Abstract
Boron (B) is a microelement and has been demonstrated to alleviate cadmium (Cd) stress and inhibit Cd uptake in wheat. However, the effect of B on accumulation of Cd and mineral elements accumulation in wheat are rarely investigated. A hydroponics experiment was performed with different treatments (CK for without B and Cd application, Cd + B, preB + Cd for B, Cd, and B was added 24 h earlier than Cd added) to explore the effect of B on the growth, subcellular component distribution of Cd, and the mineral distribution. Here, the dry weight of shoot and root were decreased under Cd application compared with CK treatment, while increased under B application, especially under preB + Cd treatment. The root parameters showed a similar trend, including surface area, root length and volume, and tips. The Cd concentrations increased under Cd application in root and shoot, while decreased under B application, especially under preB + Cd treatment in the root. In addition, the B concentration showed a decreasing trend under Cd stress, especially in roots. Subcellular component analysis showed that more than 50% Cd was distributed in soluble fractions in the root, while more than 40% Cd of cell wall (CW) fractions in the shoot, respectively. This suggests that CW fractions and soluble fractions are the main Cd storage sites. The correlation analysis was also discussed among B, Cd, and other elements. Thus, we concluded that Cd toxicity was alleviated under B application in wheat by inhibiting Cd uptake, non-organ distribution, and changing nutrient absorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Wheat is a main source of calories and play an important role in feeding the human population. Therefore, it is important to ensure the dietary safety of wheat grains. Cd contamination and toxicity to wheat have been widely reported for the past few years (Rezapour et al. 2019; Zhang et al. 2020a, 2020b). Various human activities (e.g., application of phosphate fertilizers, organic matter, wastewater irrigation, etc.) can cause Cd pollution in agricultural soils. Once the Cd is absorbed by the root system, it can migrate through the xylem to shoot and accumulate in the grain. Under Cd stress, the plant can produce some toxic symptoms, like inducing reactive oxygen species and competing for nutrient transporters, damaging photosystemII, decreasing photosynthetic pigments, destructs chloroplast structures, which causes water stress, nutrient imbalance, and death (Çatav et al. 2020; Qin et al. 2020a). However, there were complex mechanisms for plants to alleviate Cd toxicity, such as preventing Cd uptake by root cells and transport to shoot, detoxifying Cd by increasing antioxidant enzyme activity and sulfur-containing ligands, sequestering Cd by cell wall fixation and vacuole isolation (Singh et al. 2016; Abbas et al. 2017). Some agronomic strategies could also reduce Cd accumulation and toxicity in wheat, such as soil removal and replacement (Wang et al. 2011; Uraguchi & Fujiwara 2012), phytoremediation (including hyperaccumulator and low accumulation variety), crop rotation, gene regulation and inorganic amendments (Rizwan et al. 2016; Zhang et al. 2020a, b; Yang et al. 2022). Moreover, the application of soil amendment (like mercapto-modified palygorskite) could prevent Cd bioaccumulation by the thiols and bacteria community composition (Li et al. 2021b). Glutathione (GSH), as a short peptide consisting of three amino acids, was reported that alleviate Cd stress by regulating the Cd transporter genes and GSH synthesis gene expression (Qin et al. 2018; Li et al. 2021a).
Among these strategies, inorganic amendments are low-cost and effective approaches, which can balance plant nutrition and inhibit Cd absorption. For example, some essential nutrient elements, like calcium (Ca), manganese (Mn), iron (Fe), and zinc (Zn), efficiently compete with Cd in uptake (Duan et al. 2018; Qin et al. 2020a, 2023). Some beneficial elements, like selenium (Se) and silicon (Si), have been proved to inhibit Cd uptake and alleviate Cd stress in crops (Hu et al. 2014; Wang et al. 2015; Ma et al. 2015). The mechanisms are summarized to include (1) promoting plant growth to dilute toxic effects, (2) competition for transmembrane transport, (3) improving the homeostasis of antioxidant enzymes, (4) reducing Cd ion toxicity by binding phytochelatin and cysteine-rich peptides (Semane et al. 2007; Qin et al. 2018, 2020a; Fahad et al. 2015).
