. The majority monosaccharides are glucose, xylose, and galactose; however, as observed in Table 1, rhamnose, fructose, and arabinose can also be found. In cyanobacteria, EPS possess a complex nature owing to the presence of uronic acids, pyruvic acid, O-methyl, O-acetyl, and sulfate groups [39]. Owing to this complex nature, cyanobacterial EPS are not characterized as well as those of other microorganisms [44]. EPS also possess sulfate groups, which are known to confer some characteristics to EPS, such as antiviral activity. Other components such as Ca2+ and Mg2+ were also found in EPS. These are involved in multiple crosslinkages between sugar molecules, comprising unique PS chains and resulting in high consistency and stability of EPS [45].
EPS from the cyanobacterium Synechocystis sp. VRUC 165 presented a high uronic content (16.20 mol %), exhibited a hydrophilic nature, and promoted the formation of ionic bonds with charged molecules [46, 47]. EPS from the microalgae Chlamydomonas reinhardtii were characterized in terms of their chemical composition, revealing the presence of galacturonic acid, ribose, arabinose, xylose, glucose, galactose, and rhamnose sugars. Furthermore, functional groups such as pyruvate and uronic acid were also detected [48].
The chemical composition can also vary according to the genus. EPS isolated from microalgae Dunaliella tertiolecta was characterized as linear (1,4)-α-D-glucan and its structure resembled that of amylose. EPS demonstrated that only glucose units and PS were the major component of EPS. Conversely, protein was present only as an impurity [49]. EPS from the cyanobacterium Nostoc calcicola RDU-3 revealed a composition of glucose, galactose, rhamnose, xylose, and ribose monosaccharides [43]. Nine different monosaccharides were found in EPS produced by Cyanothece sp. CCY 0110: mannose, glucose, galactose, xylose, arabinose, rhamnose, and fucose, as well as the acidic hexoses, galacturonic and glucuronic acids [50].
Some authors have described how the monosaccharide composition has the potential to influence the EPS function. It is known that rhamnose and fucose confer hydrophobic properties to PS [51]. In Phaeodactylum tricornutum, the secreted fractions of mannose, glucose, and galactose constituted the biofilm mucilage, suggesting involvement in cell adhesion [52]. Many functional groups, such as proteins, carbohydrates, and nucleic acids, can complex with the heavy metals. Charged groups, such as carboxyl and hydroxyl, and negatively-charged components, such as glucuronic and galacturonic acids, in the EPS composition could be beneficial for heavy metal adsorption by aiding in to the formation of organo-metal complexes [40, 53]. EPS produced by Nostoc muscorum was demonstrated to efficiently sequester cadmium [Cd(II)] from aqueous solutions [54], while Colica et al. [55] observed that EPS produced by Cyanothece sp. CE4 removed chromium [Cr(VI)] in wastewater.
Biosynthesis mechanisms
PS and EPS biosynthesis in microalgae and cyanobacteria have not been thoroughly investigated yet; therefore, the information available is limited. According to Markou et al. [15], carbohydrate biosynthesis in microalgae and cyanobacteria occurs during the Calvin Cycle, via the usage of NADPH and ATP and intervention of ribulose (1,5-biphosphate carboxylase oxygenase/RuBisCo). PS sulfation was found to take place in the Golgi apparatus of microalgae [56].
A hypothetical pathway EPS biosynthesis in cyanobacteria was proposed by Pereira et al. [44] (Fig. 2). Based on the Wzy-dependent mechanism, the pathway occurs in four steps: (I) the activation and conversion of monosaccharides in sugar nucleotides, via the cytoplasm, by glycosyltransferases; (II) the assembly of the repeating units by sequential addition of sugars onto a lipid carrier (referred to as undecaprenyl diphosphate); (III) the polymerization of repeating units, situated at the periplasmic face of the plasma membrane (via Wzy and Wzc proteins); and (IV) the extracellular export across plasmatic membrane by a junctional pore complex (JPC).
Mechanism of extracellular polymeric substance (EPS) biosynthesis in cyanobacteria. Glycosyltransferases transfer the nucleotide sugars to a lipid carrier and are assembled at the interface between the cytoplasm and plasmatic membrane. The protein Wzx flips the sugar units across the membrane and subsequently the protein Wzy assembles the polyssacharide. The new polymer is translocated by Wzc and Wzb proteins. Finally, the polymer is translocated across the outer membrane through the lipoprotein Wza. Created with BioRender.com
Very little is known about the biosynthesis machinery for sulfated EPS. A study performed by Maeda et al. [57] with Synechocystis sp. PCC 6803 identified a set of genes responsible for the sulfated EPS (synechan) biosynthesis and a possible pathway. The authors identified two genes for sulfotransferases (xssA, xssE), followed by eight glycosyltransferase genes (xssB, xssC, xssG, xssI, xssM, xssN, xssO, xssP). EPS polymerization follows the Wzy pathway (Fig. 3). Despite the pathway presenting similarities to the one previously described by Pereira et al. [44], the presence of sulfotransferases indicated the biosynthesis of sulfated EPS. Sulfotransferases transfer a sulfo group from the sulfo donor, 3′-phosphoadenosine 5′-phosphosulfate, to the hydroxyl (single bond -OH) and amino (single bond -NH2) positioning of the saccharide residues, which are present in PS [58].
Mechanism of sulphated extracellular polymeric substance (EPS) biosynthesis in Synechocystis sp. PCC 6803. XssB, XssC, XssG, XssI, XssH, XssN, XssD, and XssP represent glycosyltransferases. The polysaccharide polymerization is represented by XssH (Wzy flippase), XssF (Wzy polymerase), and XssK (polysaccharide co-polymerase, PCP). XssT represents the OPX gene, an outer-membrane export. Created with BioRender.com
Antiviral properties of PS and EPS
In recent years, the emergence of distinguished viral infections has significantly increased. Respiratory diseases caused by viral pathogens, including the influenza virus, respiratory syncytial virus (RSV), coronavirus, adenovirus, and rhinovirus, are the leading cause of 80% of all acute morbidities [59]. Although antiviral agents and vaccines are available to cure or protect humans from virus infections, viral isolates could either be drug-resistant or not neutralized by existing vaccines; therefore, new antiviral compounds are urgently needed [17].
The antiviral properties of PS and EPS depend on the molecular weight, sugar residue composition, sulfation level, distribution of sulfate groups along the PS backbone, and protein moieties, as the antiviral activity is the result of complex interactions of many structural features [47]. A high molecular weight (~ 500 kDa) typically presents greater antiviral activity, as a larger chain is more likely to recognize and interact with numerous copies of the viral attachment protein(s) and crosslink virions [60]. In some cases, low-molecular-weight-sulfated carbohydrates can also possess strong antiviral activities, particularly when the sulfate content is high [61]. Salih et al.[9] observed that the chemical structure of PS can have an impact on the degree of their antiviral activity. While sulfated PS with a higher degree of sulfation were seen to be more bioactive as antiviral agents than those with lower degrees, the non-sulfated PS were effectively inactive.
