Abstract
The deposition of the airborne microorganisms onto cultural heritage is associated closely with the subsequent biodeterioration. In this study, a systematic investigation was carried out to detect the seasonal variation and diversity of airborne fungal concentration at the World Cultural Heritage Site Maijishan Grottoes in western China. A bio-aerosol sampler was deployed to collect samples over four seasons in 2016. The culturable airborne fungi were isolated, purified and then identified with the extraction of genomic DNA, PCR amplification of ITS rRNA region, sequencing, and phylogenetic analysis. The concentrations of culturable fungi ranged from 216 to 1389 CFU/m3, which varied seasonally with significant differences among the sampling sites. Fifteen different fungal genera were confirmed, among them, Cladosporium was the most predominant fungal genus, followed by Penicillium. The fungal community structure and their relationship with environmental factors were also delineated. The spatial–temporal differences of airborne fungi at Maijishan Grottoes were mainly due to height, rainfall, relative humidity, and temperature. The dominant genera Cladosporium and Penicillium may pose potential threats to the ancient painted sculptures and murals, and monitoring of the airborne fungi at such a heritage site could provide supporting data for the pre-warning and control of fungal outbreaks inside the caves for better management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The protective principles of cultural heritage have been gradually shifting from salvage conservation to preventive conservation in recent years. The objects for protection are also expanded from cultural heritage to its surrounding environmental conditions. Microorganisms such as bacteria, archaea and fungi may have obvious and adverse effects on the conservation of artistic-historical heritage, particularly when microclimatic conditions are favorable for microbial colonization and proliferation (Meng et al., 2017; Zhang et al., 2019). In many reported cases, overgrowth of microorganisms results in significant biodeterioration of artworks worldwide, such as the exquisite murals and painted sculptures at Mogao Grottoes, Maijishan Grottoes and Tiantishan Grottoes along the Silk Road in China (Duan et al., 2017, 2018; Ma et al., 2015) as well as the valuable pre-historical rock paintings of Altamira cave, Lascaux cave, Tito Bustillo, and La Garma caves in Europe (Bastian et al., 2010; Dupont et al., 2007; Portillo et al., 2009; Schabereiter-Gurtner et al., 2002, 2004). The excessive growth of microorganisms can result in aesthetic change and reducing the stability of the murals by colonizing and penetrating into the painting layers, triggering material loss due to organic acid corrosion, enzymatic degradation and mechanical damage by biochemical processes catalyzed by the active microorganisms (Gu, 2003; Sterflinger & Piñar, 2013).
Bioaerosols in the atmosphere of cultural heritage sites always contain high concentration of biological particles, including bacteria and fungi at historic sites that are open to public visiting (Wang et al., 2010a, 2010b). Previously, many studies were mainly focused on the airborne microbial concentration, community diversity, and distribution patterns of the urban public environments such as residential area, hospital, school and subway (Chang et al., 2015; Fang et al., 2013; Gohli et al., 2019; Hayleeyesus & Manaye, 2014; Lee et al., 2010) and, recently, more and more airborne biological research were gradually carried out in heritage sites and museums (Carlo et al., 2016; Duan et al., 2019; Pyrri et al., 2020). A series of studies revealed that when masses of tourists suddenly flooded into a cave in a short time, the original micro-environment of the cultural relics can be significantly altered due to their disturbances, such as resuspension and floating of dust particles, an increase in CO2 concentration, temperature, and relative humidity and subsequent changes of the diversity and abundance of airborne microbes (Saiz-jimenez et al., 2011; Wang et al., 2010a, 2010b; Zhang et al., 2005). These biological agents can spread with the airflow and deposited on the surface of cultural relics, because atmosphere is the primary vehicle for the transportation and dispersal of microorganisms (Nugari et al., 1993). Actually, bioaerosols represent a potential risk and threat to cultural artifacts since under favorable nutritional and microclimatic conditions, the settled biological particulate matter can develop and grow, thus triggering the selective group of microorganisms to grow and cause biodeterioration (Caneva et al., 2020). Unfortunately, in many cases, information on airborne microbes in the surrounding environments of the precious cultural heritage sites is very limited. Therefore, an investigation and monitoring of airborne microbial activities was carried out in many cultural heritage sites and even integrated into routine monitoring system (EJ, 2013).
Many studies reported that airborne microbes usually exist as bioaerosols attached to floating dust and other soil particles, most of them may be originated from both natural and anthropogenic sources, including plants, soils, vegetables, waters, sewage sludge, livestock feeding, agricultural activities and transportation industry (Bonazza et al., 2016; Brodie et al., 2007; Spirin & Mikhaĭlova, 1991). There are significant differences in the concentrations, community compositions and spatial–temporal distribution patterns of airborne microbes among different types of cultural heritage and sites. Even within neighboring sites, dynamics of airborne microbes shows a pronounced daily or seasonal periodicity and fluctuations associated with local vegetation, climatic parameters and seasonal factors, pattern of the emission sources, and anthropogenic activities (Huang et al., 2002; Jones & Harrison, 2004; Maron et al., 2006). Some of the airborne fungal species, both indoors and outdoors, including the members belonging to Cladosporium, Penicillium, Alternaria, and Aspergillus are regarded as conventional opportunistic pathogens to humans and may induce illness as rhinitis, sinusitis, asthma or alveolitis and other respiratory diseases (Carlo et al., 2016; Shirakawa et al., 2003). They are also implicated for biodeterioration of a wide range of complex polymeric materials (Gu, 2003). Overall, these unexpected changes can have a strong irreversible influence on the cave microbiology and ecosystem stability (Godoi et al., 2008; Sanchez-Moral et al., 2005).
