Abstract
We investigated the accumulation of reactive oxygen species (ROS) in arbuscular mycorrhizal (AM) roots from Medicago truncatula, Zea mays and Nicotiana tabacum using three independent staining techniques. Colonized root cortical cells and the symbiotic fungal partner were observed to be involved in the production of ROS. Extraradical hyphae and spores from Glomus intraradices accumulated small levels of ROS within their cell wall and produced ROS within the cytoplasm in response to stress. Within AM roots, we observed a certain correlation of arbuscular senescence and H2O2 accumulation after staining by diaminobenzidine (DAB) and a more general accumulation of ROS close to fungal structures when using dihydrorhodamine 123 (DHR 123) for staining. According to electron microscopical analysis of AM roots from Z. mays after staining by CeCl3, intracellular accumulation of H2O2 was observed in the plant cytoplasm close to intact and collapsing fungal structures, whereas intercellular H2O2 was located on the surface of fungal hyphae. These characteristics of ROS accumulation in AM roots suggest similarities to ROS accumulation during the senescence of legume root nodules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
H2O2 is an important signalling compound regarding the interaction of plants with pathogenic microorganisms (Apel and Hirt 2004; Laloi et al. 2004) as well as many other processes referring to root biology, such as rhizobial interaction (Matamaros et al. 2003), gravitropism (Joo et al. 2001), root elongation growth (Kwak et al. 2003; Liszkay et al. 2004), and root hair elongation (Foreman et al. 2003). Regarding the rhizobial interaction in particular, reactive oxygen species (ROS) have been observed in the early and late phases of the interaction (Matamoros et al. 2003). Whereas the early ROS accumulation is specifically modulated by Nod factors (Ramu et al. 2002; Shaw and Long 2003) and has been compared to the oxidative burst after infection by pathogenic bacteria (Santos et al. 2001), the accumulation of ROS in the late phase is connected to nodule senescence (Puppo et al. 2005). The accumulation of H2O2 in the course of the arbuscular mycorrhizal (AM) symbiosis has been shown by staining using diaminobenzidine (DAB; Salzer et al. 1999). Additional findings suggesting an increase of ROS production in AM roots include increased amounts of jasmonates observed in the late phase of the symbiosis (Hause et al. 2002) and the induction of various antioxidative enzyme activities upon mycorrhization (Arines et al. 1994; Blilou et al. 2000; Lambais et al. 2003). Apart from these reports, no molecular reactions of plants or fungi to the accumulation of H2O2 in the AM symbiosis have been reported. Nevertheless, at least two phenomena specific to AM roots might be connected to the accumulation of H2O2: the induction of carotenoid biosynthesis (Strack et al. 2003), which is induced by ROS during chromoplast differentiation in Capsicum annuum (Bouvier et al. 1998), and the phenomenon of bioprotection of AM plants (Dumas-Gaudot et al. 2000; Linderman 2000), which might be an example of ROS-dependent cross tolerance (Bowler and Fluhr 2000).
The exact localization of the accumulating H2O2 is a crucial point regarding its potential biological roles in AM roots. Because the work by Salzer et al. (1999) resulted in a low structural resolution, we applied several staining techniques to address this problem. Inasmuch as the results obtained indicated the participation of the fungal symbiont in H2O2 production, we included extraradical spores and hyphae from Glomus intraradices in our analysis.
Materials and methods
Barrel medic (Medicago truncatula cv. Jemalong), tobacco (Nicotiana tabacum cv. Samsun), and maize (Zea mays cv. Garant) were grown in a greenhouse in pots filled with expanded clay (Lecaton, 2–5 mm particle size; Fibo Exclay Deutschland, Pinneberg, Germany). Seven-day-old seedlings (five plants per 500-ml pot) were inoculated with the AM fungus, G. intraradices Schenck & Smith, by the application of propagules in expanded clay (isolate 49, provided by H. von Alten from the collection of the Institut für Pflanzenkrankheiten und Pflanzenschutz der Universität Hannover, Germany). Further details of plant growth conditions have been described previously (Maier et al. 1995). Extraradical spores and hyphae of G. intraradices were isolated under a stereomicroscope from an axenic dual culture of this fungus and Ri T-DNA-transformed roots of Daucus carota L. (Chabot et al. 1992), which had been obtained from T. Boller (Basel, Switzerland).