For a better understanding of the antiviral mechanisms of PS and EPS, it is important to know about the viral life cycle (Fig. 4). The basic stages are as follows: (1) viral adsorption, (2) viral penetration, (3) reverse transcription, (4) integration, (5) transcription (6) translation, (7) assembly, (8) budding, and (9) release [32]. Several studies have indicated some antiviral mechanisms that could be useful against some diseases, such as the inhibition of the viral protein synthesis, reverse transcriptase (RT) and RNase H [62].
Drug development focuses on two strategies: targeting the infectivity of the virus and modulating the host defense system [63]. PS and EPS compounds could have antiviral action through different mechanisms: direct virus neutralization; inhibition of virus adsorption, blocking the positive charge on the cell surface through the negative charge of the sulfate group, while interfering with the adsorption process of the virus on the cell; sulfated PS have strong polyanionic properties and can interact with the surface of virus by the negative charge and, consequently, prevent the virus infection, inhibition of invasion of the cell by the virus; inhibition of the viral replication; activation of the immune system, which can be an indirect form of promoting an antiviral effect whereas some EPS and PS can enhance the host's immune system, which can accelerate the process of virus elimination [31, 64].
PS from Porphyridium spp. could potentially block several steps of the life cycle of HSV-1, indicative of an irreversible or very strong binding between HSV-1 particles and the PS. Furthermore, the results demonstrated weak inhibition of viral infection in PS-pretreated cell cultures, indicating that only PS molecules, which interact very closely with the cell membranes viral receptors, can interfere and block virus adsorption and penetration into the host cells [65].
As a recent example of a respiratory disease, COVID-19 was first identified in 2019 in China. In 2020, the virus was considered to have incited a pandemic. The newly discovered coronavirus was distinguished as SARS-CoV-2 [66]. SARS-CoV-2 is an enveloped spherical virus that belongs to the Coronaviridae family. The virus is composed of spike (S), envelope, nucleocapsid, and membrane proteins [67] (Fig. 5). Coronaviruses are enveloped positive-stranded RNA viruses that replicate in the cytoplasm of host cells. SARS-CoV-2 uses the cellular entry receptor, angiotensin-converting enzyme 2 (ACE2) (found in the lower respiratory tract of humans), to engage in the fusion of the envelope with the host cell membrane, mediated by S proteins [64, 68].
Many drugs have been investigated in the treatment of COVID-19. A review provided by Kelleni [69] exhibits a contrast between the expectations and real-world results of four repurposed COVID-19 drugs: tocilizumab (TCZ), remdesivir, favipiravir, and dexamethasone. The author recommends considering TCZ and dexamethasone for selected severe-critical cases of COVID-19 but recognizes that subsequent clinical testing will be required. The use of TCZ was studied in 65 patients, of which 32 were treated with TCZ; during the 28-day follow-up, 69% of TCZ-treated patients experienced a clinical improvement, compared to 61% of patients receiving standard treatment. However, the rate of infection and pulmonary thrombosis proved to be similar between the two groups [70]. Limited evidence on the beneficial effects regarding mortality exist for remdesivir, and little or no effect on the duration of liberation from invasive mechanical ventilation was observed in a study with 7142 participants over 28 days. The authors concluded that there was insufficient data available to examine the effect of remdesivir in patients with SARS-CoV-2 [71]. The use of favipiravir in moderate COVID-19 patients presented a higher clinical recovery rate on day 7, and it was reported to be more effective in reducing the incidence of fever. However, according to the authors, side effects on hematopoietic tissues must be considered, as well as more clinically valid evidence in confirming the positive value of this antiviral agent [72]. Ahmed [73] showed that dexamethasone decreased the mortality risk from 40–28% in ventilated patients, and from 25–20% for patients on oxygen therapy, over a 28-day period. Dexamethasone use did not cause any significant side effects but was ineffective in mild cases. However, according to The Oxford RECOVERY Trial, the data about potential adverse effects, However, according to The Oxford RECOVERY Trial, the data about potential adverse effects and overall efficacy 290 in patients with comorbidities were insufficient[74].
The drugs chloroquine (CQ) and hydroxychloroquine (HCQ) were investigated by various research groups as a treatment for COVID-19 [64, 75, 76]. It was observed that CQ was effective in the control of SARS-CoV-2 infection in vitro, interfering with both entry and post-entry phases of the SARS-CoV-2 infection in Vero E6 cells. The drug was able to synergistically modulate immune activity in conjunction with improving its antiviral activity [77, 78]. Another in vitro study suggested that HCQ could block the entry and post-entry stages of SARS-CoV-2 [79].
Despite these in vitro studies having demonstrated promising results, controversy regarding the efficacy and safety of these treatments arose. A study evaluating the efficacy of HCQ in the treatment of hospitalized patients with COVID-19 demonstrated a significant and positive response in the primary endpoints (transfers to the intensive care unit, need for mechanical ventilation, and in-hospital death) [80]. A clinical trial conducted in several hospitals throughout China to test the efficacy and safety of CQ or HCQ in the treatment of COVID-19-associated pneumonia showed that CQ inhibited the exacerbation of pneumonia, improved lung-image findings, and no severe adverse reactions were noted [75]. However, according to a review published by Chen et al. [77], no significant differences in clinical symptoms, inflammatory biomarkers, length of hospital stay, and overall mortality were observed between the HCQ/CQ group and the control group. Furthermore, high-dose CQ was associated with the incidence of gastrointestinal disorders and cardiovascular lethality.
Another review found that treatment with HCQ was associated with increased mortality in patients with COVID-19 from all currently available randomized clinical trial evidence [81].
Until now, the drugs that were tested against SARS-CoV-2 do not clearly show evidence of safety and efficacy; some of which potentially have serious side effects. As such, natural sources of bioactive molecules, such as the PS and EPS of microalgae and cyanobacteria, have arisen as an interesting option in the search for antiviral compounds. The sulfated PS extracted from Arthospira platensis were tested against pseudotyped viruses of MERS-, and SARS-CoV-2. The authors believe that these components could possibly bind to the S protein while playing a role in the inhibition of coronavirus entry [82]. Yim and colleagues [83] suggested that sulfated PS could bind to the SARS-CoV-2 S protein, inhibiting virus entry into the human cell. Owing to the polyanionic nature, PS and EPS can interact with the positively charged regions of S protein.
As demonstrated in this review, some factors can affect the antiviral activity of PS and EPS. Molecular weight, and total PS and fucose content were identified as important factors in the inhibitory effect induced by PS on SARS-CoV-2, such that PS with a high molecular weight, and total carbohydrate and fucose content had the potential to promote the blockage of virus entry into the cell [83].
