Abstract
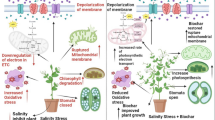
Abiotic stressors are one of the major impediments to plant growth, which results in a large loss of yield and productivity for plant producers. The objective of this research was to comprehend the remediation potential of wood-chip biochar and gallic acid by perusing its effectiveness on biomass, yield, anatomical, physiological and antioxidant enzyme activities of Solanum melongena L under salinity and boron stress. Pot experiment was designed with biochar (5 g kg−1) amendment to soil and seed pre-soaking with gallic acid (2 mM) under induced salinity (NaCl 120 mM) and boron stress (25 mg kg−1 boric acid). Results evaluated that salinity and boron negatively affect plant growth, yield, photosynthetic pigments, and antioxidant defense system. However, biochar and gallic acid treatment under stress-enhanced germination percentage (100–73%), total biomass (TB = 1.83), absolute growth rate (AGR), relative water content (RWC) 115%, plant height stress tolerance index (PHSTI), plant dry mass stress tolerance index (DMSTI) 36% and decreased mean germination time. Likewise, both varieties produce highest fruit yield such as flower number (FLN = 7.3 per plant), fruit number per plant (FRNP = 7.0), fruit size (FRS = 7.5 cm), fresh fruit weight per plant (FRFWP = 28.4 g) and fruit dry weight per plant (FRDWP = 24.6 g) in growth regulators under stress. Leaf chlorophyll ‘a’, ‘b’ content, soluble sugar, protein, superoxidase dismutase (SOD), peroxidase (POD) increased rapidly whereas malondialdehyde (MDA), hydrogen peroxide (H2O2) and glycine betaine dramatically lowered in biochar and gallic acid treatments. Anatomical traits showed that stomata size and density were increased (p < 0.05) with growth stimulators under combined abiotic stress. In conclusion, the application of biochar and gallic acid may be a promising strategy to reduce the negative impacts of abiotic stressors by improving the biomass, yield, physiological, and antioxidant defense of Solanum melongena.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Egg-plant (Solanum melongena L.) is the second most valuable fruit in the family Solanaceae after tomato with different shapes, color and size, low production cost, widely grown and consumed in Southeast Asia and southern parts has increased its popularity in Pakistan (Diaz-Perez and Eaton 2015). Over the world its cultivation area is about 1.86 million hectares (FAO 2018) whereas in Pakistan, brinjal occupies an area of 9044 hectares with an average yield of 88,148 tons hectares (Habib et al. 2015). It is deemed to be rich in fibers and help in regulating sugar level in blood. The phenolics and anthocyanins produced by eggplant inhibits the enzymes involved with type 2 diabetes, response to environmental stresses to generate defense system against infection caused by pathogens and ultraviolet radiation (Nino-Medina et al. 2017; Abbas et al. 2021).
Global climate change is presently deemed as the most destructive hazard to the natural world, getting a substantial consideration from researchers, farmers, and programmed makers due to its major impact on farming. The problem has gotten worse as a result of continual increases in the complexity and unpredictability of environmental conditions as well as global climate change (Uddin et al. 2021; Rodriguez et al. 2007). Such worse environmental factors included abiotic stressors regarded as ultimate constraint to crop yield. Among these stressors salinity is the utmost acute environmental stress presently influencing the agriculture (1128 million hectares land), a foremost hazard to the world food security (Ullah et al. 2022; Wicke et al. 2011).
A high boron concentration reduces plant chlorophyll contents, net absorption rate, lipid oxidation, membrane permeability, and antioxidant enzymes. Hydrogen peroxide (H2O2), a harmful cytotoxic chemical that acts as an intermediary signaling molecule to affect the expression of genes relevant to antioxidant defense systems, is a secondary messenger in stress-response indicators created by redox reactions in plants (Xing 2018). The lethal levels of boron can cause the generation of reactive oxygen species (ROS), like hydrogen peroxide ((H2O2), superoxide (O2−), and hydroxyl radicals (−OH) which can significantly stimulate oxidative stress in plants (Metwally et al. 2018). Upregulation of antioxidant defense mechanism in the plant cell by both non-enzymatic antioxidants (ascorbate, carotenoids, alpha-tocopherol, and glutathione), and enzymatic antioxidants such as peroxidase (POD), superoxide dismutase (SOD), mechanisms to decompose H2O2 into useful water and oxygen (O2) takes place in plant tissues/cells. These enzymatic and non-enzymatic antioxidants increase the ability of plants to withstand salt stress by oxidizing co-substrates (Landi et al. 2019; Yang et al. 2019; Eraslan et al. 2007).
Biochar a black biomass of carbon, low-cost porous pyrogenous matter formed at high temperature from organic wastes (crop deposit, animal, or poultry manure etc.) under zero or inadequate oxygen environments in a closed furnace at ≤ 700 °C through pyrolysis (Lutfunnaha et al. 2021). In recent years, biochar has gained significant attention for its use in combating global climate change through the sequestration of atmospheric CO2 into soil. In addition to its appropriate carbon content, biochar also has the potential to be more effective at supplying plants with nutrients and minerals in highly weathered, nutrient-poor, and degraded soils than in well-structured, high-quality nutrient-rich soils (Chaganti et al. 2015; Tsai et al. 2012).
Gallic acid (GA) used in phytoextraction of different metals by chelating mechanism helps the solubilization of Ni, Zn, and Cd from contaminated soil (Volf et al. 2012). Maintenance of crop capacity to high growth rate, RWC, and photosynthetic capability was estimated in gallic acid treated plants under induced abiotic stress in Glycine max L. (Yildiztugay et al. 2017). GA-induced tolerance in rice seedlings against NaCl stress was examined by enhancing the activities of H2O2-scavenging enzymes such as POD, SOD, CAT, and APX, hence protect cell membranes from oxidative damages caused by ROS (Ozfidan-konakci et al. 2015). Currently, there is no published scientific report on the possible preventive roles of gallic acid and biochar on individual and combined effect of salinity and boron stress in egg-plant. So, the present study was conducted to explore the germination, agronomic and physio-biochemical attributes of egg-plant under induced abiotic stressors.
Materials and Methods
Biochar Preparation and Physico-Chemical Analysis Through SEM and EDX
Biochar produced from hardwood of Vachellia nilotica L. was prepared in furnace with a thermocouple at average temperature of 500–550 °C for 24–48 h by pyrolysis. Well powdered biochar was analyzed with exposure to a gold glaze put on Spi coating segment for morphological characteristics using scanning electron microscope (JSM5910-JEOL-JAPAN) following the procedure of Lalay et al. (2021). Furthermore, energy dispersive X-ray spectroscopy (INCA200/Oxford instruments, U.K) was used for elemental analysis including total carbon (C), oxygen (O), nitrogen (N), sulphur (S), chlorine (Cl), calcium (Ca), magnesium (Mg), aluminum (Al), silicon (Si), iron (Fe), cupper (Cu) and zinc (Zn). Biochar suspension with water (1:10 w/v) was used to determined pH by the method of Li et al. (2016). Cation exchange capacity (CEC) of biochar was measured through ammonium acetate method following the standard protocol modified by Thomas et al. (1982). Electrical conductivity (EC) of biochar was evaluated by the methodology of Gaskin et al. (2008).
Site Description and Seed Sterilization
Pot experiments were performed at University of Peshawar (34° 1′ 33.3012'' N and 71° 33′ 36.4860'' E.), KPK Pakistan lies in Iranian plateau with 513 mm of mean annual rainfall in 2021. Soil texture was determined by hydrometer method established by Gee and Bauder (1986) whereas, EC of the saturated soil was measured at 25 °C by calibrated EC meter (BANTE, DDS-12DW, China). Exchangeable sodium percentage of soil was measured by using the methodology of Page et al. (1982). Soil and farmyard elemental analysis i.e. calcium (Ca), aluminum (Al), potassium (K), silicon (Si), oxygen (O), iron (Fe) and zinc (Zn) were accessed by energy dispersive X-ray spectroscopy. The seeds of two varieties (Neelam & BSS 513) of Solanum melongena L. were collected from National Institute of Food and Agriculture (NIFA) Pakistan, and were surface sterilized with 0.1% mercuric chloride solution and 70% ethanol for five minutes followed by thorough washing with deionized water (Warwate et al. 2017).
Experimental Design and Growth Conditions
Pots were carefully kept at a nursery in a complete randomized block design (CRBD) at a space of 5 cm away from each other with a net plot size of 3.0 × 2.0 m for proper air passage and were kept safe from rain. The experiment was carried out in green house with 2 × 2 × 2 (two varieties, two level of abiotic stress, treated and non-treated soil with biochar and seed with gallic acid). Both varieties of eggplant with 12 treatments in triplicates was divided into three groups. Group-1 was taken as control (untreated by stresses), Group-2 was primed with 2 mM solution of GA solution (3, 4, 5-triphydroxyl-benzoic acid) before sowing for 24 h (Yildiztugay et al. 2017), and Group-3 was treated with biochar mixed in ratio of 5:1 g kg−1 soil at the time of sowing. Treatments were designed as;
-
T1 = Control (Untreated).