Boron was first discovered as an essential trace element for plants in 1923 (Warington 1923). The main function of B in plants is thought to be a component of the cell wall, where it cross-links pectin polysaccharides through diol bonding of two rhamnogalacturonan II molecules (Kobayashi et al. 1996). Boron is mainly present in the form of boric acid (H3BO3) and borate (B(OH)4−), and the effectiveness is mainly affected by pH and water runoff in the soil environment (Klochko et al. 2006). Boron availability in soil is limited in many parts of the world, including USA, Brazil, Japan and China, while B toxicity often naturally occurs in soils of arid and semi-arid regions or anthropogenic activities, such as fertilization and irrigation (Parks and Edwards 2005; Yan et al. 2006; Camacho-Cristobal et al. 2008). In plant, boron has also been showed that B was involved in several physiological metabolic processes, such as photosynthesis, nitrogen (N) and carbon (C) metabolism, cell division and elongation, etc. (Shireen et al. 2018). Therefore, the symptoms of B deficiency and toxicity were reported include stunted leaf and root elongation, unhealthy flower development, oxidative damage, hormone homeostasis disordered, reduction in crop yield and quality (Tanaka and Fujiwara 2008; Hua et al. 2020; Chen et al. 2023). The range between deficiency and toxicity of B is narrow, while B application can ameliorate abiotic stress, such as salinity, drought, aluminum (Al) excess and biotic stresses (García-Sánchez et al. 2020). In addition, boron, as an essential mineral nutrient for plant growth, also affects the state of macronutrients (N, phosphorus P, potassium K, Ca, magnesium Mg, and S), micronutrients (Fe, Mn, Zn, copper Cu, and molybdenum Mo), beneficial elements (sodium Na, Se, and Si), and toxic elements (Cd and Al) (Long and Peng 2023). Actually, it has also been reported that B can inhibit Cd accumulation to alleviate Cd toxicity in wheat and rice (Chen et al. 2019; Qin et al. 2020b). Studies showed that Cd mitigation with B application by enhancing more Cd-binding sites on cell wall fraction and isolation of soluble fraction, and increase ionic soluble pectin, antioxidant system (Wu et al. 2020a, b; Riaz et al. 2021a, b). Here, we further analyzed Cd uptake and subcellular distribution under Cd stress and/or with B application in wheat seedlings. We also discussed the different nutrient element concentrations and the correlation among elements. These results explore the alleviating Cd absorption and toxicity with B application and provide a perspective on the nutrient status of B application under Cd stress.
2 Materials and Methods
2.1 Experimental Conditions and Treatments
A conventional and Cd-tolerant wheat variety, Zhengmai 379, was screened and used as materials for a hydroponic experiment at Henan Agricultural University, Zhengzhou, China (Zhang et al. 2022). Wheat seeds were surface-sterilized in 0.5% Na-hypochlorite for 15 min and then rinsed carefully with deionized water. The seeds were germinated on a plastic seedling tray on deionized water. Uniform 7-day-old wheat seedlings were transferred to the a plastic container (26 cm × 17 cm × 7 cm, length/width/height; 15 seedlings/container) filled with 4 L nutrient solution, and growth in a controlled chamber with photoperiod 16 h/8 h day/night, light intensity 200 µmol m− 2 s− 1, temperature 25℃/20℃, and relative humidity 75%. The full nutrient solution containing (µmol L− 1): Ca(NO3)2·4H2O 4000, NH4H2PO4 1000, KNO3 6000, MgSO4·7H2O 2000, H3BO3 46.2, ZnSO4·7H2O 0.8, MnCl2·4H2O 9.1, CuSO4·5H2O 0.3, FeNaEDTA 100, and (NH4)6Mo7O24·4H2O 0.2. Half-strength nutrient solution was applied for the first 7 d and then changed to full strength solution for every 4 d until harvest. Treatments with four times replicated were set as follows: (1) CK (no B and Cd added), (2) Cd, (3) Cd + B, (4) preB + Cd (24 h pretreatment before Cd exposure). CdCl2 was used as Cd treatment. The B and Cd were added at the first full strength solution (after 7 d of half-strength nutrient solution treatment) with 5 µmol L− 1 and 46.2 µmol L− 1 selected from the earlier experiments of Qin et al. 2020b); Al-Huqail et al. (2020); Zhang et al. (2022), respectively. In addition, B and Cd were added on 6 d and 7 d after treatment with half-strength nutrient solution for preB + Cd treatment, respectively. The root and shoot samples rinsed were harvested and rinsed for further analysis after 30 d culture.
2.2 The Determination of Root Parameters
Root parameters were scanned and calculated according to Qin et al. (2019). Briefly, wheat was harvested and its roots were scanned using a root scanner (Win RHIZO 2009; Canada). The image of the root was analyzed by ImageJ software. The root parameters, including total root length, surface area, root volume, average diameter, and number of root tips, were also calculated by ImageJ software.
2.3 Isolation of Cell Wall Fractions, Organelle Fractions and Soluble Fractions
Different subcellular fractions were separated by differential centrifugation referenced to Qin et al. (2017). The fresh of root and shoot samples (0.5 g) were homogenized in 12 mL extraction buffer, which contains 0.25 mol L− 1 sugar, 0.05 mol L− 1 Tris-HCl, 0.01 mol L− 1 cysteine, and 0.001 mol L− 1 MgCl2. The differential centrifugation was used to separate subcellular fractions, where 2.0 × 103 g and 10 min for cell wall fractions, 1.3 × 104 g and 50 min for organelle fractions and soluble fractions. The above steps were performed on ice.
