Abstract
Long-term imbalance in fertilization has resulted in a serious decline in potassium (K) fertility in the Yangtze River Delta region of China. Understanding of the complex responses of soil microbial communities and enzyme activities to different K fertilizer measures while maintaining soil K balance can provide a scientific basis for rational application of K fertilizer. Two field experiment sites (JY, loam soil with high pH and K fertility; GD, silty loam soil with low pH and K fertility) were selected to study the effects of K fertilizer management on enzyme activities and bacterial communities under soil K balance condition. K fertilizer treatments included no K fertilizer (K0), straw return combined with K fertilizer (SRK), and inorganic K fertilizer only (IK). Soil bacterial communities were examined using MiSeq sequencing. Wheat yield, soil nutrient contents, and enzyme activities were higher in the SRK than in the IK and K0 treatments. Available K was the most important factor affecting wheat yield. The SRK and IK treatments significantly altered bacterial communities and enzyme activities, which in turn affected the cycling of soil nutrients. The positive effect of the SRK treatment on wheat yield, enzyme activities, and potential bacterial functions in silty loam soil was greater than that in loam soil, which was closely related to soil texture, pH, and K fertility. The SRK measure is a promising approach to maintain crop yield and soil fertility and more necessary to be adopted in silty loam soil with low pH and K fertility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil potassium (K) is an essential nutrient for plant growth (Pettigrew 2008; Song et al. 2020) and can improve the quality and quantity of agricultural products (Zhao et al. 2014). Large amounts of soil K are absorbed by crops, owing to continuous improvement in crop yield, consequently resulting in gradual depletion of soil K (Niu et al. 2013). K deficiency has occurred in many regions of the world and has become a global problem (Zhao et al. 2014). In China, farmers pay more attention to nitrogen (N) fertilizer than to K fertilizer, resulting in K deficiency in many farmlands (Chen et al. 2019). Therefore, soil K deficiency has become an important factor limiting agricultural production in China.
Application of inorganic K fertilizer and crop residues are two effective methods used to alleviate the degradation of soil K fertility (Zhao et al. 2014; Bai et al. 2015). Crop straw is an important organic fertilizer resource that contains many nutrients, of which K is the most abundant one (Bai et al. 2015). China produces large amount of crop straw every year, and approximately 80% of the total K exists in the straw of cereal crops (e.g., rice, wheat, oilseed rape, and maize) (Chen et al. 2019). Straw incorporation can return a large amount of plant K to the soil. It has been reported that China needs to import a large amount of inorganic K fertilizer every year (Yu et al. 2010). Excessive inorganic K fertilizer input cannot lead to further increases in grain yield but will result in resource waste and low use efficiency (Song et al. 2020). Therefore, the effective utilization of crop straw resources is of great practical significance.
Rice–wheat system is one of the most popular cropping systems in the Yangtze River region, which is an important agricultural production region in China (Zhang et al. 2021). However, long-term imbalance in fertilization has resulted in a serious decline in K fertility in the region (Chen et al. 2019). In rice–wheat system, a large amount of crop straw is left in the field after harvest. Farmers typically burn crop straw in the field, which is a convenient and efficient way to remove crop residues (Huang et al. 2021a). However, open burning of crop straw has been reported to pollute the atmospheric environment (Yan et al. 2020) and result in loss of plant’s essential nutrients (Kumar et al. 2019). Therefore, local governments prohibit the in situ burning of crop straw and recommend using straw fertilizer resource by returning crop straw to the field.
Many studies have shown that crop straw return can increase soil organic matter, improve soil fertility and yield stability, and reduce inorganic fertilizer application (Li et al. 2010; Zhang et al. 2016; Chen et al. 2017; Sharma et al. 2021). Soil microorganisms play key roles in straw decomposition and soil nutrient cycling (Zhao et al. 2016; Tian et al. 2019; Xie et al. 2022). Soil bacteria are involved in a series of soil biochemical processes (e.g., decomposition of soil organic matter, transformation of fertilizer, and N fixation). Crop straw return acts as a substitute for inorganic fertilizers and directly or indirectly affects the composition and structure of soil microbial communities by altering soil properties (Zhu et al. 2019; Yan et al. 2020). Ceja-Navarro et al. (2010) found that crop straw return increased the abundance and diversity of beneficial soil bacteria, which is important to soil health and crop growth. In a long-term experiment (> 25 years), Yuan et al. (2013) reported that straw incorporation combined with inorganic K fertilizer increased soil microbial biodiversity compared with the application of inorganic fertilizers alone. Overall, there is a strong interaction between straw decomposition, soil microorganisms, and soil nutrient cycling (Heimann and Reichstein 2008; Zhong et al. 2018).
Soil microorganisms also play a key role in the formation of soil enzymes, which are involved in various biochemical processes in soil ecosystems (Jarosch et al. 2019; Eslaminejad et al. 2020), such as promoting the mineralization of soil organic matter and decomposition of crop residues (Huang et al. 2021a). Previous studies have reported that crop straw return significantly affects soil enzyme activities by changing soil properties and microbial metabolic functions (Zhang et al. 2016; Zhao et al. 2016). For instance, straw return can increase soil organic carbon, which has significant correlation with the activities of sucrase and cellulase (Ge et al. 2010; Huang et al. 2021a). Zhang et al. (2016) showed that the incorporation of maize straw increased soil phosphatase and urease activities, which are closely related to the availability of N and phosphorus (P) in the soil. In addition, Zhang et al. (2016) also found a positive correlation between crop yields and enzyme activities when crop straw was returned to the field.
Long-term neglect of K fertilizer management in rice–wheat cropping system in the Yangtze River Delta region has inhibited further improvement of crop yield and caused negative impact on food security (Chen et al. 2019). In recent years, several studies have reported the effects of using straw K instead of inorganic K fertilizer on soil physical properties (Zhang et al. 2014, 2016), chemical properties (Chen et al. 2017), microbial community (Zhu et al. 2019), and crop yield (Zhang et al. 2021). It has also been reported that long-term straw return without inorganic potassium fertilizer will lead to the continuous loss of soil K, thereby resulting in yield decline (Zhao et al. 2014; Bai et al. 2015). Therefore, ensuring soil K balance (the input of K fertilizer is equal to the aboveground K absorption of crops) is an effective measure for maintaining soil K fertility and crop yield stability. However, information on the effects of different K fertilizer management measures on soil biological properties (e.g., microbial communities and enzyme activities) under soil K balance condition in rice–wheat system is still limited. Therefore, the most effective K fertilizer application measure for high yield and maintenance of soil fertility need more clarification.
We established a fixed-site field experiment with rice–wheat rotation under soil K balance condition over 6 years (2012–2018) for 12 seasons in JY County (loam soil with high K fertility) and GD County (silty loam soil with low K fertility) in the Yangtze River region. The wheat yield, soil chemical properties, and soil biological properties were investigated during the wheat season of 2018 (last wheat season). We hypothesized that (i) wheat yield, soil nutrient contents, bacterial communities, and enzyme activities would have different responses to long-term straw return and inorganic K fertilizer application under soil K balance condition and (ii) these responses would vary in different soil types. The aim of this work is to provide a scientific basis for rational application of K fertilizer and sustainable development of agricultural ecosystem.