-
T2 = 25 mg kg-1 Boric acid.
-
T3 = 120 mM NaCl.
-
T4 = 25 mg kg-1 Boric acid + 120 mM NaCl.
-
T5 = 5 g kg-1 Biochar.
-
T6 = 5 g kg-1 Biochar + 25 mg kg-1 Boric acid.
-
T7 = 5 g kg-1 Biochar + 120 mM NaCl.
-
T8 = 5 g kg-1 Biochar + 25 mg kg-1 Boric acid + 120 mM NaCl.
-
T9 = 2 mM Gallic acid.
-
T10 = 2 mM Gallic acid + 25 mg kg-1 Boric acid.
-
T11 = 2 mM Gallic acid + 120 mM NaCl.
-
T12 = 2 mM Gallic acid + 25 mg kg-1 Boric acid + 120 mM NaCl.
Before seed sowing, 72 pots were well ploughed and filled with 2 kg silt clay (1:2) soil along with farmyard manure. Ten seeds of each variety were sown in earthen pots of 18 cm top and bottom diameter and 20 cm height placed 10 cm apart in triplicates. Pots were thinned after a week of seed germination and 5 healthy seedlings were maintained. Data counted for germination parameters was taken from 1st day to 10th day before application of stress. After 15 days of post germination, plants were subjected to salinity stress of 120 mM NaCl solution (Ozfidan-konakci et al. 2015) and boron stress by applying boric acid powder of 25 mg kg−1 soil (Metwally et al. 2018). After induced stress on 25th day, plants were up-rooted for the measurements of agronomic studies such as roots were gently removed and then washed distilled water to remove adhered dust particles. After absorbing moisture from the root surface, the fresh weights of root and shoot was measured. Shoot and root length, leaf area via dry weights were determined after drying in an oven at 30 °C for 72 h until the weight became constant whereas undamaged fresh leaf of plants per treatment in replicates were collected and stored in refrigerator at 4 °C of photosynthetic pigments, osmoprotectants, and plant antioxidant enzymes through spectronic UV-1700 Shimadzu, Japan. Some of the fresh leaves from each treatment were morphological studies through SEM for the analysis of stomatal physiology. The crop was then left until flowering and fruit formation. After day 75 of germination, harvesting was carried out with the collection of purple color fruits for each replication and were weighed for fresh and then oven-dried to a constant dry weight for 72 h at 30 ℃. Best precautions were carried out throughout the experiment including weeding approximately once a week and no pest or disease were seen in the experimental period.
Determination of Germination and Growth Parameters
Germination Energy (GE)
Number of germinated seeds were recorded on daily basis according to the formula given by Shumaila and Ullah (2020).
Here X1 to Xn (emerged seeds on day first till nth day), Y1 to Yn (days count from number sowing nth count).
Final Germination Percentage (FG%)
Final germination percentage was defined by the method of Al-Ansari Ksiksi (2018).
Timson Germination Index (TGI)
Timson germination index was evaluated by following the standard formula of Al-Ansari and Ksiksi (2018).
“G” (seed germination percentage), “T” (time of germination).
Coefficient of Velocity of Germination (CVG)
The CVG described the speed of seed germination. The value will be found highest when the required for germination is lower. If all the seeds emerged on first day then the highest CVG value (100%) could be possible (Shumaila and Ullah 2020).
“N” (number of seeds germinated), “T” (number of days).
Mean Germination Time (MGT)
Mean germination time denotes the minimum time for germination. Lower rate of mean germination time will give the highest population germinated (Shumaila and Ullah 2020)
where “f” is the number of seeds germinated on day x.
Germination Index (GI)
Germination index gives reported highest to the seeds germinated on first day while minimum to those germinated later (Shumaila and Ullah 2020).
where n1, n2... n10 (number of germinated seeds on first, second day till day10th) while 10, 9... and 1 (weights of the number of germinated seeds).
Germination Rate Index (GRI)
Germination rate index suggests the percentage of germination calculated by the proposed formula of Shumaila and Ullah (2020).
“G1” (germination percentage × 100), “GX” (final germination percentage × 100).
Time to 50% Germination (T50%)
Time to 50% germination was counted from day of seed sowing until 50% of seed has been emerged by the formula represented by Vujosevic et al. (2018)
“N” denotes the final germinated seeds number, nj and ni represents the cumulative germinated seeds by adjacent counts at times tj and ti, respectively, such as ni < N/2 < nj
Absolute Growth Rate (AGR)
Ghule et al. (2013) represented the formula for calculating the absolute growth rate that denotes increase in rate of growth variable during time “t” and was calculated through differential coefficient of ‘W’ with time ‘t’. Height and mass of the crop was measured two times (vegetative and flowering stage) during growing season and then the mean value for each treatment was measured.
where H1 and H2 are the heights (cm) of plant measured at t1 and t2 from soil level to the tip of the plant whereas; and W1 are W2 are the dry weight (g) of plant from time t1 and t2, respectively. Time was expressed in days.
Relative Growth Rate (RGR)
The data obtained by plant biomass separated into root, stem and leaves after harvest and were used to measure the relative growth rate indicated the rate of growth per unit dry weight (Ghule et al. 2013). Dry mass of the plant was measured two times (vegetative and flowering stage) after oven-drying at 30 °C for 72 h.
where ‘loge’ is the natural logarithms whose calculated value is 2.3026. the parameter was measured in g/plant/day.
Crop Growth Rate (CGR)
It was determined by following the standard formula represented by Kul et al. (2021)
where, W1 and W2 are dry weights of plant taken at time t1 and t2, respectively.
Relative Water Contents (RWC)
Relative water contents calculated by estimating the fresh leaf weight in grams and then kept it in oven for drying for 72 h. However, leaf saturated weight was determined by soaking in distilled water for 18 h (Kul et al. 2021).
where, “Wf” denotes leaf fresh weight, (Wd) its dry weight and (Ws) is the leaf saturated weight.
Total Biomass
Total biomass of the plant was calculated by the dry mass of root, stem and leaf after drying at 30 °C for 72 h in oven.
Stress Tolerance Index
Stress tolerance index is a useful tool for determining the high yield and stress tolerance potential of genotypes. Stress tolerance indices for different growth parameters were calculated using following formulae (Amin et al. 2014).
Stomata Index (SI)
Stomata index was calculated by the method of Dubberstein et al. (2021).
Determination of Yield Parameters
Crop yield was determined by collecting the fruits after 75 days of germination period for fresh weight analysis and then oven dried at 30 °C for 72 h in oven. Yield parameters included;
-
1.
Flower number per plant.
-
2.
Fruit number per plant.
-
3.
Fruit size.
-
4.
Fruit fresh weigh.
-
5.
Fruit dry weight.
Size of the fruit was measured with the help of digital vernier caliper at three different positions and measurement was the average of three fruits per plant. Fruits were kept for 72 h in oven at 30 ℃ for dry mass analysis.
Physiological and Biochemical Attributes
Determination of Chlorophyll Content
Chlorophyll contents were evaluated by the standard methodology of Zou et al. (2017). Fresh leaves (0.2 g) were grounded in mortar and pestle in 80% acetone and incubated for 24 h in the dark followed by centrifugation at 2000 rpm. The absorbance was recorded at 649 nm for chlorophyll “a” content, and 663 nm for chlorophyll “b” content through spectrophotometer against 80% acetone blank.
Determination of Soluble Sugar Content (SSC)
Soluble sugar content was determined by a modified protocol of Bouzroud et al. (2018) with little modifications. Fresh leaves of 0.5 g were grounded with 10 ml deionized water followed by centrifugation at 10,000 × g for 10 min and homogenized by adding 1 ml of 30% phenol. Take 0.1 ml supernatant and samples were incubated for 20 min. Concentrated sulphuric acid of 5.0 ml of was mixed with the samples after incubation and optical density were recorded through UV detector at 420 nm.
Determination of Glycine Betaine Content (GB)
Glycine betaine content (GB) content in leaf was extracted and quantified by using the methodology of Shah et al. (2021). In detail, 0.5 g frozen leaf was chopped in 10 ml distilled water. The reaction buffer for measuring GB was filtered and the filtrate was diluted with 2 ml H2SO4 solution. After the homogenate was centrifuged at 10,000 × g for 20 min, cold potassium-iodide iodine (KI–I2) was mixed with the supernatant. 1 ml supernatant was collected and measured for its optical density at 365 nm. The parameter was examined using the protocol of Shah et al. (2021).
