Abstract
Monitoring dynamics of airborne fungal species and controlling of harmful ones are of vital importance to conservation of cultural relics. However, the evaluation of air quality and the community structure characteristics of microorganisms, especially fungi, in the atmosphere of archives is in a stage of continuous exploration though more than 4,000 archives were constructed in China. Seventy-two air samples were collected in this study under different spatial and weather conditions from the archives of Kunming Medical University, located in the Kunming metropolitan area, Yunnan province, southwestern China. A total of 22 airborne fungal classes, 160 genera and 699 ASVs were identified, the species diversity is on the rise with the strengthening of air circulation with the outside space, and thus the intensive energy metabolism and activity were found in the spaces with window and sunny weather, except for the higher lipid synthesis of indoor samples than that of outdoor ones. Furthermore, there were significant differences in fungal community composition and abundance between sunny and rainy weathers. A considerable number of species have been identified as indicator in various environmental and weather conditions of the archives, and temperature and humidity were thought to have significant correlations with the abundance of these species. Meanwhile, Cladosporium and Alternaria were the dominant genera here, which may pose a threat to the health of archive professionals. Therefore, monitoring and controlling the growth of these fungal species is crucial for both conservation of paper records and health of archive professionals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fungi are the second largest biological group in the world after insects in terms of quantity, and they are ubiquitous in our living environment. The amount of fungi in the air reflects the quality of air of the region, and it is one of the biological indicators of indoor air quality [1]. Archives are an important source for recording history, and the protection of archives is an important approach to extend the life of archival materials and to achieve long-term preservation of historical materials. Paper records kept in archives can be easily damaged by chemical, physical, and biological factors. Paper degradation caused by fungi is a very important cause of damage of archival materials [2]; this is because their environment contains high concentrations of organic substances. Moreover, some fungal species involved in paper biodegradation may also pose a risk to library/archive professionals [3]. Microorganisms can enter the archive environment through air flow and humans. When these microorganisms settle on the surface of the paper, they form a micro-ecosystem to prevent the normal flow of air on the paper surface [4]. In this situation, the paper surface absorbs moisture from the air, which is conducive to microbial adhesion and subsequent biofilm formation [5]. Subsequently, at a certain level of humidity and temperature in the archive environment, the degradation of paper is accelerated through the growth of microorganisms such as fungi.
In the air of archive warehouses, the dominant fungal genera are Aspergillus, Penicillium, Chaetomium, Trichoderma, and Rhizopus [6]; Aspergillus species include A. fumigatus, A. niger, A. flavus, A. earthi, A. oryzae, and other species [3, 7]. By secreting enzymes, these fungi in the air decompose cellulose, lignin, starch, and other sugars in archival materials as soon as they colonize these materials, thereby converting them into acidic substances, which causing double harm to paper records [8, 9]. For example, Aspergillus has a high decomposition ability, and it can degrade cellulose, starch, and proteins, thus posing a high risk to paper and photo archives [10]. Previous studies have shown that Chaetomium sp., Fusarium sp., and Geotrichum sp. are specific pollutants present in the air of archive environments [11], and the first two species are involved in the biodegradation of paper [12]. Because these species can produce enzymes to degrade cellulose, they have a strong cellulose decomposition activity. Presently, many studies have investigated the impact of indoor air microorganisms on human health; however, only few studies have assessed the impact of air microorganisms on goods [13,14,15,16], particularly the impact of microorganisms in archive environment on paper archives. As environmental microorganisms could destroy various archives with historical significance [12], it is crucial to study the species and diversity of microorganisms in the indoor air of museums and archives to protect cultural heritage.
Generally, the humidity in the archive room should be regulated between 45% and 60% [17]. When the environmental humidity for archive preservation increases, on the one hand, its hygroscopicity also increases, which enhances the absorption of harmful gases in the environment, particularly acidic gases, and indirectly induces paper damage [18]; on the other hand, this situation also promotes the contact of oxygen and metal ions, resulting in oxidation reaction and thereby accelerating the aging of archival materials [17]. When the humidity of the storage environment exceeds 65%, it not only accelerates the reduction of paper strength but also induces fading of handwriting and matter printed with organic inks in the paper record. A high level of humidity in the archive environment also provides favorable conditions for the propagation and growth of various microorganisms and thus accelerates the invasion of paper records in archives. In contrast, at the low level of ambient humidity, the moisture on the archive paper will evaporate, and the moisture content will not reach the normal level; consequently, the internal structure of the paper will be damaged, which will make the paper hard and brittle [18].
Most researchers hold that many microbial communities overlap between indoor and outdoor environments; however, some researches have emphasized that the microbial community composition of indoor are different from those of outdoor microbial communities [19]. Shin et al. analyzed the internal transcribed spacer (ITS) readings of fungi in indoor and outdoor air; they found that the proportion of Basidiomycetes in outdoor fungi was 59.8%, but that in indoor fungi was 73.5%. Furthermore, the proportion of Ascomycetes in outdoor fungi was 35.1%, as compared to 23.5% in indoor fungi [20]. The differences in the composition of fungal communities in indoor and outdoor environments may be related to the differences in their relative humidity. Kotay et al. also confirmed that fungal growth is significantly positively correlated with higher indoor humidity, particularly in areas with water resources, such as kitchens and toilets; this is because humid conditions are conducive to establishing a stable microbial community [21]. Haas et al. reported that more airborne fungi were found in buildings with humidity issues than in standard buildings [22]. Bamba et al. investigated the impact of temperature and humidity on airborne fungi in houses with semi-basements surrounded by a natural forest and found that airborne fungal abundance was strongly correlated with the relative humidity level. A relative humidity level of higher than 70%, which is most suitable for fungal growth [23].
