Abstract
The effect of plant species on their root-associated arbuscular mycorrhizal fungi (AMF) under salt stress is well studied, but how cultivars modulate this association remains largely unexplored. To fill in such a gap in knowledge, this study investigates how durum wheat cultivars shape their AMF communities in relation with plant physiological traits. Six durum wheat cultivars were grown in semi-arid areas and irrigated with three salinity levels (6, 12, and 18 dS m−1). The interaction between cultivar and salinity had a considerable impact on AMF status, plant physiological traits, and grain yield (GY). In particular, Maali (modern variety) exhibited the highest belowground inputs (mycorrhizal root colonization, spore density, and spore morphotype number) at 6 and 12 dS m−1, while a clear prevalence was obtained for Agili Glabre (landrace) at 18 dS m−1. Furthermore, these two cultivars were distinguished by a low yield stress susceptibility index and a high GY. Some AMF genera (e.g., Glomus, Funneliformis, and Paraglomus) seem to interact with most cultivars, while some others including Acaulospora and Septoglomus preferred to colonize Agili Glabre cultivar. This study indicates the contribution of durum wheat cultivar in operating the AMF diversity. Under both conditions (6 and 12 dS m−1), the partial least square structural equation modelling (PLS-SEM) showed that AMF colonization had an indirect effect on GY through C metabolism, expressed mainly by δ13Cflag leaf and δ13Cgrain. These findings highlight that durum wheat cultivar is a determinant factor in AMF symbiosis performance, therefore of salt-tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Future agricultural crop productivity is jeopardized by soil salinization and the use of poor quality of water irrigation, particularly in arid and semi-arid climates (Shahid et al. 2018). Durum wheat (Triticum durum Desf.), a worldwide grown crop and the main component of pasta industrial chain, is a salt-sensitive glycophyte species (Munns et al. 2006). From 6 dS m − 1, wheat growth and grain yield were reduced by 7.1 percent for every dS m − 1 increase in salinity, with a significant yield reduction at 15 dS m − 1 (Chinnusamy et al. 2005). To cope with this abiotic stress, there is an increasing trend towards research and development of a viable alternative methods based on several beneficial microorganisms, particularly arbuscular mycorrhizal fungi (AMF, phylum Glomeromycota).
The roots of most vascular plants (> 80%) can establish mutualistic symbiotic associations with AMF (Brundrett and Tedersoo 2018), helping them to improve plant’s resilience to salt stress to consequently prevent their yield losses (Abdel Latef and Miransari 2014; Evelin et al. 2019; Yasmeen et al. 2019). AMF are distributed across a wide range of ecosystems, even in extreme conditions such as saline soils (Becerra et al. 2014). Compared to non-colonized plants, AMF alleviate salt stress-induced damage on colonized plants by modulating nutritional, physiological, and biochemical pathways through (i) improving water and nutrient uptake (Evelin et al. 2019), (ii) decreasing Na+ concentration to maintain homeostasis (Begum et al. 2019), and (iii) removing reactive oxygen species (ROS) by inducing the activities of antioxidant enzymes (e.g., superoxide dismutase, catalase, glutathione reductase, and peroxidase) or by increasing the accumulation of antioxidant compounds (e.g., ascorbate and glutathione) (Ganugi et al. 2019; Gupta et al. 2021). The soil AMF attributes under salt-affected environments has been the subject of several studies, but the results remained controversial (Barin et al. 2013; Juniper and Abbott 2006). In fact, salinity harmful effects on AMF fitness were observed with considerable evidence on sporulation, root colonization, spore density and richness, and community structure and composition (Melo et al. 2017; Torrecillas et al. 2013). On the other hand, several publications reported either no adverse effect or even boosting effects of salinity on AMF functions (Yano-Melo et al. 2003; Yamatou et al. 2008).
The AMF-induced salt tolerance of crops could significantly differ with AMF species as well as host species and cultivar identity (Estrada et al. 2013; Ganugi et al. 2021; Mao et al. 2014; Zhu et al. 2001). Moreover, a wheat cultivar genetic basis was among the specific linkage to AMF mycorrhization (Hetrick et al. 1992). Only little information is currently available on the AMF diversity within wheat roots under salt stress, and how cultivars control the fungal communities inside their roots. In non-stressed conditions, modern high-yielding wheat cultivars showed a putative loss of susceptibility to AMF in conventional agroecosytems as a result of their ability to uptake mineral nutrients, in particular phosphate, without the aid of their symbionts, compared to landraces (Hetrick et al. 1992; Zhu et al. 2001), whereas recent works revealed that modern plant breeding programs did not lead to the suppression of AMF colonization in wheat (De Vita et al. 2018; Ganugi et al. 2021). In the current study, the effect of durum wheat cultivars on AMF status was assessed through grain yield performance, tolerance index, and physiological features in plants, including the stable isotopes of carbon (12C, 13C) and nitrogen (15 N, 14 N) in addition to the mycorrhizal attributes. The stable C isotope composition (δ13C) of dry matter reflects C fractionation during photosynthetic carbon dioxide (CO2) fixation with traces of local environmental conditions (Lawlor and Cornic 2002). The N isotope composition is governed by N15 isotope composition (δ15N) of the N source and by various physiological and metabolic processes within the plant (Evans and Poorter 2001). In particular, δ13C and δ15N were proposed as a predictive criterion for durum wheat yield under drought and salinity (Yousfi et al. 2012). Nonetheless and probably due to the higher complexity of the N cycle, the δ15N is more complicated trait, much less understood and more rarely used than δ13C (Spangenberg et al. 2021). To our knowledge, this is the first study that describes the relationship between mycorrhizal proliferation and δ13C and δ15N in durum wheat cultivars under salt stress conditions.
To assess with a high efficiency and to reflect the complex fungal community interaction within durum wheat cultivars under salt stress, open-field tests were conducted in three locations differing by their irrigation water salinity (6, 12, and 18 dS m−1). Thus, six durum wheat cultivars (landraces and modern varieties) and AMF attributes such as root colonization, spore density, and diversity were evaluated after 2 years of saline water application. In addition, the relationship between AMF and durum wheat C and N metabolism was studied. Here, we addressed the following questions:
-
(1)
How the durum wheat cultivars affect the mycorrhizal status within salinity levels?
-
(2)
Does AMF play under the three irrigation conditions the same relevant role to alleviate the salt stress of durum wheat? Does landraces benefit more from AMF colonization than modern varieties that we hypothesized that have lost sensitivity to these symbionts but are even more productive?
-
(3)
Is there a cultivar-specific adaptive symbiosis depending on the degree of salinity?
-
(4)
Is there an effect of mycorrhizal colonization on yield performance following a change in C and N metabolism, as assessed by C and N contents, δ13C, and δ15N in flag leaves and grains? Does this relationship changes along salinity gradients in the field?
2 Materials and Methods
2.1 Durum Wheat Cultivars
Six (06) durum wheat (Triticum durum Desf.) cultivars were used: 3 Tunisian landraces (Bayadha, Souri, and Agili Glabre) chosen based on available information about genetic diversity (Ben Salem et al. 1997) and 3 Tunisian high yielding modern varieties (Razzek, Karim, and Maali) that are the current most cultivated (Table S1). Considering their salt tolerance performance, Bayadha and Razzek are considered as salt-sensitive cultivars, Souri and Karim are moderately tolerants, while Agili Glabre and Maali are recognized as salt-tolerant cultivars (Ben Salem et al. 1997; Boudabbous et al. 2022).
2.2 Experimental Sites
Three (3) experimental sites (St) located in the central area of Tunisia were chosen (Table 1, Fig. 1 and 2). Those regions have a typically Mediterranean semi-arid climate and present very similar weather conditions. Before sowing, the physico-chemical properties of soil (0–20 cm) were analyzed as described by Boudabbous et al. (2020, 2022). Bulk density was determined by the cylinder method (Blake and Hartge 1986).
2.3 Experimental Design and Field Management
The field experiments were irrigated with salty water at three concentration gradients (Table 1): medium irrigation water salinity (IWS1 = 6 dS m−1) in St1 (Echbika), severe irrigation water salinity (IWS2 = 12 dS m−1) in St2 (Barrouta), and very severe salinity irrigation water salinity (IWS3 = 18 dS m−1) in St3 (Sidi Bouzid) (Scianna 2002). The experiments were established in two consecutive cropping seasons (2010/11 and 2011/12). The field trials were conducted in a randomized complete block design with three replicates/treatment (n = 3). The treatments were defined by a factorial combination of 6 cultivars and 3 salinity levels. The IWS1 was considered as control due to the poor quality of water irrigation used in St2 and St3. The chemical properties of water and the technical practices are described in Table 1.