For staining with DAB, fungal hyphae and spores and sectioned or disrupted roots were incubated for 1 h, intact roots for 2–4 h at room temperature (RT) in DAB buffer (1 mg/ml DAB buffered in 100 mM sodium citrate, pH 3.7). Stained samples were transferred into 10% lactic acid. Roots were washed with 10% lactic acid, incubated with the same solution for 10 min at 94°C, and disrupted under a stereomicroscope. A Nikon Optiphot 2 was used for bright-field microscopy. Photographs were taken using the Sensia II film (Fujifilm), and the slides were scanned and processed using the program Photoshop 4.0 (Adobe). Peroxidase activity in mycorrhizal roots was determined according to Thordal-Christensen et al. (1997). Root sections or disrupted roots were preincubated for 15 min in DAB buffer and subsequently incubated for 2 min in solutions containing 0, 0.1, 1, and 10 mM H2O2. The reaction was stopped by the addition of 96% ethanol; then the samples were transferred to 10% lactic acid and analyzed by bright-field microscopy. Control samples were treated with a DAB buffer containing 10 mM ascorbate.
For staining with dihydrorhodamine 123 (DHR 123), samples were incubated in situ on microscopic slides. Fungal hyphae, spores, root sections and disrupted roots were stained for 10–30 min and directly analyzed by confocal laser scanning microscopy (CLSM). Control samples were treated with water containing no dye. Intact roots were stained for 2 h, shortly washed with water, and then analyzed by CLSM. According to Bestwick et al. (1997), the effects of 8 μM diphenylene iodonium chloride (DPI, an inhibitor of NADPH oxidases), 25 μg/ml catalase (from bovine liver), 3 mM NaN3, and 3 mM NaCN (inhibitors of peroxidases) were tested by preincubating the material for 30 min at RT in the various agents (or water as a control) and subsequent replacement of this solution by the DHR 123 staining solution. CLSM was performed using a Zeiss LSM 410 equipped with a Kr/Ar laser (excitation at 488 nm). Fluorescence of oxidized rhodamine 123 (filter setting: HFT 488, NFT 545 and LP 505) as well as bright-field micrographs were collected simultaneously. For the construction of “extended depth of focus” images, series of at least ten optical sections were collected and superimposed using the “3D for LSM” program (Zeiss, Jena, Germany).
For staining with CeCl3 and electron microscopy, samples were treated according to Bestwick et al. (1997) and Pellinen et al. (1999). Small pieces of the mycorrhizal roots were dissected, incubated in 5 mM CeCl3 (in 50 mM MOPS, pH 7.2) for 1 h at RT, and subsequently washed several times with water. Control samples were incubated in water for the same time. The samples were fixed in 1.25% glutardialdehyde and 1.25% paraformaldehyde in 50 mM sodium cacodylate buffer (pH 7.2) for 1 h at RT. After rinsing with buffer, the samples were postfixed with 1% osmium tetroxide in the buffer for 45 min, rinsed again, and dehydrated in a graded ethanol series. Ethanol was substituted by epoxy resin (Spurr 1969) and samples were polymerized at 70°C. Sections were visualized with a Zeiss EM 900 electron microscope.
Results
The presence of peroxidases, necessary for the oxidation of DAB and DHR 123 by H2O2, was checked by staining of root sections according to Thordal-Christensen et al. (1997). The application of 1 or 10 mM H2O2 resulted in a more or less uniform staining within 2 min. Addition of 10 mM ascorbate to the DAB buffer reduced staining intensity significantly (data not shown).
