Abstract
Wheat (Triticum aestivum L.) has relatively high tolerance to cadmium (Cd), but the underlying mechanisms are poorly understood. Growth and physiological parameters of wheat exposed to different Cd concentrations (0, 0.5, 5 and 50 µM) were characterized. The fresh weight, leaf chlorophyll and carotenoid concentrations and photosynthesis parameters did not differ among Cd treatments, suggesting relatively high Cd tolerance in wheat. However, the soluble sugar concentrations increased with the increasing Cd concentration and the soluble protein concentrations decreased in both shoots and roots, suggesting that the Cd application promoted nitrogen metabolism over carbon metabolism. In addition, the higher concentrations of MDA, GSH and AsA and activities of antioxidant enzymes (SOD, POD, and CAT) were observed in leaves and roots in the Cd50 treatment. Our results reveal that wheat can tolerate Cd by enhancing the antioxidant enzymes activities and increasing the concentration of ascorbate and glutathione.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
During the last few decades, heavy metal contamination, especially for cadmium (Cd), in the environment has been increasingly severe, causing toxicity and diminished output of crops (Rizwan et al. 2016a). Under Cd stress, the initial symptom in plants is usually chlorosis, suggesting the photosynthetic apparatus is particularly susceptible to Cd and a reduction of photosynthesis is a common response in plants (Burzyński and Kłobus 2004). In addition to photosynthesis, Cd toxicity might also alter a battery of physiological and biochemical processes including seed germination, gas exchange, stem and root growth, enzyme activity, hormonal balance, nutrient assimilation, protein synthesis, and DNA replication, with a resultant decrease in biomass production (Gallego et al. 2012). Cd exerts toxicity through four proposed mechanisms, including (i) competiting for cation absorption, (ii) disrupting the structure and function of proteins, (iii) displacing essential cations from specific binding sites, and (iv) enhancing generation the reactive oxygen species (ROS) (Dalcorso et al. 2013). However, plants can resist Cd toxicity by a range of mechanisms, such as reducing Cd uptake and root to shoot translocation, and detoxifying/sequestering Cd (Singh et al. 2016). Moreover, the plant antioxidant defense system plays a crucial role in combating the Cd damage. Antioxidants comprise ROS-removing enzymes such as superoxide dismutase (SOD), catalases (CAT), guaiacol peroxidase (GPX), ascorbate peroxidase (APX), glutathione reductase (GR), and non-enzymatic systems such as ascorbic acid (ASA) and glutathione (GSH) (Shah et al. 2001; Thounaojam et al. 2012). SOD is an enzyme involved in dismutating superoxide radicals in all the cellular compartments (Fridovich 1989). APX is located in the cytosol and chloroplast and is involved in scavenging of H2O2 (Willekens et al. 1997) CAT is an oxidoreductase enzyme catalyzing decomposition of H2O2 into H2O and O2 during photorespiration (Morita et al. 1994). AsA and GSH are hydroxyl radical scavengers and may also chelate Cd in the cytoplasm (Wu et al. 2015).
Wheat (Triticum aestivum) is a globally important crop being the staple food for humans and livestock. Cd is highly toxic to wheat (Jin et al. 2010; Rizwan et al. 2016a). The objectives of the present study were to characterize the responses of antioxidant enzyme and the ascorbate–glutathione cycle to Cd stress in wheat. The results from our study will improve the understanding of Cd tolerance mechanisms in wheat and help develop strategies for alleviating Cd toxicity in wheat cultivation.