2 Materials and Methods
2.1 Experimental Sites
The two experimental fields under rice–wheat rotation management were conducted over six growing seasons from 2012 to 2018 in Jiangyan County (JY, 32°26′ N, 120°05′ E) in Jiangsu Province and Guangde County (GD, 31°03′ N, 119°27′ E) in Anhui Province. Both sites are typical rice–wheat rotation areas in the Yangtze River Delta region and are characterized by a subtropical monsoon climate. The 30-year (1970–2000, http://worldclim.org/version2) mean annual temperature and precipitation for JY and GD were 15.2 °C, 980 mm and 15.8 °C, 1172 mm, respectively. The soil at the experimental site of JY was loam soil (43.8% sand (0.05–2 mm), 47.9% silt (0.002–0.05 mm), and 8.3% clay (< 0.002 mm)). At the beginning of the experiment, the topsoil (0–20 cm) had total N of 0.5 g kg−1, total P of 1.01 g kg−1, available P of 28.6 mg kg−1, available K of 75.4 mg kg−1, and pH value of 7.66. The GD experimental field had a silty loam soil (24.1% sand (0.05–2 mm), 59.7% silt (0.002–0.05 mm), and 16.2% clay (< 0.002 mm)). In the 0–20-cm soil layer, the total N, total P, available P, available K, and pH value were 0.81 g kg−1, 0.46 g kg−1, 23.6 mg kg−1, 55.2 mg kg−1, and 5.51, respectively. Local farmers in the two sites were used to transplant rice in late June and harvest rice in early October and sow winter wheat in late October and harvest in early June of the following year.
2.2 Experimental Design
The field experiments in both sites began with the rice season in June 2012 and ended with the wheat season in June 2018. In both sites, rice was transplanted by hand at 20 × 20 cm spacing, and the wheat was sown manually at a rate of 195 kg ha−1 with a row spacing of 25 cm. The varieties of rice and wheat used throughout the experimental period were Wuyunjing 24 (Oryza sativa L.) and Yangmai 20 (Triticum aestivum L.), respectively. In the field experiments, the application rates of N and P fertilizers were in accordance with the local practices of farmers in the region, and the application rate of K fertilizer in each treatment was approximately equal to the amount of K absorbed by the aboveground crop (except in the case of the treatment without K fertilizer).
At the JY site, the experiment included three K fertilizer treatments: (a) no inorganic K fertilizer and straw incorporation (K0), (b) straw return combined with inorganic K fertilizer application (SRK), in which, to balance the K levels, the amount of inorganic K fertilizer applied was approximately equal to the amount of K absorbed by the grain in the last crop season, and (c) inorganic K fertilizer only (IK), in which all the aboveground parts produced in the last crop season were completely removed, and, therefore, to balance K levels, the amount of inorganic K fertilizer applied was approximately equal to the amount of K absorbed by the crop aboveground in the last crop season. P and K fertilizers were applied as basal fertilizers during both crop seasons. During the rice season, the application rates of N and P fertilizers were 270 kg N and 90 kg P2O5 ha−1, respectively. The N fertilizer was split applied as basal, tillering, jointing, and panicle fertilizers at ratios of 40, 20, 28, and 12%, respectively. During the wheat season, the application rates of N and P2O5 were 225 and 120 kg ha−1, respectively. The N fertilizer was applied as basal, tillering, and jointing fertilizers at ratios of 40, 30, and 30%, respectively. The field trial was arranged in a randomized block design with four replicates, and the plot size was 33 m2 (4.6 × 7.18 m).
The field experiment at the GD site was the same experimental design as those at the JY site. P and K fertilizers were applied as basal fertilizers, and N fertilizer was split-applied in two split doses (67% as basal fertilizer and 33% as panicle fertilizer) for both the rice and wheat seasons. The amounts of N and P fertilizers applied were 180 kg N and 120 kg P2O5 ha−1, respectively, for both rice and wheat seasons. All treatments were replicated four times in a randomized block design, with a plot size of 30 m2 (6 × 5 m). All plots are separated by cement to prevent nutrient interactions between plots for both JY and GD.
The amount of inorganic fertilizer and straw applied in different treatments in each crop season at both sites from 2012 to 2018 are listed in Table 1. In both sites, inorganic basal fertilizers were broadcast manually and plowed into the soil by rotary tillage before rice transplanting or wheat sowing. In the K0 and IK treatments, all the crop straws were removed from the plots after harvesting. In the SRK treatment, the crop straw was air-dried and then manually chopped into approximately 5-cm-long pieces after the harvest of rice and wheat. The chopped straw was manually plowed into the 0–20-cm soil layer before rice transplanting or wheat sowing. The inorganic fertilizer sources used in this work for both sites were as follows: N fertilizer (urea, 46% N), P fertilizer (calcium superphosphate, 5.24% P), and K fertilizer (potassium chloride, 49.8% K). All other field management measures, including irrigation and pest control, were consistent with the local practices of farmers in the region.
2.3 Sampling and Measurements
At the maturity stage in each crop season, grain yields were determined from the entire plot of each treatment at 14% moisture content.
We collected bulk soil (inter-row without plants) during the wheat filling stage (early May 2018) at both sites. The topsoil (0–15 cm) from the bulk soil was collected using a soil core sampler (5 cm diameter, 0–20 cm depth). Nine soil cores were randomly collected from each plot and combined into a single sample. Fresh soil samples were sieved through a 2-mm mesh to eliminate visible stones and plant residues. Each sample was divided into three subsamples. One subsample was stored at − 40 °C for DNA extraction and microbial biomass carbon (MBC) determination. The second subsample was stored at 4 °C for analysis of soil enzyme activities. The third subsample was air-dried in the laboratory for analysis of soil chemical properties.
Soil enzyme activities (urease, sucrase, alkaline phosphatase (for the JY site), acid phosphatase (for the GD site), and cellulase) were determined using an analysis kit (Solarbio Science & Technology Co, Beijing, China), following the protocol described in the manufacturer’s instructions.
Soil MBC was determined using the chloroform fumigation extraction method (Song et al. 2022). Both 5 g fumigated and control samples were extracted with 0.5 mol L−1 K2SO4, after which the solutions were determined using a C/N analyzer (Multi N/C 2100, Analytik Jena AG, Germany). Soil MBC concentration was determined as the difference in organic C of fumigated and non-fumigated soil samples with a conversion factor of 0.45 (Yan et al. 2021).
Soil pH was determined using a pH meter (Mettler Toledo FE28, Switzerland) with a 1:2.5 ratio of soil to distilled water. Soil ammonium nitrogen (NH4+-N) and nitrate nitrogen (NO3−-N) were extracted using 2 M KCl solution at a soil/water ratio of 1:8, and their contents were determined using a continuous-discrete analyzer (SmartChem 200; Westco Scientific Instruments, Brookfield, CT, USA) after shaking for 1 h. Available phosphorus (AP) was extracted with 0.5 M NaHCO3 (pH = 8.5) and determined using the molybdenum blue method. Available K (AK) was determined via a flame photometer after extraction with 1 M NH4OAc.
Air-dried soil samples were passed through a 0.15-mm sieve to measure soil organic matter (SOM), total nitrogen (TN), and total phosphorus (TP). SOM content was measured using the potassium dichromate method and determined using the titration method. TN content was measured using the Kjeldahl method. TP content was extracted after H2SO4–HClO4 digestion and determined using the molybdenum blue method.