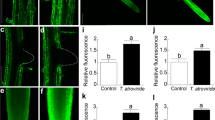
Staining of AM roots using DAB resulted in essentially similar patterns regarding all three plant species examined (Fig. 1a–c). Bright field microscopy of disrupted or sectioned roots allowed the observation of highly resolved mycorrhizal structures. Disruption before or after the staining procedure had no significant effect regarding structural details, but was essential to obtain well-resolved micrographs. We observed a strong staining of the central cylinder—independent of the mycorrhizal colonization—and of fungal structures like hyphae or vesicles. Arbuscules were stained to variable degrees in all three plant species. A particularly strong staining of arbuscules correlated with arbuscular clumping in some cases in M. truncatula. In Z. mays and N. tabacum, staining intensity of arbuscules was variable as well, but structural differences between these arbuscules were less obvious (Fig. 1a–c). In Z. mays, apocarotenoid-containing yellow droplets were observed in several cases near arbuscules intensively stained by DAB (Fig. 1c).
Staining of ROS using DAB (a–c) or DHR 123 (d–q). All CLSM micrographs are presented as single sections, except for d and e where several single sections have been combined resulting in an “extended depth of focus” presentation. Micrographs (g–r) represent a combination of green fluorescence with a small amount of light from the bright-field channel. CLSM of AM roots (M. truncatula) not treated with DHR 123 resulted in no fluorescence under the conditions used (r). In this case, the amount of light from the bright-field channel was increased to allow a better presentation. a–f Mechanically disrupted (a, b, d, e) or sectioned (c, f) roots from M. truncatula (a, d), N. tabacum (b, e) and Z. mays (c, f) showed a strong staining of the central cylinder (independent of the AM colonization) and a clear staining of fungal structures (black and white arrows). Yellow droplets (yellow arrows in c) in maize root sections stained by DAB reveal the accumulation of apocarotenoids in the close vicinity of arbuscules (black arrows in c). Accumulation of ROS in fungal hyphae isolated from an axenic dual culture (j–o) or analyzed in disrupted roots from M. truncatula (g, h) or N. tabacum (i) often was detected in the hyphal wall (h–l) and in the fungal cytoplasm (g, h, k–o). Cytoplasmic labelling appeared to be particularly pronounced during septum formation (k, m), after mechanical damage (n), or after preincubation in cyanide (o). Accumulation of ROS in fungal spores (p, q) was restricted to the wall of the spores (p), except for spores damaged mechanically, showing a generalized staining (q). All bars represent 20 μm
Staining of disrupted AM roots by DHR 123 essentially resulted in a similar pattern in comparison to staining with DAB (Fig. 1d–f). Differences in staining intensity regarding various forms of arbuscules in M. truncatula, however, were less pronounced using DHR 123 instead of DAB. The use of CLSM resulted in highly resolved micrographs and allowed the localization of some of the labelling within intercellular fungal hyphae (Fig. 1g,h). Arbuscular structures, however, were too small and labelled too intensively to allow a differentiation between a plant or a fungal location of the accumulating ROS.
Spores and hyphae from G. intraradices were isolated manually from a mycorrhizal D. carota root culture. The material was stained weakly by DAB (data not shown), but incubation in DHR 123 resulted in a clear and intensive labelling (Fig. 1j–q). Fungal spores were labelled exclusively at the spore wall (Fig. 1p), unless they were damaged by applying mechanical pressure, which resulted in a strong labelling of the spore wall and the spore cytosol (Fig. 1q). Regardless of their intraradical (Fig. 1g–i) or extraradical (Fig. 1j–p) location, fungal hyphae were often labelled at the hyphal walls. The lack of hyphal wall labelling in Fig. 1m and n, might be due to an unsuitable position of the optical section (note the variable intensity of hyphal wall labelling in Fig. 1j) or by an overlapping of the strong fluorescence from the fungal cytosol. In several cases, labelling of the hyphal cytosol was detectable, in particular, at the sites of obvious mechanical damage (imposed during the isolation procedure, Fig. 1j), during septum formation (Fig. 1k,m), or after treatment with NaCN (Fig. 1o).