Materials and Methods
Zhengmai 379, a commonly-grown cultivar in the Huanghuai winter wheat area in China, was used in this experiment. A hydroponic experiment was conducted in a controlled environment chamber and seed germination and cultivation were as described in our previous study (Qin et al. 2017b). The nutrient solution contained 0.5 mM KH2PO4, 0.5 mM K2HPO4, 0.5 mM Mg(NO3)2⋅7H2O, 1 mM KNO3, 1.0 mM Ca(NO3)2⋅4H2O, 0.125 mM KCl, 50 µM H3BO3, 12 µM MnSO4⋅H2O, 0.7 µM CuSO4⋅5H2O, 1 µM ZnSO4⋅7H2O, 100 µM FeNaEDTA and 0.25 µM Na2MoO4⋅2H2O. Twelve seedlings of uniform size were cultured in a plastic container filled with 2 L of nutrient solution. Cd was applied as CdCl2 at four final concentrations: 0 (control), 0.5 µM (Cd0.5), 5 µM (Cd5) and 50 µM (Cd50). Each treatment was replicated four times. The nutrient solutions were renewed every 3 days and adjusted to maintain pH at 6.5 ± 0.1 with HCl or NaOH. After 25 days of treatment, plants were harvested and divided into roots and shoots, and oven dried or stored fresh at − 80°C for further analyses.
Approximately 300 mg of each sample was digested with a mixture of 6 mL of nitric acid:perchloric acid (4:1, v/v). Cd was measured by flame atomic absorption spectrometry (ZEEnit 700, Analytik Jena AG, Germany) as described by Xin et al. (2016). Chlorophyll and carotenoid contents were determined by a spectrophotometer (TU-1901, Beijing, China). Just before harvest, net photosynthetic rate (Pn) stomatal conductance (Gs), transpiration rate (Tr) and intercellular CO2 concentration (Ci) were measured by a portable photosynthesis system (Li-6400P, Li-Cor, USA) according to Qin et al. (2017a).
The extent of lipid peroxidation was estimated by determining malondialdehyde (MDA) formation using the thiobarbituricacid (TBA) method (Ci et al. 2009). Briefly, 0.5 g of fresh shoot or root samples was homogenized with 2 mL of 5% v/v trichloroacetic acid (TCA) and centrifuged at 10,000×g for 10 min. Then, 1 mL 0.5% v/v TBA in 20% v/v TCA was added to 1 mL supernatant and incubated in boiling water bath for 30 min, followed by an immediate cooling on ice to stop the reaction. Thereafter, the mixture was centrifuged at 10,000×g for 5 min. The absorbance was determined at 450, 532 and 600 nm, and MDA concentration was estimated by the formula: MDA (µmol/g FW) = 6.45 (OD532-OD600)–0.56 OD450.
Soluble sugars and soluble protein in root and leaf tissues were assayed as described by Qin et al. (2017a). GSH was assayed by an enzyme recycling procedure (Anderson 1985). Fresh shoot or root samples (0.5 g) were homogenized in 5 mL acidic extraction buffer [5% w/v potassium–phosphoric acid in 1 mmol L−1 ethylenediaminetetraacetic acid (EDTA)]. The mixture was centrifuged at 14,000×g for 10 min. The supernatant was collected for analysis of GSH at 412 nm using a spectrophotometer (TU-1901, Beijing, China). Results were expressed as µmol GSH g−1 fresh weight.
Antioxidant enzyme activities (SOD, CAT and POD) in roots and leaves were determined spectrophotometrically. Fresh tissues (0.5 g) were ground in liquid nitrogen to a fine powder using a mortar and pestle. The powder was transferred to a pre-cooled (4°C) mortar and pestle with 2 mL of 50 mmol L−1 phosphate buffer (pH 7.0), containing 0.1 mmol L−1 EDTA, 4% (w/v) polyvinylpolypyrrolidone and 0.3% v/v Triton X-100. The homogenate was centrifuged at 15,000×g AT 4°C for 20 min. The supernatant was used for assays of the antioxidant enzyme activities (Beauchamp and Fridovich 1971; Aebi 1984; Zhang et al. 2012).
All data were statistically analyzed using SPSS 20.0 software. Statistical evaluation was performed with ANOVA (LSD-test, p ≤ 0.05), and the results were expressed as the mean values ± SE (standard error) of four biologic replicates.
Results and Discussion
Wheat shoot FW was not significantly affected by the Cd treatments (Table 1), but root FW decreased significantly by 24% at the Cd50 treatment compared with the Cd0 treatment. As expected, the Cd concentration and content in shoots and roots increased significantly by the Cd treatment. For the increasing Cd application rates, the Cd concentrations were 7.5, 8.2 and 5.0-fold higher in roots than shoots, and the Cd contents were 2.2, 2.4 and 1.5-fold higher in roots than shoots.
