Abstract
Salicylic acid (SA) is a signaling molecule and behaves as an antioxidant that induces stress tolerance in plants against abiotic stress. The present study explored the rice seedling response to V stress and the role of SA in improving the V stress tolerance of rice seedlings. The rice seedlings were sown in Petri dishes and incubated in a climate-controlled chamber for 4 days without light for germination. After that, the rice seedlings were shifted into hydroponic solution and allowed to grow for 18 days in hydroponic solution. The roots of 21-day-old rice seedlings were pretreated with SA (200 μM) for 3 days, and exposed to V (35 mg L−1) stress for 7 days. After 7 days of V stress, rice seedlings were harvested to determine the root attributes, photosynthetic assimilation, reactive oxygen species (ROS), ascorbate-glutathione (AsA-GSH) pathway enzymes, antioxidant and glyoxalase enzyme activities, and plant growth parameters. The findings disclosed that pretreatment of rice seedlings with SA had a high SPAD index, chlorophyll pigment content, and photosynthetic assimilation resulted in better growth compared to non-SA-pretreated rice seedlings. Strikingly, SA sustains the V homeostasis by inhibiting the accumulation of V from rice root to shoot. Besides this, pretreatment of SA increased the superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione S-transferase (GST), and ascorbate-glutathione (AsA-GSH) pathway enzymes, and also enhanced the ascorbate (AsA) and glutathione (GSH) level, and minimized the hydrogen peroxide (H2O2) and superoxide anion (\({\mathrm{O}}_2^{-\bullet }\)) of rice seedlings, by regulating the gene expression of antioxidant enzymes (OsCuZnSOD1, OsCaTB, OsGPX1, OsAPX1, OsGR2, and OsGSTU37). Furthermore, SA reduced methylglyoxal toxicity and enhanced glyoxalase enzyme activity by upregulating the genes expression of glyoxalase genes (OsGLYI-1 and OsGLYII-2) under V stress condition. Considering these findings demonstrated that SA may be utilized to reduce V availability to rice seedlings while also improving rice seedling growth and V stress resistance.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Vanadium contamination of arable agricultural land is an important risk to food security (Altaf et al. 2021a). The presence of V in the earth crust fluctuates from 10 to 220 mg kg−1, while soil under human use contains comparatively high V content (Wazalwar et al. 2011). Anthropogenic activities such as burning of fossil fuels, mining, industrial wastes, and phosphoric fertilizers are the dominant sources of V-polluted soils (Taner 2002; Devi et al. 2021; Tiwari et al. 2021). Vanadium causes significant irreversible changes in plant such as a reduction in growth and leaf necrosis, water imbalance, chlorophyll deprivation, antioxidant system destruction, and cell structure demolition, ultimately reduces crop quality and production (Altaf et al. 2022; Aihemaiti et al. 2019; García-Jiménez et al. 2018; Gokul et al. 2018; Imtiaz et al. 2018a). Elevated V concentrations influence later root formation and tempt leaf chlorosis due to chlorophyll destruction (Imtiaz et al. 2017; Altaf et al. 2021b). V ≥ 35 mg L−1 persuades the reactive oxygen species (ROS), which increases the enzymatic activity and ultimately causes cell death in rice plants (Altaf et al. 2020a). Higher V concentrations than a threshold level cause suppression or disruption of essential nutrient uptake (Aihemaiti et al. 2019), especially phosphorus uptake in plants (Imtiaz et al. 2017). The situation is especially critical in southwest China, where V pollutes 26.49% of agricultural land (Yang et al. 2017), and is classified as a dangerous pollutant to humans alongside lead (Pb), arsenic (As), and mercury (Hg) (Naeem et al. 2007). Vanadium affects human health through soil-plant-food cycle connection; therefore, V has received attention in recent decades, and it is necessary to use some eco-friendly substances on an urgent basis to minimize or stop its uptake and also induce stress tolerance in plants against V stress.
Salicylic acid (SA) is naturally occurring plant hormone that performs as a major signaling molecule; promotes several bio-physiological processes such as seed germination, photosynthesis, stomatal closure, cell growth, water metabolism, ion transmembrane transport, and flowering; and also improves the stress tolerance under both biotic and abiotic stresses (Nazar et al. 2011; Zhao et al. 2005). Scientists have studied the physiological role of SA in plants in recent years and have widely stated that it induces tolerance to salt and drought stress (Hayat et al. 2010; Mutlu et al. 2013). Now, scientists consider SA to be a signaling molecule that produces the plant response to abiotic stresses (Jini and Joseph 2017). The majority of recent studies have focused on the use of SA to improve plant growth against abiotic stress by increasing plant tolerance to stresses (Faried et al. 2017; Gondor et al. 2016a).
Salicylic acid is widely used to alleviate a series of abiotic stresses such as heavy metal stress, salt stress, drought stress, temperature stress, and ozone stress (Metwally et al. 2003; Nazar et al. 2011; Zhao et al. 2005). The first response of plants to heavy metals is the formation of ROS that significantly damages the chloroplast and the photosynthetic efficiency (Imtiaz et al. 2018b; Sharma et al. 2020). Reactive oxygen species causes toxicity by interacting with pigments, proteins, nucleic acids, and lipids and producing lipid peroxidation, enzyme inactivation, and membrane damage. Plants regulate the enzymatic and non-enzymatic machineries to reduce ROS damage and sustain the equilibrium between ROS production and damage to sustain cellular redox homeostasis (Horváth et al. 2007).
The response of SA depends upon various factors such as plant types, application method, application time, SA application concentration, and concentration inside the target plant (Horváth et al. 2007). Salicylic acid pretreatment scavenging the free radical produced by ROS by promoting the antioxidant enzymes and gene expression of OsWRKY45 that decreased the hydrogen peroxide (H2O2) accumulation in rice (Chao et al. 2010). Salicylic acid apply as a foliar application (600 μM) alleviated the cadmium (Cd) toxic effect on potatoes by ameliorating the chlorophyll content, relative water content, and proline content, and declining the H2O2, malondialdehyde (MDA), and superoxide anion (\({O}_2^{-\bullet }\)) (Li et al. 2019). Salicylic acid recovers the oxidative injury in bean plants by reducing the H2O2 and MDA content under Cd stress (Saidi et al. 2013). Pretreatment of SA improves photosynthesis and mung bean growth against salt stress through enhancing the activities of antioxidant enzymes, which comprise SOD, CAT, GPX, GR, and APX (Khan et al. 2014). Salicylic acid pretreatment reduced cell membrane injury to water-deficit conditions (Bandurska 2005). Salicylic acid increased the tolerance against copper (Cu) stress by increasing the root and shoot growth, and protein content, while declining the MDA, and proline contents of bean seedlings (Zengin 2014). Furthermore, SA pretreatment also reduced the Hg toxic effect on root by and increased the root growth of alfalfa against Hg stress (Zhou et al. 2009). Salicylic acid mitigates ROS and methylglyoxal (MG) induces oxidative stress by stimulating the activities of antioxidant and glyoxalase enzymes and improves the rice growth under selenium (Se) stress (Mostofa et al. 2020). Mostly, SA has been used to counter the adverse effects of heavy metals, e.g., Cu stress in common beans (Zengin 2014), Cd stress in maize (Krantev et al. 2008), and Pb stress in rice (Jing et al. 2007) by improving the photosynthetic efficiency and growth. These reports clearly show that SA has the potential to stimulate the plant growth and development processes against abiotic stresses, but there is no report available regarding the potential of SA to combat the V toxicity in plants.
Rice is a staple food for half of the world population and is globally grown (Reyes and Chin 2009). Rice is the cheapest source of fiber and protein, which inhibits cancer infection in the human body (Tan and Norhaizan 2017). Vanadium not only adversely damages the plant growth (Yuan et al. 2020) but also causes body weight reduction and cancer in human beings via the food chain (Ghosh et al. 2015). However, V contamination in the soil continuously expands due to industrialization, and the chances of V accumulation in food are increasing day by day. Therefore, it is necessary to search for an effective way to alleviate the damaging effects on rice growth produced by V stress.
In view of previous reports, SA has been widely used to manage the plant growth under several stresses; we assumed that SA might alleviate the toxic effects of V in rice. Thus, the current research was directed to study a sequence of morphological, physiological, and biochemical mechanism linked with (i) seedling growth and biomass production, (ii) root morphology, (iii) photosynthetic activities, (iv) ROS metabolism, (v) enzymatic and non-enzymatic activities, (vi) detoxification of MG, (vii) V uptake and translocation, and (vii) osmolytes accumulation in rice under V stress. Besides this, we also assessed the several gene expression levels linked with antioxidant and glyoxalase systems to explore the mechanism of how SA protects rice plants from V stress by regulating these systems. To best our knowledge, firstly we report the potential role of SA to alleviate the V toxicity in rice seedlings.