China has more than 4,000 archives of various kinds, with a collection of 208.86 million volumes and a construction area of more than 10 million square meters. The scale of urbanization and the process of industrialization in China have accelerated significantly. At the same time, the urban population is increasing, and air pollution is becoming more and more serious. The harmful gases and dust in the air are also becoming increasingly adversely affected by the materials made in the archives. At present, the evaluation of air quality in archive warehouses is in a stage of continuous exploration in China and even countries around the world, and it is urgent to study the air quality of archive warehouses when indoor air quality has become a major issue of global concern. Archivists are mainly concerned with the effects of humidity, temperature, harmful gases, light and dust on archival preservation, and relatively little research has been done on the community structure characteristics of microorganisms, especially fungi, in the atmosphere of archives. Kunming is a metropolitan city located in a low latitude (25 °N, 103 °E) and high altitude (1892 m) area in Yunnan, southwestern China. A four-season spring climate is formed by the effect of atmospheric circulation, geographical location, and altitude [24]. The average annual temperature is 15 °C; July has the highest average temperature of 19.7 °C, January has the lowest average temperature of 7.5 °C. The annual precipitation is 1035 mm, and the absolute humidity level is 74%. May to October is the rainy season, and the precipitation in this period accounts for approximately 85% of the whole year. The dry season is from November to April of the following year, and the precipitation in this period accounts for only approximately 15% of the whole year [25]. These climatic conditions are very suitable for microbial growth; hence, it is essential to understand the microbial taxa in archives, particularly the fungal taxa, which is crucial for microbial control and protection of the archive. The Kunming Medical University Archives keeps all kinds of archives, including party masses, administration, teaching, accounting, foreign affairs, scientific research projects, infrastructure, equipment, publications, audio and video records, physical objects, and literature of famous people, with a total of more than 40,000 volumes and more than 190,000 items. Presently, there are relatively few studies on the community structure characteristics of microorganisms in the environment of archives, and these studies have mainly concerned about the effects of humidity, harmful gases, light, dust and temperature on archive preservation; only few researchers have investigated the effect of aerial microorganisms on archives. Therefore, the present study used ITS sequencing (1) to analyze fungal diversity and community composition in the air in the Kunming Medical University Archives in different environmental conditions; (2) to examine the effects of weather conditions and spatial transformations on fungal communities and metabolite conversion in the context of diversity of microbial species within the microenvironment niche; (3) to identify indicator species in the archives in various environmental and weather conditions in order to evaluate the air quality in the archive warehouse. The results of this study will enhance our understanding of airborne fungal biodiversity in archives and provide new ideas and directions for archival preservation strategies.
Materials and methods
Sampling
The sampling place was located in the Kunming Medical University Archives. To determine the effect of ventilation and weather conditions on microbial diversity in the archives and the differences between microbial diversity inside and outside the archives, we sampled Room 114 with windows, Room 112 and Room 111 without windows, and the corridor outside Room 114. We chose a week when wet and dry seasons alternately occurred and collected samples on three sunny days (April 27, 28, and 29) and a rainy day (May 5). The area of Room 114 is approximately 200 m2, where paper archives are kept; we selected three sampling sites (A, B, and C) to collect aerial microorganisms. The areas of Room 112 and Room 111 are approximately 50 m2 each; physical archives are kept in Room 111, and a part of paper archives are kept in Room 112. We selected one sampling site (D and E) in each room to collect aerial microorganisms and chose one sampling site (F) in the corridor outside Room 114 for collecting outdoor aerial microorganisms. The samples were numbered according to the sampling time. The samples collected on April 27 were recorded as A1, B1, C1, D1, E1, and F1, the samples collected on April 28 were recorded as A2, B2, C2, D2, E2, and F2, and so on. Three parallel samples were collected for each sampling site and each sampling time for five min. Microbial aerosol samples were collected using the sampler (model: VWY.SAS SUPER100, air flow: 100 L/min) with a sterile filter membrane (glass fiber microporous filter membrane, pore size: 0.22 μm, diameter: 80 mm).
DNA extraction and PCR amplification of the microbiome
We selected the cetyltrimethylammonium Ammonium Bromide (CTAB) method to extract the DNA of samples from different sources. The quality of the extracted DNA was confirmed by Agarose Gel Electrophoresis, and the amount of the extracted DNA was quantified by an ultraviolet spectrophotometer. The ITS2 region of the fungal rRNA gene was amplified by PCR using the forward primer ITS1FI2 (5ʹ- GTGARTCATCGAATCTTTG-3ʹ) and the reverse primer ITS2 (5ʹ- TCTCCGCTTATTGC-3ʹ). Amplified in a total of 25 µL reaction volume, including 50 ng DNA template, 2.5 µL forward and reverse primers, 12.5 µL 12× main mixture, and 7.5 µL dd H2O. In the initial stage, the temperature was kept at 98 ℃ for 30 s to activate DNA polymerase and then maintained at 98 ℃ for 10 s to denature DNA. The reaction temperature was then reduced to 54 ℃ for 30 s and then increased to 72 ℃ for 45 s to extend DNA fragments. After 32 cycles, the temperature was kept at 72 ℃ for ten min. Then, the obtained products were stored in a refrigerator at 4 ℃. The PCR products were mixed in a certain proportion based on the sequencing quantity requirements of each sample.
Sequencing
We used the ITS sequencing method in this study. The obtained PCR products were confirmed with 2% agarose gel electrophoresis, and an AMPure XT bead recovery kit was used for recovery. The purified PCR products were evaluated with an Agilent 2100 bioanalyzer (Agilent, USA) and the library quantitative kit of Illumina (Kapa Biosciences, Woburn, MA, USA). After gradient dilution of all qualified online sequencing libraries, they were mixed based on the required sequencing number in a certain proportion; this was followed by denaturation of the libraries into a single chain through NaOH for online sequencing. A NovaSeq 6000 sequencer was used for 2 × 250 bp double-end sequencing, with the NovaSeq 6000 SP reagent kit (500 cycles) as the corresponding reagent.
Community diversity analysis
After high-throughput sequencing, PEAR (v0.9.6) [26] was used for sequence splicing before further analysis, fqtrim (v0.9.4) [27] was used to filter out low-quality sequences, Vsearch (v2.3.4) [28] was used to remove chimeric sequences, and qiime2 (v2019.7) [29] was used for denoising to obtain amplicon sequence variant (ASV) feature sequences, and singleton ASVs were removed.
The calculated results of the diversity index and the abundance index based on ASV indicated the bacterial community of each sample. The alpha diversity comprised the Chao1 index, Shannon and Simpson indices, Good’s coverage index, and rarefaction curve. The Chao1 index was used to assess the number of ASVs and species abundance in the samples (the higher the Chao1 index, the greater was the species richness). Shannon and Simpson indices revealed the microbial diversity of each sample (the higher the Shannon and Simpson indices, the higher was the community diversity). Good’s coverage index represented the adequacy of the sequencing data (the larger the value, the more sufficient was the sequencing data). The rarefaction curves were used to indicate the sequencing quality and the species richness in the samples. For beta diversity, principal coordinate analysis (PCoA) was used to determine the relationship between the microbial composition of samples. Linear discriminant analysis (LDA) combined with effect size (LEfSe) analysis was used to distinguish taxa with significant differences between groups. Redundancy analysis (RDA) was used to evaluate the relationship between microbial community structure and temperature and relative humidity factors in the environment. The RDP and unite database [30, 31] were used to annotate the species with the confidence threshold was 0.7, and the species abundance in each sample was counted based on the ASV (feature) table. In order to obtain information on the function components of the community, we conducted PICRUSt2 [32] functional prediction analysis based on the feature sequences of fungal samples, using KEGG and COG databases [33, 34]. All data were analyzed on the Lianchuan Biological Cloud Platform (https://www.omicstudio.cn/tool.).