2.4 Soil and Root Sampling Procedure
Throughout this investigation, the flowering growth stage (Z65) was chosen for soil and root samplings. In fact, maximum durum wheat root growth occurs at Z65 leading to highest levels of AM colonization (Urbanavičiūtė et al. 2022). Under each rhizosphere’s cultivar, five soil cores (20 cm diameter × 30 cm depth) were collected after two cropping seasons of saline water application (i.e., 2011/12 cropping season). Sampling points were located between intra-row durum wheat plants and from the center of inter-row spaces in each plot. Because spores can have an aggregate distribution, the five samples per cultivar and per plot were pooled in a plastic bag to form a composite sample (~ 1 kg per plot) to assess the AMF spore density (Koske and Halvorson 1981). The roots were collected in Z65 using the same sampling method.
2.5 Assessed Parameters
2.5.1 Root Mycorrhizal Colonization
Freshly fine root fragments were cut into 1 cm pieces, rinsed with distilled water, and cleared in 10% KOH at 90 °C for 30 min. Roots were then rinsed several times with deionized water, acidified with HCl (1%) for 3 min, and stained with Trypan Blue (0.05%, w/v) in lacto-glycerol (1:1:1, lactic acid:glycerol:water) at 90 °C for 1 h (Phillips and Hayman 1970). Before observation under the microscope, the roots were de-stained in lactoglycerol solution for a few minutes to remove the excess stain. The mycorrhizal colonization_ (RC, %), including hyphal, vesicular, and arbuscular abundance, were microscopically (× 100 to × 400) examined using the magnified intersection method (McGonigle et al. 1990).
2.5.2 Indigenous AMF Spore Isolation, Quantification, and Morphological Identification
One hundred grams of soil samples per replicate and per cultivar were used for spore isolation by wet-sieving and decanting method followed by sucrose centrifugation (Gerdemann and Nicolson 1963). Spores and debris were collected on 38 µm, 150 µm, and 500 µm sieves with tap water. After centrifugation, the supernatant was poured through a 50-mm mesh and quickly rinsed with tap water and then placed in a 9-cm Petri dish for examination under a binocular stereomicroscope (× 40, × 60). Spore density (SD 100 g−1 soil) was evaluated by counting the number of spores in 100 g of soil sample.
Morphological identification of AMF was performed based on the genus through stereoscopic microscope (40 × magnifications). Isolated spores were counted, transferred to an individual glass slide by wet needle, and then mounted in polyvinyl alcohol-lactic- glycerol (PVLG) either with or without Melzer’s reagent (Schenck and Pérez 1990). Spores were identified using morphological criteria and compared to a culture database established by INVAM (http://invam.cag.wvu.edu/). The following parameters were derived based on morphotype identification:
-
(1)
Spore morphotype number (SMN): total number of taxon or morphotype.
-
(2)
Frequency of occurrence (FO): percentage of samples containing a particular taxon or morphotype among all samples, reflecting the distribution status.
-
(3)
Relative spore density (RD): ratio of spore density of a particular taxon or morphotype to the total density of spores, showing comparing sporulation of different morphotypes in a particular soil.
-
(4)
The importance value (IV): evaluation of the dominance of AMF species based on FO and RD, calculated as IV = (FO + RD)/2. An IV ≥ 50% indicates that a taxon (genus or species) is dominant; 10% < IV < 50% applies to common taxa; and IV ≤ 10% indicates that a taxon is rarely recovered (Melo et al. 2019).
2.5.3 Leaf Area and Chlorophyll Content
During 2011/12 cropping season, the flag leaf area (FLA, cm2) was measured at the flowering growth stage (Z65) from five flag leaves per plot. Measurements were performed using a portable laser leaf area meter (CID Bio-Science, CI-202, USA). The chlorophyll content (SPAD, SPAD value) of the flag leaf was measured in 10 plants per plot using a portable meter (Minolta SPAD 502 m, Plainfield, IL, USA).
2.5.4 Total C and N and Stable C and N Isotope Analyses
The total C (%) and N (%) and the stable C (δ13C, ‰) and N (δ15N, ‰) isotope signatures were analyzed in flag leaves at the flowering stage (Z65) and mature grain samples of St1 (IWS1 = 6 dS m−1) and St2 (IWS2 = 12 dS m−1) during 2011/12 cropping season. For each plot, five flag leaves as well as mature grains were dried at 70 °C for 24 h. Powdered leaf and grain samples and reference materials (∼1 mg) were weighed into tin capsules and measured with an elemental analyzer (Flash1112EA; Thermo Finnigan, Bremen, Germany) coupled with an isotope ratio mass spectrometer (Delta CIRMS, Thermo Finnigan) operating in continuous flow mode in order to determine the total C and N content and the stable C and N isotopes. Measurements were conducted at the Scientific Facilities of the University of Barcelona. The stable isotope compositions were reported in the delta (δ) notation as variations in the molar ratio (R) of the heavy isotope to light isotope of the element E (hE/lE, i.e., 13C/12C and 15 N/14 N) in the sample relative to an international standard:
The standard for δ13C was the Pee Dee Belemnite (PDB) calcium, and that for δ15N was the molecular nitrogen in air (Air-N2).
2.5.5 Grain Yield and Yield Stress Susceptibility Index
Plants were harvested one month after the grain has reached physiological maturity at (Z92) around mid-May, 2012. For each experimental unit, an area of 1 m2 was manually harvested and grain yield (GY, t ha−1) was determined. The yield stress susceptibility percentage index (SSPI) was calculated as reported by Mekliche et al. (2015):
SPPI combines the relative performance of a genotype under saline stress conditions (IWS2 and IWS3) with its GY under control conditions (IWS1).
2.6 Data Analysis
Physiological, agronomic, and soil variables were compared using analysis of variance (ANOVA) and Tukey post hoc test at 5% significance. Linear regression was used to test the relationships between mycorrhizal RC, GY, and SSPI.
To investigate the relationships between all measured variables among the studied durum wheat cultivars, multivariate analysis was only conducted for IWS1 and IWS2 because some physiological variables (C and N contents and the stable C and N isotopes in flag leaves and mature grains) were not determined at IWS3. The principal component analysis (PCA) was conducted to determine which traits explain most the variation and to determine different groups of cultivars associated with major traits. PCA analysis was assessed using the Factoextra package (Kassambara and Mundt 2020). All statistical analyses were performed using the RStudio software version 3.6.0 (R Core Team 2019).
Partial least square structural equation modelling (PLS-SEM) was performed to identify causal relationships between AMF root colonization, physiological parameters, and grain yield of durum wheat cultivars. In this model, AMF root colonization, physiological parameters, and grain yield were defined as latent variables. Each latent variable was composed of a block of indicator variables (Table S2). Multi-collinearity between indicator variables was measured by evaluating the Variance Inflation Factor (VIF) (Hair et al. 2014a). The model was performed in a formative mode with the support of the SmartPLS 3.2.8 Pro software (Ringle et al. 2015). The evaluation criteria for the models included coefficient of determination (R2), cross-validated redundancy (Q2), and effect size (f2) were also assessed to determine the model’s predictive accuracy and its relevance (Hair et al. 2011, 2014b).
3 Results
3.1 Effect of Salinity on AMF Traits and Diversity
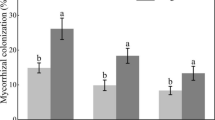
In the present study, the roots of all durum wheat cultivars from the three locations were colonized by AMF with colonization rates ranging from 8.23% to 54.93% (Table S3). Salinization of irrigation water affected significantly (all p < 0.001) the root colonization (RC), the spore density (SD), and the number of spore morphotypes (SMN). Compared to the control (IWS1), RC and SD reached their maximum at IWS2 with an increase rate of 42.74 and 63.95%, respectively. At IWS3, RC maintained the same tendency as in control conditions, while the SD was decreased by 14.56%. The average number of morphotypes was 2.00 using IWS1, increased to 2.83 with IWS2, and then slightly declined with IWS3 to achieve 2.44 (Table S3).
Altogether, 6 genera of AMF were identified (Table 2). Paraglomus (frequency of occurrence (FO) of 66.66 and 83.33%, respectively) and Funneliformis (FO of 66.66 and 83.33%, respectively) were the most often detected genera at IWS1 and IWS3, whereas at IWS2, Glomus had the highest FO of 100%, followed by Septoglomus and Rhizophagus (66.66% each). Considering the AMF sporulation, the highest RD was recorded for Glomus at both IWS2 (46.63%) and IWS3 (61.79%). Nonetheless, Funneliformis showed the best value at IWS1 (30.94%). According to the IV, dominant AMF genera were principally found at IWS3 for Paraglomus (65.67%), Glomus (64.22%), and Funneliformis (59.62%), while Glomus (71.31%) was the only dominant genus at IWS2. All other genera were common under the three salinity levels, except the two genera Septoglomus and Rhizophagus which remained seldom at IWS3.