Calcium-spirulan (Ca-SP)
Spirulan in its ionic form (calcium or sodium) is a sulfated PS. This compound was isolated and characterized in 1996 from the cyanobacterium, A. platensis [84]. It consists of saccharide constituents, xylose, glucuronic acid, and galacturonic acid, as well as disaccharide repeating units, O-hexuronosyl-rhamnose and O-rhamnosyl-3-O-methylrhamnose. Sulfated constituents were found in the calcium-spirulan (Ca-SP) structure through X-ray and Fourier-transform infrared (FTIR) spectroscopy [85]. These results suggested that Ca-SP is a sulfated PS chelating calcium ions, and mainly composed of rhamnose and fructose. Majdoub et al. [18] extracted a Spirulan-like compound of 199 kDa from S. platensis. The sulfate content was 20% weight/dry weight. Lee et al. [85] also showed that replacing calcium ions with sodium and potassium ions maintained the antiviral activity.
Hayashi et al. [86] described the antiviral activity of Ca-SP. In this study, the authors demonstrated that this PS inhibited the replication of several enveloped viruses including HSV-1, human cytomegalovirus, measles virus, mumps virus, influenza A virus, and HIV-1. The authors also demonstrated that Ca-SP had a high selectivity index for all enveloped viruses. In fact, Ca-SP could inhibit virus replication by inhibition of virus binding to the host cell, and the subsequent virus-cell fusion step [86]. However, it was inactive against nonenveloped viruses. Mechanistic analysis later indicated that Ca-SP blocks the attachment and penetration of HSV-1 into mammalian epithelial cells with a potency that proved to be comparable to the antiviral drug acyclovir [87].
Nostoflan
Nostoflan is an acidic PS without sulfate residues. It was isolated for the first time in China from the cyanobacterium Nostoc flagelliforme. Nostoflan is mainly composed of glucose, xylose, and galactose (approximately 2:1:1), with trace amounts of mannose and arabinose [20]. Other kinds of water-soluble PS were extracted from Nostoc sphaeroides Kütz, which were composed of mannose, glucose, xylose, galactose, and glucuronic acid, and possessed an average molecular weight of 1.31×105g/mol [85].
Nostoflan was demonstrated to have antiviral activity, but only towards enveloped viruses that bind to the carbohydrate receptor of cells, thus acting as inhibitor of virus-cell interaction [20, 88]. In another study, nostoflan was shown to exert an inhibitory effect on the binding process of HSV-1, but not on the virus penetration process, which occurs immediately after virus binding [89].
Other EPS and PS
The sulfated PS, p-KG03, produced by the microalgae Gyrodinium impudium, is a galactose homopolysaccharide conjugated to uronic acid. Its antiviral activity against influenza A was tested and discovered to be directly associated to its interaction with viral particles [17].
EPS extracted from A. platensis showed the presence of sulfated PS, in addition to a small group of other uncharacterized PS and possibly protein components. The antiviral activity of the EPS against koi herpesvirus was tested at a concentration greater than 18 µg.mL−1, wherein it suppressed the viral replication in common carp brain (CCB) cells, even after 22 days post-infection [90]. Radonic et al. [91] demonstrated how the antiviral activities of sulfur-containing anionic EPS from A. platensis, named TK V3, and from Porphyridium purpureum, against the enveloped vaccinia virus and ectromelia virus ultimately decreased the multiplicity of infection.
PS and EPS extraction
An important step for obtaining intracellular PS and EPS is the extraction of these compounds. They are derived from the cells and different methodologies have been described in the literature, in addition to various purification methodologies. Conventional extraction processes of these compounds are often conducted from dry biomass with organic or aqueous solvents [92]. These methods are associated with several limitations though, such as long extraction times, usage of large amounts of solvents, and the co-extraction of undesirable components, resulting in increased downstream processing costs [93]. Extraction and purification techniques that are effective and economically favorable must be considered [94]. To obtain a product without salts and proteins, chromatographic techniques [95], tangential microfiltration, and dialysis [96] have been tested.
The cell wall disruption is the primary step for the recovery of PS and may be the most critical step. Cell lysis methods are divided into non-mechanical (chemical and enzymatic hydrolysis) and mechanical methods, such as hot water extraction (HWE), high-speed homogenization, sonication, pulsed electric field, and high-pressure homogenization (HPH) [97] (Table 2). The optimization of these methodologies is considered an important step to PS extraction because high temperature and long extraction times can damage the structure of the macromolecules, as well as alter their pharmacological activity [97]. However, the use of physical methods can cause reduction of polymer size. In the presence of residual amounts of proteins, and when submitted to temperatures over 100°C, undesirable toxic molecules are formed. Examples of this include such combinations between free radicals and carbohydrate derivatives [98]. In chemical methods, when the acid hydrolysis is controlled, oligomers of varied molecular weight are produced [99]. The disadvantage of chemical methods are the strong acids or bases and elevated temperatures, leading to PS degradation.
To obtain PS from A. platensis, different extraction methodologies have been tested. The cell wall of A. platensis is composed mainly of murein, and the trichome is further enveloped by a thin mucilaginous sheath, and thus, these structures could become barriers for the water extraction methodologies. According to the authors, it is necessary to use a combination of HWE and HPH [103]. In another study, 7 cycles in a HPH system were deemed necessary for complete cell disruption of A. platensis. However, proteins were found to be extracted almost entirely from the cells, concomitantly with PS, and thus, a purification step was necessary to quantify and characterize the PS [97].
EPS extraction is commonly carried out by chemical methods using EDTA, NaOH, and organic solvents that are mixed with the biomass. Some authors have reported the use of formaldehyde and glutaraldehyde as fixative agents to protect the microalgae during the extraction process. These agents react with hydroxyl, amino groups, and carbonyl of the outer cellular layers of membrane, preventing cell lysis [39, 104]. The use of NaOH in the extraction process neutralizes acid groups on the surface of EPS, reduces the repulsion force of EPS while increasing the solubility of EPS in water [105]. Recovering EPS from the culture media generally requires alcohols, such as methanol, ethanol, or isopropanol. This method allows the selective concentration of EPS, but presents a disadvantage: microalgae and cyanobacteria, generally are cultivated in culture media with salts and these can co-precipitate with EPS [106]. Therefore, to increase the purification of EPS, an additional step is required.
The choice of the best methodology for cell disruption and product recovery needs to preserve the structure as well maintaining its bioactivity. For example, PS of high molecular weight and long main chain usually have a compacted structure with high viscosity and low solubility. Treatment with ultrasound can cause a breakage of glycosidic linkages, along with weakening of the microstructural network. Consequently, this would result in decreasing the viscosity and increasing the solubility. The destruction of inter and intra-molecular bonds result in more hydrophilic groups exposed to water molecules [107, 108]. PS that exhibit a high solubility can easily penetrate multiple cell membrane barriers and have greater bioactivity [32].
The HPH methodology has been used as an alternative to diminish the volume of organic solvent, make cell disruption more effective, and subsequently, increase the yield of the product. In the literature, HPH is mainly intended for lipids [109] and proteins [93]. Its application for the recovery of water-soluble PS is relatively new. Therefore, the effects on PS structure are still poorly documented [97].