2.4 Elements Concentration Analysis
The harvested plants were divided into roots and shoots. The samples were weigh and oven-dried at 70℃ to constant weight. The dried tissues were digested in a Microwave Digestion System with HNO3:HClO4 (4:1, volume ratio). Cadmium concentration of digestion solution was determined by graphite furnace atomic absorption spectrometry (GFAAS PinAAcie900T, USA). The concentrations of B, phosphorus (P), potassium (K), magnesium (Mg), calcium (Ca), iron (Fe), copper (Cu), manganese (Mn), and zinc (Zn) were measured by Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES, Varian 710ES, USA).
2.5 Statistical Analysis
All data showed means ± standard error (SE) of four independent replicates. Statistical data were analyzed with SPSS 20.0 (SPSS, Chicago, IL, USA) using LSD’s multiple range test (P < 0.05).
3 Results
3.1 The Dry Weight (DW) and Root Parameters of Wheat under Different Treatments
The perspective of growth (dry weight DW) and root parameters (root length, root surface area, root volume, average diameter and root tips) are the main indexes of plant growth and stress resistance (Table 1). Compared with CK treatment, the shoot DW decreased by 43.5% (P < 0.05), 22.7% (P < 0.05) and 4.5% under Cd, Cd + B, preB + Cd treatments and the root DW decreased by 32.9% (P < 0.05) and 13.3% (P < 0.05) under Cd and Cd + B treatments, respectively. A similar trend was observed in the root parameters. The total length of root decreased by 51.30% (P < 0.05), 49.0% (P < 0.05) and 48.0% (P < 0.05) under Cd, Cd + B, preB + Cd treatments compared with CK treatment, and 45.6% (P < 0.05), 45.8% (P < 0.05) and 32.1% (P < 0.05) for root volume, 48.4% (P < 0.05), 47.3% (P < 0.05) and 40.5% (P < 0.05) for surface area, 52.8% (P < 0.05), 58.5% (P < 0.05) and 52.1% (P < 0.05) for tips, respectively.
3.2 Effect of Different Treatments on Cd Concentration and B Concentration of Wheat
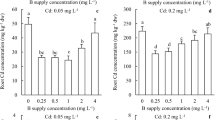
The Cd concentrations in root and shoot were significantly increased under Cd added treatments (Fig. 1). The maximum Cd concentrations of wheat root and shoot were 1014 mg/kg and 222 mg/kg under Cd treatment. The Cd concentration decreased by 21.02% and 31.90% (P < 0.05) in the root, and by 10.74% and 8.38% in the shoot at Cd + B and preB + Cd treatments compared with Cd treatment, respectively. In addition, B application obviously increased the B content in root and shoot of wheat. However, B concentration showed a decreasing trend under Cd stress, especially in roots. The transport coefficient (calculated the ratio of B concentration in shoot and root, data not shown) for B was increased with B application and decreased with Cd added compared with CK treatment, respectively.
The Cd concentration of and root (A) and shoot (B) and B concentration of root (C) and shoot (D) under CK treatment, Cd treatment, Cd + B treatment and preB + Cd treatment. Cd and B are for cadmium and boron in the ordinate, respectively. Data are means ± SE. Different lower case letters above the columns indicate statistical differences among treatments (LSD’s multiple range test at P < 0.05 level)
3.3 Different Treatments Effect on Subcellular Cd Distribution
Further analysis of the subcellular Cd distribution showed that the Cd concentrations including cell wall (CW) fractions, soluble fractions, and organelle fractions of root were higher than those of shoot under different treatments. The Cd concentrations showed that soluble fractions > CW fractions > organelle fractions in root (Fig. 2A), while CW fractions > soluble fractions > organelle fractions in shoot (Fig. 2B). In addition, it was found that Cd concentrations in CW fractions significantly decreased by 28.28% (P < 0.05) and 33.65% (P < 0.05) in root and 29.5% (P < 0.05) and 32.98% (P < 0.05) in shoot at Cd + B and preB + Cd compared with Cd treatment, respectively. Compared with Cd treatment, the Cd concentrations in soluble fractions decreased by 20.16% (P < 0.05) and 32.28% (P < 0.05) in root and 21.74% and 26.79% in shoot at Cd + B treatment and preB + Cd treatment, respectively.
Similarly, the percentage of Cd concentrations showed that soluble fractions > CW fractions > organelle fractions, and more than half of Cd is distributed in the soluble fractions in root (Fig. 2C). The percentage of Cd concentrations of shoot showed that CW fractions > soluble fractions > organelle fractions, and more than 80% of Cd is evenly distributed in the CW fractions and soluble fractions (Fig. 2D).