2 Material and Method
2.1 Plant Material, Treatment, and Growth Conditions
The rice (Oryza sativa L.) cultivar “Chao you 37” was used in this study and was bought from Hubei Tianmen Di Long Seed Industry Co. Ltd., China. The rice seed surface was disinfected with 0.1% (v/v) sodium hypochlorite (NaClO) for 15 min before sowing, and then washed with distilled water 8–10 times. A total of 50 rice seeds for each replication were sown in Petri dishes, and tap water was supplied as per the requirement to maintain the moisture for germination. The Petri dishes were incubated for 4 days in the climate-controlled chamber (RTOP-268B, Bio-Equip) at 20°C, relative humidity (RH) 75%, and supplied darkness for germination. After germination, the fifteen uniform rice seedlings were transferred into the black plastic box having half-strength Hoagland solution. After 7 days of shifting, full-strength Hoagland solution was provided to the rice seedlings until two leaf stage attained (21 days after transplanting) with light dark (14–10h), temperature (27–23°C) respectively, RH 75%, and photon flux density of 820 μmol m−2s−1 for the light duration. The nutrient medium (pH 5.6) was replenished every 3 days during the whole study.
The V concentration (35 mg L−1) was selected on the basis of our earlier studies (Altaf et al. 2020a), while we designed an experiment to evaluate at which concentration; SA improves the rice seedling growth under V (35 mg L−1) stress. The response of SA differs from species to species and also depends upon the application methods (foliar application or root application (soil conditions, hydroponic conditions, and substrate conditions)) and application timing. We pretreated the rice seedlings at two leaf stages with different SA concentrations (0–1000 μM) for 3 days, after that exposed constant V (35 mg L−1) stress for 7 days. At the time of the preliminary experiment, we noted that SA ≤ 200 μM improves the plant growth (Fig. S1), while SA > 200 μM causes growth reduction in the term biomass (fresh and dry) weight of roots and shoots under V (35 mg L−1) stress (Figs. S2 and S3). After that, we selected 200 μM SA for further studies.
The current study consisted of four treatments and six replications for each treatment in a complete randomized design: (1) 0: control (CK); (2) SA: with amendment of SA (200 μM); (3) V: with an amendment of V (35 mg L−1, NH4VO3); (4) SA + V: with pretreatment of SA and after exposed to V stress. The SA was added into Hoagland solution and treated rice seedlings at two leaf stage (21 days after transplanting) for 3 days and after that allows the seedlings to grow under V stress for 7 days as elaborated in Fig. S4. The SA (200 μM) was dissolved in ethanol (500 μM), while the CK seedlings without SA were treated with ethanol and water (500 μM L−1). After 7 days of stress, seedlings were harvested and kept at −80 °C for the measurement and analysis of different parameters.
2.2 Determination of Plant Growth and Root Morphological Parameters
For the measurement of plant growth parameters, six rice seedlings from every replication were harvested. An electric weight balance was used to estimate the roots and shoot fresh weight. Afterward, seedlings were kept in paper bags, oven-dried at 105 for 15 min, and kept at 70 °C for 72 h. After that, roots and shoots dry weight was weighed and the samples stored at 60 °C for the quantification of V content in roots and leaves (Altaf et al. 2019).
Six uniform harvested roots from every replication were washed with running water for scanning purposes and studies of root morphological attributes. The scanning of roots was done by using the Imagery Scan Screen (Epson Expression 11000XL, Regent Instruments, Canada), and root scanning softer WinRHIZO 2003a software (Regent Instruments, Canada) was operated for root scanning and measurements (Altaf et al. 2020b).
2.3 Estimation of Photosynthetic Pigments, SPAD Index, and Leaf Gas Exchange Parameters
For the determination of pigment content, fresh leaf samples (0.5 g sample−1) were treated in 10 mL of 80% acetone, centrifuged (10,000g, 10min, 4 °C), after that measured the reading at 662,645 and 470 nm by utilizing the spectrophotometer (Lambda 25 UV/VIS, PerkinElmer, USA). Pigment contents, i.e., chlorophyll (a + b) and total carotenoids were determined and calculated using the protocol defined by Lichtenthaler and Wellburn (1983).
The relative chlorophyll content of rice leaves (fully expanded) was estimated by operating the SPAD-502 Chlorophyll Meter (Minolta CameraCo., Ltd., Japan). The leaf gas exchange (net photosynthetic rate (Pn), stomatal conductance (gs), intercellular CO2 concentration, and transpiration rate (Tr)) of the rice leaves (fully expanded) were determined by operating a portable photosynthesis apparatus (LiCor-6400 LICOR Inc., Lincoln, NE, USA). The growth condition of the measuring cabinet during the recording was precise to adjust the leaf temperature 20 °C, CO2 concentration 350 μM mol−1, and photosynthetic photon-flux density 900 μM m−2s−1. A total of 6 plants were randomly selected from every treatment for taking the readings (Altaf et al. 2021c).
2.4 Determination of Total Soluble Protein, Ascorbate, and Glutathione Contents
Rice leaf and root samples (0.5g sample−1) were homogenized in 10% of phosphate buffer solution (0.1 M, pH 7.3) for the measurement of soluble protein, AsA, and glutathione (GSH) contents. The homogenates were centrifuged at 3500 g for 10 min. After the centrifugation, the supernatant was utilized for the quantification of soluble protein, AsA, and GSH content followed the descriptions of kits A045-2, BC1230, and A061-2-1 respectively, assimilated from Nanjing Jiancheng Bioengineering Institute, Nanjing, China. The wavelength of soluble protein, AsA, and GSH was recorded at 595, 265, and 412 nm respectively, by utilizing the spectrophotometer (Lambda 25 UV/VIS, PerkinElmer, USA).
2.5 Estimation of EL, Proline, MDA, H2O2, \({\mathrm{O}}_2^{-\bullet }\), and MG
Leaves and roots of the six uniform rice seedlings were harvested from every replication and washed with distilled water for the determination of EL. The method defined by Dionisio-Sese and Tobita (1998) was used to measure the EL. Electrolyte leakage was measured by applying the following formula:
where EC1 and EC2 indicate the electric conductivity before and after boiling the leaf samples respectively.
The content of proline, MDA, H2O2, and \({O}_2^{-\bullet }\)were estimated from the rice leaves and roots by using supernatant collected at the time of total protein content estimation. The proline, MDA, H2O2, and \({O}_2^{-\bullet }\)were measured by following the guidelines explain in A107-1-1, A003-3-1, A064-1-1, and A052-1-1 kits while the wavelength was recorded at 520, 530, 405, and 550, respectively by operating the spectrophotometer (Lambda 25 UV/VIS, PerkinElmer, USA). Methylglyoxal content in leaf and root was estimated according to the protocol established by Wild et al. (2012).
2.6 Histochemical Staining of H2O2 and \({\mathrm{O}}_2^{-\bullet }\)
The histochemical staining of H2O2 and \({\mathrm{O}}_2^{-\bullet }\)was accomplished according to the method established by Gong et al. (2017). For H2O2 histochemical staining, the rice leaves were incubated with 1 mg·mL−1 DAB in 50 mM Tris-acetate (pH 3.8) in the dark at 25 °C 24 h. For \({\mathrm{O}}_2^{-\bullet }\)histochemical staining, the leaves were incubated with 0.1 mg·mL−1 NBT in 25 mM K-HEPES buffer (pH 7.8) in the dark at 25 °C, 2 h. The leaves were washed five times in 80% ethanol at 70 °C in both cases (H2O2 and \({\mathrm{O}}_2^{-\bullet}\Big)\)before photographed.
2.7 Antioxidant Enzymatic Assay
Supernatants collected at the time of total protein content measurement were utilized for the analysis of enzyme activities. The SOD, CAT, APX, GR, dehydroascorbate reductase (DHAR), and monodehydroascorbate reductase (MDHAR) activities were determined from the leaf and root samples of rice seedlings by following the protocol defined in A001-4, A007-1-1, A123-1-1, A062-1-1, BC0660, and BC0650 kits, and wavelength was noted at 550, 405, 290, 340, 412, and 340 nm, respectively by operating the spectrophotometer (Lambda 25 UV/VIS, PerkinElmer, USA).
2.7.1 Glutathione-Dependent Enzymes
The enzymatic activities of glutathione-dependent enzymes were assayed from the leaves and roots of rice seedlings. The activities of glutathione S-transferase (GST) and GPX were measured by following the technique described in A004 and A005-1, and wavelength (GST and GPX) was noted at 412 nm, by using the spectrophotometer (Lambda 25 UV/VIS, PerkinElmer, USA). The protocol of Hossain et al. (2009) and Principato et al. (1987) was obeyed for the estimation of GlyI and GlyII, respectively, and extinction coefficients 3.37 and 13.6 mM−1cm−1 were used for estimation of GlyI and GlyII activities respectively.
2.8 Vanadium Determination
The determination of V accumulation in the rice leaves and root was performed according to the protocol of Hou et al. (2013) by operating the graphite furnace atomic absorption spectrophotometry (GFAAS-GTA 120).