Determination of Soluble Protein Content (SPC)
Soluble proteins in leaves were calculated by the standard method of Lowery et al. (1951). Fresh leaves (0.5 g) were chopped in 1 ml phosphate buffer solution (pH 7.0) and homogenate for 10 min. About 0.1 ml extract was taken followed by the addition of distilled water to make a total volume of 10 ml. The solution was then mixed with 1 ml reagent including [0.1 N sodium hydroxide, 0.75 g sodium carbonate, 0.37 g sodium potassium tartrate in 40 ml deionized water]. The solution was shacked for 15 min followed bt addition of 0.1 ml folin phenol reagent and incubated for 30 min. Absorbance of the samples were calculated at 650 nm using spectrophotometer.
Determination of Hydrogen Peroxide Content (H2O2)
Hydrogen peroxide content was analyzed using the methodology proposed by Shah et al. (2021). 0.5 g of frozen leaf sample was chopped in 5 ml trichloro acetic acid (TCA) followed by centrifugation for 15 min. The supernatant (0.5 ml) was mixed with 0.5 ml phosphate buffer and 1 ml potassium iodide (KI) reagent. H2O2 level was calculated using a standard curve calculated at 390 nm.
Determination of Malondialdehyde Content (MDA)
Malondialdehyde content (MDA) was determined using the methodology of Yin et al. (2022) with some modifications. Fresh leaf material (0.25 gm) was chopped in 3 ml 1.0% (w/v) trichloro acetic acid (TCA). The reaction mixture was spun in centrifuge machine at 10,000 rpm for 20 min. 1 ml supernatant was mixed with 4 ml of 0.5% (w/v) 2-thiobarbituric acid (TBA). The mixture was heated at 95 °C for 1 h and the samples were then cooled in ice bath. Optical density was measured at 532 nm.
Determination of Antioxidant Enzymes
Peroxidase (POD) and superoxide dismutase (SOD) activity levels was examined according to the proposed methods of Ma et al. (2017). 0.5 g fresh foliar material were grounded and homogenized with 2 ml solution contained [0.2 ml phosphate buffer solution (pH 7.0), 12.5 g (PVP), 4.6 g ethylene diamine tetra acetic acid (EDTA) followed by the addition of 125 ml deionized water and centrifuged at 10,000 rpm for 20 min. Total reaction mixture (3 ml) included 0.1 ml supernatant, 0.1 ml phenyl diamine (36 mg in 4 ml deionized water), 1.3 ml methyl ethyl sulphonic acid (MES), (970 mg MES dissolved in 50 ml deionized water), and a single drop of H2O2 (0.3% v/v). Absorbance of samples was observed for 3 min at 485 nm by spectrophotometer.
SOD activity was assayed by measuring the inhibition of nitro blue tetrazolium reduction. The reaction mixture of 3 ml for SOD content in 0.5 fresh leaves included 0.1 ml supernatant, 0.72 ml nitroblue tetrazolium (NBT) contained 1.89 mg NBT mixed with 30 ml deionized water, methionine 0.72 ml (58 mg methionine added in 30 ml deionized water), 0.72 ml EDTA (1.1 mg EDTA dissolved in 30 ml deionized water) and riboflavin 0.72 ml (0.02 mg riboflavin in 30 ml deionized water) followed by 30 min incubation period in dark. One unit of SOD activity was defined as an absorbance change of 1 per min in OD560nm causes inhibition of the photo reduction of NBT by 50%.
Determination of Leaf Anatomy Through SEM
One leaf per replicate was harvested and immediately submerged into liquid nitrogen for 1 min followed by 30 s submersion in methanol and 1 min submersion in hexamethyl disilazane. For leaf surface anatomy, leaves were mounted on SEM cylinder specimen mounts (JSMIT100-JEOL-JAPAN, aluminum, grooved edge, Ø32 mm). Leaves were oriented so that the adaxial surface could be examined. Mounted leaves were coated using JEOL-EC-32010CC Coating System for 30 nm of carbon. A JEOL scanning electron microscope was used to examine leaf surface anatomy including stomata size and density of each variety at high 20 kV, working distance ranging from 15 to 16 mm, magnification range from 2.20 kx to 5.00 kx with a specimen stage T = − 10°– + 90° and R = 360°, respectively.
The standard methodology of Liu et al. (2021) was applied with minor modifications for leaf anatomical studies through electron microscopy. On 15th day of salinity and boron stress treatment, mature leaves were randomly obtained from each treatment and peeled carefully at about 1 mm2 size and preserved in formalin acetic alcohol solution (90% ethanol, 5% formalin, 5% acetic acid) at 4 °C until dehydration. The cell structure of the samples was mounted in Canada balsam and the images were taken using Nikon Eclipse E600 microscope (DS-U3, Nikon, Japan). Leaf pieces from three separate plants/treatment were obtained and the values were expressed in micrometers. The trichome size was measured by microscope graticules, and the values were the mean of 3 measurements.
Statistical Analysis
The statistical analysis was a factorial design with induced salinity and boron stress. The analyses were performed in triplicate (n = 3) for various parameters including germination, agronomic, anatomical, physiological, and biochemical attributes were analyzed by Statistix 10 and IBM SPSS Statistics 22 (SPSS Inc, Chicago IL). Three-way ANOVA at significance difference (p ≤ 0.05) for all measurements, mean separation and standard deviations were compared by Tukey’s multiple range test at p ≤ 0.05 for each variety separately. Pearson correlation (R) was measured by the same software.
Results
Physico-Chemical Analysis of Soil and Biochar
In the present research work, precise surface morphological studies of soil and biochar was investigated SEM/EDX, which was processed to determine the alterations in surface forms and quantify chemical analysis. SEM micrographs revealed large sized and many pores biochar component with several cracks which enhance soil water holding capacity, araciality of carbon content in soil environment and inviting more microbes including fungi, ascomycetes and algae that help in soil fertility. Furthermore, EDX probe of SEM for elemental analysis of well fined biochar were in weigh percentage including maximum carbon content (56.33%), followed by oxygen (35.34%), silicon (3.12%), calcium (1.36%), iron (1.34%), aluminum (1.06%), nitrogen and magnesium (0.63%), chlorine (0.10%), copper (0.06%), zinc (0.04%) and sulphur content (0.01%) were determined (Table 1). Similarly, SEM showed integrated soil structure and profile with small pore, sphere like with rough surface. Physico-chemical measurement of soil through EDX probe (Fig. 1; Table 1) presented large content of oxygen (54.86%) followed by silicon (20.2%), calcium (7.86%), aluminum (7.46%), iron (6.91%) and potassium (2.47%), respectively.
Germination Parameters
Seed germination is a vital process in plant life begins with phenomena of imbibition following the activation of biochemical stage, process of cell division, cell elongation and its differentiation (Awatif and Alaaeldin 2017). However, the unavailability of soil moisture content enough for imbibition of seed and activation of metabolic events delay the initiation of seedling germination and retarding the rate of germination for a tolerant crop (Ma et al. 2016). The results in Table 2, revealed that all the traits were affected by the experimental factors and there was completely non-significant difference between control and seeds primed with 2 mM gallic acid solution and soil provided with 5 g kg−1 biochar except for TGI which was found significant in both varieties (p < 0.05) under growth stimulators. In the present study, the finding represented that imbibition of seeds with gallic acid in T12 before sowing has greatly enhanced timson germination index (0.91 ± 0.08), germination index (41.0 ± 3.7), germination rate index (1.48 ± 0.2), germination energy percentage (1.23 ± 0.05) time to 50% germination (3.27 ± 0.56) and reducing mean germination time (6.42 ± 0.36) in variety Neelam as compared to control seedlings without gallic acid priming. However, germination energy (GE) of seedlings under T2 and coefficient of velocity of germination (CVG) under T10 in gallic acid primed has been determined highest. This suggested the more potential role of gallic acid pretreatment to seeds initiated some metabolic processes for promoting early germination efficiency. However no significant differences were found among the parameter as reported in supplementary Table 1.
Similarly in variety BSS 513, timson germination index (1.10 ± 0.0) was obtained highest at T7 when the soil was amended with biochar supply soluble nutrient to germinating for activating some metabolic processes to initiate seedling formation early. In detail, maximum germination rate index (1.87 ± 0.7) and germination energy percentage (1.22 ± 0.02) has been calculated under treatment T8 in combined boron sand salinity stress whereas coefficient of velocity of germination (111 ± 6.90) was noticed highest under biochar addition to soil (T7) as compared to control, clearly suggested that biochar increased soil moisture content, physical and chemical status that indirectly enhanced seeds viability for germination. Consequently, regarding under control condition (T1) germination index (51.3 ± 2.0), germination energy (1.26 ± 0.02), time to 50% germination (3.64 ± 0.8) was increased and decreasing mean time to seedling germination (5.86 ± 0.24) was evaluated (Table 1). Results suggested that both the varieties given different responses to gallic acid and biochar treatment proposed the viability of varieties to germination indices. However, some of the treatments T7, T11 reported decreased seedlings germination indices but recorded non-significant as compared to control and growth regulators treatment mentioned in ANOVA supplementary Table 1, where interactions between genotype, between treatment, growth regulator, T x G, G x GR, T x GR, and G x T x GR were non-significant (p > 0.05) respectively.