3.2 Interactive Effect of Cultivar and Irrigation Water Salinity on AMF Traits and Diversity
The mycorrhizal RC and SD varied significantly according to cultivars and showed also a significant interaction with irrigation water salinity (all p < 0.001) (Table S3). Under medium (IWS1) and severe (IWS2) salinity conditions, Maali followed by Agili Glabre recorded constantly the best rate of RC and SD. Nonetheless, under extreme saline conditions (IWS3), Agili Glabre was noticeably the most mycorrhized cultivar showing the greatest spore abundance in its rhizosphere. At IWS1, the number of morphotypes was similar for almost all cultivars (~ 2 morphotypes), except Souri and Agili Glabre who showed the lowest SMN. However, at IWS2, Maali showed the highest SMN (~ 5 morphotypes) followed by Agili Glabre. At IWS3, the maximum AMF morphotype number was recorded under the rhizosphere of Agili Glabre (Table S3).
Under severe salinity (IWS2), roots of high yielding modern varieties (Maali, Karim, and Razzek) were more colonized by AMF and revealed higher SD and SMN compared to landraces (Agili Glabre, Souri, and Bayadha) (Table S3). However, under extreme salinity (IWS3), landraces performed well compared to modern varieties. Intriguingly, Maali underwent the most important reduction of RC and SMN.
The AMF diversity (FO, RA, and IV) varied according to cultivars as well as the interaction between cultivars and irrigation water salinity (Table 2). At IWS1, results showed that Paraglomus and Funneliformis genera were identified under the rhizosphere of the most of cultivars, while Acaulospora was found for two landraces and Glomus for two modern varieties. In contrast, Septoglomus was rarely detected and identified only for Souri. At IWS2, Glomus and Rhizophagus were registered for the most cultivars, whereas at IWS3, the most common cultivars had Glomus and Paraglomus under their rhizosphere. Interestingly, Acaulospora and Septoglomus were only identified for Agili Glabre and Rhizophagus for Razzek.
Furthermore, the highest RD was noted for Paraglomus and Acaulospora for Agili Glabre, Glomus and Rhizophagus for Maali, and Septoglomus and Funneliformis for Souri at IWS1 (Table 2). Nonetheless, at IWS2 and IWS3, a great heterogeneity was registered according to cultivars.
Regarding IV values, no AMF genus were dominant in any of the six durum wheat cultivars, except Paraglomus under the rhizosphere of Karim (Table 2).
3.3 Salinity and the Interactive Cultivars and Salinity Effects on Measured Physiological Traits
Significant variations (p < 0.01) among the irrigation water salinity were obtained for all tested physiological traits, except Cflag leaf, δ13Cflag leaf, and δ13Cgrain (Table S4). The use of brackish water negatively affected FLA, leaf chlorophyll content, Cflag leaf, Nflag leaf, δ13Cflag leaf, δ15Nflag leaf, Cgrain, Ngrain, and δ15Ngrain, but increased the δ13Cgrain even though there is no significant difference between salinity levels. FLA, chlorophyll content, δ13Cflag leaf, Ngrain, δ15Ngrain, and δ13Cgrain significantly (p < 0.05) varied according to durum wheat cultivars (Table S4). Nonetheless, significant variations (p < 0.05) were only obtained for FLA, chlorophyll content, Nflag leaf, δ15Nflag leaf, and δ15Ngrain. Overall, Souri followed by Bayadha showed the highest value of FLA under the three saline levels, while Maali exhibited the best SPAD value. Otherwise, the general trend revealed that the four cultivars, Souri, Maali, Bayadha, and Agili Glabre, showed the highest values for C and N content and C and N isotopic composition in both flag leaves and grain. Results showed clearly that landraces had higher FLA, C and N contents, and stable C and N isotope in flag leaves and grains compared to modern varieties.
3.4 Effect of Salinity and Cultivar on Grain Yield and Yield Stress Susceptibility Index
The present investigation showed a considerable variation (p < 0.001) of GY and SSPI according to salinity treatment (Table S4). The use of brackish water negatively affected the GY by 9.25 and 28.79% for IWS2 and IWS3, respectively. Otherwise, the increase in salinity increased the SSPI by 212.65%.
A significant genotypic variation (p < 0.05) was also observed for grain yield and yield stress susceptibility index (Table S4). At IWS1 and IWS2, modern varieties out-yielded the landraces, with Maali being the best performing cultivar. However, at IWS3, Maali (modern variety) followed by Agili Glabre (landrace) showed the highest best yield. Considering SSPI, Agili Glabre, Bayadha, and Maali could be considered as salt-tolerant cultivars at IWS2. The most performant cultivars at IWS3 were Agili Glabre, Souri, and Maali.
3.5 Relationship between AMF Root Colonization, Grain Yield, and Yield Stress Susceptibility Index
Significant positive relationships were found between GY and RC for all salinity levels of water irrigation (r = 0.53, p = 0.023; r = 0.55, p = 0.020; r = 0.54, p = 0.020) at IWS1, IWS2, and IWS3, respectively (Fig. 3). However, only at IWS3 (r = − 0.50, p = 0.033) was the correlation between SSPI and RC significantly negative.
3.6 Association between AMF, Physiological and Grain Yield Traits
Two principal component analyses (PCAs) were generated to study the relationships between AMF, physiological, and grain yield traits of the six durum wheat cultivars. Eigenvalues of the studied traits are reported for each irrigation water salinity (Table S5). Under irrigation water stress of 6 dS m−1, the first two PCA explained 54.2% of the total variability (Fig. 4a, Table S5). The PC1 was mainly and positively associated with FLA, δ15Nflag leaf, δ13Cgrain, and δ15Ngrain and negatively with GY. PC2 was highly and positively associated with δ13Cflag leaf and SMN and negatively with RC. The results revealed clear differences among cultivars. In fact, Maali, Karim, and Razzek showed the highest GY, SPAD, and SMN. However, Souri and Agili Glabre were characterized by high FLA, δ15Nflag leaf, δ15Ngrain and SD values and low GY. Otherwise, Bayadha had higher Cflag leaf, Ngrain, and C isotopic compositions in flag leaves and grains which were negatively correlated with GY and RC. Under IWS2, the first two axes of the PCA explained 52.7% of the total variation (Fig. 4b, Table S5). The PC1 exhibited a negative association with SD, GY, and RC and positive association with Ngrain, FLA, and δ13Cgrain. However, PC2 displayed a positive association with SPAD and Nflag leaf, and a negative association with SSPI. The PCA results showed that cultivars, Agili Glabre and Maali had the highest GY and AMF traits (RC, SD, and SMN). Karim and Razzek had higher SSPI and δ15Nflag leaf, while Bayadha and Souri had higher Ngrain, FLA, SPAD, and Cflag leaf. In contrast to Agili Glabre and Maali, these cultivars had low GY and AMF traits.
Principal component analysis (PCA) of six durum wheat cultivars submitted to two levels of irrigation water salinity: IWS1 = 6 dS m−1 (a) and IWS2 = 12 dS m−1 (b). Symbols indicate the different wheat cultivars and arrows represent the variables: RC, root colonization; SD, spore density; SMN, spore morphotype number; FLA, flag leaf area; SPAD, leaf chlorophyll content; Cflag leaf, carbon content of flag leaves; Nflag leaf, nitrogen content of flag leaves; δ13Cflag leaf, stable carbon isotope composition of flag leaves; δ15Nflag leaf, stable nitrogen isotope composition of flag leaves; Cgrain, carbon content of mature grains; Ngrain, nitrogen content of mature grains; δ13Cgrain, stable carbon isotope composition of mature grains; δ15Ngrain, stable nitrogen isotope composition of mature grains; GY, grain yield; SSPI, stress susceptibility percentage index
3.7 Grain Yield as Affected by AMF Root Colonization and Physiological Parameters under Water Salinity
PLS-SEM analysis was performed to examine significant effects between AMF root colonization and physiological parameters as explanatory variables of wheat grain yield across different genotypes. The priori model included SD and SMN as measured variables of AMF root colonization, but these variables had high VIF (variance inflation factor) values and were therefore removed. All retained variables had low VIF (< 5). Figure 5 illustrates the final PLS model. The significance of the path coefficients and the loading of the variables are shown in Tables S6 and S7, respectively. At IWS1, the results showed that wheat yield is largely explained (R2 = 0.80) by its latent variables with a predictive relevance (Q2 = 0.45) (Fig. 5a). There was a direct positive effect of AMF root colonization (+ 0.34, p < 0.01) on flag leaf nitrogen which was stronger than the indirect effects mediated through flag leaf carbon and grain carbon (− 0.48 × + 0.56 × − 0.49 = + 0.14, p < 0.1). On the other hand, AMF root colonization had significant positive effect on leaf flag nitrogen (+ 0.34, p < 0.1) which affect positively grain nitrogen (+ 0.54, p < 0.05), whereas this later had not significant effect on wheat yield (Table S6). At IWS2, AMF root colonization as well as physiological parameters explained a large variance (R2 = 0.75) of the wheat yield with a predictive relevance (Q2 = 0.62) (Fig. 5b). In this second PLS-SEM, AMF root colonization had only significant direct positive effect on wheat yield (+ 0.27, p < 0.1), while its indirect effects were not significant. Both grain carbon and grain nitrogen directly influenced wheat yield (− 0.52, p < 0.01 and − 0.37, p < 0.05) (Table S6).