2.9 Gene Expression Analysis
Total RNA from the leaf and root samples were extracted at the seventh day after V stress by the Trizol method according to protocol recommended by the manufacturer. Total RNA concentration was confirmed by using the Nanodrop 2000 spectrophotometer (Thermo, Germany), and the quality and purity of the total RNA were checked by agarose gel electrophoresis. For complementary DNA (cDNA) synthesis, extracted RNA was reverse-transcribed by RT-PCR using the Vazyme HiScript II Q RT SuperMix Kit (Vazyme, China), following the instructions described by the manufacturer. The qrt-PCR was carried out on 96-well plates by operating qTOWER3 QPCR system (Analytik jena AG, Germany) using FastStart Essential DNA Green Master Kit (Roche, USA) following the manufacturer’s instructions. OsUbiquitin (OsUBQ) gene was utilized as a reference gene, as formerly used in rice gene expression profiling against metal stress (Mostofa et al. 2020). The delta-delta Ct (2−ΔΔCT) formula was used to quantify the relative gene expression level (Livak and Schmittgen 2001). The primers used in this study are listed in Table T1.
2.10 Statistical Analysis
The obtained data was statistically analyzed by using the statistical software (IBM SPSS 22.0, IBM Corporation, New York, USA). The data in the figures are presented as mean ± standard errors (SEs) of six replications. Data was analyzed using the one-way ANOVA and Duncan multiple tests (P ≤ 0.05). The graphical representation was done with origin v.2018b.
3 Results
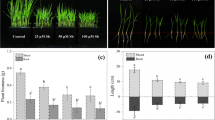
Rice seedlings exposed to V (35 mg L−1) for 7 days caused drastic changes, including shorter root length, leaf decolorization, chlorosis, 2nd leaf tip burning, and yellowing of whole plants (Fig. 1A–B). In contrast, pretreatment of SA dismissed the V-caused phytotoxic changes, and ameliorated the phenotypic growth under V stress seedlings as compared to only V-stressed seedlings. Nevertheless, the pretreatment of SA without V stress did not induce any distinct variation in the phenotypically appearance as compared to untreated CK seedlings. Moreover, SA pretreatment to V-stressed seedlings sustained the leaves’ green color and timid chlorosis, and maintained the healthy and longer roots as compared to only V-stressed seedlings (Fig. 1A–B).
(A) Effect of pretreatment of salicylic acid (SA) on rice seedling performance under vanadium (V) stress and without V stress. Whereas the CK, control seedlings; SA, seedlings pretreated with 200-μM salicylic acid; V, seedlings treated with 35 mg L−1 vanadium; SA+V, seedlings pretreated with salicylic acid (200 μM) + exposed 35 mg V L−1. (B) Response of root morphology of rice seedling pretreated with SA grown under V stress. (C) SA effect on V accumulation in roots and leaves of rice seedlings grown under V stress conditions
3.1 Plant Growth and Root Morphology
To assess the SA effect on V homeostasis, we measured the V accumulation in rice roots and leaves during the investigation. The maximum V was deposited in root than leaves (Fig. 1C). However, SA pretreatment inhibited the V uptake by the root as compared to only the V stressed seedlings (Fig. 1C). It was also noted that V was markedly more deposited in the roots than leaves.
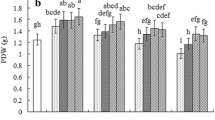
Rice seedlings exposed to V stress markedly inhibited their growth in terms of reduced root and shoot fresh weight, and dry mass (Fig. 2). As displayed in Fig. 3, rice plants under V stress showed a significant reduction in shoot fresh weight (−50.5%), root fresh weight (−48.4%), dry shoot weight (−62.6%), and root dry weight (−72.9%) as compared to CK seedlings. Conversely, SA supplementation alleviates the adverse effect of V and considerably enhances all growth and biomass-related parameters of rice seedlings.
Effect of pretreatment of salicylic acid (SA) on (A) fresh shoot weight, (B) dry shoot weight, (C) fresh root weight, and (D) dry root weight of rice seedlings grown in nutrient solution under vanadium (V) stress. Whereas the CK, control seedlings; SA, seedlings pretreated with 200-μM salicylic acid; V, seedlings treated with 35 mg L−1 vanadium (V); SA+V, seedlings pretreated with salicylic acid (200 μM) + exposed 35 mg V L−1. Value (means ± S.E) of every treatment was acquired from six replications (n=6). Different lower case letters indicate significant difference between the treatments at P < 0.05
Root characteristics of rice seedlings were grown in nutrient solution with or without pretreatment of salicylic acid (SA). Whereas the CK, control seedlings; SA, seedlings pretreated with 200-μM salicylic acid; V, seedlings treated with 35 mg L−1 vanadium (v); SA+V, seedlings pretreated with salicylic acid (200 μM) + exposed 35 mg V L−1. Value (means ± S.E) of every treatment was acquired from six replications (n=6). Different lower case letters indicate significant difference between the treatments at P < 0.05
Root architecture plays an important role in better plant growth. The findings of these experiments indicated that root characteristics (root length, root surface area, root volume, root forks, root tips, and root crossing) were significantly reduced by V stress as compared to CK. Interestingly, in SA-pretreated rice seedlings subjected to V stress, the root characteristics were ameliorated compared with V-stressed seedlings (Fig. 3).
3.2 Pigment Content, SPAD Index, and Leaf Gas Exchange Parameters
Vanadium stressed seedlings sharply decreased the chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoid content by 57.7%, 41.5%, and 50.4%, respectively as compared to CK seedlings. In contrast, SA supplementation intensely upgraded the photosynthetic pigments in V-stressed seedlings, as indicated by 79.2%, 33.3%, and 74.6%, enhanced Chl a, Chl b, and carotenoid content, respectively, when compared to the V-stressed seedlings (Fig. 4A, B, and C). It was also observed that relative chlorophyll content (SPAD index) was drastically reduced from 31.8 in CK seedlings to 18.5 in V-stressed seedlings, while SA supplementation helped to reduce the leaf damage (Fig. 4D).
The response of photosynthetic pigments, photosynthetic gas exchange, and relative chlorophyll content (SPAD index) of rice seedlings were grown in nutrient solution with or without pretreatment of salicylic acid (SA). Whereas the CK, control seedlings; SA, seedlings pretreated with 200-μM salicylic acid; V, seedlings treated with 35 mg L−1 vanadium (V); SA+V, seedlings pretreated with salicylic acid (200 μM) + exposed 35 mg V L−1. Value (means ± S.E) of every treatment was acquired from six replications (n=6). Different lower case letters indicate significant difference between the treatments at P < 0.05
All the gas exchange parameters significantly reduced under V stress; however, the reduction of Pn, Tr, Ci, and Gs was eased by SA pretreatment. The Pn, Tr, Ci, and Gs were declined by 44.5%, 58.0%, 52.1%, and 40.2% under V stress respectively as compared to CK. However, pretreatment with SA retrieved the Pn, Tr, Ci, and Gs by 18.1%, 43.4%, 53.3%, and 39.1% respectively, in V-stressed seedlings as compared to only V-stressed seedlings (Fig. 4E–H).
3.3 Oxidative Damage
Second leaf rice seedlings were stained with DAB and NBT to envision the production of H2O2, and \({O}_2^{-\bullet }\) respectively. The rice seedling under V stress showed deep black circle and dark brown polymerization products, which were representatives of \({O}_2^{\bullet -}\) and H2O2 respectively. Conversely, pretreatment of SA noticeably inhibited the over accumulation of \({O}_2^{-\bullet }\) and H2O2 in leaves under V stress seedlings (Fig. 5A and B). Vanadium stress augmented the ROS accumulation in terms of H2O2 and \({O}_2^{-\bullet }\) in leaves/roots of rice seedlings by 136.2/84.6% and 121.1/170.1% respectively, as compared with CK (Fig. 5C and D). Strikingly, SA applicant reduced H2O2 and \({O}_2^{-\bullet }\) contents in leaves/roots as by 33.3/17.1% and 34.2/24.4% respectively, as compared with only V-stressed seedlings.
Histochemical staining to determine (a) hydrogen peroxide (H2O2) and (b) superoxide anion \(\left({O}_2^{-\bullet}\right)\) utilizing the diaminobenzidine (DAB) and nitroblue tetrazolium (NBT), respectively. Effect of pretreatment of SA on oxidative biostress markers (H2O2, \({\mathrm{O}}_2^{-\bullet }\), MDA, and EL) in leaves and roots of rice seedlings grown in nutrient solution with or without vanadium stress (V). Whereas the CK, control seedlings; SA, seedlings pretreated with 200-μM salicylic acid; V, seedlings treated with 35 mg L−1 vanadium (V); SA+V, seedlings pretreated with salicylic acid (200 μM) + exposed 35 mg V L−1. Value (means ± S.E) of every treatment was acquired from six replications (n=6). Different lower case letters indicate significant difference among the treatments at P < 0.05
To access the cell membrane integrity under V stress, we measured the MDA and EL in rice seedlings. In comparison to CK, V toxicity intensely harmed the cellular integrity in rice leaves/roots, as proofed by enhanced MDA content (120.2/62.2%) and high EL (74.8/99%), respectively (Fig. 5E and F). However, SA pretreatment exhibited a significant decline in MDA and EL in leaves/root, as much as 24.1/17.7% and 19.0/35.5%, respectively, compared to that of V-stressed plants.