As reported previously, soil addition of Cd affected the seedling growth of wheat, even at low Cd rates (< 3.3 mg kg−1) (Lin et al. 2007). Naeem et al. (2016) also reported that root and shoot relative dry matter decreased in most wheat cultivars, but some cultivars did not exhibit any toxic symptoms, even at 45 µM Cd concentration. These findings indicated that the wheat cultivar used in the study reported here was relatively tolerant to Cd, with some toxicity obvious in roots only.
Cd did not significantly influence concentration of chlorophyll a and b, but carotenoids concentration was lower in the Cd50 compared with C0 and Cd0.5 treatments (Table 2). There was no significant difference in leaf Pn among the four Cd treatments, and no consistent pattern of the Cd effect on Gs, Ci and Tr.
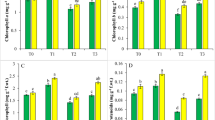
Compared with the Cd0 treatment, the leaf soluble sugar concentrations increased significantly in the treatments Cd5 (by 34%) and Cd50 (by 72%) (Fig. 1a), and the root soluble sugar concentrations increased significantly by 67% and 108% at Cd5 and Cd50, respectively (Fig. 1b). Similarly, Costa and Spitz (1997) reported that the soluble carbohydrate concentrations, ragginose and mannose in Lupinus albus increased with increasing Cd concentrations. Compared with the Cd0, the leaf soluble protein concentrations decreased significantly in the treatments Cd5 (by 12%) and Cd50 (by 20%) (Fig. 1c), and the root soluble sugar concentrations decreased significantly by 22% and 33% at Cd5 and Cd50, respectively (Fig. 1d). These results suggest a nutritional imbalance between proteins and sugars in Cd-treated plants (Gutiérrez et al. 2007; Costa and Spitz 1997).
Concentration of soluble sugars (a: leaves, b: roots) and soluble proteins (c: leaves, d: roots) in winter wheat grown for 25 days under different Cd concentration treatments. Means (± SE), n = 4. Bars with different letters are significantly different at p < 0.05 (LSD’s test). The same applied for Figs. 2 and 3
The Cd50 treatment was associated with increases in MDA concentration of 26% in leaves (p < 0.05) (Fig. 2a) and 397% in roots (p < 0.05) (Fig. 2b) compared with the Cd0 treatment (Fig. 2b). These findings were in agreement with the other reports on the Cd effects in wheat (Sun et al. 2007; Wu et al. 2015), indicating that Cd toxicity was severe in root. The treatments Cd0.5, Cd5 and Cd50 increased GSH concentration significantly (by 84%, 103% and 401% respectively) (p < 0.05), compared with Cd0 (Fig. 2c). In roots, there were no significant differences in GSH concentration between Cd0 and Cd0.5 (Fig. 2d). Cd5 and Cd50 treatments increased GSH concentration significantly [by 62% and 71% respectively (p < 0.05)], compared with the Cd0 treatment (Fig. 2d). The AsA concentration in leaves (Fig. 2e) and roots (Fig. 2f) increased gradually with increasing Cd concentration in nutrient solution, and the highest leaf and root AsA concentrations were recorded in Cd50 treatment. The Cd5 and Cd50 treatments were associated with significantly (p < 0.05) increased AsA concentrations in leaves (by 36% and 49%, respectively), compared with Cd0 (Fig. 2f). As reported, Cd-tolerant genotypes had higher concentrations of GSH and AsA than Cd-sensitive genotypes (Wang et al. 2011; Wu et al. 2015). These results suggested that increasing GSH and AsA concentrations may protect wheat from increasing concentration of Cd (Wu et al. 2015; Rizwan et al. 2016a).