Partial least square structural equation modeling (PLS-SEM) for the effects of arbuscular mycorrhizal fungi (AMF) root colonization and physiological parameters on durum wheat grain yield under two levels of irrigation water salinity: IWS1 = 6 dS m−1 (a) and IWS2 = 12 dS m−1 (b). The number on the solid arrows between latent variables indicate the path coefficients, while the number on the dashed arrows between each latent variable and indicator variables represent the loadings. Positive and negative effects are shown by blue and orange lines, respectively. FLA, flag leaf area; SPAD, leaf chlorophyll content; Cflag leaf, carbon content of flag leaves; Nflag leaf, nitrogen content of flag leaves; δ13Cflag leaf, stable carbon isotope composition of flag leaves; δ15Nflag leaf, stable nitrogen isotope composition of flag leaves; Cgrain, carbon content of mature grains; Ngrain, nitrogen content of mature grains; δ13Cgrain, stable carbon isotope composition of mature grains; δ15Ngrain, stable nitrogen isotope composition of mature grains
4 Discussion
In our investigation, a salinity effects on below- and aboveground traits of durum wheat were obtained. Several studies showed discrepant results among salinity effects on mycorrhizal status (i.e., root colonization, spore density and morphotypes) as negative (Klinsukon et al. 2021) or positive (Bencherif et al. 2015; Silva-Flores et al. 2019) effects. Our results showed that mycorrhizal set up was observed at IWS2 of 12 dS m−1 with a better mycorrhizal RC, SD, and SMN. AMF sporulation could be an efficient reproductive strategy to survive under stress conditions by developing dormant structures (Cao et al. 2020). Otherwise, the present study revealed that IWS3 (18 dS m−1) was a critical salinity to AMF species in the studied areas. In fact, as compared to IWS2, RC and SMN were altered, although they are still greater than those under IWS1 (6 dS m−1) except for SD which was more affected. This decline could either be an outcome of (i) direct effects of salinity on spore germination, hyphal growth in soil, and hyphal spreading after initiating the infection (Alrajhei et al. 2022) and (ii) indirect effects of salinity on durum wheat root development which could be severely affected since 15 dS m−1 (Chinnusamy et al. 2005) leading to reduced root and hyphal contact in soil (Juniper and Abbott 2006), and/or an increase in the H2O2 accumulation in the mycorrhized roots which might ultimately lead to arbuscular degradation (Fester and Hause 2005).
Based on reviewed literature (Pal et al. 2017; Parihar et al. 2019) and our results, we suggested that irrigation water salinity affects the mycorrhizal abundance and diversity. Salinity might regulate the diversity of soil fungal communities due to changes in soil properties (e.g., total carbon and nitrogen, and bulk density) induced by soil salinization (Adenan et al. 2021). Our results revealed that Glomus and Paraglomus were the most abundant genera respectively in severe (IWS2) and extreme salinity (IWS3), showing that they might be particularly adaptable to this semi-arid ecosystem characterized by harmful environmental circumstances including salinity (Sitieneia et al. 2015; Zhang et al. 2019). The same results were reported by Mosbah et al. (2018) who showed that the genus Glomus was dominant in semi-arid and arid climates due to its resilience to elevated temperature. The predominance of AMF species belonging to the order Glomerales was also documented in salt-affected soils in China (Krishnamoorthy et al. 2020), in particular the Glomus genus (Lumini et al. 2020; Malik et al. 2022; Zhong et al. 2022). Contrarily to our findings, a set of studies reported that Funneliformis was the less abundant taxa in saline soils (Becerra et al. 2014; Floc’h et al. 2022). According to Yang and Sun (2020), the abundance of mycorrhizae species might be explained by the fact that some species develop endospores and could therefore survive in severe salinity environments.
Glomus was the most abundant specie with the highest relative spore density under high salinity at IWS2 and IWS3. Those observations could be explained in part by the highest sporulation rate of this genus leading to a faster recovery and thus a better adaptability to severe conditions (Zhang et al. 2019). Paraglomus was the most abundant at IWS1. This funding is in line with those of Guana et al. (2020) who found that AMF species from Paraglomus were more dominant under fresh water compared to seawater. In contrast, according to Symanczik et al. (2015), Paraglomus may prefer undisturbed environments (e.g., salinity or water stress).
The abundance and diversity of root-associated biota, as well as the plant’s physiological traits, were all altered by irrigation water salinity. Expectedly, durum wheat FLA and chlorophyll content substantially decreased in response to salinity. According to Soni et al. (2021), the decrease in chlorophyll content might be due to reduced activity of the aminolevulinic acid synthase enzyme responsible for the chlorophyll synthesis or increased activity of the chlorophyll-degrading enzyme, chlorophyllase. It is well documented that salinity alters the photosynthetic machinery and transpiration, thereby restricting plant biomass (Centritto et al. 2003).
Salt stress decreased the C and N contents as well as their stable isotopic compositions in both flag leaves and mature grains, except δ13Cgrain. During photosynthetic C fixation, C3 plants usually discriminate against 13C in favor of lighter 12C, leading to a lower δ13C. Although the difference was not significant between the two salinity levels, the harmful conditions increased δ13Cgrain, as usually reported elsewhere (Araus et al. 2003; Sanchez-Bragado et al. 2020; Yousfi et al. 2012). As suggested by Ben-Jabeur et al. (2022), the increase in δ13C under saline or water scarcity conditions might be ascribed to (i) a restriction in CO2 diffusion to carboxylation sites due to decreased stomatal and mesophyll conductance and/or a likely alteration of Rubisco activity causing decreases in CO2 fixation, which leads to increases in grain 13C, and (ii) an inability of the photosynthetic machinery to use internal CO2, implying a greater sensitivity of the photosynthetic apparatus. However, in the current study, δ13Cflag leaf decreased slightly under severe saline conditions, but the difference was not significant. This result suggests that symbiosis with AMF could enhance carbon metabolism during the flowering stage to increase the carbohydrate content in leaves and to improve their remobilization to grains which might explain the non-significant difference in Cflag leaf at IWS1 and IWS2. This result corroborates with those of Eroğlu et al. (2020) who demonstrated that AMF increased wheat grain yield under salt stress through modulating carbon use efficiency.
Regarding nitrogen metabolism, the heavier stable isotope 15 N is discriminated against 14 N, leading to a lower 15 N/14 N ratio, and thus, a higher δ15N (Sanchez-Bragado et al. 2017). Salt stress affects nitrogen metabolism by decreasing N uptake, assimilation, and fractionation, resulting in δ15N and N content decrease (Sanchez-Bragado et al. 2017), while others reported an increase in δ15N which suggests that salt affects plant δ15N in a different way (Handley et al. 1997; Lopes and Araus 2006). Alteration of C and N metabolism as well as the photosynthetic activity contributed subsequently to the decrease in final GY.
In addition to the specific effect of salinity, genotypic variation among the below-ground and aboveground traits of durum wheat with respect to salinity levels was recorded. The wheat effects on the establishment of mycorrhizal symbiosis are widely examined in optimal conditions (Dupont 2018; Garcıa de Leon et al. 2020). The present study adds a new perspective on host effects on durum wheat cultivar salt stress tolerance. This finding corroborates with previous studies (Mao et al. 2014). Besides, others studies indicated that mycorrhizal traits (e.g., AM colonization, arbuscular colonization, extraradical hyphal density, spore abundance, or AMF diversity) inside roots and in soils did not significantly vary across cultivars with a differential agronomic performance (Hildermann et al. 2010; Mao et al. 2014).
Under severe to very severe salinity conditions, Maali and Agli Glabre exhibited the greatest number of morphotypes under their rhizosphere, respectively. These two cultivars seem to favor the proliferation of a maximum number of AMF genera. Notably, Acaulospora and Septoglomus were detected under the rhizosphere of Agili Glabre at IWS3. These AMF genera seem to act in coordination to improve durum wheat’s resilience to salinity stress. Similarly, Mao et al. (2014) reported that 3 out of 16 AMF phylotypes interacted with most wheat cultivars, while some phylotypes preferred to colonize specific cultivars. Our findings respond to the addressed question and clearly highlight the contribution of durum wheat cultivar in structuring AMF communities inside roots, after the irrigation with severe to extreme saline water (> 12 dS m− 1). Genotypic differences among durum wheat were among the interactions with specific AM species (Stefani et al. 2020). The variation in AMF community structure was attributed to cultivar genetic component that might be related to (i) genes of host plant affecting the structure of roots (Van Geel et al. 2021), (ii) receptivity to fungal colonization, and (iii) physiological function of the AMF associations, particularly the degree to which the fungal symbionts act as a carbon storage and nutrient source for their host plants (Walder et al. 2012; Watts-Williams et al. 2019).