The Cd concentration of cell wall (CW) fractions, organelle fractions, and soluble fractions in the root (A) and shoot (B) and percentage of Cd concentration in the root (C) and shoot (D) under different treatments. Cd is for cadmium in the ordinate. Data are means ± SE. Different lower case letters above the columns indicate statistical differences among treatments for the same fraction (LSD’s multiple range test at P < 0.05 level)
3.4 Different Treatments Effect on the Nutrient Concentration of Wheat
To better understand the nutrient concentrations under B to alleviate the Cd toxicity, the concentrations of P, K, Zn, Fe, Mn, Ca, Mg and Cu were measured (Fig. 3). On the whole, there was a similar concentration in root and shoot for P, K, and Zn, higher concentration in root for Fe and Cu, and higher concentration in the shoot for Ca, Mg, and Mn, respectively. Our data showed that the P concentrations were the minimum value under CK treatment and increased with Cd application. The P concentrations decreased by 16.49% (P < 0.05) and 17.23% (P < 0.05) in root, and by 5.53% and 9.88% (P < 0.05) in shoot at Cd + B and preB + Cd treatments compared with Cd treatment, respectively. Similarly, there were maximum for Ca, Fe, and Cu under Cd treatment, and the concentrations of Ca, Fe, and Cu were decreased under Cd + B and preB + Cd treatments compared with Cd treatment, respectively. However, the concentrations of Mg, Zn and Mn showed a decreasing trend under Cd application compared with CK treatment.
The concentrations of phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), manganese (Mn), copper (Cu), and zinc (Zn) under different treatments. Data are means ± SE. Different lower case letters above the columns indicate statistical differences among treatments for the same tissue (LSD’s multiple range test at P < 0.05 level)
3.5 The Correlation Coefficients of Different Nutrient Elements in root and Shoot
The correlation analysis of different nutrient elements in root and shoot was carried as shown in Tables 2 and 3. In the root, cadmium was negatively correlated with K, Ca (P < 0.05), Mg (P < 0.01), Mn (P < 0.01) and Zn (P < 0.01), while Cd was positively correlated with B, P, Fe (P < 0.01) and Cu (P < 0.01), respectively (Table 2). The negative correlation was observed between B with all the other elements (including P, K, Ca, Mg, Fe, Mn Cu, Zn, and Cd) in the shoot. And the negative correlation was observed among Cd with B, K, Mg (P < 0.01), Mn (P < 0.01), and Zn (P < 0.05) in the shoot, while a positive correlation was observed among Cd with P (P < 0.01), Ca, Fe and Cu, respectively (Table 3).
4 Discussion
In recent years, soil Cd pollution situation is not optimistic due to some human factors in China, especially soil around mines or non-ferrous smelteries (Zhao et al. 2015; Jiang et al. 2021; Shi et al. 2022). As we all know that B is an essential micronutrient, while Cd is indeed a harmful toxic heavy metal for plant growth and development, where Cd competitive nutrient absorption and inhibits plant growth (Qin et al. 2020a). Meanwhile, the previous research showed that B application could improve oxidative stress and suppress Cd uptake to alleviate Cd accumulation and toxicity in rice (Chen et al. 2019). Boron could inhibit Cd uptake by inhibition of the Cd transporters expression in wheat (Qin et al. 2020b). Here, the root and shoot DW were significantly decreased under Cd added treatment, while increased under B treatment compared with Cd treatment, especially under preB treatment. Similar results were found for root growth parameters, including root length, root volume, surface area, and tips. The results suggested that Cd, as a non-essential element, inhibits root and shoot growth in wheat, while B can inhibit the absorption of Cd and alleviate the toxicity of Cd in wheat. Due to B being cross-linked with the RGII of the cell wall (CW) and the CW being the main interception site for Cd (Loix et al. 2017; Guo et al. 2018). We speculated that there is a complex competition relationship between B and Cd absorption. In the present study, the results suggest that the Cd concentrations in root and shoot were significantly increased under Cd treatment, while decreased under B application treatments. In addition, B application significantly increased the concentration of B in root and shoot of wheat, while showing a decreasing trend under Cd stress. According to the Cd subcellular data, soluble fraction and cell wall fraction are the main storage site of Cd, respectively. Similarly, many plants have been reported that most of the Cd were stored in the soluble fraction in root, such as hot pepper, rapeseed, and barley (Wu et al. 2005, 2020a; Xin and Huang 2014). Our previous study has also found that the soluble fraction and cell wall fraction were the main storage sites of Cd in wheat (Qin et al. 2021). Boron application could increase Cd in the cell wall fraction, but decrease Cd in the soluble fraction of hot pepper root (Wu et al. 2020a; Huang et al. 2022). However, the subcellular distribution of Cd were not significantly influenced by B treatments in our experiment.