Another important strategy to reduce Cd stress is enhancing the antioxidative enzymes, including SOD, CAT and APX (Ci et al. 2009; Rizwan et al. 2016b). In the study presented here, SOD activities increased significantly (p < 0.05) in the Cd5 (by 19% in leaves and 74% in roots) and Cd50 treatments (by 41% in leaves and 115% in roots) compared with the control (Cd0) (Fig. 3a, b). In the Cd0.5, Cd5 and Cd50 treatments, CAT activities in leaves increased by 131%, 198% and 507%, respectively (Fig. 3c), and in roots by 122%, 235% and 511%, respectively, compared with Cd0 treatment (Fig. 3d). The APX activities in leaves and roots also increased with an increase in the Cd application. Compared with the C0 control, in the Cd5 and Cd50 treatments APX activities in leaves increased by 19% and 38% respectively (Fig. 3e), and in roots by 195% and 318%, respectively (Fig. 3f). These results indicated that the antioxidant system varied among wheat genotypes to reply the Cd tolerance and was also demonstrated to increase with increasing Cd supply (Fig. 4), suggesting that the antioxidant system in wheat was activated by Cd application in adose-dependent manner. Furthermore, it also demonstrated that antioxidant enzyme activities may contribute to Cd tolerance in wheat.
Cd exposure induced-oxidative stress that activated the tolerance and detoxification mechanisms in wheat cells according to the description of Singh et al. (2016). Glu glutamine, Cys cysteine, AsA ascorbic acid, GSSG oxidized glutathione, Gly glycine, H2O2 hydrogen peroxide, O2− superoxide radicals, ROS reactive oxygen species, GS glutathione synthetase, GPX glutathione peroxidase, APX ascorbate peroxidase
In conclusion, different wheat varieties have different Cd tolerance strategies. Our results suggest that the wheat used in our experiment had considerable tolerances to Cd. The tolerance mechanism can be explained by the high-efficiency in AsA–GSH cycle and antioxidant enzyme activities. Further studies focusing on the molecular mechanisms are still necessary.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 113(4):548–555. https://doi.org/10.1016/S0076-6879(85)13073-9
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Burzyński M, Kłobus G (2004) Changes of photosynthetic parameters in cucumber leaves under Cu, Cd, and Pb stress. Photosynthetica 42(4):505–510. https://doi.org/10.1007/S11099-005-0005-2
Ci D, Jiang D, Dai T, Jing Q, Cao W (2009) Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere 77(11):1620–1625. https://doi.org/10.1016/j.chemosphere.2009.08.062
Costa G, Spitz E (1997) Influence of cadmium on soluble carbohydrates, free amino acids, protein content of in vitro cultured Lupinus albus. Plant Sci 128(2):131–140. https://doi.org/10.1016/S0168-9452(97)00148-9
Dalcorso G, Manara A, Furini A (2013) An overview of heavy metal challenge in plants: from roots to shoots. Metallomics 5(9):1117–1132. https://doi.org/10.1039/c3mt00038a
Fridovich I (1989) Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem 264(14):7761–7764
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46. https://doi.org/10.1016/j.envexpbot.2012.04.006
Gutiérrez RA, Lejay LV, Dean A, Chiaromonte F, Shasha DE, Coruzzi GM (2007) Qualitative network models and genome-wide expression data define carbon/nitrogen-responsive molecular machines in Arabidopsis. Genome Biol 8(1):R7. https://doi.org/10.1186/gb-2007-8-1-r7
Jin CX, Zhou QX, Fan J (2010) Effects of chlorimuron-ethyl and cadimum on biomass growth and cadimum accumulation of wheat in the phaiozem area, Northeast China. Bull Environ Contam Toxicol 84:395–400. https://doi.org/10.1007/s00128-009-9635-6
Lin R, Wang X, Luo Y, Du W, Guo H, Yin D (2007) Effects of soil cadmium on growth, oxidative stress and antioxidant system in wheat seedlings (Triticum aestivum L.). Chemosphere 69(1):89–98. https://doi.org/10.1016/j.chemosphere.2007.04.041