HWE can also be used, although, it is known that the degradation of bioactive compounds may occur when cells are subjected to higher temperatures. The antiviral activity of PS, when extracted in cold and hot water, from Spirulina, was tested against HSV-2 and VSV. The authors reported that PS could inhibit the cell entry of HSV-2. However, HWE of Arthospira did not inhibit VSV entry. This could be explained by the degradation of the bioactive compounds. Furthermore, the authors tested different extracts of S. maxima. They found that the highest antiviral activity was observed with that extracted in methanol–water (3:1), indicating that the antiviral activity could be owing to polar compounds [110]. The HWE of A. platensis was reported to inhibit HIV-1 replication in two T-cell lines, which could be the result of PS binding to the CD4 receptor. This would disrupt CD4 and the glycoprotein gp120 interactions, which are crucial for viral infection [111].
Prospects
PS and EPS produced by microalgae and cyanobacteria have strong antiviral effects against diverse virus strains in vitro [17, 90, 103]. However, a relatively low number of clinical trials have been carried out to evaluate the antiviral activities of PS and EPS in humans. PS and EPS have been associated with serious side effects, such as antithrombin activity, restricting their clinical application as antiviral agents [20]. The administration of PS with a clotting agent, like thrombin, could be an option. Baba et al.[112] showed that it is possible to reduce the antithrombin activity without the sulfated PS losing its antiviral activity. The antiviral and antithrombin activity of sulfated PS may be influenced differently by the number, distribution, and spatial configuration of the sulfate groups, and thus, further studies are required. Development of antiviral therapeutic agents from microalgae and cyanobacteria must fulfill the criteria regarding safety, biocompatibility, and efficiency [12].
According to Huleihel et al. [65], PS are complex molecules with high molecular weight, and they are unable to pass through the different barriers of the body, or even through cell membranes. Owing to the absence of enzymes capable of degrading and digesting these molecules, they have the potential to accumulate in the body, potentially causing cytotoxic effects. Finally, their mechanism of action has not been elucidated, which causes more concerns about their use in humans. Low-molecular-weight anionic compounds are generally seen to possess better bioavailability than high-molecular-weight compounds [61]. According to Yamaoka et al. [113], the molecular weight or size is one of the most important factors affecting their biological fate in the body. These investigations could possibly assist in establishing the use of PS as a natural antiviral [114].
Conclusions
PS and EPS, derived from microalgae and cyanobacteria, especially when sulfated, exhibit good inhibitory effects on a great variety of viruses. Most of the studies on the antiviral effects of marine PS have been performed in vitro or in vivo (in mouse model systems). The limited number of clinical trials may create difficulty in the approval process of PS and EPS as pharmaceutical drugs. Furthermore, extraction and purification methodologies and conditions must be improved to increase PS and EPS yields whilst also being green and low cost. Cyanobacteria and microalgae PS and EPS have significant potential to be utilized for the development of new antiviral drugs against several viruses, including SARS-CoV-2. The development of effective drugs and vaccines against future coronavirus infections and other highly pathogenic viruses will be essential to reduce the devastating impacts on human life and global healthcare systems.
Data Availability
The authors declare that there is not data supporting the results or available.
References
Rajão DS, Pérez DR (2018) Universal vaccines and vaccine platforms to protect against influenza viruses in humans and agriculture. Front Microbiol 9:123. https://doi.org/10.3389/fmicb.2018.00123
Carbone DA, Pellone P, Lubritto C, Ciniglia C (2021) Evaluation of microalgae antiviral activity and their bioactive compounds. Antibiotics 10:746. https://doi.org/10.3390/antibiotics10060746
Panggabean JA, Adiguna SP, Rahmawati SI, Ahmadi P, Zainuddin EN, Bayu A, Putra MY (2022) Antiviral activities of algal-based sulfated polysaccharides. Molecules 27:1178. https://doi.org/10.3390/molecules27041178
Phélippé M, Gonçalves O, Thouand G, Cogne G, Laroche C (2019) Characterization of the polysaccharides chemical diversity of the cyanobacteria Arthrospira platensis. Algal Res 38. https://doi.org/10.1016/j.algal.2019.101426
Hayashi K, Hayashi T, Kojima3 I (1996) A Natural sulfated polysaccharide, calcium spirulan, isolated from Arthospira platensis: In: Vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. Mary Ann Liebert, Inc. AIDS Research and Human Retroviruses, 12. https://doi.org/10.1089/aid.1996.12.1463
Hasui M, Matsuda M, Okutani K, Shigeta S (1995) In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int J Biol Macromol 17:93–297. https://doi.org/10.1016/0141-8130(95)98157-t
Perumal Ulagalanthaperumal E, Perumal EU (2020) Algae: A potential source to prevent and cure the Novel Coronavirus-A review collection isolation and germplasm development of microalgae view project Freshwater Biodiversity Observation Network (FW BON) View project Government Arts College for Men Algae: A Potential Source to Prevent and Cure the Novel Coronavirus-A review. Int J Emerg Technol 11:479–483
Shah S, Famta P, Shahrukh S, Jain N, Vambhurkar G, Srinivasarao DA, Raghuvanshi RS, Singh SB, Srivastava S (2023) Multifaceted applications of ulvan polysaccharides: Insights on biopharmaceutical avenues. Int J Biol Macromol 234:123669. https://doi.org/10.1016/j.ijbiomac.2023
Salih AEM, Thissera B, Yaseen M, Hassane ASI, El-seedi HR, Sayed AM, Rateb ME (2021) Marine sulfated polysaccharides as promising antiviral agents: A comprehensive report and modeling study focusing on sars cov‐2. Mar Drugs 19. https://doi.org/10.3390/md19080406