Differences in grouping
To compare the effects of weather, humidity, ventilation, and other environmental conditions on the microbial community in the archives, we divided the samples into four groups: Type I: the samples were divided into two groups according to whether the room was windowed (W) and windowless (NW); Type II: the samples were divided into two groups based on whether they were collected in indoor (Indoor) and in the corridor (Outdoor); Type III: the samples were divided into sunny (Sunny) and rainy (Rainy) groups according to weather conditions; and Type IV: the samples were classified into four groups by combining weather and ventilation conditions: indoor sunny (IS), outdoor sunny (OS), indoor rainy (IR), and outdoor rainy (OR). We analyzed the microbial community composition and diversity differences among these different groups.
Results
Sequencing quality
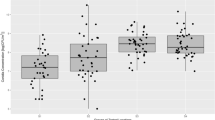
The rarefaction curves showed that when the number of sequencing bands approached 20,000, the curves tended to be stable (Fig. 1). The Chao1 index did not increase with the increase in sequencing numbers, which suggested that the sequencing data were deep enough to represent the information of most species in the samples.
Community composition and diversity analysis
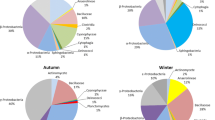
A total of 72 samples were collected, of which seven samples had very low readings; hence, they were removed from the downstream analysis. Finally, a total of 5,280,359 effective sequences of the fungal ITS rRNA gene were obtained, and these sequences were clustered into 699 fungal ASVs with 100% sequence similarity. After comparison with the RDP database [31], a total of six phyla, 22 classes, and 160 genera of fungal communities were identified. Ascomycota (77.5%) was the most abundant phyla, while the other phyla included Basidiomycota (20.4%), Zygomycota (1.5%), Olpidiomycota (0.1%) and Chytridiomycota (0.1%). As shown in Fig. 2., the five classes with higher relative abundance were Dothieomycetes (46.3%), Ustilaginomycotina (12.1%), Sordoniomycetes (10.8%), Euronomycetes (26.6%), followed by Malassezia (13.5%), Alternaria (4.2%), Paraphoma (3.7%) and Candida (3.2%). A total of 218 ASVs were not classified into specific genera.
A series of indices were calculated and compared to show the diversity of fungi in air samples (Table 1), including the diversity index (Shannon/Simpson), the richness index (Chao1), and the sample coverage index (Good’s coverage). As shown in Table 1, the value of Good’s coverage index for each sample was 1, indicating that the quantity of sequencing was big enough to analyze the diversity of fungal communities. Group OR had the richest diversity, because its Shannon index (2.53) was the highest, while group IR had the lowest Shannon index (1.49) and the lowest diversity. Fungal diversity in the room with windows (group W) was slightly higher than that in the room without windows (group NW). Group Indoor showed lower diversity than group Outdoor. Furthermore, fungal diversity in sunny weather (group Sunny) was higher than that in rainy weather (group Rainy). The fungal diversity performed a gradually increasing trend from an indoor windowless area to an indoor windowed area to an outdoor area.
Although group W and group NW had 260 and 130 unique ASVs, respectively, principal coordinate analysis (PCoA) did not clearly separate the two groups (Fig. 3.), and the results showed no significant difference between the two groups (p = 0.311, ANOSIM). PCoA also showed that the fungal diversity in group Indoor was lower than that in group Outdoor; however, the differences between the two groups were not significant (p = 0.393, ANOSIM). At the same time, the results of PCoA showed a significant difference between the sunny and rainy weather groups (p = 0.014, ANOSIM). Fungal diversity was highly similar between group IS and group OS. However, a marginally significant difference (p = 0.06, ANOSIM) in diversity was observed between group OR and group IR. All these results were driven by the Jaccard distance.
Principal coordinate analysis (PCoA) of fungal communities for four types of grouping based on Jaccard distance. (a) windowless room (NW) and windowed room (W) samples; (b) indoor and outdoor samples; (c) sunny and rainy samples; (d) indoor rainy (IR), outdoor rainy (OR), indoor sunny (IS), and outdoor sunny (OS) samples
The shared and unique genera in different environmental conditions
The shared and unique microbiota between each subgroup were analyzed at the ASV level (Fig. 4.). In Type I grouping, the samples from the windowed and windowless rooms shared 235 ASVs, with 260 unique microbial ASVs in the windowed group, which was twice that in the windowless group. In type II grouping, the indoor and corridor samples shared 235 ASVs, with 424 unique microbial ASVs in the indoor samples and 74 in the outdoor samples. In type III grouping, the sunny and rainy samples shared 193 ASVs, with 407 and 99 unique ASVs detected in sunny and rainy samples, respectively. In type IV grouping, the samples of groups IS and OS shared 153 ASVs, with 292 and 67 ASVs specific to groups IS and OS, respectively. The ASVs shared by groups IR and OR were 45, and the number of ASVs specific to group IR was 130, which was approximately twice that of group OR (68).
The unique genera collected in the windowed room were Leptospora, Rhodotorula, Ampelomyces, Khuskia, Valsa, and Erysiphe, while the unique genera of the windowless rooms were Candida, Bjerkandera, Phialophora, Hypoxylon, and Mycosphaerell. The same genera detected in group W and group NW were Cladosporium/Davidiella, Malassezia, Alternaria, Cryptococcus, Aspergillus, Acremonium, Golovinomyces, and Phoma. Compared to the outdoor corridor, the endemic genera collected in inside the archive rooms were Paraphoma, Ampelomyces, Aspergillus, Bjerkandera, Khuskia, Acremonium, Phialophora, Hypoxylon, Mycosphaerella, Valsa, Phoma, and Erysiphe, while those of the corridor genera detected in group Indoor and group Outdoor were Davidiella/Cladosporium, Malassezia, Alternaria, Candida, Cryptococcus, Kabatiella, and Rhodotorula.
Regarding weather conditions, the unique genera in sunny weather were Alternaria, Cryptococcus, Ampelomyces, Phialophora, Kabatiella, Lecanicillium, Hypoxylon, Valsa, Phoma, and Erysiphe, while those of the rainy weather were Paraphoma and Hypocrea. The same genera detected in these two groups were Davidiella/Cladosporium, Malassezia, Candida, Acremonium, and Rhodotorula.
The analysis of the combination of ventilation and weather conditions showed that the unique genera of group IS were Acremonium, Khuskia, Trichosporon, Didymella, and Erysiphe, while the unique genera of Group OS were Fusarium, Calycina, and Schizophyllum. The shared genera between the two groups included Malassezia, Davidiella/Cladosporium, Ampelomyces, Cryptococcus, Lecanicillium, Valsa, Alternaria, Kabatiella, Rhodotorula, Aspergillus, Candida, and Trametes. Compared to group OR, the unique genera of group IR were Alternaria, Golovinomyces, Cryptococcus, and Valsa, and the unique genera of group OR were Ampelomyces, Khuskia, and Acremonium. The shared genera between these two groups were Hypocrea, Didymella, Schizophyllum, Malassezia, Leptospora, Davidiella/Cladosporium, Lecanicillium, Candida, Aspergillus, and Trametes.