Maijishan Grottoes, a famous Buddhist cave temple located on the ancient Silk Road in West China, are known for their beautiful scenery, numerous ancient caves and precious artworks. Unfortunately, the painted sculptures preserved in the caves are severely threatened by several types of diseases, which mainly attributed to humid climatic conditions, increasing human disturbance and infestation by visible and invisible organisms. Some of the priceless artworks of the Maijishan Grottoes are now facing increasing risk and threat from microbial activities, especially fungal outbreaks to cause discoloration and obvious damage of the initial paints or materials. To date, little is known about the characteristics of airborne fungal community of Maijishan Grottoes, namely concentration, community structure, distribution pattern and seasonal dynamics. The objectives of this study were to conduct a scientific investigation to reveal the seasonal dynamics of airborne fungi at four sampling sites of Maijishan Grottoes and determine the composition of the fungal community over time. The results are useful data for the environmental monitoring, pre-warning and preventive protection management of this historical site.
2 Materials and methods
2.1 Sampling sites
Maijishan Grottoes are situated in the Maijishan Mountains, ca. 30 km southeast of the Tianshui city in Gansu Province, China. Maijishan Grottoes are surrounded by the Qinling Mountains (Fig. 1a). The Grottoes have a semi-humid continental climate with an average annual temperature of 10.4 °C, relative humidity of 69.2% and precipitation of 680 mm. The local vegetation belongs to a temperate deciduous broad-leaved forest ecosystem. The caves of the Maijishan Grottoes were initially built from the Northern Wei (386–534 AD) to the Qing Dynasty (1636–1912 AD) over a period of approximately 1600 years, with 221 caves, 10,632 painted sculptures and nearly 1000 m2 of murals preserved to date. Maijishan Grottoes are a recognized World Cultural Heritage Site known for its beautiful scenery, numerous caves, precious artistry and artwork, and a long history. In 1961, Maijishan Grottoes were declared as one of the first group of national relic protection unit by the State Council of the P.R. China. In 2014, the site was added to the World Heritage List as one of the key historical sites of the “Silk Roads, the Route Network of the Chang'an-Tianshan Corridor”.
2.2 Sampling methods
Four sampling sites were selected for the aerosol analyses in four seasons (April, June, October, and December) in 2016, including one outdoor site and three caves (Fig. 1b, c, d, Table 1). Buck Bio-Culture Model B30120 sampler (A.P. Buck Inc, Orlando, Fl, USA), was used to collected airborne and suspended materials, including culturable and non-culturable fungi in the atmosphere of the Maijishan Grottoes. At each sampling site, the sampler was installed 1.5 m above ground level with a supporting platform. Airflow of the sampler was set at 90L/min and sampling was carried out for 2 min, with three parallel repetitions. For each sampling, the bio-aerosol sampler loaded with 90 mm Petri dishes containing Potato Dextrose Agar (PDA) medium. The exposed dishes were incubated at 25 °C for 7–15 days.
2.3 Fungi enumeration and identification
Colony forming units (CFU) on the PDA agar plates were enumerated, and fungal concentrations were expressed as per cubic meter of air (CFU/m3). CFU/m3 was calculated as:
In the equations, C is the airborne fungi concentration of culturable ones; T is the total colonies on the PDA agar plates; t is the sampling time; F is the airflow rate.
After incubation, microbial colonies were counted and fungi were preliminary identified according to different morphological characteristics including shape, size, color, and refractivity, etc., afterward, the isolates were purified and identified using a molecular method as described below.
Isolates of pure culture biomass were homogenized in liquid nitrogen and then genomic DNA was extracted using a commercially available extraction Kit (Tiangen Co., Beijing, China) according to the manufacturer's protocols. The internal transcribed spacer (ITS) region of fungal rRNA genes was amplified with the following universal primer set: ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') (White et al., 1990). The reaction mixture (25 μL) consisted of 2.5 μL 10 × PCR buffer, 1-unit Taq polymerase (Tiangen Co., Beijing, China), 0.2 mM dNTPs, 2.5 mM MgCl2, 0.2 μM each primer, and 2.5 μL (ca. 10 ng DNA) of templates. The PCR program included an initial denaturation at 94 °C for 5 min; 30 cycles at 94 °C for 40 s, annealing at 55 °C for 40 s and an extension at 72 °C for 40 s; and a final extension step at 72 °C for 10 min. The PCR products were detected by electrophoresis on a 1.0% agarose gel.
The similarities among PCR products were determined by restriction fragment length polymorphisms (RFLP) analysis. The PCR products were digested with the double restriction endonucleases BsuR I and Hinf I (MBI, Fermentas). And then, the digested fragments were differentiated into several clusters according to their spectral patterns on a 2.5% agarose gel.