In addition, our data showed that Cd promotes the accumulation of P, Ca, Fe and Cu in wheat, while B application inhibited the absorption of these elements under Cd stress. The concentration of Mn and Mg were significantly decreased under Cd treatments regardless of B application. Some elements, like K and Zn, were significantly decreased under Cd + B and preB + Cd treatments. Previous studies also showed that the content of mineral elements, like Mn, Mg, K and Zn, decreased with the increase of Cd concentration (Qin et al., 2023). Further, the correlation analysis of different elements showed that the correlation between elements was different in root and shoot under different treatments. For B, it was found that B was negatively correlated with K, Mg, Mn, and Zn both in root and shoot, while B was positively correlated with P, Ca, Fe, Cu, and Cd in the root, respectively. These results suggest that there is a significant negative correlation among boron and other elements (including Cd) absorption. Similar results have been reported in the previous study, such as B and Cd, B and Ca, K, P, S, Mo, and Zn (Pommerrenig et al. 2019; Qin et al. 2022). In addition, the preferential distribution of B can balance shoot tissues grow and crop nutrition in wheat (Huang et al. 2001; Takano et al. 2008). Therefore, proper B nutrition plays an important role in the balance of mineral elements in plants. For Cd, a negative correlation was found among Cd with K, Mn, and Zn both in root and shoot, while positive correlation with P, Fe, and Cu, respectively. The previous report has also found that there was a dramatic reduction in accumulation and transportation to shoot for P, Ca, K and Mn with Cd treatments in wheat (Zhang et al. 2002; Çatav et al. 2020). Studies have shown that some ion elements can effectively alleviate Cd uptake and toxicity, like macro-elements N, K, P, and medium-trace-elements Ca, Mg, S, and Fe, Cu, Mn, Mo, cobalt (Co), and Zn (Qin et al. 2023; Singh et al. 2016; Hua et al. 2020). The divalent cations, like Mn, Fe, and Zn, could compete with Cd uptake with the same transporters, like family protein for IRT, NRAMP, ZIP (Zhao et al. 2022; Thomine et al. 2003; Sasaki et al. 2014). Other elements, like N, P, K, Ca, and S, could change the rhizosphere environment to affect Cd uptake (Wang et al. 2020; Hussain et al. 2021). As described, these results provide support for the relationship between nutrient absorption and Cd toxicity alleviated by B application. However, further investigation is need on B alleviates Cd toxicity by regulating oxidative mitigation or transporters, especially the functional verification of Cd transporter with high affinity in wheat.
5 Conclusion
This study explored the growth, root parameters, B and Cd uptake, and other nutrition uptake under Cd stress with B application. The results showed that the growth parameters were inhibited by Cd added, while relieved by B application. The Cd concentrations were significantly increased under Cd treatment, while decreased with B application, especially in the root. Further, it was found that Cd was mainly stored in root soluble fractions and shoot cell wall fractions by subcellular components analysis. The element concentration and correlation analysis indicated B has a negative correlation with most elements including Cd in wheat. However, balancing nutrient elements and alleviating Cd toxicity need further study.
References
Abbas T, Rizwan M, Ali S, Zia-ur-Rehman M, Qayyum MF, Abbas F, Hannan F, Rinklebed J, Ok YS (2017) Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol Environ Saf 140:37–47. https://doi.org/10.1016/j.ecoenv.2017.02.028
Al-Huqail AA, Nasir Khan M, Ali HM, Siddiqui MH, Al-Huqail AA, AlZuaibr FM, Al-Muwayhi MA, Marraiki N, Al-Humaid LA (2020) Exogenous melatonin mitigates boron toxicity in wheat. Ecotoxicol Environ Saf 201:110822. https://doi.org/10.1016/j.ecoenv.2020.110822
Camacho-Cristobal JJ, Rexach J, Gonzalez-Fontes A (2008) Boron in plants: deficiency and toxicity. J Integr Plant Biol 50:1247–1255. https://doi.org/10.1111/j.1744-7909.2008.00742.x
Çatav ŞS, Genç TO, Oktay MK, Küçükakyüz K (2020) Cadmium toxicity in wheat: impacts on element contents, antioxidant enzyme activities, oxidative stress, and genotoxicity. Bull Environ Contam Toxicol 104:71–77. https://doi.org/10.1007/s00128-019-02745-4
Chen D, Chen D, Xue R, Long J, Lin X, Lin Y, Jia L, Zeng R, Song Y (2019) Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J Hazard Mater 367:447–455. https://doi.org/10.1016/j.jhazmat.2018.12.111