2.4 Soil DNA Extraction and Illumina MiSeq High-Throughput Sequencing
DNA was extracted from 0.5 g soil samples using the FastDNA SPIN Kit for Soil (MP Biomedicals, Santa Ana, CA) according to the manufacturer’s instructions. The quality of the genomic DNA was checked on 1% agarose gels, and the concentration and purity were determined using a NanoDrop ND-2000 spectrophotometer (Thermo Scientific, Wilmington, USA). The bacterial 16S rRNA gene V4-V5 hypervariable region was amplified using the primer pair 515F and 907R (Hu et al. 2021). The qualified DNA samples were delivered to Shanghai Majorbio Bio-Pharm Technology Co. Ltd. for high-throughput sequencing of the 16S rRNA gene on the Illumina MiSeq PE 300 platform (Illumina, San Diego, CA, USA). PCR reaction for each DNA sample was performed in 20-μL reaction mixtures containing 4 μL 5 × FastPfu buffer, 2 μL dNTPs (2.5 μM), 0.8 μL Forward (5 μM) and Reverse (5 μM) Primers, 0.4 μL FastPfu polymerase, 0.2 μL BSA, 10 ng DNA template, and double distilled water (final volume up to 20 μL). The PCR condition was as follows: initial denaturation at 95 °C for 3 min, amplified for 30 cycles (denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 45 s), then a final extension at 72 °C for 10 min. The products were checked using a 2% agarose gel and then purified using an agarose gel DNA purification kit (AxQPrepDNA). Sequences obtained in this study were submitted in the National Genomics Data Center (NGDC) Genome Sequence Archive (https://bigd.big.ac.cn/gsub/) with accession number CRA006805.
2.5 Processing of Illumina Sequencing Data
The raw FASTAQ data were merged with FLASH software (Magoc and Salzberg 2011) and then processed using Quantitative Insights into Microbial Ecology (QIIME, v. 1.9.1) (Caporaso et al. 2010). Sequences with low quality (below the average quality score of 25) and those < 300 bp were discarded (Bao et al. 2019). A total of 885,979 high-quality bacterial reads were obtained in the present study. The chimeras were detected and filtered by the UCHIME algorithm (Edgar et al. 2011). The qualified sequences for bacterial reads were clustered into operational taxonomic units (OTUs; > 97% sequence identity) using the UCLUST method. The most abundant sequence within each cluster was selected as a representative sequence for that OTU. SILVA 132 database (http://www.arb-silva.de/download/archive/qiime/) was used to assign taxonomic identities for bacterial OTUs. Representative sequences were aligned using PyNAST. Non-bacterial sequences (containing < 0.001% of total sequences) were discarded, and the remaining sequences of all samples were then rarefied to the same sequencing depth of 18,000 (the lowest sequence read depth among samples) for beta (β) diversity comparisons (Xiang et al. 2018).
2.6 Statistical Analysis
One-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test (P < 0.05) was performed to test the effects of different K fertilizer treatments on crop yield, soil chemical properties, enzyme activities, bacterial α-diversity indices, and bacterial potential functions using the SPSS software (version 22.0; IBM Software, Chicago, IL, USA). Non-metric multidimensional scaling (NMDS) ordination plots based on Bray–Curtis dissimilarities were used to display the differences in bacterial community composition. The permutational multivariate analysis of variance (PERMANOVA) was used to determine the significant differences in bacterial communities among treatments using the “Adonis” function. The correlation between the soil properties and bacterial communities was calculated using the Mantel test. These statistical analyses (NMDS, PERMANOVA, and Mantel test) were implemented using the “vegan” package (Version 2.4–2) in the R environment (version 4.0.2, http://cran.r-project.org). The relationships between soil nutrients and enzyme activities were determined using Pearson correlation analysis in R version 4.0.2. In addition, Pearson correlation heatmaps were drawn with the “pheatmap” package (Version 1.0.12) in R version 4.0.2. Automatic linear modeling was performed to predict the importance of soil properties on wheat yield using the SPSS software (version 22.0) (Sun et al. 2015). The potential bacterial functions were generated using PICRUSt2 program based on Kyoto Encyclopedia of Genes and Genomes (KEGG) database (Chen et al. 2020; Wu et al. 2022).
Sustainable yield index (SYI) of wheat yield was evaluated according to the method outlined by Zhang et al. (2021).
where Ymean is the mean value of wheat yield during 2012–2018 for each treatment, Ysd is the standard deviation of wheat yield, and Ymax is the maximum wheat yield over 2012–2018 for each treatment.
3 Results
3.1 Wheat Yield
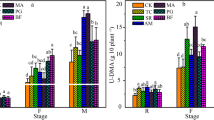
Wheat yield after 6 consecutive years is shown in Fig. 1a. The result showed that the effect of different K treatments on wheat yield was very significant (P < 0.05). In the JY site, the wheat yields under the SRK and IK treatments were 16.0% and 13.6% higher than those under the K0 treatment, respectively. The wheat yield in the SRK treatment was 2.17% higher than that in the IK treatment. In the GD site, wheat yield differed significantly in the order as follows: K0 < IK < SRK after 6 consecutive years. Wheat yield was significantly (P < 0.05) higher in the SRK and IK treatments than in the K0 treatment, with increases of 942% and 770%, respectively. In addition, the results in Fig. 1b showed that there was no significant difference (P > 0.05) in SYI among different treatments in the JY site. However, SYI in the K0 treatment was less than zero, and SYI in the SRK treatment was significantly higher (P < 0.05) than that in the IK treatment for GD.
Effects of different K fertilizer treatments on the wheat yield (a) and sustainable yield index (b) after 6 consecutive years in a rice–wheat cropping system. Vertical bars are standard error (n = 4). Different lowercase letters above the bars indicate significant differences among fertilization treatments (P < 0.05). K0, no inorganic K fertilizer and straw incorporation; SRK, straw return combined with inorganic K fertilizer application; IK, inorganic K fertilizer only. JY, Jiangyan; GD, Guangde
3.2 Soil Chemical Properties
The soil chemical properties of the different K fertilizer treatments are presented in Table 2. In the JY site, soil NO3−-N showed no significant difference among all treatments (P > 0.05). The SRK and IK treatments increased soil NH4+-N by 90.6% and 41.0%, TN by 23.3% and 9.1%, AK by 105% and 95.3%, TP by 6.9% and 0.6%, AP by 18.5% and 5.8%, and SOM by 13.2% and 0.5%, respectively, compared with the K0 treatment. IK treatment significantly (P < 0.05) increased NH4+-N and AK compared with K0 treatment, while TN, TP, AP, and SOM showed no significant differences between the IK and K0 treatments (P > 0.05). In the GD site, soil NH4+-N and TP showed no significant differences among all treatments (P > 0.05). The SRK and IK treatments increased soil NO3−-N, TN, AK, and SOM compared with the K0 treatment, with increases of 32.1% and 8.5%, 11.3% and 1.9%, 48.6% and 22.8%, and 17.6% and 4.5%, respectively. The SRK treatment significantly (P < 0.05) increased soil AP by 27.1% compared with K0 treatment. The improving effect of the SRK treatment on soil fertility was better than that of the IK treatment. In addition, the SRK treatments increased pH, while IK treatment decreased pH compared to K0 treatment in both sites.