Cloning was performed with the pGEM-T Vector System (Tiangen Co., Beijing, China) following manufacturer’s instructions, and the ligation product was subsequently transformed into E. coli DH5α cells, which allows Blue-White selection for successful ligation. The transformants were inoculated into LB medium containing ampicillin (100 mg ml−1), X-Gal (20 mg ml−1) and IPTG (200 mg ml−1). Positive clones were identified by PCR amplification with the pGEM-T vector primer pairs (T7/SP6) using the same program as the used for ITS amplification.
Fungal cultures of expected clones were sequenced by Shanghai Majorbio Bio-technology Co., Ltd. (Shanghai, China). A total of 41 obtained fungal sequences (ca. 600 bp) were then analyzed using the National Center for Biotechnology Information (NCBI) Blast program (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The most similar sequences were extracted from the GenBank database. A phylogenetic neighbor-joining tree was constructed using the MEGA software 7.0. The sequences retrieved during this study could be accessed under the numbers MH042763-MH042803.
2.4 Environmental parameters
The meteorological data were collected from monitoring at four sampling sites of the Maijishan Grottoes for the entire duration of one year (from April 16, 2016, to April 16, 2017). Temperature (T, °C) and relative humidity (RH, %) were collected by HOBO® U23-001 Temp/RH data logger (Onset Computer Corporation, Contoocook, NH, USA). Data of local rainfall were provided by Institute of Maijishan Grottoes Art, Dunhuang Academy. For all environmental parameters, the values used in data analyses were 10-day averages from before and after the sampling days.
2.5 Statistical analysis
All the experimental data were analyzed by one-way analysis of variance (ANOVA) using SPSS version 16.0. The relationships between airborne fungi concentration and environmental parameters were tested using Pearson correlation analysis. The Shannon–Wiener diversity index of fungal communities was generated using the Vegan packages in R (4.0.0). Canonical correlation analysis (CCA) for airborne fungal communities and environmental parameters were then analyzed using CANOCO version 4.5.
3 Results
3.1 Fungal concentration
Considering all four sampling sites, the concentration range of culturable fungi was from 216 to 1389 CFU/m3, the mean and median were 645 ± 47 CFU/m3 and 594 CFU/m3, respectively (Table 2). The mean concentration was 445 ± 64 CFU/m3 at MJO, 702 ± 63 CFU/m3 at MJ29, 631 ± 76 CFU/m3 at MJ9, and 804 ± 131 CFU/m3 at MJ4. In general, significantly higher fungal concentrations were found in MJ29 and MJ4 than in MJO (p < 0.05), but no significant difference was found between MJ9 and MJO (p > 0.05).
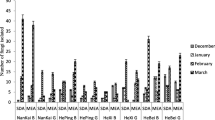
The population of culturable airborne fungi showed an obvious seasonal variation pattern at the four sites (Fig. 2). The highest concentration was observed in autumn with 741 CFU/m3 at MJO, summer with 970 CFU/m3 at M29, autumn with 835 CFU/m3 at MJ9, summer with 1389 CFU/m3 at MJ4, respectively. The lowest concentration was detected in spring with 216 CFU/m3 at MJO, 354 CFU/m3 at MJ9 and 363 CFU/m3 at MJ4, and in winter with 583 CFU/m3 at MJ29, respectively. In general, the total concentration of airborne fungi in summer and autumn was higher than those in winter and spring, and the data show normal distribution patterns in the four seasons.
The relationships between airborne fungal concentrations of the different sampling sites and their surrounding environmental parameters (temperature, relative humidity and rainfall) were determined by Pearson correlation analysis (Table 3). Average seasonal values of environmental parameters from the four sampling sites are shown in Table S1. The results show that the airborne fungal concentration was positively correlated to T for all four sampling sites, but only MJ4 showed a significant correlation (p < 0.05). RH was positively correlated to the fungal concentrations at MJ29, MJ9 and MJ4, and negatively to fungal concentration at MJO, but the correlations were all non-significant, especially for MJO. The fungal concentration at MJO was positively correlated to the rainfall.
3.2 Phylogenetic analysis of the airborne fungal communities
The composition of the airborne fungal communities was determined by rRNA phylogenetic analysis based on the ITS region. The phylogenetic tree is shown in Fig. 3. In this study, a total of 41 sequences were obtained and classified into 15 different fungal genera corresponding to the sequences deposited into GenBank database, and among them, 13 genera were affiliated to Ascomycota and the remaining two were Basidiomycota. Cladosporium (72.56%) was the most frequently encountered fungal genus, followed by Penicillium (16.35%), Epicoccum (3.14%), Alternaria (3.0%) and Pestalotiopsis (1.20%), these five fungal genera accounted for 96.26% of the whole community (Fig. 4). The rest of the members, including Leptosphaerulina, Aspergillus, Bjerkandera, Didymella, Phoma, Periconia, Phanerochaete, Bullera, Dothideomycete, and Botrytis were collectively accounted for only 3.74% of the whole fungal community.
The Shannon–Wiener index represents diversity of airborne fungal communities. The changes in the Shannon–Wiener index values of fungal communities at the four sampling sites are shown in Fig. 5. The mean value of the Shannon–Wiener index differed among the four sampling sites (0.93 at MJO, 0.88 at MJ29, 0.52 at MJ9, 0.51 at MJ4) and fluctuated markedly among different seasons. The highest diversity index values were as follows: MJO in autumn (1.26), MJ29 in autumn (1.50), MJ9 in winter (0.98), and MJ4 in spring (0.79). The lowest diversity index value was at MJ9 in autumn (0.11).