Growth Parameters
Plant growth parameters (Table 3) are of significant measures to observe the effect of abiotic stresses. Salt stress (NaCl) alone or in combination with boron stress considerably suppressed leaf RWC in variety Neelam (47%) and BSS 513 (55%). However, applied biochar treatment to soil significantly (p < 0.05) enhanced leaf RWC by 115% and 66% reporting the ability of biochar to conserve water in soil and thus enhance the vigor and viability of plant under stress. Similarly, RGR (0.880) and CGR (0.69) was non significantly reduced by boron stress with gallic acid pretreated seeds in variety Neelam as compared to control (T1). However, in variety BSS 513 it was found a little bit decreased under salinity stress by applied biochar as compared to combined effect of salt and boron stress where the same traits were measured highest thus declaring the stress modulating ability of biochar amendment to soil. Plant biomass was obtained greater at significant level (p < 0.05) by applying biochar application in Neelam variety (1.443 ± 0.3) and gallic acid pretreatment to seed in variety BSS 513 (1.8367 ± 0.19). However, the biomass of different organs as root, stem and leaf biomass was also examined. Increased salinity stress affected the total dry mass of plant reducing biomass production to 0.453 ± 0.08 in variety Neelam. On the other hand, boron stress effectively reduced the biomass in BSS 513 (0.356 ± 0.11) more significantly without the growth regulators (p < 0.001). Such results reported the affective role of biochar and gallic acid enhancing total biomass of plant, respectively.
Rendering to absolute growth rate of plant with respect to plant height (AGR-I) and its dry mass (AGR-II) was found highest by biochar amendment under boron stress (0.15 ± 0.04, 0.038 ± 0.05) and decreased non significantly when salinity stress (T2) was applied in variety Neelam. Furthermore, gallic acid primed seeds reported increased plant height (0.20 ± 0.05) and dry mass in T10 (0.036 ± 0.180) by alleviating the adverse effect of boron stress in BSS 513 and found lower under combined salt and boron stress. These results successfully representing the positive and distinguishing effect of both varieties under different growth regulator treatments and under different stress situations. The enhancement in plant height stress tolerance index (PHSTI) was highly significant (p < 0.05) and more pronounced by biochar application in Neelam variety (36.60 ± 0.0) and gallic acid (36.30 ± 0.0) in BSS 513. The non-significant decrease in stress-tolerance capability of Solanum melongena L. under stress treatment which were greatly decreased by applied growth regulators as compared to control without growth stimulators suggesting the semi-tolerance ability of the specie under induced abiotic stress. Contrarily, dry mass stress tolerance index (DMSTI) of plant was significantly (p < 0.05) enhanced in biochar treatment (T5) in Neelam variety (9.300 ± 4.70) and observed minimum under salinity stress in gallic acid presoaked seeds. However, in variety BSS 513 highest DMSTI was recorded in T12 (13.00 ± 14.0) where gallic acid pretreatment alleviate the toxicity of both combined boron and salt stress while minimum value has been reported under combined stresses without growth regulators (p < 0.05). Results undoubtedly presented the crucial role of biochar and gallic acid by enhancing the stress tolerance index of the specie to give more height and biomass production. ANOVA results in supplementary Table 1, revealed that all the interaction between genotype, between treatments, between growth regulators and their relations with each other T x G, G x GR, T x GR, and G x T x GR were found non- significant (p < 0.05) for the measured traits except PHSTI and DMSTI that indicated positive and significant interactions.
Yield Parameters
Crop yield must be increased markedly over the upcoming years to keep pace with global food demand led by growing population. Abiotic stresses decrease growth and yield of crop by affecting plant metabolism. Results obtained from the designed experiment (Table 4) showed that flower number (FN) per plant measured enhanced under biochar treatment (T5) to 7.33 ± 0.57 in Neelam variety and reduced by boron stress (T10). Control condition favored maximum flower number (3.6 ± 1.1) in BSS 513 while combine boron and salt stress decreased FLN non significantly. The highest fruit number per plant (FRNP) accessed when plant was exposed to combined salinity and boron stress under treatment T12 in both varieties (7.0 ± 1.0, 5.6 ± 4.6) with biochar application in Neelam and gallic acid presoaked seed in BSS 513. Based on these findings, the growth regulators have an affective potential in stimulation of various mechanisms that increase plant yield. Nonetheless, biochar amendment to soil upgrades the fruit size (FRS) taken in centimeters under control treatment (7.44 ± 6.6) in Neelam variety and decreased in T4. Likewise, large sized fruits in BSS 513 were found under boron stress with biochar formation (T6) whereas salinity stress decrease the size of fruit, respectively. Fruit weight less than 10 g is of no economic importance. Plants of both varieties responded differently to biochar and gallic acid such as soil treated with biochar under combined salt and boron stress (T8) increased fruit fresh weight per plant (FFWP) and fruit dry weight per plant (FDWP) by 19.47 ± 3.72 and 14.70 ± 3.72 g in Neelam. On the other hand, variety BSS 513 represented increase in FFWP by 28.40 ± 1.27 under gallic acid treatment (T9) and FDWP in combined born and salt stress by 24.6 ± 2.19 with same growth regulator. However, at combined abiotic stresses (T4), minimum FFWP (7.23 ± 0.25) and FDWP (2.30 ± 0.90) in Neelam variety has been found in comparison to control treatment. An effective decrease in FFWP (7.73 ± 0.9) and FDWP (2.36 ± 0.9) assumed under interactive effect of salt and boron stress, respectively. It has been proposed that improvement in fruit yield of plant by biochar and gallic acid might be correlated with enhancement in leaf chlorophyll content and thus improving plant performance under suboptimal growth conditions. There was no significant treatment effect found on egg-plant fruit number, fresh weight, dry weight as compared to control as shown by statistical data (Supplementary Table 1).
Physiological and Biochemical Attributes
Apparent morphological and physiological attributes of egg-plant are related to its ability in maintaining cellular metabolism, water uptake and oxidative stress under abiotic stresses lowered the physical injuries and physiological disturbance need to be quite understood. The present results of photosynthetic components including chlorophyll ‘a’ and chlorophyll ‘b’ reported maximum efficiency of both the pigments (chlorophyll ‘a’ and chlorophyll ‘b’) in control (T1) followed by gallic acid treatment along with salinity stress (T11) in variety Neelam (Fig. 2) while in variety BSS 513, changes in the photosynthetic pigments were markedly observed under biochar along with combined salinity and boron stress (T8) respectively. However, a noticeable lowest content of chlorophyll capacity observed in T8 (variety Neelam) and T3, T4 in variety BSS 513. Besides, the addition of biochar and GA alleviated the damage of salinity and boric acid stress on photosynthetic capacity such that the values reached the control. F-ratio in supplementary Table 1, showed that both chlorophyll ‘a’ and ‘b’ significant approach in terms of growth regulators (GR) and interactions between G x T x GR at p < 0.05.
Role of biochar (5 g kg−1) and gallic acid (2 mM) on a chlorophyll ‘a’ b chlorophyll ‘b’c soluble sugar and d soluble protein in foliar (mean ± standard) under induced salinity (120 mM NaCl) and boron (25 mg kg.−1) stress. Vertical bars stand for standard errors with least significance difference among mean values at p < 0.05
In salt and boric acid, soluble sugar content increased and was measured to a greater extent in gallic acid than biochar. (Fig. 2). In other words, gallic acid treatment with salinity and boric acid stress (T12) in both varieties increased sugar content and decreased in salinity stress in variety Neelam whereas in contrast with variety BSS 513, sugar content has been found reduced in boric acid stress, respectively. GA as compared to biochar treatment to salinity and boric acid stressed plants more effective in enhancing the sugar content. The interactions were found significant (p < 0.05) between genotype and between growth regulators (Supplementary Table 1). Also, highest value of soluble protein content (Fig. 2) of leaf treated with biochar in combined salinity and boric acid stress observed in variety Neelam. Besides, the addition of gallic acid increased protein content after salinity exposure in variety BSS 5113. Moreover, decreased in protein level measured in control (T1) in both varieties clearly suggested the best significant (p < 0.001) interactions between all treatments and application of growth regulators (Supplementary Table 1).
The influence of biochar and gallic acid on oxidative damage caused by salinity and boron stress was studied by detecting GB content (Fig. 3). In results, biochar application significantly increased GB under boron stress alone compared to control (untreated) in Neelam. However, exposure to gallic acid followed by biochar greatly reduced GB in combined salinity and boron stresses. In contrast, the BSS 513, maximum GB content was recorded by gallic acid application under born stress (T10) while significant reduction in GB level has been observed in combined salinity and born stresses (T12). ANOVA, supplementary Table 1, showed that growth regulators interactions were non-significant but were found more significant (p < 0.001) and positive when interacted with applied stress (G x T x GR).