Overall, the shared genera under all these environmental conditions were Davidiella/Cladosporium, Malassezia, Alternaria, Candida, Aspergillus, Lecanicillium, and Mycosphaerella. In the group Indoor, the eight genera with higher relative abundance were Davidiella/Cladosporium, Malassezia, Alternaria, Paraphoma, Cryptococcus, Candida, and Ampelomyces (Fig. 5). However, in the group Outdoor, the eight genera with higher relative abundance were Trametes, Hypocrea, Malassezia, Davidiella/Cladosporium, Didymella, Candida, Schizophyllum, and Kabatiella. From indoor to outdoor areas, the dominant genera have shifted from Davidiella/Cladosporium, Malassezia, and Alternaria to Trameters, Hypocrea, and Malassezia, while Trameters and Hypocrea were almost non-existent in the indoor environment. In the group Indoor, Paraphoma, Ampelomys, Bjerkandera, Valsa, Khuskia, Philophora, Hypoxylon, Acremonium, Phoma, and Erysiphe were almost non-existent in the outdoor environment. This result indicated a significant difference in microbial composition between indoor and outdoor environments. From the weather conditions, the eight genera with higher relative abundance in sunny samples were Davidiella/Cladosporium, Malassezia, Alternaria, Cryptococcus, Ampelomyces, Candida, Khuskia, and Trametes. The eight genera with higher relative abundance in rainy samples were Davidiella/Cladosporium, Paraphoma, Hypocrea, Bjerkandera, Candida, Didymella, Malassezia, and Mycospaerella. The genus with the highest relative abundance in these two groups was Davidiella/Cladosporium, however, Cryptococcus, Trametes, Hypoxylon, Khuskia, Valsa, Kabatiella, Ampelomyces, Rhodotorula, and Erysiphe in the group Sunny almost did not exist in the group Rainy. This result indicated that there has been a significant change in the composition of microbial communities in the air from sunny to rainy weather.
Comparison of fungal communities in samples in different groups
The number of dominant fungal genera (Cladosporium/Davidiella (26.03%), Malassezia (11.55%), and Alternaria (3.79%)) was higher in the indoor environment (group Indoor) than in the corridor (group Outdoor), which instead showed a higher presence of Trametes (8.20%) and Hypocrea (8.19%). The archives room with windows (group W) showed a higher number of Cladosporium/Davidiella (24.09%), Malassezia (15.04%), and Ampelomyces (3.54%) than the windowless rooms (group NW), which instead exhibited a higher presence of Cladosporium/Davidiella (29.31%), Alternaria (7.66%), and Paraphoma (7.14%). In sunny days (group Sunny), we collected the highest number of Cladosporium/Davidiella (21.74%), Malassezia (12.85%), and Alternaria (4.21%), while in rainy days (group Rainy), we found that the dominant genera were Cladosporium/Davidiella (25.45%), Paraphoma (9.39%) and Hypocrea (6.15%). The dominant genera of group IS were Malassezia (18.50%) and Davidiella/Cladosporium (16.44%), while group OS were Trametes (10.93%), Malassezia (8.52%), and Davidiella/Cladosporium (10.26%). The dominant genera of group IR were Davidiella/Cladosporium (48.17%) and Leptospora (5.63%), while those of group OR were Hypocrea (32.74%), Didymella (21.26%), and Schizophyllum (5.29%). At the same time, we found that the relative abundance of Cladosporium/Davidiella in the group IR was significantly higher than that in the group OR. However, in group OR, Cladosporium/Davidiella was almost nonexistent, while Hypocrea and Didymella were significantly increased (Fig. 5e).
As mentioned earlier, PCoA was used to establish beta diversity differences between the groups. Similarly, Linear discriminant analysis (LDA) combined with effect size (LEfSe) analysis was used to distinguish taxa with significant differences between groups W and NW, Indoor and Outdoor, and Rainy and Sunny as biomarkers for each group (Fig. 6).
As shown in Figs. 4 and 20 ASVs were significantly different between groups W and NW, with 10 ASVs in each group. At the species level, Cladosporium haloolerans, Ampelomyces sp. LK 2010, Stemphylium solani, Aspergillus penicillioides, and Phoma bellidis were significantly enriched in group W, while Bjerkandera adusta was enriched in group NW. We found significant differences in 37 ASVs between groups Indoor and Outdoor, wherein Massarina corticola, Wallemia ichthyophaga, Mycocalicium victoriae, Acrophialophora fusispora, Malassezia slooffiae, Udeniomyces megalosporus, Pyrenochaeta gentianicola, Phaeosphaeria spartinicola, Chaetomium brasiliense, and Trametes versicolor were more abundant in group Outdoor than group Indoor. At the species level, there were no significantly enriched species in the group Indoor, but at the family level, there were three families that were significantly enriched: Mycosphaerellaceae, Saccharomycetaceae and Erysiphaceae. A total of 56 ASVs were significantly different between groups Rainy and Sunny, 45 and 11 fungal groups were significantly enriched, respectively. At the species level, Golovinomyces asterum, Schizophyllum commune, Engyodontium album, Eutypella caricae, Acremonium brachypenium, Fusicolla acetilerea, Aspergillus penicillioides, Phlebia livida subsp tuberculata, Leptosphaeria microscopica, Botryosphaeria dothidea, Spiromastix warcupii, Podosphaera leucotricha, and Plectosphaerella cucumerina were significantly enriched in rainy day samples. In contrast, Candida membranifaciens and Fusarium asiaticum were significantly enriched in sunny samples.
Effects of temperature and relative humidity on fungal community structures
We conducted redundancy analysis (RDA) to evaluate the relationship between microbial community structure and temperature and relative humidity factors in the environment (Fig. 7). The results showed that the first and second axes accounted for 24.75% of the variance in fungal communities. The correlation between environmental temperature and the fungal community was highly significant (r² = 0.6448, p = 0.005). The correlation between RH and the fungal community was significant (r² = 0.5465, p = 0.016) too. Aspergillus and Davidiella/Cladosporium showed a positive correlation with RH and a negative correlation with temperature. In contrast, Alternaria, Malassezia, and Cryptococcus showed a negative correlation with RH and a positive correlation with temperature. Based on the collected samples, the fungal community was sensitive to humidity, which is indicated by the closer vertical distance between the samples collected on rainy days (A4, B4, and C4) and the RH arrow.