DHR 123 is not only oxidized in a peroxidase-dependent way by H2O2, but also non-enzymatically by other highly reactive oxygen species like peroxynitrite (Kooy et al. 1994; Crow 1997). Fluorescence developed within a short time after the addition of the dye and was stable for more than 30 min. Disruption of the roots during the staining procedure was a prerequisite to obtain micrographs of good quality. CLSM analysis of intact AM roots incubated for prolonged periods in DHR 123 did not reveal significant structural differences in comparison to disrupted roots, excluding the possibility of major artefacts caused by the disruption procedure. Autofluorescence of fungal structures was observed at higher wavelengths and did not interfere with CLSM analysis (Fig. 1r). The nature and origin of the accumulating ROS was examined by preincubation in catalase, DPI, NaCN and NaN3 (Bestwick et al. 1997). Clear effects were observed in the case of isolated hyphae and spores treated with catalase, NaN3 and NaCN. Catalase and NaN3 were effective only at elevated concentrations (25 μg/ml and 30 mM, respectively), NaCN showed clear effects at 3 mM final concentration (Fig. 1o). Catalase eliminated hyphal fluorescence, leaving a residual fluorescence of spore walls (data not shown), NaCN and NaN3 eliminated fluorescence of spore and hyphal walls, but increased cytosolic fluorescence often connected with clear signs of cytosolic decomposition (Fig. 1o). In contrast to hyphae and spores, incubation of disrupted roots with the compounds mentioned did not lead to clear results. This may be due to difficulties in diffusion of the various compounds. In addition, it appears problematic to compare fluorescence intensities from different layers of tissue in different preparations.
Electron microscopical analysis revealed a significant formation of precipitates indicative for the accumulation of H2O2 only in AM roots from Z. mays (Fig. 2). Electron micrographs of AM roots from M. truncatula or N. tabacum only showed minor differences between samples treated with CeCl3 and samples treated with water. In AM roots from Z. mays, deposition of precipitates was particularly pronounced within the cytosol of root cortical cells in the close vicinity of intact (Fig. 2a,a′) or collapsed (Fig. 2b,b′) arbuscular branches and at the surface of intercellular fungal hyphae (Fig. 2c,c′). Control samples treated with water instead of the CeCl3 staining solution did not show significant staining (Fig. 2d–f,d′–f′).
Electron microscopical localization of H2O2 in AM roots of Z. mays after staining by CeCl3. General views (a–f) and corresponding details (a′–f′) of sections from stained (a–c) and unstained (d–f) roots are presented. Signals specific to staining by CeCl3 were observed within the plant cytoplasm (CY) close to intact arbuscular branches (AB, a, a′) and collapsing arbuscular branches (CA, b, b′), as well as on the surface of intercellular fungal hyphae (FH, c, c′). These signals were not observed in sections of unstained roots showing corresponding structures (d–f, d′–f′). Cell walls (CW) and plant vacuoles (PV) were not stained. Bars represent 0.5 μm
Discussion
Salzer et al. (1999) provided first evidence for the accumulation of ROS in AM roots of M. truncatula using DAB for staining. They reported the accumulation of H2O2 in root cortical cells containing arbuscules—especially when containing clumped forms—and close to fungal hyphae starting to penetrate a root cortical cell. Further findings suggesting an increase of ROS production in AM roots include increased amounts of jasmonates observed in the late phase of the AM symbiosis (Hause et al. 2002) and the induction of various antioxidative enzyme activities upon mycorrhization (Arines et al. 1994; Blilou et al. 2000; Lambais et al. 2003).