3.4 Proline, Protein, and Antioxidant Metabolites
Vanadium stress markedly enhanced in proline and total GSH content in leaves/root by 322.1/272.3% and 97.6/103.6%, respectively, as compared with CK seedlings. In contrast, SA pretreatment significantly reduced the proline and GSH content of leaves/roots by 31.8/23.0% and 23.2/17.8%, respectively, as compared to only V stress seedlings (Fig. 6A and B). As displayed in Fig. 6C and D, V-stressed seedlings showed a reduction in protein and total AsA contents of leaves/roots by 57.2/53.3% and 39.8/32.1%, respectively, as compared to CK, while SA pretreatment improved the protein and total AsA contents of leaves/roots of rice seedlings as compared to only V-stressed seedlings (Fig. 6).
Effect of pretreatment of rice seedlings with salicylic acid (SA) on protein, proline, and non-antioxidant enzymes (AsA and GSH) in the leaves and roots under vanadium (V) stress. Whereas the CK, control seedlings; SA, seedlings pretreated with 200-μM salicylic acid; V, seedlings treated with 35 mg L−1 vanadium (V); SA+V, seedlings pretreated with salicylic acid (200 μM) + exposed 35 mg V L−1. Value (means ± S.E) of every treatment was acquired from six replications (n=6). Different lower case letters indicate significant difference between the treatments at P < 0.05
3.5 Antioxidant Enzymes
In comparison with CK seedlings, SOD, CAT, GPX, and GST activities were decreased in the leaves/root of rice seedlings by 54.5/55.9%, 52.7/45.5%, 35.8/31.0%, and 58.0/48.2%, respectively, under V stress. Pretreatment of SA under V-stressed seedlings resulted in a notable enhancement of SOD, CAT, GPX, and GST by 59.2/54.4%, 54.2/50.5%, 29.4/26.2, and 71/46%, respectively, in leaves/roots as compared to only V-stressed seedlings (Fig. 7A–D).
Effect of pretreatment of salicylic acid (SA) on antioxidant, and glyoxalase defense system, and methylglyoxal contents of leaves and roots under vanadium (V) stress. Whereas the CK, control seedlings; SA, seedlings pretreated with 200-μM salicylic acid; V, seedlings treated with 35 mg L−1 vanadium (V); SA+V, seedlings pretreated with salicylic acid (200 μM) + exposed 35 mg V L−1. Value (means ± S.E) of every treatment was acquired from six replications (n=6). Different lower case letters indicate significant difference between the treatments at P < 0.05
The activities of APX, DHAR, MDHAR, and GR participating in the AsA-GSH cycle were measured in leaves and roots of rice seedlings with and without SA against V stress environment (Fig. 7E–H). V stress caused significant reduction in APX, DHAR, MDHAR, and GR by 48/48.4%, 45.9/48%, 64.5/48.3%, and 50/57.2% in leaves/roots respectively, with compared to CK seedlings while pretreatment of seedlings with SA elevated the APX, DHAR, MDHAR, and GR activities in leaves/root by 56.1/28.8%, 47.2/49.1%, 105.8/32.2%, and 43/46.8, respectively, under V stress as compared with only V-stressed seedlings (Fig. 7E–H). These results confirmed that V-induced oxidative damage could be minimized with SA by synchronized accomplishment of antioxidant enzymes and AsA-GSH equilibrium, which helped to increase tolerance to V stress.
3.6 MG and Gly Enzymes
Rice seedlings exposed to V stress caused a remarkable enhancement in MG level by 108.4/138.9% in leaves/roots respectively, relatively to CK seedlings. Pretreatment of roots with SA under V stress resulted in reduction in MG level by 30.5/23.9% in leaves/roots respectively, as compared to only V stress seedlings (Fig. 7I). The activities of Gly I were reduced in leaves/roots by 42.1/40.5%, respectively, while MG contents were increased in comparison with CK seedlings (Fig. 7J). Similarly, Gly II activity was also reduced in leaves/roots by 60.3/28.5%, respectively, over CK seedlings (Fig. 7K). Pretreatment of roots with SA in nutrient medium played magic role to eliminate the MG toxicity under V stress by enhancing Gly I (36.3/39%) and Gly II (76.1/30.3%) in the leaves/roots respectively, as compared to CK (Fig. 7I–K).
3.7 Relative Gene Expression
Next, we measured the transcriptional levels of antioxidant and glyoxalase-associated genes in V-stressed seedlings with or without SA pretreatment by using the qrt-PCR (Fig. 8). Vanadium stress induced a significant decline in the transcript level of the SOD representative gene CuZnSOD1 gene in leaves/root by 61/50.3%, respectively, as compared to CK seedlings (Fig. 8A). Conversely, SA-pretreated rice seedlings exposed to V stress caused maximum transcript levels of OsCuZnSOD1 in leaves/roots by 54.8/42.7%, respectively, as compared to V stress seedlings (Fig. 8A). In the case of the CAT representative gene, OsCATB expression is reduced by 68.5/72.2% in leaves/roots, respectively, with comparison to CK seedlings (Fig. 8B). However, rice seedlings pretreated with SA under V stress strikingly increased the OsCATB expression level by 77/88.3% in leaves/roots, respectively, as compared to only V stress seedlings (Fig. 8B). The expression level of GPX and GST representative genes, namely OsGPX1 and OsGSTU37 respectively, was measured, indicating decreased OsGPX1 transcript level (by 62.2/65.8% in leaves/roots, respectively) in V-stressed seedlings as compared to CK seedlings (Fig. 8C and D), while transcript level of OsGSTU37 was also reduced only V-stressed seedlings by 67.9/70.8% in leaves/roots, respectively, as comparison to CK seedlings (Fig. 8D). On the other hand, rice seedlings pretreated with SA increased the transcript level of OsGPX1 and OsGSTU37 by 44.8/141% and 55.1/193% in leaves/roots respectively, under V stress as compared to only V-stressed seedlings (Fig. 8C and D). The transcript level of APX and GR representative genes OsAPX2 and OsGR2 was lowered in V-stressed seedlings by 271/60.6% and 41.5/63% in leaves and roots, respectively, as compared to CK seedlings (Fig. 8E and F), while pretreatment of SA enhances the transcript level of OsAPX2 and OsGR2 by 72.5/48.9% and 38.1/87.5% in leaves/roots, respectively, in comparison to on V-stressed seedlings (Fig. 8E and F).
Relative transcript abundance of different antioxidant and glyoxalase enzyme coding genes in the leaves and roots of rice seedlings under vanadium (V) stress with or without salicylic acid pretreatment. Whereas the CK, control seedlings; SA, seedlings pretreated with 200-μM salicylic acid; V, seedlings treated with 35 mg L−1 vanadium (V); SA+V, seedlings pretreated with salicylic acid (200 μM) + exposed 35 mg V L−1. Value (means ± S.E) of every treatment was acquired from six replications (n=6). Different lower case letters indicate significant difference between the treatments at P < 0.05. The delta-delta Ct (2−ΔΔCT) formula was used to quantify the relative gene expression level (Livak and Schmittgen 2001)
Furthermore, glyoxalase system representative genes OsGLYI-1 and OsGLYII-2 were also studied (Figure 9). Our findings imply that the transcript level of OsGLYI-1 was remarkably decreased in the leaves/roots of V-stressed seedlings by 77/73.6%, respectively, as compared to CK seedlings (Fig. 8G). Moreover, pretreatment of rice seedlings with SA increased the transcript level in leaves/roots under V stress by 158.8/47%, respectively, as compared to only V stress seedlings (Fig. 8G). The results of the OsGLYII-2 gene showed the V stress rapidly reduced the transcript level of rice leaves/roots by 77/73.6%, respectively, as compared to CK seedlings, while the pretreatment of SA increased the transcript level in leaves and roots in rice seedlings under V stress as compared to only V-treated seedlings (Fig. 8H).
4 Discussion
Vanadium toxicity reduced the plant growth and yield, as earlier reported in various plant species such as rice, chickpea, watermelon, and mustard (Altaf et al. 2020a; Imtiaz et al. 2018b; Imtiaz et al. 2016; Nawaz et al. 2018). In the present study, V toxicity markedly declined the rice growth in terms of biomass yield reduction (root and shoot) and leaf necrosis, while pretreatment of SA improved the rice seedling growth. The cause for the reduction in biomass yield could be that under V stress, the roots were unable to transport adequate water and minerals to the shoot. Salicylic acid improved plant growth by reducing the metal toxicity effect which has also been observed in several crops such as rice, wheat, and maize (Gondor et al., 2016b; Mostofa et al. 2019; Moussa and El-Gamal 2010; Singh et al. 2015). Salicylic acid supplementation can effectively increase cell division and elongation, which may be one of the factors contributing to increased rice growth under stressful environment.
Our data also demonstrated that V mainly accumulated in roots followed by leaves. These findings propose that V amply existed as an easily available form in the nutrient solution, which was rapidly uptake by roots and accumulated to aerial parts of the plant. Similar to our results, others scientists also confirmed that V was more deposited in roots of watermelon (Nawaz et al. 2018), tomato (Vachirapatama et al. 2011), chickpea (Imtiaz et al. 2017), and mustard (Imtiaz et al. 2018b). In contrast, SA behaved antagonistically when interacting with V, restricting the excessive V uptake by rice roots, thereby minimizing V over-disposition in leaves. Salicylic acid intervened restriction of uptake and deposition of heavy metals has also been observed in several crops, such as maize and wheat (Gondor et al. 2016b; Shakirova et al. 2016; Singh et al. 2017).