The community structures of culturable fungal compositions varied greatly among different sampling sites. The most prevalent fungal genera are presented in Fig. 6. Cladosporium was the most dominant airborne fungal genus at all four sampling sites. In spring, Cladosporium was observed at the sites of MJO, MJ9 and MJ4 with 68.97%, 85.07% and 90.08%, respectively, and significantly higher than that at MJ29 (28.57%), and lower than the largest genera Penicillium (51.79%) and followed by Alternaria (19.64%). In addition, Pestalotiopsis was the second largest genus with 20.69% at MJO. In summer, the proportion of Penicillium had increased sharply to a higher level with 95.31% at MJ29, and Cladosporium held the dominating position of the largest genus at the sites of MJ9 and MJ4 with 94.03% and 96.81%, respectively. Meanwhile, the proportion of Cladosporium slightly decreased to 54.55% at MJO, Epicoccum increased significantly to 36.36%. In autumn, the proportions of Cladosporium at these three sites of MJO (53.13%), MJ9 (96.84%) and MJ4 (94.50%) were similar to their proportions in summer, and the two genera of Pestalotiopsis (20.83%) and Epicoccum (13.54%) were also detected at MJO. Meanwhile, Penicillium was not observed at MJ29 and Cladosporium reappeared (54.90%), followed by Leptosphaerulina (16.67%), Aspergillus (15.69%) and Epicoccum (11.76%). In winter, Cladosporium was the most prevalent genus at the two sites of MJO (66.67%) and MJ4 (91.30%), that showed very close to their abundance in spring, and the proportion of Cladosporium was significantly increased to 80.77% at MJ29. In addition, Cladosporium has changed to the secondary position, which decreased significantly to 36.36% at MJ9, and Alternaria (54.55%) increased as the genus with the highest proportion.
3.3 The contributions of environmental parameters to airborne fungal community structure
The relationships between fungal community structure and environmental parameters were delineated by Canonical correlation analysis (CCA). The results showed that the structure of culturable fungal communities varied greatly among the different sampling sites (Fig. 7). The contribution of the environmental parameters to the fungal community distribution was ranked as height (0.35) of the sampling sites, rainfall (0.29), T (0.09) and RH (0.07) in descending order.
4 Discussion
Nowadays, aerobiology has become an important discipline for monitoring, developing prevention and control strategies for the biological deterioration of cultural heritage (Caneva et al., 2020). Fundamental steps for prevention and management are the knowledge of biological agents (quantitative and qualitative) and of factors affecting their circulation, survival and growth (Balocco et al., 2014). In this study, the temporal–spatial dynamics of airborne fungal communities were investigated at the world cultural heritage site Maijishan Grottoes. The results showed that a seasonal fluctuation pattern of the fungal concentration in the atmosphere was observed and the relatively higher fungal concentration was recorded in summer and autumn than winter and spring. Furthermore, even within the same season, the airborne fungal concentration varied among the different sampling sites, possibly due to the micro-meteorological conditions between sites, such as temperature, RH, and wind directions (Tanaka et al., 2015). In addition, the proliferation of airborne fungi in the caves should not be ignored. Pearson correlation analysis revealed that there was a positive correlation between fungal concentrations in the atmosphere and temperature at all sampling sites, and the site MJ29 showed a significant correlation (p < 0.05). Meanwhile, the three sampling sites that are located on the mountain housing the grottoes, namely MJ29, MJ9, and MJ4, all showed a positive correlation between airborne fungal concentration and RH, but a negative correlation at MJO only. In addition, a positive correlation between the airborne fungal concentration and rainfall was detected at MJO. At the Maijishan Grottoes and its surrounding regions, the rainfall was mainly accumulated in summer and autumn. Although rainfall can reduce the atmospheric particle concentrations as well as microbial spores in the atmosphere, the warmer climate in summer and autumn with the rainfall can stimulate the germination and proliferation of the microbial spores. During the whole monitoring period, airborne fungal concentration among the sampling sites ranged from 216 to 1389 CFU/m3, and the mean value was 645 ± 47 CFU/m3, lower than the microbial concentration loading in some famous heritage sites, e.g., Mogao Grottoes in China (Wang et al., 2010a). This is probably attributed to the surrounding environment of high-density vegetation in the Maijishan Grottoes, which is surrounded by the West Qinling Mountains, for which airborne microbes could be absorbed and attached on the surfaces of plant leaves (Lymperopoulou et al., 2016).
The airborne fungal concentration at MJO was lower than those at the three caves that are located on the mountain (MJ29, MJ9, and MJ4), and that’s mainly due to a relatively small area of space and higher visitor density at these three caves. As the only entrance for visitors, MJO may suffer severe disturbance from numerous tourists, e.g., rising of airborne microbial concentration, but these disturbances were significantly alleviated by outdoors with open air stream movement. Additionally, based on the latest classification criteria for ecological disturbance of cave environment, a cave may already be infested by fungi as a result of massive visits, water, or other types of spillage, etc., when the concentration reaches between 500 and 1000 CFU/m3. A cave is under an irreversible ecological disturbance and conditions if a persistent population of CFU/m3 is above 1000 (Porca et al., 2011). In recent years, a huge number of tourists have been attracted by the Maijishan Grottoes, resulting in a constant disturbance of the micro-environmental conditions, which, in many cases, is harmful to the conservation of cultural heritage sites and management practice (Bastian et al., 2010; Jurado et al., 2009). Therefore, it is most important that the concentration of airborne fungi is maintained to a relative lower level at these sites, so that the caves can be prevented from severe threats from fungal outbreaks.