Role of biochar (5 g kg−1) and gallic acid (2 mM) on a glycine betaine b hydrogen peroxide c malondialdehyde in foliar (mean ± standard) under induced salinity (120 mM NaCl) and boron (25 mg kg.−1) stress. Vertical bars stand for standard errors with least significance difference among mean values at p < 0.05
Key Stress Indicators Analysis (H2O2 & MDA)
H2O2 content in the leaves of egg-plant treated with boric acid and combined abiotic stress salinity stress (T2 & T4) increased than control and decreased with gallic acid with similar stress treatments (T12) in variety Neelam. In contrast, the BSS 513 revealed low level of H2O2 through gallic acid application under salinity stress followed by biochar addition to soil with similar stress condition. Moreover, biochar and gallic acid application with their interaction to combined salinity and boron stresses (G x T x GR) decreased H2O2 accumulation non-significantly in both varieties (Fig. 3; Supplementary Table 1). Our current experiment study proposed that lipid peroxidation (MDA) of leaf was affected by salt interaction (Fig. 3) that amplified MDA content that was more pronounced in the absence of growth regulators in both varieties. An effective decrease in the trait was assumed with gallic acid primed seedlings followed by biochar amendment under interactive salt and boron stress in variety Neelam and salinity stress in BSS 513. Present results marked that salinity stress might have caused toxic injury on membranes and was well alleviated by gallic acid pre-soaking technique. In contrast, a non-significant increase by biochar application to soil was measured under salinity (T7) than control condition in BSS 513. Besides this, gallic acid successfully decreased the level of MDA over induced boron stress alone (T10). Results in supplementary Table 1, declared significant (p < 0.05) F-ratio with respect to interactions between genotype, between treatment and their interaction with each other (G x T x GR).
Antioxidant Enzymes (POD, SOD)
The scavenging activity of antioxidant enzymes in leaves was examined to show the impact of gallic acid and biochar in reducing salinity and boric acid stress (Fig. 4). The total SOD activity was significantly highest than compared to control in Neelam variety with biochar treatment under boron followed by boric acid stress while reduced level determined in control. Additionally, in variety BSS 513, a considerable increase in SOD activity was seen after gallic acid treatment under salinity and then boric acid treatment. Similar to this, spectroscopic examination revealed that the Neelam variety's leaves with gallic acid displayed the highest POD activity up to a substantial level under combined boric acid and salt stress. The reduced POD level was noticed in salinity stress with gallic acid application non-significantly. In detail, enhancement in POD content of BSS 513 variety exhibited with gallic acid alone followed by biochar with mutual salinity and boric acid stresses. Results proposed that both the varieties respond differently under distinct growth regulators. However, both regulate the ability of plant antioxidant systems to scavenge free radicals. Results showed that plants become more resilient to environmental challenges when their antioxidant enzyme activity is elevated in stressful conditions. However, ANOVA in supplementary Table 1, proposed markedly significant results in terms of G x T x GA, T x GR, G x GR, between treatment at p < 0.05, respectively.
Leaf Surface Study Through SEM and Electron Microscopy
Plants alter their stomata closure to minimize water loss and a moderate absorption of CO2 for changing circumstances (Richardson et al. 2017). Leaf structure analysis (Fig. 5a–d; Table 5) obtained from the micrographs of SEM (JSMIT100-JEOL-JAPAN), showed that gallic acid treatment (T10) significantly increased stomata length and width of 240 µm and 165 µm under salinity stress in Neelam variety and under combined salt and boron stress (T12) by 143 µm and 177 µm in variety BSS 513. In detail, the variation in stomata density (STD) has been calculated maximum by 181 per mm2 in Neelam and 244 per mm2 in BSS 513 when gallic acid and biochar treatment was applied under control. However, both stresses in combine form decreased the trait more negatively in treatment T4. Contrarily, the biochar treatment to soil enhanced leaf stomata size and density in both varieties significantly p < 0.05 ranges from 28.9 µm to 35.4 µm whereas lowered up to 18 µm by combined salt and boron stresses (T4) without the amendment of soil with biochar and seed priming with gallic acid in Neelam variety and under treatment T12 in BSS 513 with application of growth regulators.
a Changes in leaf stomata length (STL)), stomata width (STW), stomata density (STD) of Solanum melongena L. in variety Neelam through SEM under induced salinity (120 mM NaCl) and boron (25 mg kg−1) stress. b Changes in leaf stomata length (STL)), stomata width (STW), stomata density (STD) of Solanum melongena L. in variety Neelam through SEM under induced salinity (120 mM NaCl) and boron (25 mg kg−1) stress. c Changes in leaf stomata length (STL)), stomata width (STW), stomata density (STD) of Solanum melongena L. in variety BSS 513 through SEM under induced salinity (120 mM NaCl) and boron (25 mg kg−1) stress. d Changes in leaf stomata length (STL)), stomata width (STW), stomata density (STD) of Solanum melongena L. in variety BSS 513 through SEM under induced salinity (120 mM NaCl) and boron (25 mg kg−1) stress
A rise in density and size of trichome may contribute a significant role in stress tolerance mechanism of plant. Nonetheless, results (Fig. 6a–d; Table 5) showed a noticeable increase in trichome length by biochar application under boron stress (T6) was ranging from 3125 µm in Neelam variety and 2401 µm with soaked seeds by gallic acid in BSS 513 thus decreasing the harsh effects of combined stresses (T12). In detail, an increased level has been noticed under boron stress that led to enhance in trichome size by biochar treatment as compared to control. Data clearly confined the adverse effect of combined stress condition on leaf photosynthetic aperture under untreated growth regulator or biochar applications. Nonetheless, stomata density showed a noticeable increase by biochar application under boron stress (T6) was ranging from 3125 µm in Neelam variety and 2401 µm with soaked seeds by gallic acid in BSS 513 thus decreasing the harsh effects of combined stresses (T12). The data for stomata and trichome analysis was confirmed by the evaluations via F-ratio analysis of all the traits with significant level at p < 0.05 in terms of interactions between treatment, between genotype, between growth regulators and between G x T, G x GR, T x GR, G x T x GR respectively (supplementary Table 1).
a Changes in trichomes length (TL) of Solanum melongena L. in variety Neelam through light microscopy under induced salinity (120 mM NaCl) and boron (25 mg kg−1) stress. b Changes in trichomes length (TL) of Solanum melongena L. in variety Neelam through light microscopy under induced salinity (120 mM NaCl) and boron (25 mg kg−1) stress. c Changes in trichomes length (TL) of Solanum melongena L. in variety BSS 513 through light microscopy under induced salinity (120 mM NaCl) and boron (25 mg kg−1) stress. d Changes in trichomes length (TL) of Solanum melongena L. in variety BSS 513 through light microscopy under induced salinity (120 mM NaCl) and boron (25 mg kg−1) stress
Correlation and Regression Analysis
Regression and correlation (Supplementary Table 2, 3) exemplified a positive and significant (p < 0.05) relation between the chlorophyll ‘b’ content of leaf in both varieties of S. melongena L. via growth regulators was r = 0.12, SSC and SPC were calculated r = 0.34 whereas as SOD, H2O2 and MDA measured r = 0.67, r = 0.41, r = 0.33 respectively. However, a non-significant and negative relation between chlorophyll ‘a’ content, POD and GB has been observed. Contrarily, by applied abiotic stresses, all the traits were responded negatively and non-significant except for the SPC, SOD, H2O2. Correlation analysis of physiological and biochemical attributes determined a positive and significant correlation between chlorophyll and SPC, SOD correlate with SPC at p < 0.01 whereas, GB also evaluated significantly in correlation with SPC (p < 0.05). However, MDA and H2O2 significantly correlate with antioxidant enzymes SOD, POD and SPC at p < 0.05, respectively.
Discussion
Biochar Analysis
The present study was conducted to investigate the effects biochar and gallic acid on salt and boron stress mitigation in Solanum melongena L. Biochar, a carbon rich organic compound generally when added to soil increase its pH, EC, ion exchange capacity, many organic compounds including cellulose, lignin, and enhance crop yield. This high carbon content is due to elevated temperature of pyrolysis procedure leading to fix the carbon content enhanced while oxygen decreased (Hao et al. 2013). SEM/EDX results showed more cracks and porous nature of biochar along with high EC, pH, and carbon content as compared to oxygen. Our investigation is in lineage to the results of Hao et al. (2013) and Mendez et al. (2013) who worked and analyzed that large pore with cracks generated due to high temperature pyrolysis that enhance carbon stability in soil. Ahmad et al. (2012) work was also in agreement to our results who examined the decrease oxygen/carbon ration with high temperature hence results in hydrophilic surface of biochar. Elemental analysis via EDX exposed element content and nutrients to our crops may be a useful for plants in time of growth and yield. Thus, biochar is a promising technique to improve soil physical structure, fertility, nutrients availability seed germination and growth (Nafees et al. 2021).