Root size and architecture are important factors that play a major role in the nutrient uptake efficiency of crops (Postma and Lynch 2012). Alteration in root structure improves plant growth by increasing the uptake of nutrients and utilization efficiency (Wissuwa et al. 2016). The adverse effect of V inhibited the root growth is testified in rice (Altaf et al. 2020a). In this study, V inhibited the root growth by disturbing the root attributes, while pretreatment of SA improved the root growth due to improvement in root-related attributes. It may have caused a reduction in root growth due to cell growth impairment (Altaf et al. 2020a). The role of SA in improving the root-related attributes has been reported in barley (Metwally et al. 2003), wheat (Moussa and El-Gamal 2010), and rice (He et al. 2010) under heavy metal stress.
Heavy metal-induced photosynthetic reduction directly damaged the functioning of photosynthesis machinery (Carpentier 2001), as well as stomatal closure (Sheoran et al. 1990), and decreased the efficiency of CO2 uptake and assimilation (Seregin and Kozhevnikova 2006). The photosynthesis rate reflects the performance of plant growth that is directly connected with chlorophyll and carotenoid contents (Jahan et al. 2019; Jiang et al. 2017). Generally, a higher level of chlorophyll leads to better plant growth. In the present research work, high V concentration in leaves significantly reduced the SPAD index, photosynthetic assimilation, stomatal conductance, intercellular CO2, transpiration rate, and level of chlorophyll and carotenoids, eventually produced leaf chlorosis and poor plant growth. Similar to our results, V inhibited the photosynthetic rate and chlorophyll pigment destruction in several crops such as rice, chickpea, and mustard (Altaf et al. 2020a; Imtiaz et al. 2018b; Imtiaz et al. 2016). Heavy metals disturb the activity of enzymes involved in chlorophyll biosynthesis, such as aminolaevulinic acid dehydratase and protochlorophyllide reductase. Heavy metal stress also has a negative effect on the intake of magnesium, which is an essential component of the chlorophyll molecule, resulting in a considerable reduction in chlorophyll synthesis (Sela et al. 1989). The decline in gas exchange characteristics during metal stress is ascribed to stomatal deformation, which causes suppression of gas exchange and hence lower conductance (Anjum et al. 2016). In contrast, the pretreatment of SA enhanced the photosynthetic rate and reduced the chlorophyll pigment losses by protecting the photosynthetic apparatus from the V toxic effect. This protective response of SA might also play an important role in improving the rice growth against V stress condition, as the rice seedlings pretreated with SA were able to sustain the photosynthetic rate and chlorophyll pigments under V stress as compared to only V stress seedlings. Similarly, results of improving the photosynthetic rate and chlorophyll pigment contents by SA under metal stress have been reported in mustard and lemon balm under nickel stress (Soltani Maivan et al. 2017; Zaid et al. 2019), maize and peppermint under Cd stress (Ahmad et al., 2018a; Krantev et al. 2008), and mustard under Pb stress (Kohli et al. 2018). Photosynthetic processes also control the protein and sugar content in plants against stressful circumstances (Simkin et al. 2019). In this research work, we also noted a positive association of photosynthetic rate and photosynthetic pigments with protein in rice seedlings pretreated with SA under V stress. These findings suggested that SA improved the photosynthetic activity to sustain the protein, which might support cob V toxicity by supplying metabolites and energy through several biochemical pathways.
The plant faced environmental stresses that triggered the overproduction of ROS, which disturbs the metabolic processes. Excessive production of ROS response as toxic to cellular parts such as DNA, lipids, and protein, causing membrane injury and cell death in plants (Altaf et al. 2021d). Vanadium induced excessive ROS production by disrupting the equilibrium between generation detoxification of ROS in plants. V-treated rice seedlings accumulated high ROS (H2O2 and \({O}_2^{-\bullet }\)) and MDA in leaf tissues. These biomolecules are very likely to disturb the ion exchange capacity of the cell membrane as well as all metabolic activities that are linked to cell membrane stability (Zahra et al. 2018). The excessive accumulation of these compounds produced oxidative damage in rice seedlings, which is consistent with results in mustard (Imtiaz et al. 2018b) and rice (Yuan et al. 2020). Besides this, SA supplementation in V-stressed seedlings significantly decreased the H2O2,\({O}_2^{-\bullet }\), MDA, and EL, possibly due to higher enzymatic activities of AsA-GSH cycle and less accumulation of V in root and leaves. Similarly, SA facilitated to reduce the H2O2, \({O}_2^{\bullet -}\), MDA contents, and EL level have been reported in rice under Se stress (Mostofa et al. 2020), and mustard under Ni stress.
Plants produce GSH and AsA, the most abundant soluble antioxidants in cells, to trigger the antioxidant defense system and allow plants to respond to environmental challenges (Nanda and Agrawal, 2016; Foyer and Noctor, 2011). These antioxidant defense system metabolites scavenge ROS, consequently stabilizing the cellular redox state (Noctor et al. 2018; Altaf et al. 2021e). Our findings related to non-enzymatic compounds showed that total AsA contents were drastically reduced, while GSH content precipitously enhanced in the rice leaves of V-stressed seedlings, showing their distinguishing response to V toxicity. It is possible that V-induced reduction in AsA content by increasing the GSH level as a similar pattern was also observed in rice under Cu and Se stress (Mostofa et al. 2020; Mostofa et al. 2014). The ROS produced due to a contemporized antioxidant defense system might confer lipid peroxidation, as manifest by enhanced production of reactive aldehydes like MG and MDA. These increments were more supported by increased enzyme activities of GSH-dependent like GPX and GST, which are recognized to be persuaded for detoxifying several aldehydes that are over accumulated against a stressful environment (Hossain et al. 2012). Additionally, glutathione-S-transferase, which is activated by GSH, contributes to the protection of the cell membrane against the oxidation of lipids and proteins (Hausladen and Alscher et al. 2017).
Plants rapidly respond to negative effects caused by heavy metals and metalloids such as arsenic and Cd by regulating endogenous SA content (Lu et al., 2018; Singh et al. 2017). Likewise, SA application improved the SA cellular level, resulted in an upgraded protection apparatus under oxidative stress produced by arsenite in rice and Cd in Lemna minor (Lu et al. 2018; Singh et al. 2017). The dismutation of \({O}_2^{\bullet -}\) to H2O2 is catalyzed by SOD activity, which is widely regarded as the most important component of defense, and it plays a significant role in the antioxidant system (Jomova et al. 2011). Actually, the addition of SA in nutrient medium successfully initiated the ROS metabolic pathways by increasing the SOD and CAT enzyme activities, which both collectively act for the elimination of H2O2 and \({O}_2^{\bullet -}\) (Czarnocka and Karpiński 2018). The excessive ROS elimination by SOD and CAT was also made easy by AsA-GSH cycle, which also displayed a defensive role by holding the high activities of AsA-GSH cycle in SA-treated seedlings under V stress (Fig. 7A–H).
The antioxidant system plays a key role in diminish ROS and inducing metal tolerance in plants (Ahmad et al. 2018b). Salicylic acid has reportedly increased enzyme activity in several plant species under metal stress (El Dakak and Hassan, 2020; Faraz et al., 2020; Majumdar et al. 2020). Desynchronizing of antioxidant systems by V is also observed in the AsA-GSH cycle, in which the activities of APX, GR, DHAR, and MDHAR were altered. One of the most important enzymes associated with the AsA-GSH cycle is APX, which is found mostly in the plastid stroma and membrane and is responsible for scavenging H2O2 through the AsA-GSH cycle in plants (Ghosh and Biswas 2017). Glutathione reductase activity helps to restore GSH and enhances cellular antioxidant capability, as GSH works as a scavenger of ROS in stressed plants, increasing their tolerance to oxidative damage (Bela et al. 2015). DHA is converted to AsA via DHAR activity (Suekawa et al. 2017). In the present study, the activities of APX, GR, DHAR, and MDHAR were found to be reduced in the roots and leaves of V-stressed rice seedlings, as previous were recorded in rice seedlings (Singh et al. 2015), peas (Rodríguez-Ruiz et al. 2019), and soybeans (Chandrakar et al. 2016) under heavy metal stress. Impeded APX activity can result in an excess of H2O2 buildup in many subcellular compartments, resulting in lipid and protein damage (Singh et al. 2015). On the other hand, SA supplementation upregulated the AsA-GSH cycle enzyme activities under V stress conditions, as previous noted in rice (Singh et al. 2017) and maize (Kaya et al. 2020). In the current study, SA addition to V-treated plants upregulated the enzymatic and non-enzymatic activities, which established that SA recovers oxidative injury induced by V stress in rice by upraising the defense system of antioxidants.