3.3 Soil Biological Properties
Compared with K0, the SRK and IK treatments showed the potential to increase enzyme activities and MBC. In the JY site (Fig. 2a), the enzyme activity levels were ranked in the order as follows: K0 < IK < SRK. The activities of urease, sucrase, alkaline phosphatase, and cellulase increased by 14.7, 47.9, 40.9, and 235%, respectively, in the SRK treatment and 2.9, 21.9, 20.0, and 58.2%, respectively, in the IK treatment compared with those in the K0 treatment. Compared with the IK treatment, the SRK treatment increased urease activity by 11.4%, sucrase activity by 21.4%, alkaline phosphatase activity by 17.4%, and cellulase activity by 111%. The SRK and IK treatments increased soil MBC by 38.3% and 21.5% compared with the K0 treatment. In the GD site (Fig. 2b), the enzyme activity levels were ranked in the order as follows: K0 < IK < SRK. Compared to those in the K0 treatment, the activities of urease, sucrase, acid phosphatase, and cellulase increased by 22.1, 248, 27.3, and 227%, respectively, in the SRK treatment and 7.21, 13.6, 5.83, and 36.2%, respectively in the IK treatment. Compared with the IK treatment, the SRK treatment increased urease activity by 14.1%, sucrase activity by 207%, alkaline phosphatase activity by 20.3%, and cellulase activity by 140%. Soil MBC was higher in the SRK and IK treatments than in the K0 treatment, with increases of 16.5% and 8.46%, respectively.
Radar chart showing the responses of enzyme activities and microbial biomass carbon (MBC) to different fertilization treatments after 6 consecutive years at Jiangyan (a) and Guangde (b). Different lowercase letters associated with the datapoints on the same perpendicular line indicate significant differences among fertilization treatments (P < 0.05). K0, no inorganic K fertilizer and straw incorporation; SRK, straw return combined with inorganic K fertilizer application; IK, inorganic K fertilizer only
3.4 Soil Bacterial Community and Potential Functions
The bacterial communities among different K fertilizer treatments were evaluated at the OTU level. PERMANOVA analysis indicated that fertilization treatments had significant effects on bacterial communities (JY: R2 = 0.1338, P = 0.011; GD: R2 = 0.2517, P = 0.002) (Fig. 3a and b). NMDS analyses based on Bray–Curtis distance also revealed that samples from different treatments were clearly separated, and samples from the same treatment could be gathered together.
Non-metric multidimensional scaling (NMDS) analysis based on Bray–Curtis dissimilarity display differences in the bacterial community compositions for Jiangyan (a, JY) and Guangde (b, GD). Different symbols represent the samples under different treatments. Differences of bacterial community compositions among treatments were tested by Adonis analysis. K0, no inorganic K fertilizer and straw incorporation; SRK, straw return combined with inorganic K fertilizer application; IK, inorganic K fertilizer only
The bacterial functions (N-cycling and P-cycling) were predicted using PICRUSt2 program. Through comparing with Kyoto Encyclopedia of Genes and Genomes (KEGG) database, a total of 7992 functional genes were obtained, including 30 N-cycling and 4 P-cycling gene families. For JY (Table 3), the SRK and IK treatments increased the putative abundances of nxrA and nxrB, involved in nitrification; nirK, norB, and nosZ involved in denitrification; nasB, nasA, narB, and nirA involved in assimilatory nitrate reduction; narI involved in dissimilatory nitrate reduction; and glnA involved in Glutamine synthesis compared with the K0 treatment.
For GD (Table 4), the SRK treatment increased the putative abundances of narI, nirK, norB, norC, and nosZ involved in denitrification; nasB, nasA, narB, and nirA involved in assimilatory nitrate reduction; nirB involved in dissimilatory nitrate reduction; gdh2 and cynS involved in organic N decomposition; glnA involved in glutamine synthesis; and ppa involved in P-cycling compared with the K0 treatment. The IK treatment increased the putative abundance of nirS involved in denitrification compared with the K0 treatment. In addition, the differences in the total abundances of gene families in the same function were compared in Table S1. The relative total abundances of gene families involved in N-cycling and P-cycling in GD were less than those in JY. In the JY site, the SRK treatment increased the putative gene abundance of nitrification by 6.88%, denitrification by 0.31%, assimilatory nitrate reduction by 1.67%, dissimilatory nitrate reduction by 0.21%, and P-cycling by 0.17% compared with the IK treatment. In the GD site, the SRK treatment increased the putative gene abundance of nitrification by 2.90%, denitrification by 22.4%, assimilatory nitrate reduction by 33.0%, dissimilatory nitrate reduction by 10.1%, and P-cycling by 1.42% compared with the IK treatment.
3.5 Correlation Analysis of Bacterial Communities Against Soil Properties and Wheat Yield
The results of automatic linear modeling displayed apparent differences in the importance of soil properties to wheat yield after 6 consecutive years (Fig. 4). Predictive importance of each soil property to wheat yield in JY was ranked in the order as follows: pH < NO3−-N < SOM < TP < AP < TN < MBC < NH4+-N < AK. In the GD site, the predictive importance of each soil property to wheat yield was in the following order: pH < TP < NH4+-N < AP < TN < NO3−-N < SOM < MBC < AK.
Predictive importance of soil properties to wheat yield after 6 consecutive years for Jiangyan (a, JY) and Guangde (b, GD) determined by automatic linear modeling. TN, total nitrogen; AK, available potassium; TP, total phosphorus; AP, available phosphorus; SOM, soil organic matter; MBC, microbial biomass carbon
Results of the Mantel test showed the correlation between the bacterial community structure and soil properties (Table 5). The highest to the lowest Pearson’s correlation scores of the Mantel test in the JY site are listed as follows: cellulase activity, SOM, urease activity, NH4+-N, alkaline phosphatase activity, TP, AK, AP, MBC, TN, sucrase activity, pH, and NO3−-N. The bacterial community structure was significantly (P < 0.05) correlated with soil properties (except for NO3−-N). In the GD site, the bacterial community structure had a significant (P < 0.05) correlation with soil cellulase activity, MBC, urease activity, sucrase activity, AK, pH, NO3−-N, acid phosphatase activity, AP, SOM, and TN.
Pearson’s correlation analysis showed the relationship between soil nutrients and enzyme activities after 6 consecutive years. In the JY site (Fig. 5a), SOM, MBC, and TP were significantly positively correlated with urease activity. pH, TN, SOM, MBC, NH4+-N, AK, and AP had a significant positive correlation (P < 0.05) with cellulase activity. TN, SOM, MBC, TP, AK, and AP had a significant positive correlation (P < 0.05) with sucrase activity. TN, SOM, MBC, TP, NH4+-N, AK, and AP had a significant positive correlation (P < 0.05) with alkaline phosphatase activity. In the GD site (Fig. 5b), TN, AK, AP, pH, NO3−-N, SOM, and MBC showed significant positive correlations (P < 0.05) with sucrase activity. Urease, cellulase, and acid phosphatase activities were significantly (P < 0.05) correlated with all soil properties.