Liewert I, Ehrig K, Alban S (2017) Effects of fucoidans and heparin on reactions of neutrophils induced by IL-8 and C5a. Carbohydr Polym 165:462–469. https://doi.org/10.1016/j.carbpol.2017.02.051
Sanniyasi E, Venkatasubramanian G, Anbalagan MM, Raj PP, Gopal RK (2019) In vitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds) (Dictyota bartayesiana J.V.Lamouroux and Turbinaria decurrens Bory). Sci Rep 9. https://doi.org/10.1038/s41598-019-47917-8
Bai RG, Tuvikene R (2021) Potential antiviral properties of industrially important marine algal polysaccharides and their significance in fighting a future viral pandemic. Viruses 13:1817. https://doi.org/10.3390/v13091817
Templeton DW, Quinn M, Van Wychen S, Hyman D, Laurens LML (2012) Separation and quantification of microalgal carbohydrates. J Chromatogr A 1270:225–234. https://doi.org/10.1016/j.chroma.2012.10.034
Donot F, Fontana A, Baccou JC, Schorr-Galindo S (2012) Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr Polym 87:951–962. https://doi.org/10.1016/j.carbpol.2011.08.083
Markou G, Angelidaki I, Georgakakis D (2012) Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl Microbiol Biotechnol 96:631–645. https://doi.org/10.1007/s00253-012-4398-0
Tang DYY, Khoo KS, Chew KW, Tao Y, Ho SH, Show PL (2020) Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour Technol 304:122997. https://doi.org/10.1016/j.biortech.2020.122997
Yim JH, Kim SJ, Ahn SH, Lee HK (2007) Characterization of a novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodinium impudicum KG03. Bioresour Technol 98:361–367. https://doi.org/10.1016/j.biortech.2005.12.021
Majdoub H, Mansour M, Ben, Chaubet F, Roudesli MS, Maaroufi RM (2009) Anticoagulant activity of a sulfated polysaccharide from the green alga Arthrospira platensis. Biochim Biophys Acta Gen Subj 1790:1377–1381. https://doi.org/10.1016/j.bbagen.2009.07.013
Rajasekar P, Palanisamy S, Anjali R, Vinosha M, Elakkiya M, Marudhupandi T, Tabarsa M, You SG, Prabhu NM (2019) Isolation and structural characterization of sulfated polysaccharide from Arthospira platensis and its bioactive potential: In vitro antioxidant, antibacterial activity and Zebrafish growth and reproductive performance. Int J Biol Macromol 141:809–821. https://doi.org/10.1016/j.ijbiomac.2019.09.024
Kanekiyo K, Lee JB, Hayashi K, Takenaka H, Hayakawa Y, Endo S, Hayashi T (2005) Isolation of an antiviral polysaccharide, nostoflan, from a terrestrial cyanobacterium, Nostoc flagelliforme. J Nat Prod 68:1037–1041. https://doi.org/10.1021/np050056c
Netanel Liberman G, Ochbaum G, Mejubovsky-Mikhelis M, Bitton R, (Malis), Arad S (2020) Physico-chemical characteristics of the sulfated polysaccharides of the red microalgae Dixoniella grisea and Porphyridium aerugineum. Int J Biol Macromol 145:1171–1179. https://doi.org/10.1016/j.ijbiomac.2019.09.205
Gaignard C, Macao V, Gardarin C et al (2018) The red microalga Flintiella sanguinaria as a new exopolysaccharide producer. J Appl Phycol 30:2803–2814. https://doi.org/10.1007/s10811-018-1389-2
Zhang J, Liu L, Chen F (2019) Production and characterization of exopolysaccharides from Chlorella zofingiensis and Chlorella vulgaris with anti-colorectal cancer activity. Int J Biol Macromol 134:976–983. https://doi.org/10.1016/j.ijbiomac.2019.05.117
Trabelsi L, M’sakni NH, Ouada B et al (2009) H. Partial characterization of extracellular polysaccharides produced by cyanobacterium Arthrospira platensis. Biotechnol Bioproc E 14:27–31. https://doi.org/10.1007/s12257-008-0102-8
Volk RB, Venzke K, Blaschek W (2007) Structural investigation of a polysaccharide released by the cyanobacterium Nostoc insulare. J Appl Phycol 19:255–262. https://doi.org/10.1007/s10811-006-9131-x
Ge H, Xia L, Zhou X et al (2014) Effects of light intensity on components and topographical structures of extracellular polysaccharides from the cyanobacteria Nostoc sp. J Microbiol 52:179–183. https://doi.org/10.1007/s12275-014-2720-5
Roussel M, Villay A, Delbac F, Michaud P, Laroche C, Roriz D et al (2015) Antimicrosporidian activity of sulphated polysaccharides from algae and their potential to control honeybee nosemosis. Carbohydr Polym 133:213–220
Di Pippo F, Ellwood NTW, Gismondi A, Bruno L, Rossi F, Magni P, de Philippis R (2013) Characterization of exopolysaccharides produced by seven biofilm-forming cyanobacterial strains for biotechnological applications. J Appl Phycol 25:1697–1708. https://doi.org/10.1007/s10811-013-0028-1
Villay A, Laroche C, Roriz D, El Alaoui H, Delbac F, Michaud P (2013) Optimisation of culture parameters for exopolysaccharides production by the microalga Rhodella violacea. Bioresour Technol 146:732–735. https://doi.org/10.1016/j.biortech.2013.07.030
de Jesus CS, de Jesus Assis D, Rodriguez MB, Menezes Filho JA, Costa JAV, de Souza Ferreira E, Druzian JI (2019) Pilot-scale isolation and characterization of extracellular polymeric substances (EPS) from cell-free medium of Arthospira sp. LEB-18 cultures under outdoor conditions. Int J Biol Macromol 124:1106–1114
Chen L, Huang G (2018) The antiviral activity of polysaccharides and their derivatives. Int J Biol Macromol 115:77–82. https://doi.org/10.1016/j.ijbiomac.2018.04.056
Wang W, Wang SX, Guan HS (2012) The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar Drugs 10:2795–2816. https://doi.org/10.3390/md10122795
Xie L, Shen M, Hong Y, Ye H, Huang L, Xie J (2020) Chemical modifications of polysaccharides and their anti-tumor activities. Carbohydr Polym 229. https://doi.org/10.1016/j.carbpol.2019.115436
Shalaby EA, Shanab SMM, Singh V (2010) Salt stress enhancement of antioxidant and antiviral efficiency of Arthospira platensis. J Med Plants Res 4:2622–2632. https://doi.org/10.5897/jmpr09.300
Mendhulkar VD, Shetye LA, Khot O (2020) Modulation of the anti-cancer activity of sulfated polysaccharides, synthesized in Arthospira platensis, due to varying degree of sulfation induced by nutrient and physical stress. J Biologically Act Prod Nat 10:275–284. https://doi.org/10.1080/22311866.2020.1806729
Keidan M, Broshy H, Van Moppes D, Arad S (2006) Assimilation of sulphur into the cell-wall polysaccharide of the red microalga Porphyridium sp. (Rhodophyta). Phycologia 45:505–511. https://doi.org/10.2216/05-57.1