Chen Z, Bai X, Zeng B, Fan C, Li X, Hu B (2023) Physiological and molecular mechanisms of Acacia melanoxylon stem in response to boron deficiency. Front Plant Sci 14:1268835. https://doi.org/10.3389/fpls.2023.1268835
Duan MM, Wang S, Huang DY, Zhu QH, Liu SL, Zhang Q, Zhu HH, Xu C (2018) Effectiveness of simultaneous applications of lime and zinc/iron foliar sprays to minimize cadmium accumulation in rice. Ecotoxicol Environ Saf 165:510–515. https://doi.org/10.1016/j.ecoenv.2018.09.037
Fahad S, Hussain S, Khan F, Wu C, Saud S, Hassan S, Ahmad N, Gang D, Ullah A, Huang J (2015) Effects of tire rubber ash and zinc sulfate on crop productivity and cadmium accumulation in five rice cultivars under field conditions. Environ Sci Pollut Res 22:12424–12434. https://doi.org/10.1007/s11356-015-4518-3
García-Sánchez F, Simón-Grao S, Martínez-Nicolás JJ, Alfosea-Simón M, Liu C, Chatzissavvidis C, Pérez-Péreze JG, Cámara-Zapata JM (2020) Multiple stresses occurring with boron toxicity and deficiency in plants. J Hazard Mater 397:122713. https://doi.org/10.1016/j.jhazmat.2020.122713
Guo JJ, Tan X, Fu HL, Chen JX, Lin XX, Ma Y, Yang ZY (2018) Selection for cd pollution-safe cultivars of Chinese kale (Brassica alboglabra L. H. Bailey) and biochemical mechanisms of the cultivar-dependent cd accumulation involving in cd subcellular distribution. J Agric Food Chem 66:1923–1934. https://doi.org/10.1021/acs.jafc.7b05123
Hu Y, Norton GJ, Duan G, Huang Y, Liu Y (2014) Effect of selenium fertilization on the accumulation of cadmium and lead in rice plants. Plant Soil 384:131–140. https://doi.org/10.1007/s11104-014-2189-3
Hua T, Zhang R, Sun H, Liu C (2020) Alleviation of boron toxicity in plants: mechanisms and approaches. Crit Rev Environ Sci Technol 51:2975–3015. https://doi.org/10.1080/10643389.2020.1807451
Huang L, Bell RW, Dell B (2001) Boron supply into wheat (Triticum aestivum L. Cv. Wilgoyne) ears whilst still enclosed within leaf sheaths. J Exp Bot 52:1731–1738. https://doi.org/10.1093/jexbot/52.361.1731
Huang Y, Huang B, Shen C, Zhou W, Liao Q, Chen Y, Xin J (2022) Boron supplying alters cadmium retention in root cell walls and glutathione content in Capsicum annuum. J Hazard Mater 432:128713. https://doi.org/10.1016/j.jhazmat.2022.128713
Hussain B, Umer MJ, Li J, Ma Y, Abbas Y, Ashraf MN, Tahir N, Ullah A, Gogoi N, Farooq M (2021) Strategies for reducing cadmium accumulation in rice grains. J Clean Prod 286:125557. https://doi.org/10.1016/j.jclepro.2020.125557
Jiang Z, Guo Z, Peng C, Liu X, Zhou Z, Xiao X (2021) Heavy metals in soils around non-ferrous smelteries in China: Status, health risks and control measures. Environ Pollut 282:117038. https://doi.org/10.1016/j.envpol.2021.117038
Klochko K, Kaufman AJ, Yao W, Byrne RH, Tossell JA (2006) Experimental measurement of boron isotope fractionation in seawater. Earth Planet Sci Lett 248:276–285. https://doi.org/10.1016/j.epsl.2006.05.034
Kobayashi M, Matoh T, Azuma J (1996) Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol 110:1017–1020. https://doi.org/10.1104/pp.110.3.1017
Li XZ, Jia R, Lu XZ, Xu YM, Liang XF, Shen LF, Li BY, Ma C, Wang N, Yao C, Zhang SM (2021) The use of mercapto-modified palygorskite prevents the bioaccumulation of cadmium in wheat. J Hazard Mater 417:125917. https://doi.org/10.1016/j.jhazmat.2021.125917
Li GZ, Chen SJ, Li NY, Wang YY, Kang GZ (2021a) Exogenous glutathione alleviates cadmium toxicity in wheat by influencing the absorption and translocation of cadmium. Bull Environ Contamin Toxicol 107:320–326. https://doi.org/10.1007/s00128-021-03283-8
Loix C, Huybrechts M, Vangronsveld J, Gielen M, Keunen E, Cuypers A (2017) Reciprocal interactions between cadmium-induced cell wall responses and oxidative stress in plants. Front Plant Sci 8:1867. https://doi.org/10.3389/fpls.2017.01867
Long Y, Peng J (2023) Interaction between boron and other elements in plants. Genes 14:130. https://doi.org/10.3390/genes14010130
Ma J, Cai H, He C, Zhang W, Wang L (2015) A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol 206:1063–1074. https://doi.org/10.1111/nph.13276
Parks JL, Edwards M (2005) Boron in the environment. Criti Rev Environ Sci Technol 35(2):81–114. https://doi.org/10.1080/10643380590900200
Pommerrenig B, Eggert K, Bienert G (2019) Boron deficiency effects on sugar, ionome, and phytohormone profiles of vascular and non-vascular leaf tissues of common plantain (Plantago major L). Int J Mol Sci 20(16):3882. https://doi.org/10.3390/ijms20163882