In plant–microbe interactions, ROS are important signalling compounds (Apel and Hirt 2004; Laloi et al. 2004) and direct effectors (Mellersh et al. 2002) involved in various stress and defence reactions. The compounds may be produced by apoplastic peroxidases, by plasma membrane located NADPH dehydrogenases, or by the action of various cytoplasmic sources of ROS, most notably chloroplasts or mitochondria (Apel and Hirt 2004). An increase in cytoplasmic ROS may be either due to an increased production of such compounds or to a reduced efficiency of protective, antioxidative systems. Depending on the cellular location, increased amounts of ROS may result in the activation of various signalling cascades, in direct toxic effects for the pathogens or in modifications of apoplastic structures directed against the pathogen, as well as in cellular senescence similar to programmed cell death as in the case of the hypersensitive response or the senescence of root nodules (Neill et al. 2002; Apel and Hirt 2004).
To gain information regarding the possible roles of ROS in AM roots, we addressed the following questions: Which of the two symbiotic partners is actually producing the accumulating ROS? Which cellular compartment is mainly involved in the accumulation? Is the accumulation of ROS connected to a specific stage of the symbiosis?
We used three different staining methods for answering these questions. Staining by CeCl3 is due to the formation of insoluble cerium peroxides, staining by DAB and DHR 123 relies on the peroxidase-dependent oxidation of these compounds by H2O2. DHR 123 can be oxidized, in addition, by highly reactive oxygen species in peroxidase-independent reactions (Kooy et al. 1994; Crow 1997). Staining by CeCl3 was apparently less sensitive when compared to staining by DAB or DHR 123, revealing strong and reliable signals only for AM roots from Z. mays (Fig. 2). This observation suggests either a particularly good penetration of Ce3+ in maize root preparations or particularly high levels of H2O2 in this tissue.
Regarding the organism producing the accumulating ROS, staining by DHR 123 revealed that a certain amount can be produced by the fungus. At least in the case of intercellular fungal hyphae, accumulating ROS could be clearly localized within the fungal cytosol. This observation is supported by our experiments with isolated fungal hyphae and spores, isolated from a root culture of D. carota colonized by G. intraradices, showing that the fungal production of ROS is a rather common phenomenon. Nevertheless, electron microscopical analysis of AM roots treated with CeCl3 resulted in precipitates indicative for H2O2 on the plant side but not on the fungal side of the interaction. Although this might be explained by a reduced diffusion of CeCl3 through the fungal cell wall, it rather shows that a large, if not the main part, of the accumulating H2O2 in AM roots is produced by the plant.
Regarding the cellular compartment mainly involved in the accumulation of ROS, the fungal and plant cytosol as well as the surface of intercellular fungal hyphae were identified. Electron microscopical analysis revealed, in particular, the absence of H2O2 from the plant apoplast. The presence of H2O2 in the apoplast could have been interpreted as a characteristic feature of a plant defence reaction against pathogens.
As Salzer et al. (1999) had shown, staining by DAB revealed a particularly strong labelling of collapsing arbuscules in roots from M. truncatula. Regarding roots from Z. mays and N. tabacum, differences in the staining intensity of different arbuscules were observed, but it was difficult to recognize the functional state of these arbuscules. Differences in staining intensities of different forms of arbuscules could not be observed after staining by DHR 123. This contrast to DAB-staining might be due to the oxidation of DHR 123 by additional ROS (not H2O2) or to a small dynamic range of the detection method. Electron microscopical analysis revealed the accumulation of H2O2 in the vicinity of collapsing and of intact fungal hyphae. Taking these data together, we suggest that there is a connection of ROS accumulation and the degradation of arbuscules, but the accumulation of ROS may precede the final collapse of arbuscular branches.
Regarding the accumulation of ROS within the cytoplasm of fungal hyphae, we observed a clear connection to mechanical damage, chemical stress and septum formation. Because catalase was able to quench the fluorescence of the hyphal cytosol and cell walls, the main accumulating ROS is certainly H2O2. The lacking effect of catalase at lower levels and the remaining fluorescence of spore walls might be due to a restricted diffusion of the enzyme. NaCN and, to a weaker extent, NaN3 were able to quench fluorescence in the fungal cell wall, indicating the participation of peroxidases in either the production of accumulating ROS or the oxidation of DHR 123. The strong increase of cytosolic fluorescence after the application of the compounds seems to be due to toxic effects because the cytosol in such hyphae appears to be degenerating. Although the connection of cytosolic fluorescence and mechanical damage in the case of fungal spores might be due to a restricted diffusion of the dye across the thick wall of the spores, our observations regarding fungal hyphae rather suggest that cytoplasmic accumulation of ROS is part of a general fungal stress reaction.