A heatmap showing the Pearson’s correlations between soil nutrients and enzyme activities for Jiangyan (a, JY) and Guangde (b, GD). Correlation coefficient (r) is represented on the right side of the legend with different colors. *P < 0.05 and **P < 0.01. TN, total nitrogen; AK, available potassium; TP, total phosphorus; AP, available phosphorus; SOM, soil organic matter; MBC, microbial biomass carbon
4 Discussion
Fertilization is an important method to maintain soil nutrient balance and improve soil fertility (Zhao et al. 2019). In the present study, K fertilization strategies significantly affected most soil properties after a period of 6-year field experiments (Table 2). Previous studies reported that long-term inorganic fertilizer alone significantly reduce soil pH (Ge et al. 2010; Li et al. 2021), which is consistent with this study. Soil acidity caused by inorganic fertilizers, especially N fertilizer, is mainly a result of nitrification, which leads to the formation of nitric acid. Moreover, plant roots will release H+ to the soil when they absorb cations, which will also lead to soil acidification. Although the amount of N and P fertilizer applied in IK and K0 treatments were the same, the soil pH in the IK treatment was lower than that in the K0 treatment. This might be because the IK treatment produced more roots and absorbed more cations from soil compared with the K0 treatment. Huang et al. (2021b) reported that large amounts of organic acids can be generated during straw decomposition, which could accelerate soil acidification. However, we found that the SRK treatment increased soil pH compared to the K0 and IK treatments. This might be because a large number of base cations were returned to the soil after straw decomposition, thus increasing soil pH. Han et al. (2020) found that straw return increased soil pH for a maize–rice rotation system compared with no straw return. Chen et al. (2021) reported that mulching with straw addition could alleviate soil acidification.
Many studies have shown that straw return is an important way to increase the level of soil nutrients and finally improve crop yield (Su et al. 2019; Li et al. 2021). In our experiments, the soil N content (soil TN, NH4+-N, and NO3−-N) was higher in the SRK treatment than in the K0 and IK treatments, indicating that N input was greater than N output in the SRK treatment, which is consistent with previous reports that straw return is beneficial for the accumulation of N in the soil (Sharma et al. 2021; Liu et al. 2022). To our knowledge, crop straw has a high C:N. The N of crop straw itself can be returned to the soil to increase the input of soil N. Straw return also had a positive effect on the growth of soil microorganisms. The fertilizer-N immobilization by soil microorganisms could be strengthened to promote the transformation of soil N into slowly available N sources, thereby reducing N loss and improving N-use efficiency (Zhang et al. 2016). SRK treatment increased soil TP content compared with K0 and IK treatments, probably because the P in straw was returned to the soil, thereby increasing the soil P pool. The significant increase of AP content in the SRK treatment might be related to the increase of soil phosphatase activity by straw return (Han et al. 2020). The soil AK content was higher in the SRK and IK treatments than that in the K0 treatment, corroborating the result of previous research (Zhao et al. 2014) and indicating that inorganic K fertilizer and straw return both alleviated soil K depletion. In addition, the SRK treatment increased AK content compared with the IK treatment, which was consistent with previous studies (Zhao et al. 2014; Bai et al. 2015). In contrast to soil N, soil P and K are more stable and are not lost in the form of gas. Excessive K fertilizer could be converted into non-exchangeable K and fixed in the soil (Zhao et al. 2014). Straw return acted as slowly released K fertilizer, which could reduce the fixation of K by soil. Organic acids produced by straw decomposition might promote the release of soil non-exchangeable K (Bai et al. 2015). Moreover, we found that the AK content of the SRK treatment was close to that of the IK treatment in JY. In contrast, the SRK treatment significantly increased the AK content compared with the IK treatment in GD. This might be because the finer-textured (GD, silty loam) soil fixed more K nutrients, thus reducing the soil AK content in the IK treatment.
In agricultural soils, increasing soil carbon storage is of great significance. Crop straw is a substantial C source and plays an important role in increasing soil organic matter content (Su et al. 2019). Although straw return can increase soil respiration and C consumption by improving the activity of soil microorganism group, most previous studies have reported the positive effects of straw return on soil organic carbon (Chen et al. 2021; Huang et al. 2021b; Li et al. 2021). The release of carbon from decomposed straw increased the input of soil organic carbon. Zhao et al. (2014) reported that crop straw retention enhanced soil organic carbon and it increased with increasing straw inputs. In the present study, we also found that the SRK treatment significantly improved SOM compared with K0 and IK treatments.
Crop yield is directly related to soil properties. In this work, the SRK treatment was better than the IK treatment in increasing wheat yield at both sites. These findings are in agreement with those of previous studies (Zhao et al. 2014; Bai et al. 2015). This increase was probably associated with SRK treatment, which returned a large part of the nutrients back into the soil and improved the soil physical, chemical, and biological properties (Zhao et al. 2016). Interestingly, the response of wheat yield to different K fertilizer treatments varied in different sites after 6 consecutive years. In JY, the wheat yield in the K0 treatment was 5.41t ha−1, whereas that in GD was 0.54t ha−1. This result showed that the application of N and P fertilizers without K fertilizer could not meet the growth of crop, and soil K nutrient was the main limiting factor for wheat yield in the K0 treatment in GD. There is a dynamic balance between AK and non-exchangeable K in soil, and part of non-exchangeable K could be converted into AK when the soil AK concentration was low (Li et al. 2021). Our previous research (Li et al. 2020) reported that due to the low basic K fertility of GD soil, soil K nutrient was gradually depleted with the increasing year and thus resulted in inadequate K supply for crop growth in the K0 treatment. By contrast, the non-exchangeable K in JY soil was transformed into exchangeable K in the K0 treatment. Therefore, the response of wheat yield in the GD site to K depletion was much greater than that in the JY site.
In general, the degradation of straw in soil will produce a large quantity of labile organic compounds (Zhao et al. 2016), which provide nutrients and energy for the growth of soil microorganisms. Moreover, straw return can reduce soil bulk density, increase soil aeration, and improve soil hydrothermal conditions (Zhang et al. 2016), which created favorable conditions for the growth of soil microorganisms. The results obtained in our study also indicated that long-term straw return increased soil MBC. In addition, the MBC in the IK treatment were found to be higher than that in the K0 treatment for both sites. This result might be related to the higher root residues of IK treatment.
Soil enzymes play a vital role in soil system and regulate many important biochemical processes in soil (Li et al. 2019; Liu et al. 2021). The close relations between soil microbes and soil enzyme activities are well known. Most soil enzymes are produced by soil microorganisms (Zhu et al. 2019). Previous studies have shown that straw return can increase microbial biomass, thus providing energy and a favorable environment for the increase of soil enzyme (Gianfreda et al. 2005; Sharma et al. 2021). Soil sucrase and cellulase decompose sucrose and cellulose in straw into monosaccharides, thus providing available C nutrients for soil microorganisms, which in turn stimulates the enhancement of other soil enzyme activities. Soil urease and phosphatase participate in the cycling of soil N and P, respectively. Zhu et al. (2019) and Huang et al. (2021b) reported that long-term straw return treatment increases the soil enzyme activities of cellulase, sucrase, phosphatase, and urease, which promoted the decomposition of straw and nutrient availability compared to inorganic fertilizers. In the present study, there was a significant positive correlation between soil enzyme activities and soil nutrient contents (Fig. 5). The highest enzyme activities of urease, sucrase, phosphatase, and cellulase were observed in SRK, followed by IK and K0, corroborating previous studies. The promoting effect of straw return on cellulase activity was far greater than that of straw return on the activity of other enzymes. This result might be related to the large amount of cellulose in crop straw.