Qin SY, Sun XC, Hu CX, Tan QL, Zhao XH (2017) Uptake, transport and distribution of molybdenum in two oilseed rape (Brassica napus L.) cultivars under different nitrate/ammonium ratios. J Zhejiang Univ-Sci B (Biomed Biotechnol) 18(6):512–521. https://doi.org/10.1631/jzus.B1600249
Qin S, Liu H, Nie Z, Gao W, Li C, Lin Y, Zhao P (2018) AsA-GSH cycle and antioxidant enzymes play important roles in cd tolerance of wheat. Bull Environ Contam Toxicol 101:684–690. https://doi.org/10.1007/s00128-018-2471-9
Qin SY, Guo WY, Cheng J, Qian YC, Liu HE, Gao W, Nie ZJ, Li C, Zhao P (2019) Effects of different cadmium stress on root system of winter wheat. J Henan Agri Univ 53(4):519–524. https://doi.org/10.16445/j.cnki.1000-2340.2019.04.004
Qin S, Liu H, Nie Z, Rengel Z, Gao W, Li C, Zhao P (2020a) Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: a review. Pedosphere 30(2):168–180. https://doi.org/10.1016/S1002-0160(20)60002-9
Qin S, Liu H, Rengel Z, Gao W, Nie Z, Li C, Hou M, Cheng J, Zhao P (2020b) Boron inhibits cadmium uptake in wheat (Triticum aestivum) by regulating gene expression. Plant Sci 297:110522. https://doi.org/10.1016/j.plantsci.2020.110522
Qin SY, Liu H, Mei H, Li Y, Gao W, Nie Z, Li C, Wang L, Zhao P (2021) Effect of different cadmium concentrations on growth response of wheat and uptake and subcellular distribution of cd. J Henan Agri Univ 55(6):1029–1035. https://doi.org/10.16445/j.cnki.1000-2340.20210621.001
Qin SY, Xu Y, Nie Z, Liu H, Gao W, Li C, Wang L, Zhao P (2022) Effect of boron on cadmium uptake and expression of cd transport genes at different growth stages of wheat (Triticum aestivum L). Ecotoxicol Environ Saf 241:113834. https://doi.org/10.1016/j.ecoenv.2022.113834
Qin S, Xu Y, Liu H, Zhao P (2023) Foliar application of zinc promotes cadmium absorption by increasing expression of cadmium transporter genes and activities of antioxidative enzymes in winter wheat. Pedosphere 33(4):612–621. https://doi.org/10.1016/j.pedsph.2022.07.001
Rezapour S, Atashpaz B, Moghaddam SS, Kalavrouziotis IK, Damalas CA (2019) Cadmium accumulation, translocation factor, and health risk potential in a wastewater-irrigated soil-wheat (Triticum aestivum L.) system. Chemosphere 231:579–587. https://doi.org/10.1016/j.chemosphere.2019.05.095
Riaz M, Kamran M, Fang Y, Yang G, Rizwan M, Ali S, Zhou Y, Wang Q, Deng L, Wang Y, Wang X (2021a) Boron supply alleviates cadmium toxicity in rice (Oryza sativa L.) by enhancing cadmium adsorption on cell wall and triggering antioxidant defense system in roots. Chemosphere 266:128938. https://doi.org/10.1016/j.chemosphere.2020.128938
Riaz M, Kamran M, Rizwan M, Ali S, Zhou Y, Núñez-Delgado A, Wang X (2021b) Boron application mitigates cd toxicity in leaves of rice by subcellular distribution, cell wall adsorption and antioxidant system. Ecotoxicol Environ Saf 222:112540. https://doi.org/10.1016/j.ecoenv.2021.112540
Rizwan M, Ali S, Abbas T, Zia-ur-Rehman M, Hannan F, Keller C, Al-Wabel MI, Ok YS (2016) Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Saf 130:43–53. https://doi.org/10.1016/j.ecoenv.2016.04.001
Sasaki A, Yamaji N, Ma JF (2014) Overexpression of OsHMA3 enhances cd tolerance and expression of zn transporter genes in rice. J Exp Bot 65(20):6013–6021. https://doi.org/10.1093/jxb/eru340
Semane B, Cuypers A, Smeets K, Van Belleghem F, Horemans N, Schat H, Vangronsveld J (2007) Cadmium responses in Arabidopsis thaliana: glutathione metabolism and antioxidative defence system. Physiol Plant 129:519–528. https://doi.org/10.1111/j.1399-3054.2006.00822.x
Shi J, Du P, Luo H, Wu H, Zhang Y, Chen J, Wu M, Xu G, Gao H (2022) Soil contamination with cadmium and potential risk around various mines in China during 2000–2020. J Environ Manage 310:114509. https://doi.org/10.1016/j.jenvman.2022.114509
Shireen F, Nawaz MA, Chen C, Zhang Q, Zheng Z, Sohail H, Sun JY, Cao HS, Huang Y, Bie Z (2018) Boron: functions and approaches to enhance its availability in plants for sustainable agriculture. Int J Mol Sci 19:1856. https://doi.org/10.3390/ijms19071856
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6:1143. https://doi.org/10.3389/fpls.2015.01143
Takano J, Miwa K, Fujiwara T (2008) Boron transport mechanisms: collaboration of channels and transporters. Trends Plant Sci 13(8):451–457. https://doi.org/10.1016/j.tplants.2008.05.007
Tanaka M, Fujiwara T (2008) Physiological roles and transport mechanisms of boron: perspectives from plants. Eur J Physiol 456:671–677. https://doi.org/10.1007/s00424-007-0370-8