Redundancy analysis (RDA) highlighting correlations among the top ten fungal genera and the relative humidity (RH) and temperature (T). A, B, and C represent three different sampling sites in Room 114, with the numbers 1, 2, and 3 following A, B, and C representing sunny weather and 4 representing rainy weather
Metabolic pathway prediction based on feature sequences of fungal samples
We found 10 pathways that were significantly differentially abundant between the windowed and windowless room samples. The most abundant metabolic pathways estimated in these two groups of samples were those related to the ornithine cycle (including urea cycle, coenzyme A biosynthesis I, TCA cycle I, superpathway of guanosine nucleotides de novo biosynthesis I, II, guanosine nucleotides degradation III, and guanosine ribonucleotides de novo biosynthesis). Except for guanosine nucleotides degradation III, the abundance of 9 other metabolic pathways in the windowed room was higher than that in the windowless room (Fig. 8a). The pathway with the greatest difference was TCA cycle I, with the abundance of the group W almost twice that of the group NW.
There are 8 pathways that were significantly differentially abundant between the sunny and rainy samples. The most abundant metabolic pathways estimated in these two groups of samples were those related to nucleotide synthesis (including adenosine deoxyribonucleotides de novo biosynthesis II, guanosine deoxyribonucleotides de novo biosynthesis II, and superpathway of guanosine nucleotides de novo biosynthesis I). Except for 1,3-propanediol biosynthesis and galactose degradation I, the abundance of 6 other pathways including sulfate reduction I and TCA cycle I was higher in group Sunny than in group Rainy (Fig. 8b). The pathway with the biggest difference was TCA cycle I, and the abundance of the group Sunny was three times that of the group Rainy.
We found 22 pathways that were significantly differentially abundant between the indoor and outdoor samples. The most abundant metabolic pathways estimated in these two groups of samples were those related to carbohydrate metabolism (including glycolysis III, L-rhamnose degradation III, subcross degradation III, start degradation V, and glucose and glucose 1-phosphate degradation) and lipid metabolism (including fatty acid elongation, CDP − diacylglycerol biosynthesis I, II, and phosphatidylglycerol biosynthesis). Other pathways included pathways related to energy metabolism (including aerial respiration I, and pentose phase pathway) and pathways related to metabolism of co factors (including pantothenate and coenzyme A biosynthesis I). Among all 22 pathways with significant differences, only glycolysis III, airborne respiration I, pentose phase pathway, and CDP - diacylglycerol biosynthesis I, II had a higher abundance in group Outdoor than in group Indoor, while the 16 other pathways had a higher abundance in group Indoor (Fig. 8c). In the outdoor samples, the top three pathways of relative abundance were aerobic respiration I, pentose phosphate pathway, and glycolysis III, and the abundance of these three pathways was higher than that of the indoor group; while in the indoor samples, the top three pathways of relative abundance were aerobic respiration I, fat acid elongation, and pentose phosphate pathway. Among the pathways with higher abundance, the most significant difference is in fatty acid elongation, with the abundance of the indoor group being twice that of the outdoor group.
Discussion
Diversity, community composition, and dominant groups in different environments
This study used the ITS gene sequencing method to analyze the microbial communities in different environments of the Kunming Medical University Archives. Fungal community composition, alpha and beta diversities, and environmental factors were analyzed. Based on these analyzes, certain differences were observed between the dominant groups of aerial fungi under different environmental conditions.
In the diversity analysis, the Shannon, Simpson, and Chao1 indices of group W were higher than those of group NW; however, these differences were not statistically significant. Furthermore, the fungal diversity of group W was generally higher than that of group NW. The dominant genera of group W were Cladosporium/Davidiella and Malassezia, and the genus with the lowest abundance was Paraphoma. In contrast to group W, the dominant genera in group NW were Cladosporium/Davidiella, Alternaria, and Paraphoma, and the genus with the lowest abundance was Kabatiella. Malassezia is a symbiotic fungus commonly found on the skin of humans and animals [35]; this fungus was a dominant genus in group W and group Indoor, but not in group NW and group Outdoor. Based on this finding, it is speculated that Malassezia was more dominant in indoor environments than outdoor environments, and that the windowed room has more air exchange with the outdoor environment than the windowless room, which resulting in the dilution of Malassezia in the windowed room.
For indoor and outdoor samples, the Chao1 index of the latter group was lower than that of the former group; this finding indicated that the number of species in group Indoor was greater than that in group Outdoor. However, the Shannon index and Simpson index of group Outdoor were higher than those of group Indoor; this result indicated that the microbial diversity of group Outdoor was higher than that of group Indoor. This also indicated that the evenness of indoor samples was higher than that of outdoor samples because biodiversity represents the combination of the number and evenness of species in the habitat. Although some studies have shown that the microbial communities in indoor and outdoor air are highly similar [36,37,38], our study did not exhibit the same result. We considered that there were some differences in the composition of microbial communities between indoor and outdoor air although the differences were not statistically significant; this finding was same to the results of Fang et al [39]. In group Indoor, the dominant genera were Cladosporium/Davidiella, Malassezia, and Alternaria, and the genus with the lowest abundance was Schizophyllum. In group Outdoor, the dominant genera were Trametes, Hypocrea, and Malassezia, and the genus with the lowest abundance was Mycosphaerella.
For sunny and rainy weather samples, the Shannon and Simpson indices of the sunny weather samples were higher than those of the rainy weather samples; this finding showed that the microbial diversity of the rainy weather samples was lower than that of the sunny weather samples. However, the Chao1 index of the sunny weather samples was lower than that of the rainy weather samples. This finding indicated that the number of species in the sunny weather samples was lower than that in the rainy weather samples; however, the species evenness of the sunny weather samples was higher than that of the rainy weather samples. In the sunny weather samples, the dominant genera were Cladosporium/Davidiella, Malassezia, and Alternaria, while the genus with the lowest abundance was Candida. However, in the rainy weather samples, the dominant genera were Cladosporium/Davidiella, Paraphoma, and Hypocrea, and the genus with the lowest abundance was Valsa. The reason for this difference may be that the increase in RH in the air inhibits the growth of Malassezia and Alternaria; this is because our RDA result showed that the abundance of these two genera was inversely proportional to RH.
Analysis of the functional characteristics of the dominant species
To identify the significantly different taxa occurring in different environments, we conducted LEfSe analysis to find biomarkers that enriched in each group. Cladosporium, Ampelomyces, and Pleospora were enriched in group W, while Bjerkandera and Tubeufiales were enriched in group NW. Mycosphaerellaceae, Saccharomycetaceae, and Erysiphaceae were more abundant in group Indoor, while Saccharomyces, Taphrinales, and Acrophialophora were more abundant in group Outdoor. Basidiomycota, Malasseziales, and Malassezia were enriched in group Sunny, while Ascomycota, Hypocrea, and Hypocreaceae were enriched in group Rainy. However, the specificity of all these species were not high, although the LDA value was > 3 and the P-value was 0.05.