Numerous studies have shown that fertilization plays an important role in shaping soil bacterial community structure and metabolic function through influencing the living environment (Geisseler and Scow 2014; Navarro-Noya et al. 2013; Wang et al. 2018). The changes of bacterial communities are of great significance to the decomposition of soil organic matter and nutrient dynamics and thus are essential for maintaining soil fertility and productivity in agricultural ecosystems (Li et al. 2014; Luo et al. 2019). Soil bacteria has preferences for specific ecological niches and thereby is very sensitive to changes in soil properties (Dangi et al. 2020). A study by Sun et al. (2015) showed that soil pH is the most important factor to explain the changes of soil bacterial community structure under different fertilization measures. This is because pH is the regulator of vital movement. Zhu et al. (2019) reported that soil AK plays an important role in shaping bacterial community in a double-rice cropping system after 9 years of straw incorporation and reduced inorganic fertilizer. Zhao et al. (2019) indicated that the change in soil available N may result in a significant change in the bacterial community after straw addition in different long-term fertilization soils. Su et al. (2019) indicated that soil AK, total organic carbon, and AP contents are important factors affecting soil bacterial community after long-term straw return. Due to the supplemental inputs of nutrients from straw return, soil organic carbon is the key property regulating soil microbial community structure (Chen et al. 2021). In the present study, the soil bacterial communities were significantly correlated with soil pH, TN, AK, TP, and SOM for both JY and GD, indicating that long-term application of inorganic K fertilizer and straw return altered the soil bacterial distribution.
Many studies have shown that changes in soil properties caused by fertilization can drive the shifts in soil bacterial functional profiles (Yuan et al. 2019; Zhang et al. 2019). It is generally believed that the abundance of functional gene is related to soil nutrient content (Huang et al. 2019). Huang et al. (2021b) indicated a positive relationship between soil microbial carbon metabolism ability and SOM content. In the present study, higher soil permeability of JY soil increased the degree of aeration and accelerated the decomposition of organic compounds compared with GD soil. For this reason, GD soil had a stronger C retention capacity than JY soil. Higher content of soil inorganic N content can increase the substrates for nitrification and denitrification, thus resulting in greater abundance of relevant N transformation genes (Zhang et al. 2019). In this study, the abundances of gene families involved in nitrification, denitrification, assimilatory nitrate reduction, dissimilatory nitrate reduction, organic N decomposition, and glutamine synthesis were enhanced by straw return. We also found that the N-preserving capacity of GD soil was stronger than that of JY soil (Table 2). The possible reasons for this result might be the following: (i) the abundances of gene families involved in N-cycling in GD were less than the corresponding abundances in JY; (ii) compared with that of JY, GD has a higher cation exchange capacity and negative surface charge, thus having a stronger NH4+ adsorption capacity, which decreased the level of bioavailable NH4+ in GD soil; and (iii) neutral soil is generally considered to be conducive to the growth of soil bacteria, and the soil pH of JY was slightly alkaline, whereas the soil pH of GD was acidic, which resulted in the potential N-cycling and P-cycling functions of JY being higher than those of GD. In addition, the promoting effect of SRK on the potential N-cycling and P-cycling functions in GD were greater than those in JY, indicating that straw return appeared to be more effective for improving soil biological properties in GD soil.
Previous studies have shown that plant has a strong impact on soil bacterial communities (Zhang et al. 2019; Yan et al. 2020). In the present study, the highest wheat yield was observed in the SRK treatment; thus, it had more litter, root exudates, and root residues, which promoted the activity of soil microorganisms. The activities of soil microbes and enzyme in the IK treatment were higher than those in the K0 treatment, which confirmed the importance of plants in determining the soil microbial community. In the present study, soil AK content was the most important factor affecting wheat yield. However, under the condition of extreme K deficiency in the K0 treatment, especially the K0 treatment in GD (wheat yield was almost zero), soil bacteria and enzymes still maintained a measurable level of activity, indicating that the stability of soil bacteria in changing environments was higher than that of plants (Huang et al. 2021b). Soil AK might indirectly affect soil bacterial community through plants, which was one of the reasons that the positive effect of straw return on enzyme activities and potential N-cycling and P-cycling functions in GD was greater than that in JY.
5 Conclusions
The wheat yield, soil chemical properties, soil bacterial communities and enzyme activities were investigated after 6 consecutive years under soil potassium (K) balance condition in rice–wheat system. Wheat yield with low soil K fertility was more sensitive to K fertilizer application than that with high soil K fertility. Soil available potassium (AK) was the most important factor affecting wheat yield. Soil texture, AK content, and pH had a significant impact on the soil bacterial community, thus affecting changes in enzyme activities and nutrient cycling, as well as ultimately affecting crop yield. The positive effects of straw return on wheat yield, soil nutrients, and microorganisms in silty loam soil with low pH and K fertility were greater than those in loam soil with high pH and K fertility. Overall, straw return combined with inorganic K fertilizer is a promising approach to maintain crop yield and soil fertility and more necessary to be adopted in the silty loam soil with low pH and K fertility.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- JY:
-
Jiangyan
- GD:
-
Guangde
- K:
-
Potassium
- P:
-
Phosphorus
- N:
-
Nitrogen
- NH4 +-N:
-
Ammoniac nitrogen
- NO3 −-N:
-
Nitrate nitrogen
- TN:
-
Total nitrogen
- AK:
-
Available potassium
- TP:
-
Total phosphorus
- AP:
-
Available phosphorus
- SOM:
-
Soil organic matter
- MBC:
-
Microbial biomass carbon
- K0:
-
No inorganic K fertilizer and straw incorporation
- SRK:
-
Straw return combined with inorganic K fertilizer application
- IK:
-
Inorganic K fertilizer only
- OTU:
-
Operational taxonomic unit
- NGDC:
-
National Genomics Data Center
- LSD:
-
Least significant difference
- NMDS:
-
Non-metric multidimensional scaling
- PERMANOVA:
-
Permutational multivariate analysis of variance