Morita S, Tasaka M, Fujisawa H, Ushimaru T, Tsuji H (1994) A cDNA clone encoding a rice catalase isozyme. Plant Physiol 105(3):1015–1016. https://doi.org/10.1104/pp.105.3.1015
Naeem A, Saifullah, Rehman MZ, Akhtar T, Ok YS, Rengel Z (2016) Genetic variation in cadmium accumulation and tolerance among wheat cultivars at the seedling stage. Commun Soil Sci Plant Anal 47(5):554–562. https://doi.org/10.1080/00103624.2016.1141918
Qin S, Sun X, Hu C, Tan Q, Zhao X, Xin J, Wen X (2017a) Effect of NO3 −:NH4 + ratios on growth, root morphology and leaf metabolism of oilseed rape (Brassica napus L.) seedlings. Acta Physiol Plant 39(9):198. https://doi.org/10.1007/s11738-017-2491-9
Qin S, Sun X, Hu C, Tan Q, Zhao X, Xu S (2017b) Effects of tungsten on uptake, transport and subcellular distribution of molybdenum in oilseed rape at two different molybdenum levels. Plant Sci 256:87–93. https://doi.org/10.1016/j.plantsci.2016.12.009
Rizwan M, Ali S, Abbas T, Zia-ur-Rehman M, Hannan F, Keller C, Al-Wabel MI, Ok YS (2016a) Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Saf 130:43–53. https://doi.org/10.1016/j.ecoenv.2016.04.001
Rizwan M, Meunier JD, Davidian JC, Pokrovsky OS, Bovet N, Keller C (2016b) Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ Sci Pollut Res 23(2):1414–1427. https://doi.org/10.1007/s11356-015-5351-4
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161(6):1135–1144. https://doi.org/10.1016/S0168-9452(01)00517-9
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6:1143. https://doi.org/10.3389/fpls.2015.01143
Sun RL, Zhou QX, Sun FH, Jin CX (2007) Antioxidative defense and proline/phytochelatin accumulation in a newly discovered Cd-hyperaccumulator, Solanum nigrum L. Environ Exp Bot 60(3):468–476. https://doi.org/10.1016/j.envexpbot.2007.01.004
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD, Sahoo L, Sanjib P (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53(4):33–39. https://doi.org/10.1016/j.plaphy.2012.01.006
Wang F, Chen F, Cai Y, Zhang G, Wu F (2011) Modulation of exogenous glutathione in ultrastructure and photosynthetic performance against Cd stress in the two barley genotypes differing in Cd tolerance. Biol Trace Elem Res 144:1275–1288. https://doi.org/10.1007/s12011-011-9121-y
Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van MM, Inzé D, Van WC (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. Embo J 16(16):4806–4816. https://doi.org/10.1093/emboj/16.16.4806
Wu Z, Zhao X, Sun X, Tan Q, Tang Y, Nie Z, Qu C, Chen Z, Hu C (2015) Antioxidant enzyme systems and the ascorbate-glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere 138:526–536. https://doi.org/10.1016/j.chemosphere.2015.06.080
Xin J, Zhao X, Tan Q, Sun X, Wen X, Qin S, Hu C (2016) The effects of cadmium exposure on cadmium fractionation and enzyme activities in the rhizosphere of two radish cultivars (Raphanus sativus L.). Bull Environ Contam Toxicol 98(2):290–295. https://doi.org/10.1007/s00128-016-1998-x
Zhang M, Hu C, Zhao X, Tan Q, Sun X, Cao A, Cui M, Zhang Y (2012) Molybdenum improves antioxidant and osmotic-adjustment ability against salt stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis). Plant Soil 355:375–383. https://doi.org/10.1007/s11104-011-1109-z
Acknowledgements
We thank Prof. Zed Rengel (University of Western Australia, Australia) for critical reviewing and revision of the manuscript. This work was financially supported by the Doctoral Scientific Research Foundation of Henan Agricultural University (Grant No. 30500592), National Key R&D Program of China (Grant No. 2017YFD0201700), and Henan Province Science and Technology Research Project (Grant No. 172102110060).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, S., Liu, H., Nie, Z. et al. AsA–GSH Cycle and Antioxidant Enzymes Play Important Roles in Cd Tolerance of Wheat. Bull Environ Contam Toxicol 101, 684–690 (2018). https://doi.org/10.1007/s00128-018-2471-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-018-2471-9