The airborne fungal community structures analyzed in the present study showed that large differences were detected at the four sampling sites and no unique composition structure can be distinguished with any of these sites. In our research, a total of 15 different fungal genera were detected. Among them, Cladosporium was the most predominant fungal genus, followed by Penicillium. Both of them were frequently isolated genera in the atmosphere of the Maijishan Grottoes, which is similar to previous studies carried out in caves and libraries (Fernandez-Cortes et al., 2011; Pusz et al., 2015; Vanderwolf et al., 2013). Those fungi were saprobic and could produce numerous conidia that can be easily dispersed by air (Abrusci et al., 2005). Saprobic fungi obtain their nutrients from organic matter and they play crucial roles in nutrient cycling in natural ecosystems. High abundance of Cladosporium and Penicillium at this heritage site may be associated with the abundant organic matters, which derived from the dead and decomposed forest vegetation above Maijishan Grottoes. The abundance of airborne fungi detected at the Maijishan Grottoes was also influenced by sampling sites and time of sampling during the periods. Among the four sites, MJO showed the highest Shannon–Wiener index value and MJ4 the lowest, suggesting that diversity decreased with the increase in height. Interestingly, the highest diversity values were recorded in autumn (MJO and MJ29), but the lowest diversity value was also observed in autumn (MJ9), but different location (Fig. 5).
Currently, both culture-dependent and culture-independent methods based on 16S/ITS ribosomal DNA/RNA regions of bacteria and fungi have been widely employed on identification of the airborne microbiome at historical sites. Although airborne fungal community composition varied depending on the sites-specificity and detection methods, several representative genera, especially Cladosporium, Penicillium, Alternaria and Aspergillus, are frequently detected and isolated from the air of the caves, e.g., five paleolithic decorated caves in France and cave of Nerja in southern Spain (Docampo et al., 2011; Leplat et al., 2019). Meanwhile, they were also regarded as opportunistic pathogens to humans and could cause a potential danger to tourists and working employees (Carlo et al., 2016). In addition, they are also known in biodeterioration of a wide range of polymeric materials (Gu, 2003) and possibly biodeterioration of the cultural relics (Meng et al., 2017; Savković et al., 2019; Zhang et al., 2019). However, since less than 1% of the total microbes of the natural ecosystem can be cultured in laboratory conditions, enrichment and isolation only show a very tiny fraction of the natural community population of the easily cultivable ones due to the nutritional preference of different microbes and optimal growing conditions (Schabereiter-Gurtner et al., 2001), especially considering the nutritional preference of different microbes. Thus, it is difficult to show the information of airborne microbiota comprehensively solely based on the available culture-based methods. Recently, the advances in high-throughput sequencing methods can overcome the inherent defect of the culture-based methods and can provide further deeper insights into the complete microbial world. Roche 454-pyrosequencing, Illumina sequencing and now Next Generation Sequence have been employed to investigate the temporal stability of bacterial and eukaryote communities of the indoor air. Over a period of a half year results show a stable bacterial diversity in the Louvre Museum during visit time and highlight a bacterial signature of the indoor air with the presence of ‘core species, but the stability of the eukaryotes was lower than that of bacteria (Gaüzère et al., 2014). Furthermore, these are also powerful tools to reveal the differences on microbial community structure and composition between the atmosphere and the paintings for establishing the transportation mechanism of airborne microorganisms at heritage sites. Previously, the microbial community characteristic colonizing the ancient painted sculptures of the Maijishan Grottoes was studied using the Illumina MiSeq platform to reveal a fairly rich fungal diversity on the surface of the painted layers (Duan et al., 2017). Among them, a large number of fungal sequences classified into the unclassified or no rank genera based on the current undercapacity database of 18S rRNA gene sequences, and most of the sequences were affiliated with Capnodiales (unclassified and no rank) and Ascomycota_unclassified. In the future, a combination of culture-based methods and high-throughput sequencing techniques shall be widely employed to profile the airborne microflora information of Maijishan grottoes and other heritage sites. Archaea as a phylogenetically distinguished group has been found to contribute to the deterioration of inorganic cultural heritage (Meng et al., 2017) and more new archaea are still waiting to be discovered and isolated (Zhou et al., 2019).