The current study also assessed the metabolism of MG, known as reactive aldehyde, and its overproduction in plant tissue caused toxicity at a cellular level (Mostofa et al. 2018). Glutathione-dependent Gly system (Gly I and Gly II) played a vital role in eliminating the toxic effect of MG induced under metal stress conditions (Mostofa et al. 2018). The results of this study displayed that V excessive deposition in leaves and root tissues caused by overproduction of MG (Fig. 7I) may be due to an ineffective Gly system as proposed by a substantial reduction in Gly I and Gly II activities under V stress (Fig. 7J and K). Conversely, declined in MG levels was observed V-stressed seedlings pretreated with SA, which increased the Gly I and Gly II activities (Fig. 7I–K), presenting that SA is engaged to detoxify the MG by upregulating the Gly defense system under V stress. A similar response of the Gly system was also reported in rice under Se stress (Mostofa et al. 2020).
Most of the genes studied (OsCuZnSOD1, OsCaTB, OsGPX1, OsGSTU37, OsAPX2, and OsGR2) exhibited the opposite pattern in their transcript abundance when compared with representative enzyme activities (Figs. 7A–F and 8A–F). The reason for this could be that gene expression and enzyme activity tests were performed on samples taken at the same time point (7 days after treatment). It is well established that transcript levels peak before protein levels because gene expression begins before protein accumulation (Mostofa et al. 2020). We could not rule out the possibility that separate genes code for distinct isozymes that together control the overall activity of a single enzyme, necessitating the determination of the expression levels of all genes associated with that enzyme (Mostofa et al. 2020). Thus, sampling at multiple time points for expression profiling (e.g., transcriptome analysis) and enzyme activity assays will be critical for future investigations aimed at elucidating the link between gene expression levels and enzyme activities. Nonetheless, rice seedlings pretreated with SA enhanced the transcript levels of OsCuZnSOD1, OsCaTB, OsGPX1, OsGSTU37, OsAPX2, OsGR2, OsGLYI-1, and OsGLYII-2 under V stress as compared to only V-stressed seedlings. Similar results have also been reported in rice under Se stress (Mostofa et al. 2020).
5 Conclusion
The findings in the present study led us to conclude that SA may be an efficient growth regulator for mitigating V-induced detrimental effects in rice by influencing a variety of physiological, biochemical, and molecular processes. Vanadium stress suppressed the root growth, photosynthesis, and leaf gas exchange parameters of rice plants. Vanadium accumulation in rice root and shoot was noted the primary cause of the reduction in plant growth. Salicylic acid pretreatment minimized the V accumulation in root and shoot; enhanced the antioxidant and ascorbate-glutathione (AsA-GSH) pathway enzyme activities; decreased the reactive oxygen species (ROS) and methylglyoxal (MG) contents; upregulated the antioxidant and glyoxalase-related genes; and improved the photosynthetic efficiency of the rice plants under V stress. Besides this, addition of SA along with fertilizer could be an option to overcome the V-induced toxic effect while the crops are being sown in V-polluted soil. Furthermore, field trials with cost-benefit ratio should be investigated to validate SA use as an antidote to V toxicity for minimizing yield losses in V-contaminated soils.
References
Ahmad B, Jaleel H, Sadiq Y, Khan MMA, Shabbir A (2018a) Response of exogenous salicylic acid on cadmium induced photosynthetic damage, antioxidant metabolism and essential oil production in peppermint. Plant Growth Regul 86:273–286. https://doi.org/10.1007/s10725-018-0427-z
Ahmad J, Baig MA, Ali AA, Al-Huqail AA, Ibrahim MM, Qureshi MI (2018b) Differential antioxidative and biochemical responses to aluminium stress in Brassica juncea L. cultivars. Hortic Environ Biotechnol 59:615–627. https://doi.org/10.1007/s13580-018-0068-1
Aihemaiti A, Jiang J, Gao Y, Meng Y, Zou Q, Yang M, Xu Y, Han S, Yan W, Tuerhong T (2019) The effect of vanadium on essential element uptake of Setaria viridis L. seedlings. J Environ Manage 237:399–407. https://doi.org/10.1016/j.jenvman.2019.02.054
Altaf MM, Diao XP, Ur RA, Imtiaz M, Shakoor A, Altaf MA, Younis H, Fu P, Ghani MU (2020a) Effect of vanadium on growth, photosynthesis, reactive oxygen species, antioxidant enzymes, and cell death of rice. J Soil Sci Plant Nutr 20:2643–2656. https://doi.org/10.1007/s42729-020-00330-x
Altaf MM, Diao XP, Shakoor A, Imtiaz M, Ur AR, Altaf MA, Khan LU (2021a) Delineating vanadium (V) ecological distribution, its toxicant potential, and effective remediation strategies from contaminated soils. J Soil Sci Plant Nutr 21:1–19. https://doi.org/10.1007/s42729-021-00638-2
Altaf MA, Shahid R, Ren MX, Naz S, Altaf MM, Qadir A, Anwar M, Shakoor A, Hayat F (2020b) Exogenous melatonin enhances salt stress tolerance in tomato seedlings. Biol Plant 64:604–615. https://doi.org/10.32615/bp.2020.090
Altaf MA, Shahid R, Ren MX, Khan LU, Altaf MM, Jahan MS, Nawaz MA, Naz S, Shahid S, Lal MK (2021b) Protective mechanisms of melatonin against vanadium phytotoxicity in tomato seedlings: insights into nutritional status, photosynthesis, root architecture system, and antioxidant machinery. J Plant Growth Regul 1–17. https://doi.org/10.1007/s00344-021-10513-0
Altaf MA, Shahid R, Ren MX, Altaf MM, Khan LU, Shahid S, Jahan MS (2021c) Melatonin alleviates salt damage in tomato seedling: a root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Sci Hortic 285:110145. https://doi.org/10.1016/j.scienta.2021.110145
Altaf MA, Shahid R, Ren MX, Altaf MM, Khan LU, Altaf MM, Jahan MS (2021d) Melatonin mitigates nickel toxicity by improving nutrient uptake fluxes, root architecture system, photosynthesis and antioxidant potential in tomato seedling. J Soil Sci Plant Nutr 21:1842–1855. https://doi.org/10.1007/s42729-021-00484-2
Altaf MA, Shahid R, Ren MX, Mora-Poblete F, Arnao MB, Naz S (2021e) Phytomelatonin: an overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plantar 172:820–846. https://doi.org/10.1111/ppl.13262
Altaf MA, Shahid R, Altaf MA, Ren MX, Tan K, Xiang WQ, Qadir A, Shakoor A, Altaf MM (2019) Effect of NPK, organic manure and their combination on growth, yield and nutrient uptake of chilli (Capsicum Annum L.). Horticul Int J 3:217–222. https://doi.org/10.15406/hij.2019.03.00135
Altaf MA, Shu H, Hao Y, Zhou Y, Mumtaz MA, Wang Z (2022) Vanadium toxicity induced changes in growth, antioxidant profiling, and vanadium uptake in pepper (Capsicum annum L.) seedlings. Horticulturae 8:28. https://doi.org/10.3390/horticulturae8010028
Anjum SA, Tanveer M, Hussain S, Ashraf U, Khan I, Wang L (2016) Alteration in growth, leaf gas exchange and photosynthetic pigments of maize plants under combined cadmium and arsenic stress. Water Air Soil Pollut. https://doi.org/10.1007/s11270-016-3187-2
Bandurska H (2005) The effect of salicylic acid on barley response to water deficit. Acta Physiol Plant 27:379–386. https://doi.org/10.1007/s11738-005-0015-5
Bela K, Horváth E, Gallé A, Szabados L, Tari I, Csiszár J (2015) Plant glutathione peroxidases: Emerging role of the antioxidant enzymes in plant development and stress responses. J Plant Physiol 176:192–201. https://doi.org/10.1016/j.jplph.2014.12.014
Carpentier R (2001) The negative action of toxic divalent cations on the photosynthetic apparatus. Handbook of plant and crop physiology. Marcel Dekker, New York, pp 763–772
Chandrakar V, Dubey A, Keshavkant S (2016) Modulation of antioxidant enzymes by salicylic acid in arsenic exposed Glycine max L. J Soil Sci Plant Nutr 16:3. https://doi.org/10.4067/S0718-95162016005000048
Chao YY, Chen CY, Huang WD, Kao CH (2010) Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant Soil 329:327–337. https://doi.org/10.1007/s11104-009-0161-4
Czarnocka W, Karpiński S (2018) Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic Biol Med 122:4–20. https://doi.org/10.1016/j.freeradbiomed.2018.01.011
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9. https://doi.org/10.1016/S0168-9452(98)00025-9
Devi R, Behera B, Raza MB, Mangal V, Altaf MA, Kumar R, Kumar A, Tiwari RK, Lal MK, Singh B (2021) An insight into microbes mediated heavy metal detoxification in plants: a review. J Soil Sci Plant Nutr 1-23. https://doi.org/10.1007/s42729-021-00702-x