The cytoplasmic accumulation of ROS in AM roots argues against the participation of mechanisms involved in plant pathogen interactions, like the oxidative burst, which would result in an apoplastic accumulation. The correlation of H2O2 with arbuscular degradation rather suggests interesting similarities of this phenomenon with root nodule senescence, which also involves the generation of H2O2. Regarding this symbiosis, it has been discussed that an increase in cytoplasmic H2O2 levels triggered by endogenous or by plant-derived signals, and caused by either increased production of H2O2 or by a reduced activity of antioxidant systems, induces the degradation of symbiosomes and the programmed cell death of nodule cells (Puppo et al. 2005). H2O2 in AM roots might be generated in a similar way. Although plant cells are not dying upon arbuscular disintegration, there are several features specific to AM roots, which might be induced by the accumulating ROS. One example is the activation of carotenoid biosynthesis in the late phase of arbuscular development (Fester et al. 2002; Hans et al. 2004) because H2O2 is able to induce carotenoid biosynthesis in plant organs above ground (Bouvier et al. 1998). Another possible example is bioprotection of AM-colonized plants against root pathogens and increased resistance of such plants against abiotic stress (see Dumas-Gaudot et al. 2000; Linderman 2000, for review), which may be a typical example for cross tolerance mediated by the accumulation of ROS (Bowler and Fluhr 2000).
Although the molecular reactions of AM fungi to the accumulation of ROS are not known, components of respective fungal molecular signalling cascades have already been determined for other interactions (Nathues et al. 2004). Because we have shown that in the case of G. intraradices, fungal senescence and cytoplasmic ROS accumulation are correlating, we suggest that H2O2 diffusing across the thin hyphal wall of arbuscular branches might be able to initiate the fungal programme for senescence. This way, the accumulation of ROS in the cytoplasm of arbuscule-containing cells might ultimately lead to arbuscular degradation.
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arines J, Quintela M, Vilarino A, Palma JM (1994) Protein patterns and superoxide dismutase activity in non-mycorrhizal and arbuscular-mycorrhizal Pisum sativum L. plants. Plant Soil 166:37–45
Bestwick CS, Brown IR, Bennett MHR, Mansfield JW (1997) Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9:209–221
Blilou I, Bueno P, Ocampo JA, García-Garrido JM (2000) Induction of catalase and ascorbate peroxidase activities in tobacco roots inoculated with the arbuscular mycorrhizal fungus Glomus mosseae. Mycol Res 104:722–725
Bouvier F, Backhaus RA, Camara B (1998) Induction and control of chromoplast-specific carotenoid genes by oxidative stress. J Biol Chem 273:30651–30659
Bowler C, Fluhr R (2000) The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci 5:241–246
Chabot S, Bécard G, Piché Y (1992) Life cycle of Glomus intraradices in root organ culture. Mycologia 84:315–321
Crow JP (1997) Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide 1:145–157
Dumas-Gaudot E, Gollotte A, Cordier C, Gianinazzi S, Gianinazzi-Pearson V (2000) Modulation of host defence systems. In: Kapulnik Y, Douds DD (eds) Arbuscular mycorrhizas: physiology and function. Kluwer, Dordrecht, pp 173–200
Fester T, Hause B, Schmidt D, Halfmann K, Schmidt J, Wray V, Hause G, Strack D (2002) Occurrence and localization of apocarotenoids in arbuscular mycorrhizal plant roots. Plant Cell Physiol 43:256–265
Foreman J, Demidchik V, Bothwell JH, Mylona P, Mederma H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Hans J, Hause B, Strack D, Walter MH (2004) Cloning, characterization, and immunolocalization of a mycorrhiza-inducible 1-deoxy-d-xylulose 5-phosphate reductoisomerase in arbuscule-containing cells of maize. Plant Physiol 134:614–624