The airborne fungal dynamics of the Maijishan Grottoes showed a pronounced seasonal periodicity and fluctuations. Canonical correlation analysis revealed that the airborne fungal distribution was influenced by altitude, rainfall, temperature, and RH across the four sampling sites at the Maijishan Grottoes. Among them, altitude has a greater effect on the fungal communities of the two higher floored caves (MJ4 and MJ9) than MJ29, rainfall has a stronger effect on MJ9 and MJ29 than MJ4. In addition, other environmental factors such as wind speed, wind direction, and solar radiation were also probably having an important impact on the composition and distribution of airborne fungi. Generally, wind speed and direction directly influence the transport of bioaerosols over the regions and their concentration at remote location from the source (Kathiriya et al., 2021). Both bacterial and fungal spores in the atmosphere were all sensitive to solar radiation levels, lower intensity of solar radiation may lead to the release of airborne microbial spores, but high intensity of solar radiation may decrease airborne microbial concentration (Kowalski & Pastuszka, 2017). In Mogao Grottoes, we found that wind speed and solar radiation was significantly positive correlated with airborne bacterial concentration outdoors, but have non-significant correlation with the airborne fungal concentration at the same site (Wang et al., 2010a, 2010b). Unfortunately, in this study, the above-mentioned factors were not incorporated into related analysis due to the limitations of the objective conditions. Therefore, their roles in airborne microorganism distribution at the Maijishan Grottoes still need further study in the future.
5 Conclusion
In summary, the aerobiological analysis of samples yielded a representative inventory of the airborne fungal community information at the Maijishan Grottoes of China, including their concentration, community diversity, distribution patterns, and relationship with monitored environmental parameters. Based on the information obtained, it is necessary to carry out the long-term monitoring of the airborne microorganisms and integrate it into a holistic pre-warning monitoring system. This study provides supporting information for the early detection and control of fungal outbreaks at heritage sites.
References
Abrusci, C., Martín-González, A., Del Amo, A., Catalina, F., Collado, J., & Platas, G. (2005). Isolation and identification of bacteria and fungi from cinematographic films. International Biodeterioration & Biodegradation, 56(1), 58–68.
Balocco, C., Petrone, G., & Cammarata, G. (2014). Numerical multi-physical approach for the assessment of coupled heat and moisture transfer combined with people movements in historical buildings. Build Simulation, 7, 289–303.
Bastian, F., Jurado, V., Nováková, A., Alabouvette, C., & Sáiz-Jiménez, C. (2010). The microbiology of Lascaux Cave. Microbiology, 156(3), 644–652.
Bonazza, A., De Nuntiis, P., Mandrioli, P., & Sabbioni, C. (2016). Aerosol impact on cultural heritage: Deterioration processes and strategies for preventive conservation. Wiley-VCH Verlag GmbH & Co.
Brodie, E. L., DeSantis, T. Z., Parker, J. P. M., Zubietta, I. X., Piceno, Y. M., & Andersen, G. L. (2007). Urban aerosols harbor diverse and dynamic bacterial populations. Proceedings of the National Academy of Sciences of the United States of America, 104(1), 299–304.
Caneva, G., De Nuntiis, P., Fornaciari, M., Ruga, L., Valenti, P., & Pasquariello, G. (2020). Aerobiology applied to the preventive conservation of cultural heritage. Aerobiologia, 36(1), 99–103.
Carlo, E. D., Chisesi, R., Barresi, G., Barbaro, S., Lombardo, G., Rotolo, V., Sebastianelli, M., Travagliato, G., & Palla, F. (2016). Fungi and bacteria in indoor cultural heritage environments: microbial related risks for artworks and human health. Environment and Ecology Research, 4(5), 257–264.
Chang, C., Liang, T., & Yang, L. (2015). Microbial air contamination in an intensive care unit. International Journal Public Health, 4(3), 145–151.
Docampo, S., Trigo, M. M., Recio, M., Melgar, M., García-Sánchez, J., & Cabezudo, B. (2011). Fungal spore content of the atmosphere of the Cave of Nerja (southern Spain): Diversity and origin. Science of the Total Environment, 409(4), 835–843.
Duan, Y., Wu, F., Wang, W., Gu, J. D., Li, Y., Feng, H., Chen, T., Liu, G., & An, L. (2018). Differences of microbial community on the wall paintings preserved in situ and ex situ of the Tiantishan Grottoes, China. International Biodeterioration & Biodegradation, 132, 102–113.
Duan, Y., Wu, F., Wang, W., He, D., Gu, J. D., Feng, H., Chen, T., Liu, G., & An, L. (2017). The microbial community characteristics of ancient painted sculptures in Maijishan Grottoes, China. PLoS ONE, 12(67), e0179718.
Duan, Y., Wu, F., Wang, W., He, D., Gu, J. D., Feng, H., Chen, T., Liu, G., & An, L. (2019). Spatial and temporal distribution characteristics of the airborne bacteria in the Maijishan grottoes, China. Acta Microbiologica Sinica, 59(1), 145–156.
Dupont, J., Jacquet, C., Dennetiere, B., Lacoste, S., Bousta, F., Orial, G., Cruaud, C., Couloux, A., & Roquebert, M. F. (2007). Invasion of the French paleolithic painted cave of Lascaux by members of the Fusarium solani species complex. Mycologia, 99(4), 526–533.
EJ, W. F., Wang, W. F., Chen, G. L., Zhao, L. Y., & He, D. P. (2013). Monitoring and research on microbes in the environment of the wall paintings in No. 5 of the Wei and Jin Tombs. Dunhuang Research, 6, 109–116.
Fang, Z. G., Sun, P., Ouyang, Z. Y., Liu, P., Sun, L., & Wang, X. Y. (2013). Studies on the size distribution of airborne microbes at home in Beijing. Environmental Science, 34(7), 2526–2532.