Taken as a whole, the mycorrhizal association under durum wheat rhizosphere of modern varieties was higher compared to that of landraces at severe salinity level (IWS2). Under higher salt stress conditions (IWS3), it appeared that landraces respond better than modern varieties in AMF symbiosis, especially Agili Glabre. Previous studies, conducted in non-stress conditions, speculated a positive relationship between mycorrhizal attributes and the introduction of semi-dwarfing genes Rht during breeding programs (De Vita et al. 2018). Nonetheless, our controversial results highlight revealed the enigmatic relationship suggesting that the cultivar-AMF interaction is strongly dependent on salt stress level. This resulting beneficial symbiosis could be related to the genetic markers in linkage with chromosome regions involved in AM root colonization (De Vita et al. 2018).
With our set of cultivars, distinct genotypic performances were obtained in terms of GY and salt-tolerance (SSPI). Interestingly, under very severe saline conditions (IWS3), Maali (modern variety) followed by Agili Glabre (landrace) were the best performing cultivars in terms of GY and conversely for salt tolerance. The specific salt stress tolerance of landraces was well documented. These genotypes contain genes that confer adaptability to adverse situations, making them a valuable source of genetic variety for modern plant breeding to improve abiotic or biotic stress adaption, yield performance, and quality attributes in restricted environments (Marone et al. 2022). In addition, it is well established that improved varieties are more tolerant and adapted to salinity than modern varieties (Chamekh et al. 2016). In fact, highly stable improved genotypes with a high yield potential are most salt stress tolerant compared to landraces (Chamekh et al. 2022).
A genetic variation was also obtained under salinity for FLA, chlorophyll content, δ13Cflag leaf, Ngrain, δ15Ngrain, and δ13Cgrain, as noted in previous studies (Araus et al. 2013; Omrani et al. 2022; Yousfi et al. 2012). The more productive cultivars (i.e., modern varieties) are those with lower δ13C in flag leaves and mature grains, which suggests that they exhibit higher stomatal conductance and photosynthetic activity. Otherwise, these cultivars showed a lower N content and δ15N in grains compared to landraces. It is well known that landraces are characterized by higher quality than modern varieties due to higher protein content (Ben Krima et al. 2021; Frankin et al. 2021). The natural variation of the stable nitrogen isotopes 15 N/14 N assessed through the nitrogen isotope composition (δ15N) is linked to nitrogen sources used by the plant (NH4+ uptake will induce 15 N enrichment compared to NO3−), to the activity of enzymes involved in the assimilation of ammonium (glutamine synthetase, GS) or nitrate (nitrate reductase, NR), and to the nature of compounds resulting from nitrogen fractionation.
Our study also stated a link between AMF, physiological characteristics, and grain yield. Under control (IWS1) and severe water irrigation salinity (IWS2), AMF root colonization showed a direct positive effect on wheat yield, especially for Maali with the highest RC and SD. A positive indirect effect of AMF on wheat yield was mediated through carbon in flag leaves and grains. The relationship between AMF root colonization and flag leaf carbon was negative. As previously stated, plants have a substantial impact on C allocation to mycorrhizal fungi (Thirkell et al. 2019). To improve mineral nutrients (e.g., P and N) transport to host plants, higher AMF proliferation should result in greater C demand and reduced plant storage carbon (Thirkell et al. 2019). In fact, Wang et al. (2021) showed that AMF colonization rate is negatively related to the rate of plant’s rhizodeposit decomposition, implying an exchange in carbon allocation between rhizodeposit and mycorrhizal symbiosis.
Otherwise, the negative relationship between AMF colonization and C suggests that AMF enhance the stomatal conductance to improve plant C content and salt tolerance. In fact, the salt-tolerant cultivars (e.g., Agili Glabre and Maali) with a greater AMF root colonization had a low δ13Cflag leaf. AMF symbiosis changes stomatal morphology by reducing stomatal density and increasing the size of the guard cells and stomatal pores, thereby improving the stomatal conductance and water relations of wheat leaves under salinity stress (Zhu et al. 2018). Then, cultivars characterized by high level of C in flag leaves should maintain a good level of C in grains. Cgrain content is the result of the combined C values of assimilates produced by different photosynthetic organs (flag leaf and ear) contributing to grain filling (Sanchez-Bragado et al. 2014). Otherwise, carbon in grain, which is largely described by 13Cgrain, has a negative impact on wheat yield. In fact, the most productive cultivars (Maali, Karim, and Razzek) displayed lower δ13Cgrain, suggesting higher stomatal conductance and water use efficiency (Araus et al. 1998). However, at IWS2, only Razzek and Maali exhibited a low δ13Cgrain. In contrast to previous studies using δ13C (Araus et al. 2003; Yousfi et al. 2010), tolerant cultivars (e.g., Agili Glabre, Bayadha, and Maali) did not necessarily express lower δ13C than susceptible ones with respect to salinity tolerance.
At IWS1, AMF root colonization positively affected flag leaf nitrogen, explained mostly by δ15Nflag leaf and positively related to biophysical traits and grain nitrogen. Several studies have reported that AMF colonization helps increase N uptake under salt stress conditions (Evelin et al. 2019). This might be due directly to (i) maintaining membrane stability, up-regulating of nitrate (NRT1.1, NAR2.2) and ammonium (AMT1.1 and AMT1.2) transporters and increasing NR activity (Felicia et al. 2017; Talaat and Shawky 2014) and/or indirectly to (ii) stimulating soil bacteria involved in the mineralization processes of soil organic matter (Saia et al. 2014). Nonetheless, at IWS2, this relationship between these latent variables was not significant probably due to the higher complexity of the N cycle which might explain the less intensively use of N isotopes (15 N/14 N ratio) compared to C isotopes (13C/12C ratio) in plant physiology and ecology studies (Farquhar et al. 1989; Högberg 1997). Under the same conditions (IWS2), grain nitrogen negatively affected the wheat yield. Landraces with low GY performance clearly have more N content in grain than modern varieties, with the superiority of Bayadha.
In contrast to IWS1, the PCA revealed that salt-tolerant cultivars (e.g., Agili Glabre and Maali) with better AMF proliferation showed a greater diversity after irrigation with IWS2 which explains the positive correlation between the mycorrhizal traits (i.e., RC, SD, and SMN).
5 Conclusions
This is the first report of a field-based comparison of arbuscular mycorrhizal fungi (AMF) association across durum wheat cultivars and salinity stress using stable isotopic traits. The current research showed that under extreme salinity conditions, durum wheat landrace Agili Glabre benefit more from AMF colonization than modern varieties. In fact, its rhizosphere was harbored by the maximum of AMF genera where some gather specific association with cultivar that seems to act in coordination to improve durum wheat’s resilience to salinity stress. Besides, we showed that the effect of mycorrhizal colonization on yield performance follows a change in carbon and nitrogen metabolism. More interestingly, at 6 and 12 dS m−1, AMF colonization had an indirect effect on grain yield through carbon metabolism, explained largely by stable carbon isotope composition of flag leaves and mature grains. Establishing a firm relationship between mycorrhizal partners and isotopic signatures appears to be difficult, but a better understanding of the mechanisms underlying specific cultivar–AMF receptivity under stress conditions remains important in the future‐breeding program.