Raposo MFDJ, De Morais AMMB, De Morais RMSC (2014) Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci 101:56–63. https://doi.org/10.1016/j.lfs.2014.02.013
Mishra A, Jha B (2013) Microbial exopolysaccharides. The Prokaryotes: Applied bacteriology and biotechnology. Springer, Berlin Heidelberg, pp 179–192
Bhunia B, Prasad Uday US, Oinam G, Mondal A, Bandyopadhyay TK, Tiwari ON (2018) Characterization, genetic regulation and production of cyanobacterial exopolysaccharides and its applicability for heavy metal removal. Carbohydr Polym 179:228–243. https://doi.org/10.1016/j.carbpol.2017.09.091
Sheng GP, Yu HQ, Li XY (2010) Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol Adv 28:882–894. https://doi.org/10.1016/j.biotechadv.2010.08.001
Kumar AS, Mody K, Jha B (2007) Bacterial exopolysaccharides - A perception. J Basic Microbiol 47:103–117
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
Singh S, Das S (2011) Screening, production, optimization and characterization of cyanobacterial polysaccharide. World J Microbiol Biotechnol 27:1971–1980. https://doi.org/10.1007/s11274-011-0657-y
Pereira S, Zille A, Micheletti E, Moradas-Ferreira P, De Philippis R, Tamagnini P (2009) Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol Rev 33:917–941
Arad MRASM, Ginzberg A, Huleihel M (2006) Antiviral activity of sulfated polysaccharides of marine red algae. Biomaterials from Aquatic and Terrestrial Organisms. CRC Press, pp 49–74. https://doi.org/10.1201/9781482280470
De Philippis R, Colica G, Micheletti E (2011) Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: Molecular basis and practical applicability of the biosorption process. Appl Microbiol Biotechnol 92:697–708. https://doi.org/10.1007/s00253-011-3601-z
Xiao R, Zheng Y (2016) Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol Adv 34:1225–1244
Bafana A (2013) Characterization and optimization of production of exopolysaccharide from Chlamydomonas reinhardtii. Carbohydr Polym 95:746–752. https://doi.org/10.1016/j.carbpol.2013.02.016
Geun Goo B, Baek G, Jin Choi D, Il Park Y, Synytsya A, Bleha R, Ho Seong D, Lee CG, Kweon Park J (2013) Characterization of a renewable extracellular polysaccharide from defatted microalgae Dunaliella tertiolecta. Bioresour Technol 129:343–350. https://doi.org/10.1016/j.biortech.2012.11.077
Mota R, Guimarães R, Büttel Z, Rossi F, Colica G, Silva CJ, Santos C, Gales L, Zille A, De Philippis R, Pereira SB, Tamagnini P (2013) Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110. Carbohydr Polym 92:1408–1415. https://doi.org/10.1016/j.carbpol.2012.10.070
Zhou J, Mopper K, Passow U (1998) The role of surface-active carbohydrates in the formation of transparent exopolymer particles by bubble adsorption of seawater. Limnol Oceanogr 43:1860–1871. https://doi.org/10.4319/lo.1998.43.8.1860
Willis A, Eason-Hubbard M, Hodson O, Maheswari U, Bowler C, Wetherbee R (2014) Adhesion molecules from the diatom Phaeodactylum tricornutum (Bacillariophyceae): Genomic identification by amino-acid profiling and in vivo analysis. J Phycol 50:837–849. https://doi.org/10.1111/jpy.12214
Sheng GP, Xu J, Li WH, Yu HQ (2013) Quantification of the interactions between Ca2+, Hg2 + and extracellular polymeric substances (EPS) of sludge. Chemosphere 93:1436–1441. https://doi.org/10.1016/j.chemosphere.2013.07.076
Raghavan PS, Potnis AA, Bhattacharyya K, Salaskar DA, Rajaram H (2020) Axenic cyanobacterial (Nostoc muscorum) biofilm as a platform for Cd(II) sequestration from aqueous solutions. Algal Res 46. https://doi.org/10.1016/j.algal.2019.101778
Colica G, Mecarozzi PC, De Philippis R (2010) Treatment of Cr(VI)-containing wastewaters with exopolysaccharide-producing cyanobacteria in pilot flow through and batch systems. Appl Microbiol Biotechnol 87:1953–1961. https://doi.org/10.1007/s00253-010-2665-5
Keidan M, Friedlander M, Arad S (2009) Effect of Brefeldin A on cell-wall polysaccharide production in the red microalga Porphyridium sp. (Rhodophyta) through its effect on the Golgi apparatus. J Appl Phycol 21:707–717. https://doi.org/10.1007/s10811-009-9406-0
Maeda K, Okuda Y, Enomoto G, Watanabe S, Ikeuchi M (2021) Biosynthesis of a sulfated exopolysaccharide, synechan, and bloom formation in the model cyanobacterium synechocystis sp. Strain pcc 6803. Elife 10. https://doi.org/10.7554/eLife.66538
Liu J, Moon AF, Sheng J, Pedersen LC (2012) Understanding the substrate specificity of the heparan sulfate sulfotransferases by an integrated biosynthetic and crystallographic approach. Curr Opin Struct Biol 22:550–557. https://doi.org/10.1016/j.sbi.2012.07.004
Zhang N, Wang L, Deng X, Liang R, Su M, He C, Hu L, Su Y, Ren J, Yu F, Du L, Jiang S (2020) Recent advances in the detection of respiratory virus infection in humans. J Med Virol 92:408–417. https://doi.org/10.1002/jmv.25674
Witvrouw M, De Clercq E (1997) Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. 29:497–511. https://doi.org/10.1016/s0306-3623(96)00563-0
Ghosh T, Chattopadhyay, Marschall M, Karmakar K, Mandal P, Ray B (2009) Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 19:2–15. https://doi.org/10.1093/glycob/cwn092
Biesert L, Adamski M, Zimmer G, Suhartono H, Fuchs J, Unkeibach U, Mehlhorn RJ, Hideg K, Miibradt R, Riibsamen-Waigmann H (1990) Anti-human immunodeficiency virus (HIV) drug HOE/BAY 946 increases membrane hydrophobicity of human lymphocytes and specifically suppresses HIV-protein synthesis. Springer-Verlag
Sami N, Ahmad R, Fatma T (2021) Exploring algae and cyanobacteria as a promising natural source of antiviral drug against SARS-CoV-2. Biomed J 44:54–62. https://doi.org/10.1016/j.bj.2020.11.014
Chen X, Han W, Wang G, Zhao X (2020) Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int J Biol Macromol 164:331–343. https://doi.org/10.1016/j.ijbiomac.2020.07.106
Huleihel M, Ishanu V, Tal J, Malis S, Arad (2001) Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J Appl Phycol 13:127–134. https://doi.org/10.1023/A:1011178225912
Bakhshandeh B, Jahanafrooz Z, Abbasi A, Goli MB, Sadeghi M, Mottaqi MS, Zamani M (2021) Mutations in SARS-CoV-2; Consequences in structure, function, and pathogenicity of the virus. Microb Pathog 154:104831. https://doi.org/10.1016/j.micpath.2021.104831