Fernandez-Cortes, A., Cuezva, S., Sanchez-Moral, S., Cañaveras, J. C., Porca, E., Jurado, V., Martin-Sanchez, P. M., & Saiz-Jimenez, C. (2011). Detection of human-induced environmental disturbances in a show cave. Environmental Science & Pollution Research, 18(6), 1037–1045.
Gaüzère, C., Moletta-Denat, M., Blanquart, H., Ferreira, S., Moularat, S., Godon, J. J., & Robine, E. (2014). Stability of airborne microbes in the Louvre Museum over time. Indoor Air, 24(1), 29–40.
Godoi, R. H. M., Potgieter-Vermaak, S., Godoi, A. F. L., Stranger, M., & Van Grieken, R. (2008). Assessment of aerosol particles within the Rubens’ House Museum in Antwerp, Belgium. X-Ray Spectrometry, 37(4), 298–303.
Gohli, J., Bøifot, K. O., Moen, L. V., Pastuszek, P., Skogan, G., Udekwu, K. I., & Dybwad, M. (2019). The subway microbiome: seasonal dynamics and direct comparison of air and surface bacterial communities. Microbiome, 7, 160.
Gu, J. (2003). Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. International Biodeterioration & Biodegradation, 52(2), 69–91.
Hayleeyesus, S. F., & Manaye, A. M. (2014). Microbiological quality of indoor air in university libraries. Asian Pacific Journal of Tropical Biomedicine, 4(1), 312–317.
Huang, C. Y., Lee, C. C., Li, F. C., Ma, Y. P., & Su, H. J. J. (2002). The seasonal distribution of bioaerosols in municipal landfill sites: a 3-yr study. Atmospheric Environment, 36(27), 4385–4395.
Jones, A. M., & Harrison, R. M. (2004). The effects of meteorological factors on atmospheric bioaerosol concentrations-a review. Science of the Total Environment, 326(1–3), 151–180.
Jurado, V., Fernandez-Cortes, A., Cuezva, S., Laiz, L., Cañaveras, J. C., Sanchez-Moral, S., & Saiz-Jimenez, C. (2009). The fungal colonisation of rock-art caves: Experimental evidence. Science of Nature, 96(9), 1027–1034.
Kathiriya, T., Gupta, A., & Singh, N. K. (2021). An opinion review on sampling strategies, enumeration techniques, and critical environmental factors for bioaerosols: An emerging sustainability indicator for society and cities. Environmental Technology & Innovation, 21, 101287.
Kowalski, M., & Pastuszka, J. S. (2017). Effect of ambient air temperature and solar radiation on changes in bacterial and fungal aerosols concentration in the urban environment. Annals of Agricultural and Environmental Medicine, 25(2), 259–261.
Lee, S. H., Lee, H. J., Kim, S. J., Lee, H. M., Kang, H., & Kim, Y. P. (2010). Identification of airborne bacterial and fungal community structures in an urban area by T-RFLP analysis and quantitative real-time PCR. Science of the Total Environment, 408(6), 1349–1357.
Leplat, J., François, A., Touron, S., Galant, P., & Bousta, F. (2019). Aerobiological behavior of Paleolithic decorated caves: a comparative study of five caves in the Gard department (France). Aerobiologia, 35, 105–124.
Lymperopoulou, D. S., Adams, R. I., & Lindow, S. E. (2016). Contribution of vegetation to the microbial composition of nearby outdoor air. Applied and Environmental Microbiology, 82(13), 3822–3833.
Ma, Y., Zhang, H., Du, Y., Tian, T., Xiang, T., Liu, X., Wu, F., An, L., Wang, W., Gu, J. D., & Feng, H. (2015). The community distribution of bacteria and fungi on ancient wall paintings of the Mogao Grottoes. Scientific Reports, 5, 7752.
Maron, P. A., Mougel, C., Lejon, D. P., Carvalho, E., Bizet, K., Marck, G., Cubito, N., Lemanceau, P., & Ranjard, L. (2006). Temporal variability of airborne bacterial community structure in an urban area. Atmospheric Environment, 40(40), 8074–8080.
Meng, H., Katayama, Y., & Gu, J. (2017). More wide occurrence and dominance of ammonia-oxidizing archaea than bacteria at three Angkor sandstone temples of Bayon, Phnom Krom and Wat Athvea in Cambodia. International Biodeterioration & Biodegradation, 117, 78–88.
Nugari, M. P., Realini, M., & Roccardi, A. (1993). Contamination of mural paintings by indoor airborne fungal spores. Aerobiologia, 9(2), 131–139.
Porca, E., Jurado, V., Martin-Sanchez, P. M., Hermosin, B., Bastian, F., Alabouvette, C., & Saiz-Jimenez, C. (2011). Aerobiology: An ecological indicator for early detection and control of fungal outbreaks in caves. Ecological Indicators, 11(6), 1594–1598.
Portillo, M. C., Saiz-Jimenez, C., & Gonzalez, J. M. (2009). Molecular characterization of total and metabolically active bacterial communities of “white colonizations” in the Altamira Cave, Spain. Research in Microbiology, 160(1), 41–47.
Pusz, W., Ogórek, R., Knapik, R., Kozak, B., & Bujak, H. (2015). The occurrence of fungi in the recently discovered Jarkowicka Cave in the Karkonosze Mts. (Poland). Geomicrobiology Journal, 32(1), 59–67.