El Dakak RA, Hassan IA (2020) The alleviative effects of salicylic acid on physiological indices and defense mechanisms of maize (Zea Mays L. Giza 2) stressed with cadmium. Environ Process 7:873–884. https://doi.org/10.1007/s40710-020-00448-1
Faraz A, Faizan M, Sami F, Siddiqui H, Hayat S (2020) Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica juncea L. J Plant Growth Regul 39:641–655. https://doi.org/10.1007/s00344-019-10007-0
Faried HN, Ayyub CM, Amjad M, Ahmed R, Wattoo FM, Butt M, Bashir M, Shaheen MR, Waqas MA (2017) Salicylic acid confers salt tolerance in potato plants by improving water relations, gaseous exchange, antioxidant activities and osmoregulation. J Sci Food Agric 97:1868–1875. https://doi.org/10.1002/jsfa.7989
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18. https://doi.org/10.1104/pp.110.167569
García-Jiménez A, Trejo-Téllez LI, Guillén-Sánchez D, Gómez-Merino FC (2018) Vanadium stimulates pepper plant growth and flowering, increases concentrations of amino acids, sugars and chlorophylls, and modifies nutrient concentrations. PloS one 13. https://doi.org/10.1371/journal.pone.0201908
Ghosh S, Biswas AK (2017) Selenium Modulates Growth and Thiol Metabolism in Wheat (Triticum aestivum L.) during Arsenic Stress. Am J Plant Sci 8:27. https://doi.org/10.4236/ajps.2017.83026
Ghosh SK, Saha R, Saha B (2015) Toxicity of inorganic vanadium compounds. Res Chem Intermed 41:4873–4897. https://doi.org/10.1007/s11164-014-1573-1
Gokul A, Cyster L, Keyster M (2018) Efficient superoxide scavenging and metal immobilization in roots determines the level of tolerance to Vanadium stress in two contrasting Brassica napus genotypes. S Afr J Bot 119:17–27. https://doi.org/10.1016/j.sajb.2018.08.001
Gondor OK, Pal M, Darko E, Janda T, Szalai G (2016a) Salicylic acid and sodium salicylate alleviate cadmium toxicity to different extents in maize (Zea mays L.). PloS one 11. https://doi.org/10.1371/journal.pone.0160157
Gondor OK, Pál M, Darkó É, Janda T, Szalai G (2016b) Salicylic acid and sodium salicylate alleviate cadmium toxicity to different extents in maize (Zea mays L.). PLoS One 11:e0160157. https://doi.org/10.1371/journal.pone.0160157
Gong B, Nie W, Yan Y, Gao Z, Shi Q (2017) Unravelling cadmium toxicity and nitric oxide induced tolerance in Cucumis sativus: insight into regulatory mechanisms using proteomics. J Hazard Mater 336:202–213. https://doi.org/10.1016/j.jhazmat.2017.04.058
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68:14–25. https://doi.org/10.1016/j.envexpbot.2009.08.005
He J, Ren Y, Pan X, Yan Y, Zhu C, Jiang D (2010) Salicylic acid alleviates the toxicity effect of cadmium on germination, seedling growth, and amylase activity of rice. J Plant Nutr Soil Sci 173:300–305. https://doi.org/10.1002/jpln.200800302
Horváth E, Szalai G, Janda T (2007) Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul 26:290–300. https://doi.org/10.1007/s00344-007-9017-4
Hossain MA, Hossain MZ, Fujita M (2009) Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust J Crop Sci 3:53–64
Hossain MA, Piyatida P, da Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. Pak J Bot 872875:37. https://doi.org/10.1155/2012/872875
Hou M, Hu C, Xiong L, Lu C (2013) Tissue accumulation and subcellular distribution of vanadium in Brassica juncea and Brassica chinensis. Microchem J 110:575–578. https://doi.org/10.1016/j.microc.2013.07.005
Hausladen A, Alscher RG (2017) Glutathione. In: Alscher RG, Hess JL (eds) Antioxidants in higher plants. CRC Press, Boca Raton, pp 1–30
Imtiaz M, Ashraf M, Rizwan MS, Nawaz MA, Rizwan M, MehmoodS YB, Yuan Y, Ditta A, Mumtaz MA (2018a) Vanadium toxicity in chickpea (Cicer arietinum L.) grown in red soil: effects on cell death, ROS and antioxidative systems. Ecotoxicol Environ Saf 158:139–144. https://doi.org/10.1016/j.ecoenv.2018.04.022
Imtiaz M, Mushtaq MA, Nawaz MA, Ashraf M, Rizwan MS, Mehmood S, Aziz O, Rizwan M, Virk MS, Shakeel Q (2018b) Physiological and anthocyanin biosynthesis genes response induced by vanadium stress in mustard genotypes with distinct photosynthetic activity. Environ Toxicol 62:20–29. https://doi.org/10.1016/j.etap.2018.06.003
Imtiaz M, Mushtaq MA, Rizwan MS, Arif MS, Yousaf B, Ashraf M, Shuanglian X, Rizwan M, Mehmood S, Tu S (2016) Comparison of antioxidant enzyme activities and DNA damage in chickpea (Cicer arietinum L.) genotypes exposed to vanadium. Environ Sci 23:19787–19796. https://doi.org/10.1007/s11356-016-7192-1
Imtiaz M, Rizwan MS, Mushtaq MA, Yousaf B, Ashraf M, Ali M, Yousuf A, Rizwan M, Din M, Dai Z (2017) Interactive effects of vanadium and phosphorus on their uptake, growth and heat shock proteins in chickpea genotypes under hydroponic conditions. Environ Exp Bot 134:72–81. https://doi.org/10.1016/j.envexpbot.2016.11.003
Jahan MS, Wang Y, Shu S, Zhong M, Chen Z, Wu J, Sun J, Guo S (2019) Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci Hortic 247:421–429. https://doi.org/10.1016/j.scienta.2018.12.047
Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li D (2017) Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep 7:42039. https://doi.org/10.1038/srep42039
Jing C, Cheng Z, Li LP, Sun ZY, Pan XB (2007) Effects of exogenous salicylic acid on growth and H2O2-metabolizing enzymes in rice seedlings under lead stress. Res J Environ Sci 19:44–49. https://doi.org/10.1016/S1001-0742(07)60007-2
Jini D, Joseph B (2017) Physiological mechanism of salicylic acid for alleviation of salt stress in rice. Rice Sci 24:97–108. https://doi.org/10.1016/j.rsci.2016.07.007
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107. https://doi.org/10.1002/jat.1649
Kaya C, Şenbayram M, Akram NA, Ashraf M, Alyemeni MN, Ahmad P (2020) Sulfur-enriched leonardite and humic acid soil amendments enhance tolerance to drought and phosphorus deficiency stress in maize (Zea mays L.). Sci Rep10:6432. https://doi.org/10.1038/s41598-020-62669-6
Khan MIR, Asgher M, Khan NA (2014) Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol Biochem 80:67–74. https://doi.org/10.1016/j.plaphy.2014.03.026
Kohli SK, Handa N, Sharma A, Gautam V, Arora S, Bhardwaj R, Wijaya L, Alyemeni MN, Ahmad P (2018) Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, antioxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environ Sci Pollut Res 25:15159–15173. https://doi.org/10.1007/s11356-018-1742-7
Krantev A, Yordanova R, Janda T, Szalai G, Popova L (2008) Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165:920–931. https://doi.org/10.1016/j.jplph.2006.11.014
Li Q, Wang G, Wang Y, Yang D, Guan C, Ji J (2019) Foliar application of salicylic acid alleviate the cadmium toxicity by modulation the reactive oxygen species in potato. Ecotoxicol Environ Saf 172:317–325. https://doi.org/10.1016/j.ecoenv.2019.01.078
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592. https://doi.org/10.1042/bst0110591
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lu Q, Zhang T, Zhang W, Su C, Yang Y, Hu D, Xu Q (2018) Alleviation of cadmium toxicity in Lemna minor by exogenous salicylic acid. Ecotoxicol Environ Saf 147:500–508. https://doi.org/10.1016/j.ecoenv.2017.09.015
Majumdar S, Sachdev S, Kundu R (2020) Salicylic acid mediated reduction in grain cadmium accumulation and amelioration of toxicity in Oryza sativa L. cv Bandana. Ecotoxicol Environ Saf 205:111167. https://doi.org/10.1016/j.ecoenv.2020.111167
Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281. https://doi.org/10.1104/pp.102.018457
Mostofa MG, Ghosh A, Li ZG, Siddiqui MN, Fujita M, Tran LSP (2018) Methylglyoxal–a signaling molecule in plant abiotic stress responses. Free Radic Biol Med 122:96–109. https://doi.org/10.1016/j.freeradbiomed.2018.03.009
Mostofa MG, Rahman M, Ansary M, Uddin M, Fujita M, Tran LSP (2019) Interactive effects of salicylic acid and nitric oxide in enhancing rice tolerance to cadmium stress. Int J Mol Sci 20:5798. https://doi.org/10.3390/ijms20225798
Mostofa MG, Rahman MM, Siddiqui MN, Fujita M, Tran LSP (2020) Salicylic acid antagonizes selenium phytotoxicity in rice: selenium homeostasis, oxidative stress metabolism and methylglyoxal detoxification. J Hazard Mater 122572. https://doi.org/10.1016/j.jhazmat.2020.122572