Andrew M, Jayaraman G (2021) Marine sulfated polysaccharides as potential antiviral drug candidates to treat Corona Virus disease (COVID-19). Carbohydr Res 505:108326. https://doi.org/10.1016/j.carres.2021.108326
Jafari Porzani S, Konur O, Nowruzi B (2022) Cyanobacterial natural products as sources for antiviral drug discovery against COVID-19. J Biomol Struct Dyn 40:7629–7644. https://doi.org/10.1080/07391102.2021.1899050
Kelleni MT. Tocilizumab Remdesivir, Favipiravir, and Dexamethasone Repurposed for COVID-19: A comprehensive clinical and pharmacovigilant reassessment. https://doi.org/10.1007/s42399-021-00824-4/Published
Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, Tomelleri A, Baldissera E, Rovere-Querini P, Ruggeri A, Monti G, De Cobelli F, Zangrillo A, Tresoldi M, Castagna A, Dagna L (2020) Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med 76:43–49. https://doi.org/10.1016/j.ejim.2020.05.021
Ansems K, Grundeis F, Dahms K, Mikolajewska A, Thieme V, Piechotta V, Metzendorf M-I, Stegemann M, Benstoem C, Fichtner F (2021) Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev 2021. https://doi.org/10.1002/14651858.CD014962
Ghasemnejad-Berenji M, Pashapour S (2021) Favipiravir and COVID-19: A Simplified Summary. Drug Res 71:166–170. https://doi.org/10.1055/a-1296-7935
Ahmed MH, Hassan A (2020) Dexamethasone for the Treatment of Coronavirus Disease (COVID-19): a Review. SN Compr Clin Med 2:2637–2646. https://doi.org/10.1007/s42399-020-00610-8
Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S (2020) Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol 39:2085–2094. https://doi.org/10.1007/s10067-020-05190-5
Gao J, Tian Z, Yang X (2020) Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 14:72–73. https://doi.org/10.5582/bst.2020.01047
Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 56. https://doi.org/10.1016/j.ijantimicag.2020.105949
Chen Y, Li MX, Lu GD, Shen HM, Zhou J (2021) Hydroxychloroquine/chloroquine as therapeutics for covid-19: Truth under the mystery. Int J Biol Sci 17:1538–1546. https://doi.org/10.7150/ijbs.59547
Gevers S, Kwa MSG, Wijnans E, van Nieuwkoop C (2020) Safety considerations for chloroquine and hydroxychloroquine in the treatment of COVID-19. Clin Microbiol Infect 26:1276–1277. https://doi.org/10.1016/j.cmi.2020.05.006
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M (2020) Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6. https://doi.org/10.1038/s41421-020-0156-0
Tsanovska H, Simova I, Genov V, Kundurzhiev T, Krasnaliev J, Kornovski V, Dimitrov N, Vekov T (2022) Hydroxychloroquine (HCQ) Treatment for hospitalized patients with COVID- 19. Infect Disord Drug Targets 22. https://doi.org/10.2174/1871526522666220303121209
Axfors C, Schmitt AM, Janiaud P, van’t Hooft J, Abd-Elsalam S, Abdo EF, Abella BS, Akram J, Amaravadi RK, Angus DC, Arabi YM, Azhar S, Baden LR, Baker AW, Belkhir L, Benfield T, Berrevoets MAH, Chen CP, Chen TC, Cheng SH, Cheng CY, Chung WS, Cohen YZ, Cowan LN, Dalgard O, de Almeida e Val FF, de Lacerda MVG, de Melo GC, Derde L, Dubee V, Elfakir A, Gordon AC, Hernandez-Cardenas CM, Hills T, Hoepelman AIM, Huang YW, Igau B, Jin R, Jurado-Camacho F, Khan KS, Kremsner PG, Kreuels B, Kuo CY, Le T, Lin YC, Lin WP, Lin TH, Lyngbakken MN, McArthur C, McVerry BJ, Meza-Meneses P, Monteiro WM, Morpeth SC, Mourad A, Mulligan MJ, Murthy S, Naggie S, Narayanasamy S, Nichol A, Novack LA, O’Brien SM, Okeke NL, Perez L, Perez-Padilla R, Perrin L, Remigio-Luna A, Rivera-Martinez NE, Rockhold FW, Rodriguez-Llamazares S, Rolfe R, Rosa R, Røsjø H, Sampaio VS, Seto TB, Shehzad M, Soliman S, Stout JE, Thirion-Romero I, Troxel AB, Tseng TY, Turner NA, Ulrich RJ, Walsh SR, Webb SA, Weehuizen JM, Velinova M, Wong HL, Wrenn R, Zampieri FG, Zhong W, Moher D, Goodman SN, Ioannidis JPA, Hemkens LG (2021) Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun 12. https://doi.org/10.1038/s41467-021-22446-z
1# JJ, 1# KT, Ajay A, Das VRA, Stalin Raj V Green tea and Arthospira extracts inhibit SARS, MERS, and SARS-2 spike pseudotyped virus entry in vitro 2 3. https://doi.org/10.1101/2020.06.20.162701
Yim SK, Kim K, Kim I, Chun SH, Oh TH, Kim JU, Kim J, Jung W, Moon H, Ku B, Jung K (2021) Inhibition of sars-cov-2 virus entry by the crude polysaccharides of seaweeds and abalone viscera in vitro. Mar Drugs 19. https://doi.org/10.3390/MD19040219
Hayashi K, Hayashi T, Kojima I (1996) A Natural Sulfated Polysaccharide, Calcium Spirulan, Isolated from Arthospira platensis: In Vitro and ex Vivo Evaluation of Anti-Herpes Simplex Virus and Anti-Human Immunodeficiency Virus Activities. Mary Ann Liebert, Inc
Lee J-B, Srisomporn P, Hayashi K, Tanaka T, Sankawa U, Hayashi T (2001) Effects of structural modification of calcium spirulan, a sulfated polysaccharide from Arthospira Platensis, on antiviral activity. 49:108–10. https://doi.org/10.1248/cpb.49.108
Hayashi T, Hayashit K, Maeda M (1996) Calcium Spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Arthospira platensis 59:83–7. https://doi.org/10.1021/np960017o
Mader J, Gallo A, Schommartz T, Handke W, Nagel CH, Günther P, Brune W, Reich K (2016) Calcium spirulan derived from Arthospira platensis inhibits herpes simplex virus 1 attachment to human keratinocytes and protects against herpes labialis. J Allergy Clin Immunol 137:197–203e3. https://doi.org/10.1016/j.jaci.2015.07.027
Besednova NN, Makarenkova ID, Zvyagintseva TN, Imbs TI, Somova LM, Zaporozhets TS (2016) Antiviral action and pathogenetic targets for seaweed sulfated polysaccharides in herpesvirus infections. Biomeditsinskaya Khim 62:217–227. https://doi.org/10.3390/md17060373
Kanekiyo K, Hayashi K, Takenaka H, Lee J-B, Hayashi T (2007) Anti-herpes simplex virus target of an acidic polysaccharide, nostoflan, from the edible blue-green alga Nostoc flagelliforme. 30:1573–1575. https://doi.org/10.1248/bpb.30.1573
Reichert M, Bergmann SM, Hwang J, Buchholz R, Lindenberger C (2017) Antiviral activity of exopolysaccharides from Arthrospira platensis against koi herpesvirus. J Fish Dis 40:1441–1450. https://doi.org/10.1111/jfd.12618