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
References
Bai YL, Wang L, Lu YL, Yang LP, Zhou LP, Lu N, Cheng MF (2015) Effects of long-term full straw return on yield and potassium response in wheat-maize rotation. J Integr Agr 14:2467–2476. https://doi.org/10.1016/S2095-3119(15)61216-3
Bao YY, Dolfing J, Wang BZ, Chen RR, Huang MS, Li ZP, Lin XG, Feng YZ (2019) Bacterial communities involved directly or indirectly in the anaerobic degradation of cellulose. Biol Fert Soils 55:201–211. https://doi.org/10.1007/s00374-019-01342-1
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Ceja-Navarro JA, Rivera-Orduna FN, Patino-Zuniga L, Vila-Sanjurjo A, Crossa J, Govaerts B, Dendooven L (2010) Phylogenetic and multivariate analyses to determine the effects of different tillage and residue management practices on soil bacterial communities. Appl Environ Microbiol 76:3685–3691. https://doi.org/10.1128/AEM.02726-09
Chen ZM, Wang HY, Liu XW, Zhao XL, Lu DJ, Zhou JM, Li CZ (2017) Changes in soil microbial community and organic carbon fractions under short-term straw return in a rice–wheat cropping system. Soil till Res 165:121–127. https://doi.org/10.1016/j.still.2016.07.018
Chen XQ, Li T, Lu DJ, Cheng L, Zhou JM, Wang HY (2019) Estimation of soil available potassium in Chinese agricultural fields using a modified sodium tetraphenyl boron method. Land Degrad Dev 31:1737–1748. https://doi.org/10.1002/ldr.3535
Chen Y, Tian W, Shao Y, Li YJ, Lin LA, Zhang YJ, Han H, Chen ZJ (2020) Miscanthus cultivation shapes rhizosphere microbial community structure and function as assessed by Illumina MiSeq sequencing combined with PICRUSt and FUNGUIld analyses. Arch Microbiol 202:1157–1171. https://doi.org/10.1007/s00203-020-01830-1
Chen QY, Liu ZJ, Zhou JB, Xu XP, Zhu YJ (2021) Long-term straw mulching with nitrogen fertilization increases nutrient and microbial determinants of soil quality in a maize–wheat rotation on China’s Loess Plateau. Sci Total Environ 755:145930. https://doi.org/10.1016/j.scitotenv.2021.145930
Dangi S, Gao SD, Duan YH, Wang D (2020) Soil microbial community structure affected by biochar and fertilizer sources. Appl Soil Ecol 150:103452. https://doi.org/10.1016/j.apsoil.2019.103452
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Eslaminejad P, Heydari M, Kakhki FV et al (2020) Plant species and season influence soil physicochemical properties and microbial function in a semi-arid woodland ecosystem. Plant Soil 456:43–59. https://doi.org/10.1007/s11104-020-04691-1
Ge GF, Li ZJ, Fan FL, Chu GX, Hou ZN, Liang YC (2010) Soil biological activity and their seasonal variations in response to long-term application of organic and inorganic fertilizers. Plant Soil 326:31–44. https://doi.org/10.1007/s11104-009-0186-8
Geisseler D, Scow KM (2014) Long-term effects of mineral fertilizers on soil microorganisms–a review. Soil Biol Biochem 75:54–63. https://doi.org/10.1016/j.soilbio.2014.03.023
Gianfreda L, Rao MA, Piotrowska A, Palumbo G, Colombo C (2005) Soil enzyme activities as affected by anthropogenic alterations: intensive agricultural practices and organic pollution. Sci Total Environ 341:265–279. https://doi.org/10.1016/j.scitotenv.2004.10.005
Han YL, Ma W, Zhou BY et al (2020) Effects of straw-return method for the maize–rice rotation system on soil properties and crop yields. Agronomy 10:461. https://doi.org/10.3390/agronomy10040461
Heimann M, Reichstein M (2008) Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451:289–292. https://doi.org/10.1038/nature06591
Hu XJ, Liu JJ, Liang AZ et al (2021) Conventional and conservation tillage practices affect soil microbial co-occurrence patterns and are associated with crop yields. Agr Ecosyst Environ 319:107534. https://doi.org/10.1016/j.agee.2021.107534
Huang R, Wang YY, Liu J, Li JC et al (2019) Variation in N2O emission and N2O related microbial functional genes in straw- and biochar-amended and non-amended soils. Appl Soil Ecol 137:57–68. https://doi.org/10.1016/j.apsoil.2019.01.010
Huang W, Wu FJ, Pan XH et al (2021a) Effects of long-term straw return on soil organic carbon fractions and enzyme activities in a double-cropped rice paddy in South China. J Integr Agr 20:236–247. https://doi.org/10.1016/S2095-3119(20)63347-0
Huang RL, Crowther TW, Sui YY, Sun B, Liang YT (2021b) High stability and metabolic capacity of bacterial community promote the rapid reduction of easily decomposing carbon in soil. Commun Biol 4:1376. https://doi.org/10.1038/s42003-021-02907-3
Jarosch KA, Kandeler E, Frossard E, Bünemann EK (2019) Is the enzymatic hydrolysis of soil organic phosphorus compounds limited by enzyme or substrate availability? Soil Biol Biochem 139:107628. https://doi.org/10.1016/j.soilbio.2019.107628
Kumar S, Sharma DK, Singh DR, Biswas H, Praveen KV, Sharma V (2019) Estimating loss of ecosystem services due to paddy straw burning in North-west India. Int J Agr Sustain 17:146–157. https://doi.org/10.1080/14735903.2019.1581474
Li ZP, Liu M, Wu XC, Han FX, Zhang TL (2010) Effects of long-term chemical fertilization and organic amendments on dynamics of soil organic C and total N in paddy soil derived from barren land in subtropical China. Soil Tillage Res 106:268–274. https://doi.org/10.1016/j.still.2009.12.008
Li CH, Yan K, Tang LS, Jia ZJ, Li Y (2014) Change in deep soil microbial communities due to long-term fertilization. Soil Biol Biochem 75:264–272. https://doi.org/10.1016/j.soilbio.2014.04.023
Li Y, Fang F, Wei JL, Wu XB, Cui RZ, Li GS, Zheng FL, Tan DS (2019) Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: a three-year experiment. Sci Rep-UK 9:12014. https://doi.org/10.1038/s41598-019-48620-4
Li CZ, Zhao XL, Liu XW, Lu DJ, Chen X, Wang HY, Zhou JM (2020) Rice and wheat yield and soil potassium changes in response to potassium management in two soil types. Nutr Cycl Agrecosys 117:121–130. https://doi.org/10.1007/s10705-020-10056-y
Li JF, Gan GY, Chen X, Zou JL (2021) Effects of long-term straw management and potassium fertilization on crop yield, soil properties, and microbial community in a rice–oilseed rape rotation. Agriculture 11:1233. https://doi.org/10.3390/agriculture11121233
Liu JA, Shu AP, Song WF et al (2021) Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 404:115287. https://doi.org/10.1016/j.geoderma.2021.115287
Liu SW, Wang MJ, Yin M et al (2022) Fifteen years of crop rotation combined with straw management alters the nitrogen supply capacity of upland-paddy soil. Soil Till Res 215:105219. https://doi.org/10.1016/j.still.2021.105219
Luo XS, Han S, Fu XQ, Li X, Wang L, Peng SB, Chen WL, Huang QY (2019) The microbial network in naturally fertile paddy soil possibly facilitates functional recruitment in the rice mature stage. Appl Soil Ecol 135:174–181. https://doi.org/10.1016/j.apsoil.2018.12.008
Magoc T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Navarro-Noya YE, Gomez-Acata S, Montoya-Ciriaco N et al (2013) Relative impacts of tillage, residue management and crop-rotation on soil bacterial communities in a semi-arid agroecosystem. Soil Biol Biochem 65:86–95. https://doi.org/10.1016/j.soilbio.2013.05.009