References
Abdel Latef AAH, Miransari M (2014) The role of arbuscular mycorrhizal fungi in alleviation of salt stress. In: Miransari M (ed) Use of microbes for the alleviation of soil stresses. Springer, New York, pp 23–38. https://doi.org/10.1007/978-1-4939-0721-2_2
Adenan S, Oja J, Juha M, Alatalo JM, Shraim AM, Alsafran M, Tedersoo L, Zobel M, Ahmed T (2021) Diversity of arbuscular mycorrhizal fungi and its chemical drivers across dryland habitats. Mycorrhiza 31:685–697. https://doi.org/10.1007/s00572-021-01052-3
Alrajhei K, Saleh I, Abu-Dieyeh MH (2022) Biodiversity of arbuscular mycorrhizal fungi in plant roots and rhizosphere soil from different arid land environment of Qatar. Plant Physiol 6:369. https://doi.org/10.1002/pld3.369
Araus JL, Amaro T, Casadesús J, Asbati A, Nachit MM (1998) Relationships between ash content, carbon isotope discrimination and yield in durum wheat. Aust J Plant Physiol 25:835–842. https://doi.org/10.1071/PP98071
Araus JL, Villegas D, Aparicio N, del Moral LFG, El Hani S, Rharrabti Y, Ferrio JP, Royo C (2003) Environmental factors determining carbon isotope discrimination and yield in durum wheat under Mediterranean conditions. Crop Sci 43:170–180. https://doi.org/10.2135/cropsci2003.1700
Araus JL, Cabrera-Bosquet L, Serret MD, Bort J, Nieto-Taladriz MT (2013) Comparative performance of δ13C, d18O and d15N for phenotyping durum wheat adaptation to a dryland environment. Funct Plant Biol 40:595–608. https://doi.org/10.1071/FP12254
Barin M, Aliasgharzad N, Rasouli-Sadaghiani MH, Moghddam M (2013) Abundance of arbuscular mycorrhizal fungi in relation to soil salinity around Lake Urmia in northern Iran analyzed by use of lipid biomarkers and microscopy. Pedobiol 56:225–232. https://doi.org/10.1016/j.pedobi.2013.09.001
Becerra A, Bartoloni N, Cofré N, Soteras F, Cabello M (2014) Arbuscular mycorrhizal fungi in saline soils: vertical distribution at different soil depth. Braz J Microbiol 45:585–594. https://doi.org/10.1590/s1517-83822014000200029
Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ashraf M, Ahmed N, Zhang L (2019) Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front Plant Sci 10:1068. https://doi.org/10.3389/fpls.2019.01068
Ben Krima S, Slim A, Gélisse S, Kouki H, Nadaud I, Pierre Sourdille P, Yahyaoui A, Ben M’barek S, Suffert F, Marcel T (2021) Life story of Tunisian durum wheat landraces revealed by their genetic and phenotypic diversity. bioRxiv. https://doi.org/10.1101/2020.08.14.251157
Ben Salem M, Boussen H, Slama A (1997) Evaluation of the resistance to hydric and caloric stress of a collection of durum wheat: search for early selection parameters. 6th Scientific Days of Plant Biotechnology Network. AUPELF-UREF, Orsay, pp 316–326 (in French)
Bencherif K, Boutekrabt A, Fontaine J, Laruelle F, Dalpè Y, Lounès-Hadj Sahraoui A (2015) Impact of soil salinity on arbuscular mycorrhizal fungi biodiversity and microflora biomass associated with Tamarix articulata Vahll rhizosphere in arid and semi-arid Algerian areas. Sci Total Environ 533:488–494. https://doi.org/10.1016/j.scitotenv.2015.07.007
Ben-Jabeur M, Chamekh Z, Jallouli S, Ayadi S, Serret MD, Araus JL, Trifa Y, Hamada W (2022) Comparative effect of seed treatment with thyme essential oil and Paraburkholderia phytofirmans on growth, photosynthetic capacity, grain yield, δ15N and δ13C of durum wheat under drought and heat stress. Ann Appl Biol 181:58–69. https://doi.org/10.1111/aab.12754
Blake GR, Hartge KH (1986) Bulk density. In: Klute A (ed) Methods of soil analysis, Part 1—Physical and mineralogical methods, 2nd Edn, Agronomy Monograph 9. American Society of Agronomy—Soil Science Society of America, Madison, pp 363–382. https://doi.org/10.1002/gea.3340050110
Boudabbous Kh, Bouhaouel I, Karmous C, Benaissa N, Trifa Y, Sahli A, Slim Amara H (2020) The variation of phosphorous content, grain yield, and rhizosphere microbial biomass among durum wheat cultivars under salinity stress. Arch Agron Soil Sci 66:1721–1734. https://doi.org/10.1080/03650340.2019.1691170
Boudabbous Kh, Bouhaouel I, Benaissa N, Jerbi M, Trifa Y, Sahli A, Karmous C, Hajer Slim A (2022) Durum wheat salt stress tolerance is modulated by the interaction between plant genotypes, soil microbial biomass, and enzyme activity. Ital J Agron 17:1942. https://doi.org/10.4081/ija.2022.1942
Brundrett MC, Tedersoo L (2018) Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol 220:1108–1115. https://doi.org/10.1111/nph.14976
Cao Y, Wua X, Zhukovaa A, Tanga Z, Wenga Y, Lia Z, Yanga Y (2020) Arbuscular mycorrhizal fungi (AMF) species and abundance exhibit different effects on saline-alkaline tolerance in Leymus chinensis. J Plant Interact 15:266–279. https://doi.org/10.1080/17429145.2020.1802524
Centritto M, Loreto F, Chartzoulakis K (2003) The use of low [CO2] to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant Cell Environ 26:585–594. https://doi.org/10.1046/j.1365-3040.2003.00993.x
Chamekh Z, Ayadi A, Karmous C, Boudabbous K, Trifa Y, Amara H, Yousfi S, Serret MD, Araus JL (2016) Comparative effect of salinity on growth, grain yield, water use efficiency, δ13C and δ15N of landraces and improved durum wheat varieties. Plant Sci J 251:44–53. https://doi.org/10.1016/j.plantsci.2016.07.005
Chamekh Z, Zouari I, Jallouli S, Ayadi S, Abdenour S, Trifa Y (2022) Breeding for salt tolerance in wheat: The contribution of carbon isotopic signatures. Czech J Genet Plant Breed 58:43−54. https://doi.org/10.17221/51/2021-CJGPB
Chinnusamy V, Jagendorf A, Zhu JK (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448. https://doi.org/10.2135/cropsci2005.0437
De Vita P, Avio L, Sbrana C, Laidò G, Marone D, Mastrangelo AM, Cattivelli L, Giovannetti M (2018) Genetic markers associated to arbuscular mycorrhizal colonization in durum wheat. Sci Rep 8:10612. https://doi.org/10.1038/s41598-018-29020-6
Dupont S (2018) Arbuscular mycorrhizal fungal communities of 31 durum wheat cultivars (Triticum turgidum var. durum) under field conditions in Eastern Canadian province of Quebec. Thesis presented in view of obtaining of Master’s degree in Biological Sciences, University of Montreal (in French)
Eroğlu ÇG, Cabral C, Ravnskov S, Bak Topbjerg H, Wollenweber B (2020) Arbuscular mycorrhiza influences carbon-use efficiency and grain yield of wheat grown under pre- and post-anthesis salinity stress. Plant Biol 22:863–871. https://doi.org/10.1111/plb.13123
Estrada B, Aroca R, Maathuis FJ, Barea JM, Ruizlozano JM (2013) Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell Environ 36:1771–1782. https://doi.org/10.1111/pce.12082
Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24:755–767. https://doi.org/10.1046/j.1365-3040.2001.00724.x
Evelin H, Devi TS, Gupta S, Kapoor R (2019) Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: current understanding and new challenges. Front Plant Sci 10:470. https://doi.org/10.3389/fpls.2019.00470
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537. https://doi.org/10.1146/annurev.pp.40.060189.002443
Felicia V, Russia P, Ingraffia R, Giambalvo D, Frenda AS, Martinelli F (2017) Arbuscular mycorrhizal symbiosis mitigates the negative effects of salinity on durum wheat. PLoS One 12:e0184158. https://doi.org/10.1371/journal.pone.0184158
Fester T, Hause G (2005) Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza 15:373–379. https://doi.org/10.1007/s00572-005-0363-4
Floc’h J-B, Hamel C, Laterrière M, Tidemann B, St-Arnaud M, Hijri M (2022) Long-term persistence of arbuscular mycorrhizal fungi in the rhizosphere and bulk soils of non-host Brassica napus and their networks of co-occurring microbes. Front Plant Sci 13:828145. https://doi.org/10.3389/fpls.2022.828145
Frankin S, Roychowdhury R, Nashef K, Abbo S, Bonfil DJ, Ben-David R (2021) In-field comparative study of landraces vs. modern wheat genotypes under a Mediterranean climate. Plants 10:2612. https://doi.org/10.3390/plants10122612