Pyrri, I., Tripyla, E., Zalachori, A., Chrysopoulou, M., Parmakelis, A., & Kapsanaki-Gotsi, E. (2020). Fungal contaminants of indoor air in the National Library of Greece. Aerobiologia, 36, 387–400.
Saiz-Jimenez, C., Cuezva, S., Jurado, V., Fernandez-Cortes, A., Porca, E., Benavente, D., Cañaveras, J. C., & Sanchez-Moral, S. (2011). Paleolithic art in peril: Policy and science collide at Altamira Cave. Science, 334(6052), 42–43.
Sanchez-Moral, S., Luque, L., Cuezva, S., Soler, V., Benavente, D., Laiz, L., Gonzalez, J. M., & Sáiz-Jiménez, C. (2005). Deterioration of building materials in Roman catacombs: The influence of visitors. Science of the Total Environment, 349(1–3), 260–276.
Savković, Ž, Stupar, M., Unković, N., Ivanović, Ž, Blagojević, J., Vukojević, J., & Grbić, M. L. (2019). In vitro biodegradation potential of airborne Aspergilli and Penicillia. Science of Nature, 106, 8.
Schabereiter-Gurtner, C., Piñar, G., Lubitz, W., & Rölleke, S. (2001). An advanced molecular strategy to identify bacterial communities on art objects. Journal of Microbiological Methods, 45(2), 77–87.
Schabereiter-Gurtner, C., Saiz-Jimenez, C., Piñar, G., Lubitz, W., & Rölleke, S. (2002). Phylogenetic 16S rRNA analysis reveals the presence of complex and partly unknown bacterial communities in Tito Bustillo cave, Spain, and on its Palaeolithic paintings. Environmental Microbiology, 4(7), 392–400.
Schabereiter-Gurtner, C., Saiz-Jimenez, C., Piñar, G., Lubitz, W., & Rölleke, S. (2004). Phylogenetic diversity of bacteria associated with Paleolithic paintings and surrounding rock walls in two Spanish caves (Llonín and La Garma). FEMS Microbiology Ecology, 47(2), 235–247.
Shirakawa, M. A., Beech, I. B., Tapper, R., Cincotto, M. A., & Gambale, W. (2003). The development of a method to evaluate bioreceptivity of indoor mortar plastering to fungal growth. International Biodeterioration & Biodegradation, 51(2), 83–92.
Spirin, V. F., & Mikhaĭlova, N. A. (1991). Microbial contamination of air at the swine-breeding farms. Gigiena I Sanitariia, 5, 34–36.
Sterflinger, K., & Piñar, G. (2013). Microbial deterioration of cultural heritage and works of art—Tilting at windmills? Applied Microbiology & Biotechnology, 97(22), 9637–9646.
Tanaka, D., Terada, Y., Nakashima, T., Sakatoku, A., & Nakamura, S. (2015). Seasonal variations in airborne bacterial community structures at a suburban site of central Japan over a 1-year time period using PCR-DGGE method. Aerobiologia, 31(2), 143–157.
Vanderwolf, K. J., Malloch, D., McAlpine, D. F., & Forbes, G. J. (2013). A world review of fungi, yeasts, and slime molds in caves. International Journal of Speleology, 42(1), 77–96.
Wang, W., Ma, X., Ma, Y., Mao, L., Wu, F., Ma, X., An, L., & Feng, H. (2010a). Seasonal dynamics of airborne fungi in different caves of the Mogao Grottoes, Dunhuang, China. International Biodeterioration & Biodegradation, 64(6), 461–466.
Wang, W., Ma, Y., Ma, X., Wu, F., Ma, X., An, L., & Feng, H. (2010b). Seasonal variations of airborne bacteria in the Mogao Grottoes, Dunhuang, China. International Biodeterioration & Biodegradation, 64(4), 309–315.
White, T. J., Bruns, T., Lee, S. J. W. T., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: A guide to methods and applications (pp. 315–322). New York: Academic Press.
Zhang, G., Gong, C., Gu, J., Katayama, Y., Someya, T., & Gu, J. D. (2019). Biochemical reactions and mechanisms involved in the biodeterioration of stone world cultural heritage under the tropical climate conditions. International Biodeterioration & Biodegradation, 143, 104723.
Zhang, G. B., Xue, P., Hou, W. F., & Guo, Q. L. (2005). The study on micro-environment of the cave affected by the visitors of the Mogao Grottoes. Dunhuang Research, 4, 83–86.
Zhou, Z., Liu, Y., Lloyd, K. G., Pan, J., Yang, Y., Gu, J. D., & Li, M. (2019). Genomic and transcriptomic insights into the ecology and metabolism of benthic archaeal cosmopolitan, Thermoprofundales (MBG-D archaea). The ISME Journal, 13(4), 885–901.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 32060258, 32060277); Science and Technology Plan of Gansu Province (No. 18JR3RA004, 20YF8WF016); Project of Gansu Cultural Relics Bureau (No. GWJ202011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest in this study.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors of this investigation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duan, Y., Wu, F., He, D. et al. Diversity and spatial–temporal distribution of airborne fungi at the world culture heritage site Maijishan Grottoes in China. Aerobiologia 37, 681–694 (2021). https://doi.org/10.1007/s10453-021-09713-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-021-09713-8