Germination, Growth and Yield Parameters
Compared to control, pretreatment gallic acid and biochar amendment to soil heightened seed germination energy, velocity of germination, germination percentage and emergence percentage. This might be due to best water holding capacity of biochar for better germination or mineral nutrients composition in biochar that released in soil thus maintaining best level of fertility to stimulate growth parameters. Our investigations are in linked with studies of Ali et al. (2021) who reported that biochar addition into the soil increased germination parameters. Similar results were determined by Zhang et al. (2021); Bua et al. (2020). Similarly, maximum T50% and decreased the time for seedling germination (MGT) has been reported by gallic acid pre-treated seeds. Although, native bacteria and soluble plant growth regulators present in biochar also have an advantage to better germination of seeds (Page-Dumroese et al. 2015). Adetunjia et al. (2021) investigated that gallic acid proved to be more effective for cabbage and lettuce seed germination.
In addition, salt stress is known to decrease RWC (Irshad et al. 2021). Our results indicated significant improvement in RWC of plant under salt stress that are well in line with the results obtained by Abbas et al. (2021) who worked on quinoa and told that high RWC with biochar addition enhance RWC of plant in saline soil. Farhangi-Abriz and Torabian (2018) also proposed highest RWC under saline stress in bean seedlings. Similarly, a non-significant reduction in RGR and CVG by employed stress with treated and untreated condition believed that both gallic acid and biochar unwarily improve in regulating such traits as varieties respond tolerantly. According to Parkash and Singh (2021) said that egg-plant is moderately sensitive to salinity stress such that the dry mass was lowed and was non-significantly different as compared biochar treated plants under same stress. In our research studies, a non-significant enhancement in AGR-I and AGR-II in biochar amendment treatment with its co-attributes under salinity. On contrary, studies by Usman et al. (2016) on tomato and Akhtar et al. (2015) on wheat, it was found that salinity stress decreased the plant height, leaf area while biochar addition enhanced significantly. Parkash and Singh (2020) also proposed enhancement occurred in plant height and leaf surface area with biochar amended treatments than the control (non-biochar). Loss in plant biomass under salinity and boron stress was significantly enhanced under growth stimulators. Similarly, PHSTI and DMSTI were accelerated due to biochar formation under stress condition suggested the ability of biochar by mitigating the adversities of saline stress. Our results confined the property gallic acid by enhancing crop yield are in lineage with finding of Gharib et al. (2018) who reported that GA up to 150 ppm concentration significantly enhanced 100-seed weight in grams of cowpea plants more than control treatment.
Physiological and Biochemical Characteristics
The decrease in chlorophyll content is directly correlated with a decrease in the photosynthetic activity of plants. Torabian et al. (2019) explained that there is reduction in ROS and DPPH activity in leaf cells due to applied biochar formation and thus enhanced chlorophyll amount in saline soil. Biochemical results reported enhancement in chlorophyll ‘a’ and chlorophyll ‘b’ content by biochar addition to soil and gallic acid successfully withhold the advertises of both stresses and improved photosynthetic pigments. Our results are in line with the findings Ran et al. (2020) proposed significant increase in chlorophyll under salinity stress. Results agree with the investigation of Klein et al. (2015); and Farghaly et al. (2021), who said that gallic acid increase the photosynthetic capacity of tomato callus under boron stress. A small content of free amino acid and proline buildup in cytoplasm can rapidly reach a great level and play a more effective role in cell osmotic potential (Farhangi-Abriz and Torabian 2017). Our results are linked to the evaluation of Kiani et al. (2021) worked in wheat and Aegilops cylindrica accompanied more chlorophyll under salt stress.
Accumulation of osmolytes under stress condition counterbalance osmotic pressure and perform significant role in redox-regulation via their capability to scavenge oxy-free radicals for most advantageous level for cellular actions (Hisyam et al. 2017). Furthermore, increase in soluble sugar content in salinity and boron stress increased in gallic acid treatment while non-significant enhancement in biochar amendment proved that plant required SSC for osmotic adjustment under abiotic stress could be further explained by the investigation of Farhangi-Abriz and Torabian (2017) in bean seedlings where no applicable changes observed in sugar concentration under salt stress. Similar results are indicated by experiment of Kanwal et al. (2018) in wheat under salinity stress and soil biochar formation. Glycine betaine (GB) and proline are the most important organic osmolytes and stress marker that accumulate in different plant species in response to environmental stresses. A remarkable increase in GB obtained under stress condition and reduced by biochar and GA application.
The dismutation of O−2 into H2O2 and oxygen by SOD whereas H2O2 into H2O through POD plays a function in cellular protecting against ROS and is a vital step in defending the cells. (Farhangi-Abriz and Torabian 2017). Higher SOD and POD activity in boron and salt indicated that gallic acid inhibit the oxidative injury that may takes part in mechanism of biomolecules synthesis by scavenging the ROS (Rosa et al. 2022). The result agrees with work of Ozfidan-konakci et al. (2015) where increased in SOD activity in Oryza sativa under gallic acid subjected to salt stress observed. Gallic acid presoaking treatment led to SOD activity enhancement in wheat seedlings (Bhardwaj et al. 2017). Sgherri et al. (2003) proposed a model that explain the enhancement in POD activity via gallic acid treatment under salinity stress. Ozfidan-konakci et al. (2015) also reported that gallic acid alone or under stress condition did not change POD activity.
Lipid peroxidation is associated with plant stress tolerance mechanism that arising from oxidative stress conditions. In this study a significant decrease in MDA content by both stresses in pre-treatment gallic acid has been observed that proposed same finding investigated by Yildiztugay et al. (2017), gallic acid application caused a decline in lipid peroxidation on membranes under temperature stress. Our findings are in accordance with the findings of Yetişsin and Kurt (2019) in maize seedlings supplemented with gallic acid cause remarkable decrease in MDA contents thus indicating a protective effect on membranes under cupper stress. Shao et al (2005) investigated reduced levels of MDA in pre-treatment with gallic acid confined the indication of high membrane stability. Similarly, gallic acid treatment reduced formation of hydrogen peroxide in stress condition were consistent with previous results representing gallic acid pre-treatment appeared to be more effective by decreasing H2O2 content in wheat cultivars under stressed conditions (Bhardwaj et al. 2017). Gallic acid enhanced plant tolerance capability to salt stress by reducing H2O2 and protect the cell membrane from oxidative in sunflower damage (Saidi et al. 2021). Phenolic compounds perform a critical role in the defense opposed to biotic and abiotic stresses (Sieminska-Kuczer et al. 2022). These secondary metabolites take effect of antioxidants in numerous plants tissues by alleviating the oxidative stress by scavenging ROS.
Anatomical Characteristics
Anatomical investigations of leaves showed that, they were sensitive to induced salt stress as compared to boron stress. As leaf plasticity, changes in morphological structure led to modulation in both physiological and biochemical attributes in plants (Liu et al. 2021). In the present experiment work, the SEM analysis showed an increase in stomata size and density by gallic acid and biochar in soil while decreased under both stresses. Our results are in alignment to the previous work on tomato plants carried out by Akhtar et al. (2014), who found that both stomatal aperture and density reduce under salinity stress be likely to decline such variables. Similar finding obtained by Nafees et al. (2022) and Akhtar et al. (2015), who found that biochar incorporation rises both stomatal aperture and density involving reduced stress level experienced by the plants. Contrarily, trichomes are epidermal cells development in aerial parts of plant importantly help in response to abiotic stress (Zhao et al. 2016). According to results obtained by light microscopy, the increased trichome length under both stresses individually and combinedly signifies by growth regulator and biochar amendment. These findings agree with the work of Khanam et al. (2018) who described that growth regulator enhanced trichome diameter in Mentha piperata L. under salt stress. These outcomes may be well recognized with aid of Passinho-Soares et al (2017) reported that growth regulators influenced quantitative and qualitative profiles of the distribution of trichomes on the leaf surface. Bose et al. (2013) found growth regulators have immense role in trichomes development.
Conclusion
Conclusively, it has been anticipated that the world's changing climate would cause abiotic stressors that will disrupt physiological and biochemical processes of plants, as a result, diminish the yield of our valuable commercial crops including Solanum melongena L. in Pakistan. Our findings showed that abiotic stress adversely effected the germination and agronomic attributes of the aforesaid plant along with the architecture of leaves by adversely lowering stomatal size and trichomes, which are subsequently favorably and dramatically improved by the applied growth regulators, notably gallic acid as compared to biochar. It is further added that, the amount of key stress indicator molecules including MDA and H2O2 were enhanced in all non-biochar and gallic acid treatments which were adversely amended by amount of these molecules. In conclusion, both the growth stimulators alleviated the negative effects of salt and boron stress in egg-plant seedlings by improving the plant growth, yield parameters, anatomy by adjusting some physiological, biochemical and antioxidant defense mechanisms.