These results revealed that Cladosporium was the dominant genus in all samples except the outdoor samples, Alternaria was another dominant genus among multiple groups of samples, and many previous studies showed similar results to the present study. Fu et al. conducted research on aerial fungi in parks, shopping malls, factory areas, and agricultural markets in four major cities in China; they found that the dominant genera were Cladosporium and Alternaria [40]. Fang et al. studied the distribution and community structure of summer air microbes in Beijing; their findings indicated that Cladosporium was the dominant fungus, which accounted for 47.2% of the total species [41]. Chang et al. found Cladosporium as the dominant fungus in their investigation of microorganisms in the air of open-air pig houses [42]. Shelton et al. also found Cladosporium and Penicillium as the dominant groups in their study of the fungi in indoor and outdoor air in the United States [43]. Cladosporium is a common indoor and outdoor mold [44], which is commonly found in decaying organic materials, soil, food, and textiles [45]. Some species of Cladosporium have been proven to have strong lignin and cellulose degradation ability [46]; thus, these fungal species pose a great risk of damage to paper archives. Alternaria is also a common aerial fungal genus, and its spores are commonly found in soil, household dust, and indoor air of buildings [47]. Alternaria can be found in any natural environments with the abundance of cellulose as these fungi feed on cellulose and cause a variety of plant diseases [48] and respiratory tract infections and allergies [49]. In a cellulose liquid medium, Alternaria can produce enhanced carboxymethyl cellulase [50]. Robl et al. confirmed that Alternaria, Aspergillus, and Trichoderma isolated from Brazilian metamorphic books are excellent producers of cellulose hydrolases [51]; therefore, we should also consider Alternaria when discussing the protection of paper archives. In addition, Aspergillus is also a target that should be focused. Although Aspergillus was not considered a dominant fungus in our present study, it deserves our attention because of its inherent characteristics. Aspergillus is a well-known fungal genus that can produce various enzymes (such as cellulase, amylase, protease, and lipase) [52,53,54,55], particularly Aspergillus niger, which has been widely studied by many scholars and can produce various compounds such as amylase, acid protease, cellulase, and pectinase [56]. This species not only has strong cellulose decomposition ability, but it also produces black brown hyphae and spores on paper materials [57], thereby causing multiple damages to paper materials. The above-mentioned three genera have been shown to cause allergic reactions and have the possibility to produce volatile toxic chemical compounds into the archive environment, which may pose a health risk to archive professionals [58].
Effect of temperature and humidity on fungal communities and suggestions for archive protection
The cooperation between paper protectors and microbiologists is a good way for managing the biodegradation of paper records, as it can provide feasible and advanced methods for protectors to evaluate the risks of microbial spoilage. Environmental factor analysis can describe the effect of environmental factors on the microbial community in the archive rooms. The availability of water is considered an important factor that determines the colonization of microorganisms on paper surfaces. As is known to all, paper is a material that can absorb moisture, and its water activity is related to microclimate parameters such as RH and temperature [2]. Therefore, protection strategies should include the evaluation of environmental microclimate parameters. Borrego et al. analyzed the relationship between microbial concentration and the T and RH (temperature and relative humidity) values; they found significant differences between the Argentine and Cuban archives. In Argentine archives, T and RH are suitable for document preservation, but the climatic conditions of the Cuban National Archives are higher than the optimal preservation conditions; hence, the concentration of microorganisms in the air is higher than that of most Argentine archives [4]. In addition to T and RH, air flow, human activities, and pH can also affect microbial community diversity. The interior surface of a well ventilated space usually contains microbial composition more similar to that of outdoor air [38]. High pedestrian flow can affect the internal microclimate of the indoor environment and cause the re-suspension of microorganisms present on the surface of objects [59]. When the humidity in the air increases, more water will combine with acidic gases in the air to produce acidic substances, causing a decrease in pH and affecting the growth of microorganisms.
For several fungi mentioned earlier that pose a significant threat to paper records, we found that humidity seems to have a greater impact on their growth compared to temperature. If the RH is below 80%, it is not conducive to the growth of Cladosporium/Davidiella, and the optimal temperature range for its growth is wide and ranges from 9 ℃ to 34 ℃. Alternaria is a cold-resistant fungus that remains dormant during prolonged drying periods and quickly reappears once the water source becomes available again. The optimal temperature for Aspergillus is approximately 28 ℃, and the minimum RH is 88%. The RDA results also show a strong positive correlation between Aspergillus and Cladosporium/Davidiella and RH. Alternaria, Malassezia, and Cryptococcus are pathogenic fungi and are related to human health. The RDA results showed that they were positively correlated with temperature, and their optimum growth temperature is about 25–37 ℃. To slow down the biodegradation of paper records and protect the health of archive professionals, we need to control relative humidity and temperature in the archive rooms to create an environment that is not conducive to fungal growth and reproduction. We suggest setting the maximum suitable temperature at 21 °C and the optimal RH at 30–50%, as higher temperature and humidity can increase microbial activity, while lower humidity can lead to drying and embrittlement of paper materials [60]. In addition, we can also use some mildewproof agents such as p-nitrophenol to inhibit the proliferation of mold and other microorganisms. The NMF-1 mildewproof agent developed in recent years is one of them. The drug is highly volatile, gas-phase insecticidal and mildewproof, and can be used safely without direct contact with paper archives [61].
The role of microbial metabolic function
The role of air microbial metabolic function has been rarely studied and reported. Our study found that the abundance of TCA cycle I in the groups W and Sunny was twice and three times higher than that in the NW and Rainy groups, respectively. This indicates that the microbial energy metabolism and microbial activity in the groups W and Sunny were higher than those in the groups NW and Rainy, and this may be the reason why the diversity of samples in groups W and Sunny was higher than that in the groups NW and Rainy. Thus, we speculated that sunny weather and well ventilated environments may be more suitable for microbial growth than rainy weather and enclosed environments. For the preservation of archive records, we cannot control the weather conditions, therefore, we hold that it is possible to reduce the opening of the archives’ windows to reduce air exchange and thus reduce the impact of microbial activities on archival materials. The relative abundance of the top five pathways in the outdoor samples was higher than that in the indoor samples, with three pathways (aerobic respiration I, pentose phosphate pathway, and glycolysis III) related to energy metabolism and the other two (CDP − diacylglycerol biosynthesis I, II) related to biofilm synthesis; this result indicated that the microbial metabolism in the outdoor samples was more vigorous, which is consistent with our previous finding that the diversity of the outdoor samples was higher than that of the indoor samples. The most significant different pathway between groups Indoor and Outdoor was atty acid elongation, which was almost twice as abundant in the indoor group as in the outdoor group. This result indicated that lipid synthesis of the indoor samples was significantly more vigorous than that of the outdoor samples, which may be related to the high abundance of Malassezia in the indoor samples. The lipase in Malassezia can decompose triglycerides and produce saturated fatty acids.