Ganugi P, Masoni A, Pietramellara G, Benedettelli S (2019) A review of studies from the last twenty years on plant–arbuscular mycorrhizal fungi associations and their uses for wheat crops. Agron 9:840. https://doi.org/10.3390/9120840
Ganugi P, Masoni A, Sbrana C, Dell’Acqua M, Pietramellara G, Benedettelli S, Avio L (2021) Genetic variability assessment of 127 Triticum turgidum L. accessions for mycorrhizal susceptibility-related traits detection. Sci Rep 1:13426. https://doi.org/10.1038/s41598-021-92837-1
Garcıa de Leon D, Vahter T, Zobel M, Koppel M, Edesi L, Davison J, Al-Quraishy S, Hozzein WN, Moora M, Oja J, Vasar M, Opik M (2020) Different wheat cultivars exhibit variable responses to inoculation with arbuscular mycorrhizal fungi from organic and conventional farms. PLoS ONE 15:5. https://doi.org/10.1371/journal.pone.0233878
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal endogone species extracted from soil by wet-sieving and decanting. Trans Brit Mycol Soc 46:235–244. https://doi.org/10.1016/S0007-1536(63)80079-0
Guana B, Zhanga H, Wangb X, Yanga S, Chena M, Houd A, Cagled GA, Hana, (2020) Salt is a main factor shaping community composition of arbuscular mycorrhizal fungi along a vegetation successional series in the Yellow River Delta. CATENA 185:104318. https://doi.org/10.1016/j.catena.2019.104318
Gupta S, Thokchom DS, Koul M, Rupam Kapoor R (2021) Arbuscular Mycorrhiza mediated mineral biofortification and arsenic toxicity mitigation in Triticum aestivum L. Plant Stress 5:100086. https://doi.org/10.1016/j.stress.2022.100086
Hair JF, Ringle CM, Sarstedt M (2011) PLS-SEM: Indeed a silver bullet. J Mark Theory Pract 19:139–152. https://doi.org/10.2753/MTP1069-6679190202
Hair JF, Sarstedt M, Hopkins L, Kuppelwieser VK (2014b) Partial least squares structural equation modeling (PLS-SEM): an emerging tool in business research. Eur Bus Rev 26:106–121. https://doi.org/10.1108/EBR-10-2013-0128
Hair JF, Hult GTM, Ringle CM, Sarstedt M (2014a) A primer on partial least squares structural equation modeling, 4th ed. Thousand Oaks, CA
Handley LL, Robinson D, Forster BP, Ellis RP, Scrimgeour CM, Gordon DC, Nero E, Raven JA (1997) Shoot d15N correlates with genotype and salt stress in barley. Planta 201:100–102. https://doi.org/10.1007/BF01258686
Hetrick BAD, Wilson GWT, Cox TS (1992) Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors. Can J Bot 70:10. https://doi.org/10.1139/b92-253
Hildermann I, Messmer M, Dubois D, Boller T, Wiemken A, Mäder P (2010) Nutrient use efficiency and arbuscular mycorrhizal root colonisation of winter wheat cultivars in different farming systems of the DOK long-term trial. J Sci Food Agric 12:2027–38. https://doi.org/10.1002/jsfa.4048
Högberg P (1997) Tansley review No 95–15N natural abundance in soil-plant systems. New Phytol 137:179–203. https://doi.org/10.1046/j.1469-8137.1997.00808.x
Juniper S, Abbott LK (2006) Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 16:371–379. https://doi.org/10.1007/s00572-006-0046-9
Kassambara A, Mundt F (2020) Factoextra: Extract and visualize the results of multivariate data analyses. R Package Version 1.0.7. https://CRAN.R-project.org/package=factoextra. Accessed 19 March 2022
Klinsukon C, Lumyong S, Thomas W, Kuyper Boonlue S (2021) Colonization by arbuscular mycorrhizal fungi improves salinity tolerance of eucalyptus (Eucalyptus camaldulensis) seedlings. Sci Rep 11:4362. https://doi.org/10.1038/s41598-021-84002-5
Koske RE, Halvorson WL (1981) Ecological studies of vesicular–arbuscular mycorrhizae in a barrier sand dune. Can J Bot 59:8. https://doi.org/10.1139/b81-193
Krishnamoorthy R, Anandham R, Senthilkumar M, Sa T (2020) Diversity and community structure of arbuscular mycorrhizal fungi in the rhizosphere of salt-affected soils. In: Sharma SK, Singh UB, Sahu PK, Singh HV, Sharma PK (eds) Rhizosphere Microbes. Microorganisms for Sustainability, vol 23. Springer, Singapore, pp. 453–470. https://doi.org/10.1007/978-981-15-9154-9_18
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:275–294. https://doi.org/10.1046/j.0016-8025.2001.00814.x
Lopes M, Araus JL (2006) Nitrogen source and water regime effects on durum wheat photosynthesis, and stable carbon and nitrogen isotope composition. Physiol Plant 126:435–445. https://doi.org/10.1071/FP04031
Lumini E, Pan J, Magurno F, Huang C, Bianciotto V, Xue X, Balestrini R, Tedeschi A (2020) Arbuscular mycorrhizal fungi characterization from saline lands in arid oases, northwest China. J Fungi 6:80. https://doi.org/10.3390/jof6020080
Malik JA, AlQarawi AA, Dar BA, Hashem A, Alshahrani TS, AlZain MN, Habib MM, Javed MM, Abd_Allah EF (2022) Arbuscular mycorrhizal fungi isolated from highly saline “Sabkha Habitat” soil alleviated the NaCl-induced stress and improved Lasiurus scindicus Henr. growth. Agriculture 12:337. https://doi.org/10.3390/agriculture12030337
Mao L, Liu Y, Shi G, Jiang S, Cheng G, Li X, An L, Feng H (2014) Wheat cultivars form distinctive communities of root-associated arbuscular mycorrhiza in a conventional agroecosystem. Plant Soil 374:949–961. https://doi.org/10.1007/s11104-013-1943-2
Marone D, Mastrangelo AM, Borrelli GM, Mores A, Laidò G, Russo MA, Ficco DBM (2022) Specialized metabolites: physiological and biochemical role in stress resistance, strategies to improve their accumulation, and new applications in crop breeding and management. Plant Physiol Biochem 172:48–55. https://doi.org/10.1016/j.plaphy.2021.12.037
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Mekliche A, Hanif-Mekliche L, Aïdaoui A, Gate P, Bouthier A, Monneveux P (2015) Grain yield and its components study and their association with normalized difference vegetation index (NDVI) under terminal water deficit and well-irrigated conditions in wheat (Triticum durum Desf. and Triticum aestivum L.). Afr J Biotechnol 14:2142–2148. https://doi.org/10.5897/AJB2015.14535
Melo CD, Luna S, Krüger C (2017) Arbuscular mycorrhizal fungal community composition associated with Juniperus brevifolia in native Azorean forest. Acta Oecol 79:48–61. https://doi.org/10.1016/j.actao.2016.12.006
Melo CD, Walker C, Krüger C, Paulo AV, Borges PAV, Luna S, Mendonça D, Fonseca HMAC, Machado AC (2019) Environmental factors driving arbuscular mycorrhizal fungal communities associated with endemic woody plant Picconia azorica on native forest of Azores. Ann Microbiol 69:1309–1327. https://doi.org/10.1007/s13213-019-01535-x
Mosbah M, Philippe DL, Mohamed M (2018) Molecular identification of arbuscular mycorrhizal fungal spores associated to the rhizosphere of Retamaraetam in Tunisia. J Soil Sci Plant Nutr 64:335–341. https://doi.org/10.1080/00380768.2018.1431012
Munns R, James RA, Läuchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57:1025–1043. https://doi.org/10.1093/jxb/erj100
Omrani S, Arzani A, Esmaeilzadeh Moghaddam M, Mahlooji M (2022) Genetic analysis of salinity tolerance in wheat (Triticum aestivum L.). PLoS One 17: e0265520. https://doi.org/10.1371/journal.pone.0265520
Pal S, Singh HB, Farooqui A (2017) Diversity of arbuscular mycorrhiza associated with long term wastewater irrigation in the peri-urban soil of Varanasi. Int J Agric Environ Biot 10:779–784. https://doi.org/10.5958/2230-732X.2017.00096.1
Parihar M, Rakshit A, Bahadur H, Rana SK (2019) Diversity of arbuscular mycorrhizal fungi in alkaline soils of hot sub humid eco-region of Middle Gangetic Plains of India. Acta Agric Scand – B Soil Plant Sci 69:386–397. https://doi.org/10.1080/09064710.2019.1582692
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161. https://doi.org/10.1016/S00071536(70)80110-3
R Core Team R (2019) A language and environment for statistical computing reference index. Version 3.6.0 (2019–04–26)
Ringle CM, Wende S, Becker JM (2015) "SmartPLS 3." Boenningstedt: SmartPLS GmbH
Saia S, Benítez E, García-Garrido JM, Settanni L, Amato G, Giambalvo D (2014) The effect of arbuscular mycorrhizal fungi on total plant nitrogen uptake and nitrogen recovery from soil organic material. J Agric Sci 152:370–378. https://doi.org/10.1017/S002185961300004X