References
Abbas Mehdi S M, Nasir M, Ashraf M, Kausar S, Iqbal N, Nazar MZK, Ahmad I, Murtaza G, Ahmad I (2021) Evaluation of best dose of micronutrients (Zinc, Iron and Boron) to combat malnutrition in brinjal (Solanum melongena L.). Pak J Agri Res 34(1):16–168
Adetunji AE, Varghese B, Pammenter N (2021) Effects of exogenous application of five antioxidants on vigor, viability, oxidative metabolism and germination enzymes in aged cabbage and lettuce seeds. S Afr J Bot 137:85–97
Ahmad M, Lee SS, Yang JE, Ro HM, Lee YH, Ok YS (2012) Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil. Ecotoxicol Environ Saf 79:225–231
Akhtar N (2014) Effect of physical and chemical mutagens on morphological behavior of tomato (Solanum Lycopersicum) CV. Plant Breed Seed Sci 70:69–79
Akhtar SS, Andersen MN, Liu F (2015) Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric Water Manag 158:61–68
Al-Ansari F, Ksiksi TA (2018) quantitative assessment of germination parameters: the case of Capsicum annuum L. Open Eco J 11:13–21
Ali L, Xiukang W, Naveed M, Ashraf S, Nadeem SM, Haider FU, Mustafa A (2021) Impact of biochar application on germination behavior and early growth of maize seedlings: insights from a growth room experiment. Appl Sci 11:1–13
Amin AA, Abouziena HF, Abdelhamid MT, Rashad ESM, Gharib AEF (2014) Improving growth and productivity of faba bean plants by foliar application of thiourea and aspartic acid. Int J Plant Soil Sci 3:724–736
Bhardwaj RD, Kaur L, Srivastava P (2017) Comparative evaluation of different phenolic acids as priming agents for mitigating drought stress in wheat seedlings. Pro. Natl Acad Sci India Sect B Biol Sci 87(4):1133–1142
Bose SK, Yadav RK, Mishra S, Sangwan RS, Singh AK, Mishra B, Srivastava AK, Sangwan NS (2013) Effect of gibberellic acid and calliterpenone on plant growth attributes, trichomes, essential oil biosynthesis and pathway gene expression in differential manner in Mentha arvensis L. Plant Physiol Biochem 66:150–158
Bouzroud S, Gouiaa S, Hu N, Bernadac A, Mila I, Bendaou N, Smouni A, Bouzayen M, Zouine M (2018) Auxin response factors (ARFs) are potential mediators of auxin action in tomato response to biotic and abiotic stress (Solanum lycopersicum). PLoS One 13:e0193517
Bua X, Xuea J, Wua Y, Mac W (2020) Effect of Biochar on seed germination and seedling growth of Robinia pseudoacacia L. In Karst Calcareous Soils. Commun Soil Sci Plant Anal 51(3):352–363
Chaganti VN, Crohn DM (2015) Evaluating the relative contribution of physiochemical and biological factors in ameliorating a saline–sodic soil amended with composts and biochar and leached with reclaimed water. Geoderma 259–260(1):45–55
Diaz-Perez TC, Eaton JE (2015) Eggplant (Solanum melongena L.) plant growth and fruit yield as affected by drip irrigation rate. HortScience 50(11):1709–1714
Dubberstein D, Oliveira MG, Aoyama EM, Guilhen JH, Ferreira A, Marques I, Ramalho JC, Partelli FL (2021) Diversity of leaf stomatal traits among Coffea canephora Pierre ex A. Froehner Genotypes Agronomy 11:1126
Eraslan F, Inal A, Gunes A, Alpaslan M (2007) Impact of exogenous salicylic acid on the growth, antioxidant activity and physiology of carrot plants subjected to combined salinity and boron toxicity. Sci Hortic 113:120–128
FAO (2018) FAOSTAT. The Food and Agriculture Organization (FAO) of United Nation.
Farghaly FA, Salam HK, Hamada AM, Radi AA (2021) The role of benzoic acid, gallic acid and salicylic acid in protecting tomato callus cells from excessive boron stress. Sci Hortic 278:109867
Farhang-Abriz S, Torabian S (2017) Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol Environ Saf 137:64–70
Farhangi-Abriz S, Torabian S (2018) Effect of biochar on growth and ion contents of bean plant under saline condition. Environ Sci Pollut Res 25:11556–11564
Gaskin JW, Steiner C, Harris K, Das KC, Bibens B (2008) Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans ASABE 51:2061–2069
Gee G W, Bauder J W (1986) Particle-size analysis. Methods of soil analysis. Part 1. 2nd ed. Agron. Monogr. 9. ASA and SSSA, Madison, WI. In A. Klute (ed.). 383– 411.
Gharib FAEL, Zeid IM, Ghazi SM, Ahmed EZ (2018) Physiological effects of ascorbic and gallic acids on growth and metabolic activities of cowpea (Vigna unguiculata L.) Plants. J Plant Physiol Pathol 6(4):1–9
Ghule PL, Dahiphale VV, Jadhav JD, Palve DK (2013) Absolute growth rate, relative growth rate, net assimilation rate as influenced on dry matter weight of Bt cotton. Int Res J Agri Econ Stat 4(1):42–46
Habib K, Khan IA, Akbar R, Saeed M, Farid A, Ali I, Alam M (2015) Response of Brinjal Solanum melongena L. genotypes against insect pests in Peshawar, Pakistan. J Entomol Zool Studies 3(3):423–427
Hao FH, Zhao XC, Wei OY, Lim CY, Chen SY, Shan YS, Lai XH (2013) Molecular structure of corncob-derived Biochar’s and the mechanism of atrazine sorption. Agron J 105:773–782
Hisyam B, Alam MA, Naimah N, Jahan MS (2017) Roles of glycine betaine on antioxidants and gene function in rice plants under water stress. Asian J Plant Sci 16:132–140
Irshad A, Rehman RN, Kareem HA, Yang P, Hu T (2021) Addressing the challenge of cold stress resilience with the synergistic effect of Rhizobium inoculation and exogenous melatonin application in Medicago truncatula. Ecotoxicol Environ Saf 226:112816
Kanwal S, Ilyas N, Shabir S, Saeed M, Gul R, Zahoor M, Batool N, Mazhar R (2018) Application of biochar in mitigation of negative effects of salinity stress in wheat (Triticum aestivum L.). J Plant Nutr 41(4):526–538
Kiani R, Arzani A, Maibody SAM (2021) Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. J Front Plant Sci 12:646221
Klein A, Keyster M, Ludidi N (2015) Response of soybean nodules to exogenously applied caffeic acid during NaCl-induced salinity. S Afr J Bot 96:13–18
Kul R, Arjumend T, Ekinci M, Yildirim E, Turan M, Argin S (2021) Biochar as an organic soil conditioner for mitigating salinity stress in tomato. Oil Sci Plant Nutr 67(6):693–706
Lalay G, Ullah S, Ahmed I (2021) Physiological and biochemical responses of Brassica napus L. to drought-induced stress by the application of biochar and plant growth promoting Rhizobacteria. Microsc Res Tech 85(4):1–15
Landi M, Margaritopoulou T, Papadakis IE (2019) Boron toxicity in higher plants: an update. Planta 250:1011–1032
Li H, Ye X, Geng Z, Zhou H, Guo X, Zhang Y, Zhao H, Wang G (2016) The influence of biochar type on long-term stabilization for Cd and Cu in contaminated paddy soils. J Hazard Mater 304:40–48
Liu Y, He Z, Xie Y, Su L, Zhang R, Wang H, Li C, Long S (2021) Drought resistance mechanisms of Phedimus aizoon L. Sci Rep 11:13600
Lowery OA, Poesenbrough NJ, Fal AL, Randall RJ (1951) Protein measurement with foline phenol reagent. J Biol Chem 193(1):265–275
Lutfunnahar SJ, Piash MI, Rahman MH (2021) Impact of MgCl2 modified biochar on phosphorus and nitrogen fractions in Coastal Saline Soil. Open J Soil Sci 11(6):331–351
Ma N, Hu C, Wan L, Hu Q, Xiong J, Zhang C (2017) Strigolactones improve plant growth, photosynthesis, and alleviate oxidative stress under salinity in rapeseed (Brassica napus L.) by regulating gene expression. Front Plant Sci 1671:1–15
Ma Z, Marsolais F, Bykova NV, Igamberdiev AU (2016) Nitric oxide and reactive oxygen species mediate metabolic changes in barley seed embryo during germination. Front Plant Sci 7:138
Mendez A, Terradillos M, Gascó G (2013) Physicochemical and agronomic properties of biochar from sewage sludge pyrolyzed at different temperatures. J Anal Appl Pyrol 102:124–130
Metwally AM, Radi AA, El-Shazoly RM, Hamada AM (2018) The role of calcium, silicon and salicylic acid treatment in protection of canola plants against boron toxicity stress. J Plant Res 131:1015–1028