Mostofa MG, Seraj ZI, Fujita M (2014) Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 251:1373–1386. https://doi.org/10.1007/s00709-014-0639-7
Moussa H, El-Gamal SM (2010) Effect of salicylic acid pretreatment on cadmium toxicity in wheat. Biol Plant 54:315–320. https://doi.org/10.1007/s10535-010-0054-7
Mutlu S, Karadağoğlu Ö, Atici Ö, Nalbantoğlu B (2013) Protective role of salicylic acid applied before cold stress on antioxidative system and protein patterns in barley apoplast. Biologia Plantarum 57:507–513. https://doi.org/10.1007/s10535-013-0322-4
Naeem A, Westerhoff P, Mustafa S (2007) Vanadium removal by metal (hydr) oxide adsorbents. Water Res 41:1596–1602. https://doi.org/10.1016/j.watres.2007.01.002
Nanda R, Agrawal V (2016) Elucidation of zinc and copper induced oxidative stress, DNA damage and activation of defence system during seed germination in Cassia angustifolia Vahl. Environ Exp Bot 125:31–41. https://doi.org/10.1016/j.envexpbot.2016.02.001
Nazar R, Iqbal N, Syeed S, Khan NA (2011) Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J Plant Physiol 168:807–815. https://doi.org/10.1016/j.jplph.2010.11.001
Nawaz MA, Jiao Y, Chen C, Shireen F, Zheng Z, Imtiaz M, Bie Z, Huang Y (2018) Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J Plant Physiol 220:115–127. https://doi.org/10.1016/j.jplph.2017.11.003
Noctor G, Reichheld JP, Foyer CH (2018) ROS-related redox regulation and signaling in plants. Semin Cell Dev Biol 80:3–12. https://doi.org/10.1016/j.semcdb.2017.07.013
Postma JA, Lynch JP (2012) Complementarity in root architecture for nutrient uptake in ancient maize/bean and maize/bean/squash polycultures. Ann Bot 110:521–534. https://doi.org/10.1093/aob/mcs082
Principato GB, Rosi G, Talesa V, Giovanni E, Uotila L (1987) Purification and characterization of two forms of glyoxalase II from the liver and brain of Wistar rats. Bba Prot St 911:349–355. https://doi.org/10.1016/0167-4838(87)90076-8
Reyes L, Chin C (2009) The new frontier. Rice Today 8:28–29
Rodriguez-Ruiz M, Aparicio-ChaconJose MV, Palma JM, Corpas FJ (2019) Arsenate disrupts ion balance, sulfur and nitric oxide metabolisms in roots and leaves of pea (Pisum sativum L.) plants. Botany 161:143–156. https://doi.org/10.1016/j.envexpbot.2018.06.028
Saidi I, Ayouni M, Dhieb A, Chtourou Y, Chaïbi W, Djebali W (2013) Oxidative damages induced by short-term exposure to cadmium in bean plants: protective role of salicylic acid. S Afr J Bot 85:32–38. https://doi.org/10.1016/j.sajb.2012.12.002
Sela M, Garty J, Tel-Or E (1989) The accumulation and the effect of heavy metals on the water fern Azolla filiculoides. New Phytol 112(1):7–12. https://doi.org/10.1111/j.1469-8137.1989.tb00302.x
Seregin I, Kozhevnikova A (2006) Physiological role of nickel and its toxic effects on higher plants. Russ J Plant Physiol 53:257–277. https://doi.org/10.1134/S1021443706020178
Shakirova F, Allagulova CR, Maslennikova D, Klyuchnikova E, Avalbaev A, Bezrukova M (2016) Salicylic acid-induced protection against cadmium toxicity in wheat plants. Environ Exp Bot 122:19–28. https://doi.org/10.1016/j.envexpbot.2015.08.002
Sharma A, Sidhu GPS, Araniti F, Bali AS, Shahzad B, Tripathi DK, Brestic M, Skalicky M, Landi M (2020) The role of salicylic acid in plants exposed to heavy metals. Molecules 25:540. https://doi.org/10.3390/molecules25030540
Sheoran I, Singal H, Singh R (1990) Effect of cadmium and nickel on photosynthesis and the enzymes of the photosynthetic carbon reduction cycle in pigeonpea (Cajanus cajan L.). Photosynth Res 23:345–351. https://doi.org/10.1007/BF00034865
Simkin AJ, López-Calcagno PE, Raines CA (2019) Feeding the world: improving photosynthetic efficiency for sustainable crop production. J Exp Bot 70:1119–1140. https://doi.org/10.1093/jxb/ery445
Singh AP, Dixit G, Kumar A, Mishra S, Kumar N, Dixit S, Singh PK, Dwivedi S, Trivedi PK, Pandey V (2017) A protective role for nitric oxide and salicylic acid for arsenite phytotoxicity in rice (Oryza sativa L.). Plant Physiol Biochem 115:163–173. https://doi.org/10.1016/j.plaphy.2017.02.019
Singh AP, Dixit G, Mishra S, Dwivedi S, Tiwari M, Mallick S, Pandey V, Trivedi PK, Chakrabarty D, Tripathi RD (2015) Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front. Plant Sci 6:340. https://doi.org/10.3389/fpls.2015.00340
Soltani Maivan E, Radjabian T, Abrishamchi P, Talei D (2017) Physiological and biochemical responses of Melissa officinalis L. to nickel stress and the protective role of salicylic acid. Arch Acker Pflanzenbau Bodenkd 63:330–343. https://doi.org/10.1080/03650340.2016.1207241
Suekawa M, Kondo T, Fujikawa Y, Esaka M (2017) Regulation of ascorbic acid biosynthesis in plants. Ascorbic Acid in Plant Growth, Development and Stress Tolerance 157–176. https://doi.org/10.1007/978-3-319-74057-7_6
Tan BL, Norhaizan ME (2017) Scientific evidence of rice by-products for cancer prevention: chemopreventive properties of waste products from rice milling on carcinogenesis in vitro and in vivo. Biomed Res Int 2017:9017902. https://doi.org/10.1155/2017/9017902
Taner M (2002) Vanadium-geology, processing and applications: Proceedings of international symposium on vanadium (p. 265). Canadian Institute of Mining, Metallurgy and Petroleum.
Tiwari RK, Lal MK, Kumar R, Mangal V, Altaf MA, Sharma S, Singh B, Kumar M (2021) Insight into melatonin-mediated response and signaling in the regulation of plant defense under biotic stress. Plant Mol. Biol 1-15. https://doi.org/10.1007/s11103-021-01202-3
Vachirapatama N, Jirakiattiku Y, Dicinoski G, Townsend AT, Haddad PR (2011) Effect of vanadium on plant growth and its accumulation in plant tissues. SJST 33:255–261 http://rdo.psu.ac.th/sjstweb/journal/cover-33-3
Wazalwar SS, Bhave NS, Dikundwar AG, Ali P (2011) Microwave assisted synthesis and antimicrobial study of Schiff base vanadium (IV) complexes of phenyl esters of amino acids. Synthesis Reactiv Inorganic, Metal-Organic, Nano-Metal Chem 41:459–464. https://doi.org/10.1080/15533174.2011.568427
Wild R, Ooi L, Srikanth V, Münch G (2012) A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: the N-acetyl-L-cysteine assay. Anal Bioanal Chem 403:2577–2581. https://doi.org/10.1007/s00216-012-6086-4
Wissuwa M, Kretzschmar T, Rose TJ (2016) From promise to application: root traits for enhanced nutrient capture in rice breeding. J Exp Bot 67:3605–3615. https://doi.org/10.1093/jxb/erw061
Yang J, Teng Y, Wu J, Chen H, Wang G, Song L, Yue W, Zuo R, Zhai Y (2017) Current status and associated human health risk of vanadium in soil in China. Chemosphere 171:635–643. https://doi.org/10.1016/j.chemosphere.2016.12.058
Yuan Y, Imtiaz M, Rizwan M, Dong X, Tu S (2020) Effect of vanadium on germination, growth and activities of amylase and antioxidant enzymes in genotypes of rice. Int J Environ Sci Technol 17:383–394. https://doi.org/10.1007/s13762-019-02451-y
Zaid A, Mohammad F, Wani SH, Siddique KM (2019) Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. Ecotoxicol Environ Saf 180:575–587. https://doi.org/10.1016/j.ecoenv.2019.05.042
Zahra S, Mahmood S, Noreen S, Akrem A (2018) Independent and combined nickel and cadium induced lipid peroxidation of biological membranes and its mitigation through antioxidant enzymes in Grewia asiatica L Pak J Life. Soc Sci 16:48–54
Zengin F (2014) Exogenous treatment with salicylic acid alleviating copper toxicity in bean seedlings. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences 84:749–755. https://doi.org/10.1007/s40011-013-0285-4
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333. https://doi.org/10.1016/j.biotechadv.2005.01.003
Zhou ZS, Guo K, Elbaz AA, Yang ZM (2009) Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of (Medicago sativa L.) plants. Environ Exp Bot 65:27–34. https://doi.org/10.1016/j.envexpbot.2008.06.001
Funding
The current work was supported by the Key Research and Development Projects of Hainan Province, China (grant number ZDYF2018122).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 751 kb)
Rights and permissions
About this article
Cite this article
Altaf, M.M., Diao, Xp., Wang, H. et al. Salicylic Acid Induces Vanadium Stress Tolerance in Rice by Regulating the AsA-GSH Cycle and Glyoxalase System. J Soil Sci Plant Nutr 22, 1983–1999 (2022). https://doi.org/10.1007/s42729-022-00788-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00788-x