Sanchez-Bragado R, Elazab A, Zhou B, Serret MD, Bort J, Nieto-Taladriz MT, Araus JL (2014) Contribution of the ear and the flag leaf to grain filling in durum wheat inferred from the carbon isotope signature: Genotypic and growing conditions effects. J Integr Plant Biol 56:444–454. https://doi.org/10.1111/jipb
Sanchez-Bragado R, Serret MD, Araus JL (2017) The nitrogen contribution of different plant parts to wheat grains: exploring genotype, water, and nitrogen effects. Front Plant Sci 7:19–86. https://doi.org/10.3389/fpls.2016.01986
Sanchez-Bragado R, Newcomb M, Chairi F, Condorelli EG, Ward RW, White JW, Maccaferri M, Tuberosa R, Araus JL, Molins MDS (2020) Carbon isotope composition and the NDVI as phenotyping approaches for drought adaptation in durum wheat: Beyond trait selection. Agron 10:1679. https://doi.org/10.3390/agronomy10111679
Schenck NC, Pérez Y (1990) Manual for the identification of VA mycorrhizal fungi, 3rd edn. Synergistic Publications, Florida
Scianna J (2002) Salt -affected soils: their causes, measure, and classification. Res Method Hort Note No.5
Shahid SA, Zaman M, Heng L (2018) Soil salinity: historical perspectives and a world overview of the problem. In: Zaman M, Shahid SA, Heng L (eds) Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques. Springer, Cham, pp 43–53. https://doi.org/10.1007/978-3-319-96190-3_2
Silva-Flores P, Bueno G, Neira J, Palfner G (2019) Factors affecting arbuscular mycorrhizal fungi spore density in the Chilean Mediterranean-type ecosystem. J Soil Sci Plant Nutr 19:42–50. https://doi.org/10.1007/s42729-018-0004-6
Sitieneia PC, Wagaraa IN, Kariukia ST, Jefwa JM, Kibiro EM (2015) Occurrence and biodiversity of arbuscular mycorrhizae fungi associated with indigenous trees in eastern Mau forest, Kenya. Sci J Microbiol 2015:1–19. https://doi.org/10.7237/sjmb/125
Soni S, Kumar A, Sehrawat N, Kumar A, Kumar N, Lata C, Mann A (2021) Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat genotypes. Saudi J Biol Sci 28:2510–2517. https://doi.org/10.1016/j.sjbs.2021.01.052
Spangenberg JE, Schweizer M, Zufferey V (2021) Carbon and nitrogen stable isotope variations in leaves of two grapevine cultivars (Chasselas and pinot noir): implications for ecophysiological studies. Plant Physiol Bioch 163:45–54. https://doi.org/10.1016/j.plaphy.2021.03.048
Stefani F, Dupont S, Laterrière M, Knox R, Ruan Y, Hamel C, Hijri M (2020) Similar arbuscular mycorrhizal fungal communities in 31 durum wheat cultivars (Triticum turgidum L. var. durum) under field conditions in eastern Canada. Front Plant Sci 11:1206. https://doi.org/10.3389/fpls.2020.01206
Symanczik S, Courty PE, Boller T, Wiemken A, Al-Yahya’ei MN (2015) Impact of water regimes on an experimental community of four desert arbuscular mycorrhizal fungal (AMF) species, as affected by the introduction of a non-native AMF species. Mycorrhiza 25:639–647. https://doi.org/10.1007/s00572-015-0638-3
Talaat NB, Shawky BT (2014) Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot 98:20–31. https://doi.org/10.1016/j.envexpbot.2013.10.00
Thirkell TJ, Pastok D, Katie JF (2019) Carbon for nutrient exchange between arbuscular mycorrhizal fungi and wheat varies according to cultivar and changes in atmospheric carbon dioxide concentration. Glob Chang Biol 26:1725–1738. https://doi.org/10.1111/gcb.14851
Torrecillas E, Torres P, Alguacil MM (2013) Influence of habitat and climate variables on arbuscular mycorrhizal fungus community distribution, as revealed by a case study of facultative plant epiphytism under semiarid conditions. Appl Environ Microbiol 79:7203–7209. https://doi.org/10.1128/AEM.02466-13
Urbanavičiūtė I, Bonfiglioli L, Pagnotta MA (2022) Diversity in root architecture of durum wheat at stem elongation under drought stress. Agron 12:1329. https://doi.org/10.3390/agronomy12061329
Van Geel M, Aavik T, Ceulemans T, Träger S, Mergeay J, Peeters G, Van Acker K, Zobel M, Koorem K, Honnay O (2021) The role of genetic diversity and arbuscular mycorrhizal fungal diversity in population recovery of the semi-natural grassland plant species Succisa pratensis. Ecol Evo 21:200. https://doi.org/10.1186/s12862-021-01928-0
Walder F, Niemann H, Natarajan M, Lehmann M, Boller T, Wiemken A (2012) Mycorrhizal networks: Common goods of plants shared under unequal terms of trade. Plant Physiol 159:789–797. https://doi.org/10.1104/pp.112.195727
Wang R, Cavagnaro TR, JiangY KC, Dijkstra FA (2021) Carbon allocation to the rhizosphere is affected by drought and nitrogen addition. J Ecol 00:1–11. https://doi.org/10.1111/1365-2745.1374
Watts-Williams SJ, Emmett BD, Levesque-Tremblay V, MacLean AM, Sun XP, Satterlee JW, Harrison MJ (2019) Diverse Sorghum bicolor accessions show marked variation in growth and transcriptional responses to arbuscular mycorrhizal fungi. Plant Cell Environ 42:1758–1774. https://doi.org/10.1111/pce.13509
Yamatou M, Ikeda S, Iwase K (2008) Community of arbuscular mycorrhizal fungi in coastal vegetation on Okinawa Island and effect of the isolated fungi on growth of sorghum under salt-treated conditions. Mycorrhiza 18:241–249. https://doi.org/10.1007/s00572-008-0177-2
Yang C, Sun J (2020) Soil salinity drives the distribution patterns and ecological functions of fungi in saline-alkali land in the Yellow River Delta, China. Front Microbiol 11:594284. https://doi.org/10.3389/fmicb.2020.594284
Yano-Melo AM, Saggin OJ Jr, Maia LC (2003) Tolerance of mycorrhized banana (Musa sp. cv. Pacovan) plantlets to saline stress. Agric Ecosyst Environ 95:343–348. https://doi.org/10.1016/S0167-8809(02)00044-0
Yasmeen T, Tariq M, Iqbal S, Saleem Arif M, Riaz M, Shahzad SM, Ali S, Noman M, Li T (2019) Ameliorative capability of plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) against salt stress in plant. In: Hasanuzzaman M, Hakeem K, Nahar K, Alharby H (eds) Plant abiotic stress tolerance. Springer, Cham, pp 409–448. https://doi.org/10.1007/978-3-030-06118-0_17
Yousfi S, Serret MD, Voltas J, Araus JL (2010) Effect of salinity and water stress during the reproductive stage on growth ion concentrations ∆13C and δ15N of durum wheat and related amphiploids. J Exp Bot 61:3529–3542. https://doi.org/10.1093/jxb/erq184
Yousfi S, Serret MD, Márquez AJ, Voltas J, Jose AJL (2012) Combined use of δ13C, d18O and d15N tracks nitrogen metabolism and genotypic adaptation of durum wheat to salinity and water deficit. New Phytol 194:230–244. https://doi.org/10.1111/j.1469-8137.2011.04036.x
Zhang Z, Wang H, Song X, Liang Z, Tang Z (2019) Arbuscular mycorrhizal fungal diversity is affected by soil salinity and soil nutrients in typical saline-sodic grasslands dominated by Leymus chinensis. Arid Land Res Manag 34:68–82. https://doi.org/10.1080/15324982.2019.1631405
Zhong F, Fan X, Ji W, Hai Z, Hu N, Li X, Liu G, Yu C, Chen Y, Lian B (2022) Soil fungal community composition and diversity of culturable endophytic fungi from plant roots in the reclaimed area of the eastern coast of China. J Fungi 8:124. https://doi.org/10.3390/jof8020124
Zhu YG, Smith SE, Barritt AR, Smith FA (2001) Phosphorus (P) efficiencies and mycorrhizal responsiveness of old and modern wheat cultivars. Plant Soil 237:249–255. https://doi.org/10.1023/A:1013343811110
Zhu X, Cao Q, Sun L, Yang X, Yang W, Zhang H (2018) Stomatal conductance and morphology of arbuscular mycorrhizal wheat plants response to elevated CO2 and NaCl stress. Front Plant Sci 9:1363. https://doi.org/10.3389/fpls.2018.01363
Acknowledgements
We are thankful to the laboratory staff of Catholic University of Louvain and Spanish AGL2013-44147. The contribution of J.L.A. was supported by the Spanish Project PID2019-106650RB-C21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boudabbous, K., Bouhaouel, I., Jerbi, M. et al. Relationships Between Mycorrhizal Attributes and Stable Carbon and Azote Isotopes in a Semi-arid Environment as Influenced by Durum Wheat Cultivars and Salinity Level. J Soil Sci Plant Nutr 22, 4327–4343 (2022). https://doi.org/10.1007/s42729-022-01031-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-01031-3