Thomine S, Lelievre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H (2003) AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J 34:685–695. https://doi.org/10.1046/j.1365-313X.2003.01760.x
Uraguchi S, Fujiwara T (2012) Cadmium transport and tolerance in rice: perspectives for reducing grain cadmium accumulation. Rice 5:1–8. https://doi.org/10.1186/1939-8433-5-5
Wang MY, Chen AK, Wong MH, Qiu L, Cheng H, Ye ZH (2011) Cadmium accumulation in and tolerance of rice (Oryza sativa L.) varieties with different rates of radial oxygen loss. Environ Pollut 159:1730–1736. https://doi.org/10.1016/j.envpol.2011.02.025
Wang S, Wang F, Gao S (2015) Foliar application with nano-silicon alleviates cd toxicity in rice seedlings. Environ Sci Pollut Res 22:2837–2845. https://doi.org/10.1007/s11356-014-3525-0
Wang M, Chen S, Zheng H, Li S, Chen L, Wang D (2020) The responses of cadmium phytotoxicity in rice and the microbial community in contaminated paddy soils for the application of different long-term N fertilizers. Chemosphere 238:124700. https://doi.org/10.1016/j.chemosphere.2019.124700
Warington K (1923) The effect of boric acid and borax on the broad bean and certain other plants. Ann Bot 37:629–672. https://doi.org/10.1093/oxfordjournals.aob.a089871
Wu F, Dong J, Qian QQ, Zhang GP (2005) Subcellular distribution and chemical form of cd and Cd-Zn interaction in different barley genotypes. Chemosphere 60:1437–1446. https://doi.org/10.1016/j.chemosphere.2005.01.071
Wu X, Song H, Guan C, Zhang Z (2020a) Boron alleviates cadmium toxicity in Brassica napus by promoting the chelation of cadmium onto the root cell wall components. Sci Total Environ 728:138833. https://doi.org/10.1016/j.scitotenv.2020.138833
Wu X, Song H, Guan C, Zhang Z (2020b) Boron mitigates cadmium toxicity to rapeseed (Brassica napus) shoots by relieving oxidative stress and enhancing cadmium chelation onto cell walls. Environ Pollut 263:114546. https://doi.org/10.1016/j.envpol.2020.114546
Xin J, Huang B (2014) Subcellular distribution and chemical forms of cadmium in two hot pepper cultivars differing in cadmium accumulation. J Agric Food Chem 62:508–515. https://doi.org/10.1021/jf4044524
Yan X, Wu P, Ling H, Xu G, Xu F, Zhang Q (2006) Plant nutriomics in China: an overview. Ann Bot 98:473–482. https://doi.org/10.1093/aob/mcl116
Yang S, Wua P, Jeyakumar P, Wang H, Zheng X, Liu W, Wang L, Li X, Ru S (2022) Technical solutions for minimizing wheat grain cadmium: a field study in North China. Sci Total Environ 818:151791. https://doi.org/10.1016/j.scitotenv.2021.151791
Zhang G, Fukamin M, Sekimoto (2002) Influence of cadmium on mineral concentrations and yield components in wheat genotypes differing in cd tolerance at seedling stage. Field Crops Res 77:93–98. https://doi.org/10.1016/S0378-4290(02)00061-8
Zhang L, Gao C, Chen C, Zhang W, Huang XY, Zhao FJ (2020a) Overexpression of rice OsHMA3 in wheat greatly decreases cadmium accumulation in wheat grains. Environ Sci Technol 54:10100–10108. https://doi.org/10.1021/acs.est.0c02877
Zhang R, Tao C, Zhang Y, Hou YH, Chang QR (2020b) Health risk assessment of heavy metals in agricultural soils and identification of main influencing factors in a typical industrial park in northwest China. Chemosphere 252:126591. https://doi.org/10.1016/j.chemosphere.2020.126591
Zhang K, Xu Y, Liu H, Zhao P, Qin S (2022) Difference study of response of different winter wheat seedling growth to cadmium. Shandong Agricul Sic 54(7):104–112. https://doi.org/10.14083/j.issn.1001-4942.2022.07.015
Zhao FJ, Ma Y, Zhu YG, Tang Z, McGrath SP (2015) Soil contamination in China: current status and mitigation strategies. Environ Sci Technol 49:750–759. https://doi.org/10.1021/es5047099
Zhao FJ, Tang Z, Song JJ, Huang XY, Wang P (2022) Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol Plant 15:27–44. https://doi.org/10.1016/j.molp.2021.09.016
Funding
This work was financed by the Chinese State Natural Science Foundation and the Scientific Technological Key Projects of Henan Province, the contracts No 32002128 and No 212102310979.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Y.F., M.X., H.L., P.Z., F.S., S.Q., and G.L. The first draft of the manuscript was written by S.Q., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, Y., Xie, M., Liu, H. et al. Effects of Boron Application on Absorption of Cadmium and Other Mineral Elements of Wheat. J Soil Sci Plant Nutr 24, 3943–3952 (2024). https://doi.org/10.1007/s42729-024-01813-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-024-01813-x