Radonić A, Thulke S, Achenbach J, Kurth A, Vreemann A, König T, Walter C, Possinger K, Nitsche A (2010) Anionic polysaccharides from phototrophic microorganisms exhibit antiviral activities to vaccinia virus. J Antivir Antiretrovir 2:051–055. https://doi.org/10.4172/jaa.1000023
Luengo E, Martínez JM, Bordetas A, Álvarez I, Raso J (2015) Influence of the treatment medium temperature on lutein extraction assisted by pulsed electric fields from Chlorella vulgaris. Innov Food Sci Emerg Technol 29:15–22. https://doi.org/10.1016/j.ifset.2015.02.012
Carullo D, Abera BD, Casazza AA, Donsì F, Perego P, Ferrari G, Pataro G (2018) Effect of pulsed electric fields and high-pressure homogenization on the aqueous extraction of intracellular compounds from the microalgae Chlorella vulgaris. Algal Res 31:60–69. https://doi.org/10.1016/j.algal.2018.01.017
Costa JAV, Freitas BCB, Moraes L, Zaparoli M, Morais MG (2020) Progress in the physicochemical treatment of microalgae biomass for value-added product recovery. Bioresour Technol 301:122727. https://doi.org/10.1016/j.biortech.2019.122727
Tiwari ON, Mondal A, Bhunia B, Bandyopadhyay T, kanti, Jaladi P, Oinam G, Indrama T (2019) Purification, characterization and biotechnological potential of new exopolysaccharide polymers produced by cyanobacterium Anabaena sp. CCC 745. Polym (Guildf) 178. https://doi.org/10.1016/j.polymer.2019.121695
Cruz D, Vasconcelos V, Pierre G, Michaud P, Delattre C (2020) Exopolysaccharides from cyanobacteria: Strategies for bioprocess development. Appl Sci (Switzerland) 10:3763. https://doi.org/10.3390/app10113763
Elain A, NkounkouC, Donnart M & K Green extraction of polysaccharides from Arthrospira platensis using high pressure homogenization. https://doi.org/10.1007/s10811-020-02127-y/Published
Gurpilhares D, de Moreira B, Bueno TR, Cinelli JdaL, Mazzola LP, Pessoa PG, Sette A LD (2016) Algae’s sulfated polysaccharides modifications: Potential use of microbial enzymes. Process Biochem 51:989–998. https://doi.org/10.1016/j.procbio.2016.04.020
Courtois J (2009) Oligosaccharides from land plants and algae: production and applications in therapeutics and biotechnology. Curr Opin Microbiol 12:261–273. https://doi.org/10.1016/j.mib.2009.04.007
Kapoore RV, Butler TO, Pandhal J, Vaidyanathan S (2018) Microwave-assisted extraction for microalgae: from biofuels to biorefinery. Biology 7:18. https://doi.org/10.3390/biology7010018
Zhao G, Chen X, Wang L, Zhou S, Feng H, Chen WN, Lau R (2013) Ultrasound assisted extraction of carbohydrates from microalgae as feedstock for yeast fermentation. Bioresour Technol 128:337–344. https://doi.org/10.1016/j.biortech.2012.10.038
Martínez JM, Delso C, Álvarez I, Raso J (2020) Pulsed electric field-assisted extraction of valuable compounds from microorganisms. CRFSFS 19:530–552. https://doi.org/10.1111/1541-4337.12512
Liu Q, Yao C, Sun Y, Chen W, Tan H, Cao X, Xue S, Yin H (2019) Production and structural characterization of a new type of polysaccharide from nitrogen-limited Arthrospira platensis cultivated in outdoor industrial-scale open raceway ponds. Biotechnol Biofuels 12. https://doi.org/10.1186/s13068-019-1470-3
Pierre G, Zhao JM, Orvain F, Dupuy C, Klein GL, Graber M, Maugard T (2014) Seasonal dynamics of extracellular polymeric substances (EPS) in surface sediments of a diatom-dominated intertidal mudflat (Marennes-Oléron, France). J Sea Res 92:26–35. https://doi.org/10.1016/j.seares.2013.07.018
Tian X, Shen Z, Han Z, Zhou Y (2019) The effect of extracellular polymeric substances on exogenous highly toxic compounds in biological wastewater treatment: An overview. Bioresour Technol Rep 5:28–42. https://doi.org/10.1016/j.biteb.2018.11.009
Delattre C, Pierre G, Laroche C, Michaud P (2016) Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol Adv 34:1159–1179. https://doi.org/10.1016/j.biotechadv.2016.08.001
Cui R, Zhu F (2021) Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci Technol 107:491–508. https://doi.org/10.1016/j.tifs.2020.11.018
Xu Y, Zhang X, Yan XH, Zhang JL, Wang LY, Xue H, Jiang GC, Ma XT, Liu XJ (2019) Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int J Biol Macromol 135:706–716. https://doi.org/10.1016/j.ijbiomac.2019.05.166
Choi WY, Lee HY (2016) Effective production of bioenergy from marine Chlorella sp. by high-pressure homogenization. Biotechnol Biotechnol Equip 30:81–89. https://doi.org/10.1080/13102818.2015.1081407
Hernández-Corona A, Nieves I, Meckes M, Chamorro G, Barron BL (2002) Antiviral activity of Arthospira maxima against herpes simplex virus type 2. Antiviral Res 56:279–85. https://doi.org/10.1016/s0166-3542(02)00132-8
Ayehunie S, Belay A, Baba TW, Ruprecht RM (1998) Inhibition of HIV-1 replication by an aqueous extract of Arthospira platensis (Arthrospira platensis). J Acquir Immune Defic Syndr Hum Retrovirol 18:7–12. https://doi.org/10.1097/00042560-199805010-00002
Baba M, De Clercq E, Schols D, Pauwels R, Snoeck R, Boeckel V, Dedem G, Van, Kraaijeveld N, Hobbelen P, Ottenheijm H, Hollander F, Den (1990) Novel Sulfated Polysaccharides: Dissociation of Anti-Human Immunodeficiency Virus Activity from Antithrombin Activity. J Infect Dis 161:208–213. https://doi.org/10.1093/infdis/161.2.208
Yamaoka T, Tabata Y, Ikada Y (1993) Body distribution profile of polysaccharides after intravenous administration. Drug Deliv 1:75–82. https://doi.org/10.3109/10717549309031345
Ray B, Ali I, Jana S, Mukherjee S, Pal S, Ray S, Schütz M, Marschall M (2022) Antiviral strategies using natural source-derived sulfated polysaccharides in the light of the COVID-19 pandemic and major human pathogenic viruses. Viruses 14. https://doi.org/10.3390/v14010035
Acknowledgments
This study was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Contributions
MBFS drafted the manuscript. CMLLT reviewed the manuscript. All authors critically revised and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Luis Augusto Nero
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva, M.B.F., Teixeira, C.M.L.L. Cyanobacterial and microalgae polymers: antiviral activity and applications. Braz J Microbiol (2024). https://doi.org/10.1007/s42770-024-01452-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42770-024-01452-5