Nafees M, Ullah S, Ahmed I (2021) Morphological and elemental evaluation of biochar through analytical techniques and its combined effect along with plant growth promoting rhizobacteria on Vicia faba L. under induced drought stress. Microsc Res Tech 84(12):2947–2959
Nafees M, Ullah S, Ahmed I (2022) Modulation of drought adversities in Vicia faba by the application of plant growth promoting rhizobacteria and biochar. Microsc Res Tech 85(5):1856–1869
Nino-Medina G, Urías-Orona V, Muy-Range MD, Heredia JB (2017) Structure and content of phenolics in eggplant (Solanum melongena) - a review. S Afr J Bot 111:161–169
Ozfidan-Konakci C, Yildiztugay E, Kucukoduk M (2015) Protective roles of exogenously applied gallic acid in Oryza sativa subjected to salt and osmotic stresses: effects on the total antioxidant capacity. Plant Growth Regul 75:219–234
Page AL, Miller RH, Keeney DR (1982) Methods of soil analysis Chemical and microbiological properties, 2nd edn. American Society of Agronomy Inc, Madison
Page-Dumroese D, Robichaud PR, Brown RE, Tirocke JM (2015) Water repellency of two forest soils after biochar addition. Am Soc Agric Biol Eng 58:335–342
Parkash V, Singh S (2021) Potential of biochar application to mitigate salinity stress in eggplant. Am Soc Hortic Sci 55(12):1–10
Passinho-Soares HC, Davida JP, De-Santana JRF, Davidc JM, Rodrigues FM, Mesquitac PRR, Oliveira FS, Bellintani MC (2017) Influence of growth regulators on distribution of trichomes and the production of volatiles in micropropagated plants of Plectranthus ornatus. Rev Bras 27:679–690
Ran C, Gulaqa A, Zhu J, Wang X, Zhang S, Geng Y, Guo L, Jin F, Shao X (2020) Benefits of biochar for improving ion contents, cell membrane permeability, leaf water status and yield of rice under saline-sodic paddy field condition. J Plant Growth Regul 39:370–377
Richardson F, Brodribb TJ, Jordan GJ (2017) Amphistomatic leaf surfaces independently regulate gas exchange in response to variations in evaporative demand. Tree Physiol 37(7):869–878
Rodríguez DJA, Weatherhead EK, Knox JW, Camacho E (2007) Climate change impacts on irrigation water requirements in the Guadalquivir river basin in Spain. Reg Environ Change 7(3):149–59
Rosa D, Elya B, Hanafi M, Khatib A, Surya MI (2022) In vitro and in silico screening analysis of artabotrys sumatranus leaf and twig extracts for α-glucosidase inhibition activity and its relationship with antioxidant activity. Sci Pharm 91:2
Saidi I, Guesmi F, Kharbech O, Hfaiedh N, Djebali W (2021) Gallic acid improves the antioxidant ability against cadmium toxicity: Impact on leaf lipid composition of sunflower (Helianthus annuus). Ecotoxicol Environ Saf 210:1–11
Sgherri C, Ranieri A, Quartacci MF (2013) Antioxidative responses in Vitis vinifera infected by grapevine fanleaf virus. J Plant Physiol 170(2):121–128
Shah W, Ullah S, Ali S, Idrees M, Khan MN et al (2021) Effect of exogenous alpha-tocopherol on physio-biochemical attributes and agronomic performance of lentil (Lens culinaris Medik) under drought stress. PLoS One 16(8):e0248200
Shao HB, Liang ZS, Shao MA, Wang BC (2005) Changes of antioxidative enzymes and membrane peroxidation for soil water deficits among 10 wheat genotypes at seedling stage. Colloids Surf B 42:107–113
Shumaila S, Ullah S (2020) Mitigation of salinity-induced damages in Capsicum annum L. (Sweet Pepper) seedlings using priming techniques: a future perspective of climate change in the region. Commun Soil Sci Plant Anal 1:1–24
Sieminska-Kuczer A, Szymańska-Chargot M, Zdunek A (2022) Recent advances in interactions between polyphenols and plant cell wall polysaccharides as studied using an adsorption technique. Food Chem 373:131487
Thomas GW (1982) Exchangeable cations. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis Chemical and microbiological properties, 2nd edn. American Society of Agronomy Inc, Madison, pp 159–165
Torabian S, Farhang-abriz S, Rathjen J (2019) Biochar and lignite effect H+atpase, H+ppase activities in root tonoplast and nutrient content of mung bean under salt stress. Plant Physiol Biochem 129:141–149
Tsai WT, Liu SC, Chen HR, Chang YM, Tsai YL (2012) Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere 89(2):198–203
Uddin S, Ullah S, Nafees M (2021) Effect of seed priming on growth and performance of Vigna radiata L. under induced drought stress. J Agric Food Res 4:100140
Ullah S, Ali S, Binte Abid A, Nafees M (2022) Modulating response of Zea mays to induced salinity stress through application of nitrate mediated silver nanoparticles and indole acetic acid. Microsc Res Tech 85(3):1135–1145
Usman ARA, Al-Wabel MI, Abdulaziz AH, Mahmoud WA, El-Naggar AH, Ahmad M, Abdulelah AF, Abdulrasoul AO (2016) Conocarpus biochar induces changes in soil nutrient availability and tomato growth under saline irrigation. Pedosphere 26(1):27–38
Volf I, Stîngu A, Popa VI (2012) New natural chelating agents with modulator effects on copper phytoextraction. Environ Eng Manag J 11:487–491
Vujosevic B, Petar C, Milosav B, Milan M, Bojan M, Dusan S, Mladen T (2018) Field performance of abnormal maize seedlings. Ratar Povrt 55(1):34–38
Warwate SI, Kandoliya UK, Bhadja NV, Golakiya BA (2017) The effect of seed priming with plant growth promoting Rhizobacteria (PGPR) on growth of coriander (Coriandrum sativum L) seedling. Int J Curr Microbiol App Sci 6(3):1926–1934
Wicke B, Smeets E, Dornburg V, Vashev B, Gaiser T, Turkenburg W, Faaij A (2011) The global technical and economic potential of bioenergy from salt-affected soils. Energy Environ Sci 8(4):2669–1681
Xing G, Pham AN, Miller CJ, Waite TD (2018) pH-dependence of production of oxidants (Cu (III) and/or HO•) by copper-catalyzed decomposition of hydrogen peroxide under conditions typical of natural saline waters. Geochim Cosmochim Acta 232:30–47
Yang R, Howe JA, Golden BR (2019) Calcium silicate slag reduces drought stress in rice (Oryza sativa L.). J Agron Crop Sci 205:353–361
Yetişsin F, Kurt F (2019) Gallic acid (GA) alleviating copper (Cu) toxicity in maize (Zea mays L) seedlings. Int J Phytoremediation 22(4):420–426
Yildiztugay E, Ozfidan-Konakci C, Kucukoduk M (2017) Improvement of cold stress resistance via free radical scavenging ability and promoted water status and photosynthetic capacity of gallic acid in soybean leaves. J Soil Sci Plant Nutr 17(2):366–384
Yin Y, Xu J, He X, Yang Z, Fang W, Tao J (2022) Role of exogenous melatonin involved in phenolic acid metabolism of germinated hulless barley under NaCl stress. Plant Physiol Biochem 170:14–22
Zhang K, Wang Y, Mao J, Chen B (2021) Effects of biochar nanoparticles on seed germination and seedling growth. Environ Pollut 256:113409
Zhao Q, Chen XY (2016) Development: a new function of plant trichomes. Nat Plants 2(7):16096
Zou M, Yuan L, Zhu S, Liu S, Ge J, Wang G (2017) Effects of heat stress on photosynthetic characteristics and chloroplast ultrastructure of a heat-sensitive and heat-tolerant cultivar of wucai (Brassica campestris L.). Acta Physiologiae Plantarum 39(1):1–10
Author information
Authors and Affiliations
Contributions
SU conceived and designed the experiments. Shumaila performed the experiments, analyzed the data, and wrote the paper. MN edited and reviewed the paper.
Corresponding author
Ethics declarations
Conflict of interest
All authors contributed equally and declare no conflict of interest. The paper is our original research work and is not published elsewhere.
Additional information
Handling Editor: M. Iqbal R. Khan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shumaila, Ullah, S. & Nafees, M. Biochar Application to Soil and Seed Pre-Soaking on Growth, Yield and Physiological Response of Solanum melongena L. Under Induced Abiotic Stresses. J Plant Growth Regul 42, 6980–7003 (2023). https://doi.org/10.1007/s00344-023-10990-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-10990-5