Hause B, Maier W, Miersch O, Kramell R, Strack D (2002) Induction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiol 130:1213–1220
Joo JH, Bae YS, Lee JS (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126:1055–1060
Kooy NW, Royall JA, Ischiropoulos H, Beckman JS (1994) Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med 16:149–156
Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22:2623–2633
Laloi C, Apel K, Danon A (2004) Reactive oxygen signalling: the latest news. Curr Opin Plant Biol 7:323–328
Lambais MR, Ríos-Ruiz WF, Andrade RM (2003) Antioxidant responses in bean (Phaseolus vulgaris) roots colonized by arbuscular mycorrhizal fungi. New Phytol 160:421–428
Linderman RG (2000) Effects of mycorrhizas on plant tolerances to diseases. In: Kapulnik Y, Douds DD (eds) Arbuscular mycorrhizas: physiology and function. Kluwer, Dordrecht, pp 345–365
Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O2·-, H2O2, and ·OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123
Maier W, Peipp H, Schmidt J, Wray V, Strack D (1995) Levels of a terpenoid glycoside (blumenin) and cell wall-bound phenolics in some cereal mycorrhizas. Plant Physiol 109:465–470
Matamoros MA, Dalton DA, Ramos J, Clemente MR, Rubio MC, Becana M (2003) Biochemistry and molecular biology of antioxidants in the rhizobia–legume symbiosis. Plant Physiol 133:499–509
Mellersh DG, Foulds IV, Higgins VJ, Heath MC (2002) H2O2 plays different roles in determining penetration failure in three diverse plant–fungal interactions. Plant J 29:257–268
Nathues E, Joshi S, Tenberge KB, von den Driesch M, Oeser B, Bäumer N, Mihlan M, Tudzynski P (2004) CPTF1, a CREB-like transcription factor, is involved in the oxidative stress response in the phytopathogen Claviceps purpurea and modulates ROS level in its host Secale cereale. Mol Plant Microb Interact 17:383–393
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5:388–395
Pellinen R, Palva T, Kangasjärvi J (1999) Subcellular localization of ozone-induced hydrogen peroxide production in birch (Betula pendula) leaf cells. Plant J 20:349–356
Puppo A, Groten K, Bastian F, Carzaniga R, Soussi M, Lucas MM, de Felipe MR, Harrison J, Vanacker H, Foyer C (2005) Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol 165:683–701
Ramu SK, Peng H-M, Cook DR (2002) Nod factor induction of reactive oxygen species production is correlated with expression of the early nodulin gene rip1 in Medicago truncatula. Mol Plant-Microb Interact 15:522–528
Salzer P, Corbiere H, Boller T (1999) Hydrogen peroxide accumulation in Medicago truncatula roots colonized by the arbuscular mycorrhiza-forming fungus Glomus intraradices. Planta 208:319–325
Santos R, Herouart D, Sigaud S, Touati D, Puppo A (2001) Oxidative burst in alfalfa–Sinorhizobium meliloti symbiotic interaction. Mol Plant-Microb Interact 14:86–89
Shaw SL, Long SR (2003) Nod factor inhibition of reactive oxygen efflux in a host legume. Plant Physiol 132:2196–2204
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Strack D, Fester T, Hause B, Schliemann W, Walter MH (2003) Arbuscular mycorrhiza: biological, chemical, and molecular aspects. J Chem Ecol 29:1955–1979
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
Acknowledgements
The authors thank Gerlinde Waiblinger and Regina Franke for their excellent technical assistance and the “Deutsche Forschungsgemeinschaft” for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fester, T., Hause, G. Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza 15, 373–379 (2005). https://doi.org/10.1007/s00572-005-0363-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-005-0363-4