Conclusion
In summary, this study revealed the fungal community structure and diversity in different environmental conditions in the archives of Kunming Medical University by using ITS sequencing. Overall, the aerial fungal diversity of windowless rooms was lower than that of windowed rooms, while the fungal diversity of the windowed rooms was lower than that of the outdoor environment; the species diversity in sunny weather was higher than that in rainy weather. We linked the investigated fungal communities with environmental microclimate parameters (temperature and relative humidity), and the result showed a strong correlation between fungal communities and temperature and RH. Cladosporium and Alternaria were the dominant genera in most of the environments we sampled, and they are also the main fungi that degrade paper materials in archives. Therefore, to restrain their growth and protect paper materials, we recommend controlling the temperature in archives at around 21 ℃ and RH at 30–50%, and we also can use some anti mold agents that do not harm the paper records.
References
Cabral JP (2010) Can we use indoor fungi as bioindicators of indoor air quality? Historical perspectives and open questions. Sci Total Environ 408:4285–4295
Cappitelli F, Pasquariello G, Tarsitani G, Sorlini C (2010) Scripta manent? Assessing microbial risk to paper heritage. Trends Microbiol 18:538–542
Tang H, Jiang J, Fan WQ, Wang C (2015) Investigation of air microorganism contamination in museum storeroom. Occup Health 31:2088–2092
Borrego S, Lavin P, Perdomo I, Gomez de Saravia S, Guiamet P (2012) Determination of indoor air quality in archives and biodeterioration of the documentary heritage. ISRN Microbiol 2012: 680598
Guiamet P, Borrego S, Lavin P, Perdomo I, de Saravia SG (2011) Biofouling and biodeterioration in materials stored at the historical archive of the Museum of La Plata, Argentine and at the National Archive of the Republic of Cuba. Colloids Surf B 85:229–234
Ren SS (2018) Research progress on microbial species in domestic libraries, archives, and museums. Res Prot Anc Books 172–178
Grinshpun SA, Reponen TWilleke K (1997) Aerosol characteristics of airborne actinomycetes and fungi. J Aerosol Sci 28:667–668
Duan DC (2017) The Study on the protection of paper relics by bacterial cellulose and chitinous; Master thesis, Liaoning University, Shenyang, China
Borrego S, Guiamet P, Gómez de Saravia S, Batistini P, Garcia M, Lavin P, Perdomo I (2010) The quality of air at archives and the biodeterioration of photographs. Int Biodeterior Biodegrad 64:139–145
Li GH, Song SL, Li XZ, Jiang JX, Cao BL (2014) Detrimental Fungi types in Paper Files in Jiangxi Province. Biol Disaster Sci 37:301–304
Silva M, Moraes A, Nishikawa MM, Gatti M, Alencar M, BrandO LE, Nóbrega A (2006) Inactivation of fungi from deteriorated paper materials by radiation. Int Biodeterior Biodegrad 57:163–167
Pinzari F, De Mico GPA (2006) Biodeterioration of paper: a SEM study of fungal spoilage reproduced under controlled conditions. Macromol Symp 238:57–66
Kumar P, Singh AB, Singh R (2022) Comprehensive health risk assessment of microbial indoor air quality in microenvironments. PLoS ONE 17:e0264226
Estensmo ELF, Smebye Botnen S, Maurice S, Martin-Sanchez PM, Morgado L, Bjorvand Engh I, Hoiland K, Skrede I, Kauserud H (2022) The indoor mycobiomes of Daycare centers are affected by occupancy and climate. Appl Environ Microbiol 88:e0211321
Felgueiras F, Mourao Z, Oliveira Fernandes E, Gabriel MF (2022) Airborne bacterial and fungal concentrations and fungal diversity in bedrooms of infant twins under 1 year of age living in Porto. Environ Res 206:112568
An XL, Xu JX, Xu MR, Zhao CX, Li H, Zhu YG, Su JQ (2023) Dynamics of Microbial Community and potential Microbial pollutants in Shopping malls. mSystems 8:e0057622
Wang DQ (2020) How to protect paper archives in archives. Inside Outside Lantai 81–82
Ma MS (2020) Research on the preservation and methods of Paper archives in Public Security organs. Office Oper 117–119
Rai S, Singh DK, Kumar A (2021) Microbial, environmental and anthropogenic factors influencing the indoor microbiome of the built environment. J Basic Microbiol 61:267–292
Shin SK, Kim J, Ha SM, Oh HS, Chun J, Sohn J, Yi H (2015) Metagenomic insights into the bioaerosols in the indoor and outdoor environments of childcare facilities. PLoS ONE 10:e0126960
Kotay S, Chai W, Guilford W, Barry K, Mathers AJ (2017) Spread from the Sink to the patient: in situ study using Green fluorescent protein (GFP)-Expressing Escherichia coli to Model bacterial dispersion from hand-washing Sink-Trap reservoirs. Appl Environ Microbiol 83:e0332716
Haas D, Habib J, Galler H, Buzina W, Schlacher R, Marth E, Reinthaler FF (2007) Assessment of indoor air in Austrian apartments with and without visible mold growth. Atmos Environ 41:5192–5201
Bamba I, Azuma M, Hamada N, Kubo H, Isoda N (2014) Case study of airborne fungi according to air temperature and relative humidity in houses with semi-basements adjacent to a forested hillside. Biocontrol Sci 19:1–9
Peng N, Zhou H, Li JL, Wang YM, Li YX (2015) Study on the variation characteristic and future Trend of Urbanization Cimate in Kunming in nearly fifty years. J Anhui Agric Sci 43:171–174
Zhuang YF, Xie KM, He YZ (2017) A study on the adaptability of the climate in Kunming region to the development of olive oil. Agric Technol 37:152–153
Zhang J, Kobert K, Flouri T, Stamatakis A (2014) PEAR: a fast and accurate Illumina paired-end reAd mergeR. Bioinformatics 30:614–620
Pertea G (2015) fqtrim: v0.9.4 release
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857
Nilsson RH, Larsson K-H, Taylor AFS, Bengtsson-Palme J, Jeppesen TS, Schigel D, Kennedy P, Picard K, Glöckner FO, Tedersoo L, Saar I, Kõljalg U, Abarenkov K (2019) The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res 47:D259–D264
Maidak BL, Olsen GJ, Larsen N, Overbeek R, McCaughey MJ, Woese CR (1997) The RDP (ribosomal database project). Nucleic Acids Res 25:109–110
Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, Huttenhower C, Langille MGI (2020) PICRUSt2 for prediction of metagenome functions. Nat Biotechnol 38:685–688
Kanehisa M (2000) KEGG: Kyoto Encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Galperin MY, Wolf YI, Makarova KS, Vera Alvarez R, Landsman D, Koonin EV (2021) COG database update: focus on microbial diversity, model organisms, and widespread pathogens. Nucleic Acids Res 49:D274–D281
Chueachavalit C, Meephansan J, Payungporn S, Sawaswong V, Chanchaem P, Wongpiyabovorn J, Thio HB (2022) Comparison of Malassezia spp. colonization between human skin exposed to high- and low-ambient air pollution. Exp Dermatol 31:1454–1461
Ye J (2021) Study on Residential Microbial Communities in Nanjing Based on High-Throughput Sequencing and Culturing Methods; Doctoral dissertation, Southeast University, NanJing, China
Ziaee A, Zia M, Goli M (2018) Identification of saprophytic and allergenic fungi in indoor and outdoor environments. Environ Monit Assess 190:574
Adams RI, Miletto M, Taylor JW, Bruns TD (2013) Dispersal in microbes: fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J 7:1262–1273
Fang ZG, Ouyang ZY (2009) Advance of airborne fungal community and the influencing factors in indoor and outdoor environments in urban ecosystem. Ecol Environ Sci 18:386–393
Fu CL, He WH, Jia JH, Sun ZM, Yuan W, Zhou YG, Yan EP (2000) Special investgation of Fungi from Four cities of China. Microbiol China 264–269
Fang ZG, Ouyang ZY, Hu LF, Wang XK, Lin XQ (2005) Community structure and ecological distribution of airborne microbes in summer in Beijin. Acta Ecol Sin 83–88
Chang CW, Chung H, Huang CF, Su HJ (2001) Exposure of workers to airborne microorganisms in open-air swine houses. Appl Environ Microbiol 67:155–161
Shelton BG, Kirkland KH, Flanders WD, Morris GK (2002) Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl Environ Microbiol 68:1743–1753
Rejc T, Kukec A, Bizjak M, GodicTorkar K (2020) Microbiological and chemical quality of indoor air in kindergartens in Slovenia. Int J Environ Health Res 30:49–62
Li X (2013) Review of morphology classification of Cladosporium. J Anhui Agric Sci 41:6254–6255
Ma R, Huang H, Bai Y, Luo H, Fan Y, Yao B (2018) Insight into the cold adaptation and hemicellulose utilization of Cladosporium neopsychrotolerans from genome analysis and biochemical characterization. Sci Rep 8:6075
Ren P, Ahearn DG, Crow SA (1998) Mycotoxins of Alternaria alternata produced on ceiling tiles. J Ind Microbiol Biotechnol 20:53–54
Xie HY (2006) Studies on investigation and morphologic and moleculai identification of the genus Alternaria Nees from some areas of China; Master thesis, Guizhou University, Guizhou, China
Grewling L, Nowak M, Szymanska A, Kostecki L, Bogawski P (2019) Temporal variability in the allergenicity of airborne Alternaria spores. Med Mycol 57:403–411
Macris BJ (1984) Production and characterization of cellulase and beta-glucosidase from a mutant of Alternaria alternata. Appl Environ Microbiol 47:560–565
Robl D, Delabona Pda S, Mergel CM, Rojas JD, Costa Pdos S, Pimentel IC, Vicente VA, da Pradella C, Padilla JG G (2013) The capability of endophytic fungi for production of hemicellulases and related enzymes. BMC Biotechnol 13:94
Zhou YH, Zhang CY (2021) Research Progress on Cellulase produced by Aspergillus Niger. Agricultural Technology & Equipment, pp 72–73
Li T (2019) Comparison of basic culture characteristics of different Aspergillus oryzae strains and heterology experession of lipase; Master thesis, Nanchang University, Nanchang, China
Fan LX (2022) Screening of Aspergillus oryzae with High Protease Activity and its Application in Doubanjiang; Master thesis, Jiangnan University, Wuxi, China
Zhang S, Qu QS, Cheng H (2018) Amylase production from solid-state fermentation of kitchen garbage by aspergillus oryzae. China Food Addit 141–146
Zhang X, Han SY (2016) Research Progress on Fermentation production of enzyme by Aspergillus Niger. Chem Bioeng (Wuhan China) 33:13–16
Simonovicova A, Hlinkova E, Chovanova K, Pangallo D (2013) Influence of the Environment on the morphological and biochemical characteristics of different aspergillus Niger Wild type strains. Indian J Microbiol 53:187–193
Pinheiro AC, Sequeira SO, Macedo MF (2019) Fungi in archives, libraries, and museums: a review on paper conservation and human health. Crit Rev Microbiol 45:686–700
Chen YP, Cui Y, Dong JG (2010) Variation of airborne bacteria and fungi at Emperor Qin’s Terra-Cotta Museum, Xi’an, China, during the Oct. 1 gold week period of 2006. Environ Sci Pollut Res Int 17:478–485
Tian ZL, Long K, YI XH, Ren SS, Zhang M (2016) A study on influence of storage environment on paper properties. China Pulp Pap Ind 37:31–33
Li XL, Tu XY, Deng F (2010) Discussion on anti mold measures in paper archives preservation. Light Ind Sci Technol 26:5–6
Funding
This research was jointly funded by the Department of Science and Technology of Yunnan Province (202101AY070001-072) to YW and National Natural Science Foundation of China (32270018) to YZ.
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.Z. and Y.W.; methodology, Q.Z., L.Z., Y. H., L.Y.; software, Q.Z., L.Z. and Y. H.; validation, Y.Z., Y.W., Q.Z. and L.Z.; formal analysis, Q.Z., L.Z. and Y. H.; investigation, Y. H., L.Y. and Q. X. Z.; resources, Y. H., L.Y. and Q. X. Z.; data curation, Q.Z., L.Z., Y. H. and L.Y.; writing—original draft preparation, Q.Z. and L.Z.; writing—review and editing, Y.Z., Y.W., Q.Z. and L.Z.; visualization, Y.Z., Y.W., Q.Z. and L.Z.; supervision, Y.Z. and Y.W.; project administration, Y.Z. and Y.W.; funding acquisition, Y.Z. and Y.W. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Responsible Editor: Melissa Fontes Landell.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Q., Wang, Y., Hou, Y. et al. Metabarcode insights into the airborne fungal diversity in the indoor and outdoor environments in archives from Yunnan, Southwestern China. Braz J Microbiol 55, 1601–1618 (2024). https://doi.org/10.1007/s42770-024-01323-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-024-01323-z