Niu JF, Zhang WF, Ru SH et al (2013) Effects of potassium fertilization on winter wheat under different production practices in the North China Plain. Field Crop Res 140:69–76. https://doi.org/10.1016/j.fcr.2012.10.008
Pettigrew WT (2008) Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol Plantarum 133:670–681. https://doi.org/10.1111/j.1399-3054.2008.01073.x
Sharma S, Singh P, Choudhary OP, Neemisha (2021) Nitrogen and rice straw incorporation impact nitrogen use efficiency, soil nitrogen pools and enzyme activity in rice-wheat system in north-western India. Field Crop Res 266:108131. https://doi.org/10.1016/j.fcr.2021.108131
Song XD, Liu F, Wu HY et al (2020) Effects of long-term K fertilization on soil available potassium in East China. Catena 188:104412. https://doi.org/10.1016/j.catena.2019.104412
Song DL, Dai XL, Guo TF et al (2022) Organic amendment regulates soil microbial biomass and activity in wheat-maize and wheat-soybean rotation systems. Agr Ecosyst Environ 333:107974. https://doi.org/10.1016/j.agee.2022.107974
Su Y, Lv JL, Yu M, Ma ZH et al (2019) Long-term decomposed straw return positively affects the soil microbial community. J Appl Microbiol 128:138–150. https://doi.org/10.1111/jam.14435
Sun RB, Zhang XX, Guo XS, Wang DZ, Chu HY (2015) Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol Biochem 88:9–18. https://doi.org/10.1016/j.soilbio.2015.05.007
Tian XP, Wang L, Hou YH, Wang H, Tsang YF, Wu JH (2019) Responses of soil microbial community structure and activity to incorporation of straws and straw biochars and their effects on soil respiration and soil organic carbon turnover. Pedosphere 29:492–503. https://doi.org/10.1016/S1002-0160(19)60813-1
Wang MP, Chen L, Li YT, Chen L, Liu ZY, Wang XJ, Yan PS, Qin S (2018) Responses of soil microbial communities to a short-term application of seaweed fertilizer revealed by deep amplicon sequencing. Appl Soil Ecol 125:288–296. https://doi.org/10.1016/j.apsoil.2018.02.013
Wu X, Hu H, Li SJ et al (2022) Chemical fertilizer reduction with organic material amendments alters co-occurrence network patterns of bacterium-fungus-nematode communities under the wheat–maize rotation regime. Plant Soil 473:605–623. https://doi.org/10.1007/s11104-022-05314-7
Xiang XJ, Gibbons SM, Li H et al (2018) Shrub encroachment is associated with changes in soil bacterial community composition in a temperate grassland ecosystem. Plant Soil 425:539–551. https://doi.org/10.1007/s11104-018-3605-x
Xie ZH, Yu ZH, Li YS et al (2022) Soil microbial metabolism on carbon and nitrogen transformation links the crop-residue contribution to soil organic carbon. Npj Biofilms Microbi 8:14. https://doi.org/10.1038/s41522-022-00277-0
Yan SS, Song JM, Fan JS et al (2020) Changes in soil organic carbon fractions and microbial community under rice straw return in Northeast China. Glob Ecol Conserv 22:e00962. https://doi.org/10.1016/j.gecco.2020.e00962
Yan MF, Li TH, Li XR, Liu Y, Zhang JB (2021) Microbial biomass and activity restrict soil function recovery of a post-mining land in eastern Loess Plateau. Catena 199:105107. https://doi.org/10.1016/j.catena.2020.105107
Yu CJ, Qin JG, Xu J, Nie H, Luo ZY, Cen KF (2010) Straw combustion in circulating fluidized bed at low-temperature: transformation and distribution of potassium. Can J Chem Eng 88:874–880. https://doi.org/10.1002/cjce.20321
Yuan HZ, Ge TD, Zhou P, Liu SL, Roberts P, Zhu HH, Zou ZY, Tong CL, Wu JS (2013) Soil microbial biomass and bacterial and fungal community structures responses to long-term fertilization in paddy soils. J Soil Sediment 13:877–886. https://doi.org/10.1007/s11368-013-0664-8
Yuan HZ, Zhu ZK, Wei XM et al (2019) Straw and biochar strongly affect functional diversity of microbial metabolism in paddy soils. J Integr Agr 18:1474–1485. https://doi.org/10.1016/S2095-3119(18)62102-1
Zhang P, Wei T, Jia ZK, Han QF, Ren XL (2014) Soil aggregate and crop yield changes with different rates of straw incorporation in semiarid areas of northwest China. Geoderma 230:41–49. https://doi.org/10.1016/j.geoderma.2014.04.007
Zhang P, Chen XL, Wei T et al (2016) Effects of straw incorporation on the soil nutrient contents, enzyme activities, and crop yield in a semiarid region of China. Soil till Res 160:65–72. https://doi.org/10.1016/j.still.2016.02.006
Zhang C, Song ZL, Zhuang DH, Wang J, Xie SS, Liu GB (2019) Urea fertilization decreases soil bacterial diversity, but improves microbial biomass, respiration, and N-cycling potential in a semiarid grassland. Biol Fert Soils 55:229–242. https://doi.org/10.1007/s00374-019-01344-z
Zhang JW, Li WW, Zhou Y et al (2021) Long-term straw incorporation increases rice yield stability under high fertilization level conditions in the rice-wheat system. Crop J 9:1191–1197. https://doi.org/10.1016/j.cj.2020.11.007
Zhao SC, He P, Qiu SJ, Jia LL, Liu MC, Jin JY, Johnston AM (2014) Long-term effects of potassium fertilization and straw return on soil potassium levels and crop yields in north-central China. Field Crop Res 169:116–122. https://doi.org/10.1016/j.fcr.2014.09.017
Zhao SC, Li KJ, Zhou W et al (2016) Changes in soil microbial community, enzyme activities and organic matter fractions under long-term straw return in north-central China. Agr Ecosyst Environ 216:82–88. https://doi.org/10.1016/j.agee.2015.09.028
Zhao SC, Qiu SJ, Xu XP, Ciampitti IA, Zhang SP, He P (2019) Change in straw decomposition rate and soil microbial community composition after straw addition in different long-term fertilization soils. Appl Soil Ecol 138:123–133. https://doi.org/10.1016/j.apsoil.2019.02.018
Zhong YQW, Yan WM, Wang RW et al (2018) Decreased occurrence of carbon cycle functions in microbial communities along with long-term secondary succession. Soil Biol Biochem 123:207–217. https://doi.org/10.1016/j.soilbio.2018.05.017
Zhu J, Peng H, Ji XH, Li CJ, Li SN (2019) Effects of reduced inorganic fertilization and rice straw recovery on soil enzyme activities and bacterial community in double-rice paddy soils. Eur J Soil Biol 94:103116. https://doi.org/10.1016/j.ejsobi.2019.103116
Acknowledgements
We thank Guoyin Yuan and Changzhou Li for their help with these experiments.
Funding
This work was financially supported by the Major State Basic Research Development Program of China (Grant No. 2013CB127401) and the National Department Public Benefit Research Foundation of China (Grant No. 201203013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, L., Wang, H. Responses of Soil Bacterial Communities and Enzyme Activities to Straw Return and Potassium Fertilization with Two Soils Under Soil Potassium Balance Condition in Rice–Wheat System. J Soil Sci Plant Nutr 23, 1845–1859 (2023). https://doi.org/10.1007/s42729-023-01142-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01142-5