Abstract
The present study was done to elucidate the effects of vanadium (V) on photosynthetic pigments, membrane damage, antioxidant enzymes, protein, and deoxyribonucleic acid (DNA) integrity in the following chickpea genotypes: C-44 (tolerant) and Balkasar (sensitive). Changes in these parameters were strikingly dependent on levels of V, at 60 and 120 mg V L−1 induced DNA damage in Balkasar only, while photosynthetic pigments and protein were decreased from 15 to 120 mg V L−1 and membrane was also damaged. It was shown that photosynthetic pigments and protein production declined from 15 to 120 mg V L−1 and the membrane was also damaged, while DNA damage was not observed at any level of V stress in C-44. Moreover, the antioxidant enzyme activities such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) were increased in both genotypes of chickpea against V stress; however, more activities were observed in C-44 than Balkasar. The results suggest that DNA damage in sensitive genotypes can be triggered due to exposure of higher vanadium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil is an integral component of terrestrial ecosystems, harboring several vital ecological functions such a primary productivity and decomposition. In recent years, heavy metal contamination in agricultural soil has attracted global attention owing to its adverse effects on environmental health, quality, and security aspect of food product (Harmanescu et al. 2011). Vanadium (V) is the fifth most abundant transition element, widely distributed Earth’s crust (approximately 10–220 mg kg−1), and abundantly found in 65 different types of minerals. Additionally, V is extensively mined in China, USA, Russia, and South Africa to meet the growing demand of its use as an important constituent of various alloys. Industrial use of V and associated fossil fuel burning have considerably increased both environmental and human health concerns of anthropogenic V emissions in past few decades (Małuszynski 2007; Wazalwar et al. 2011; Reijonen et al. 2016). Moreover, V loading in environment could be originated from V-containing fertilizer, mine-tailing leachates, and municipal and industrial sludge (ATSDR 2012).
Ecotoxicological effects of V involved various biochemical alterations related to enzymatic activity, interaction with protein, and protein-DNA unit of living organisms and plants (Anke 2004; Goc 2006). As previous few studies only elaborated the toxic effect of V on plant physiology through leaf chlorosis, chloroplast damage, shoot and root mortalities (Imtiaz et al. 2015; Xiao et al. 2012), the molecular mechanism of V toxicity to plants is still an avenue to be explored.
Programmed cell death (PCD) is a critical physiological process of plant development involving precise degradation of cellular components and/or selective cell death, being employed as plant defense strategy against metal or other environmental stress factors (Gadjev et al. 2008; Lohmann and Beyersmann 1993). PCD in plants and animals is associated with cell shrinkage, vacuolization, chromatin condensation, and DNA fragmentation (Papini et al. 2011; Wang et al. 2012; Poor et al. 2013). To the best of our knowledge, no study has investigated the vanadium-induced PCD in plants. However, several studies have reported the activation of plant defense through PCD against cadmium toxicity (Fojtova and Kovarik 2000), salt stress (Banu et al. 2009), changes in growth plant medium (Koukalova et al. 1997), and under pathogen attack (Greenberg et al. 1994).
Occurrence of PCD in both plant and animal cells is quite similar (Havel and Durzan 1996). PCD usually involved the degradation of deoxyribonucleic acid (DNA) fragments from 50 to 200 kb by an apoptotic process. In most of the cases, subsequent fragmentation will lead towards further cleavage of DNA into nucleosomal linker regions through characteristic display of DNA ladder on agarose gels. Moreover, some reports also disclose various enzymatic regulation involving several molecular mechanisms of PCD (Wyllie 1980; Walker and Sikorska 1994).
In the present study, two genotypes of chickpea, C-44 (tolerant to V stress) and Balkasar (sensitive to V stressImtiaz et al. 2015), were compared for their responses to V toxicity, in order to elucidate the difference in DNA integrity between tolerant and sensitive chickpea plants.
To our knowledge, this is the first study that investigated the V-induced DNA integrity in chickpea genotypes. This study had the following objectives: (1) to determine the effect of V on DNA of selected chickpea genotypes and (2) to investigate the effect of V on various biochemical responses, i.e., enzyme activities, electrolyte leakage, protein, and photosynthetic pigments in selected chickpea genotypes.
Materials and methods
Materials
C-44 (tolerant to V stress) and Balkasar (sensitive to V stress) seeds were purchased from Ayyub Agricultural Research Institute (AARI), Faisalabad, Pakistan. The seeds were surface sterilized with 0.1 % (v/v) sodium hypochlorite (NaClO) for 10 min and then rinsed five times with distilled water.
The growth room was climate controlled with a temperature range of 22–25 °C and relative humidity of 70 %. A 14-h photoperiod with an average photon flux density of 820 mmol m−2 s−1 was supplied by an assembly of cool-white fluorescent lamps.
Plant culture
The seeds were sown in dark on cheese cloth dipped in deionized water. After 10 days, the uniform-sized seedlings were transferred into 4-L plastic boxes (four plants per box) containing one-quarter-strength Hoagland nutrient solution (2.5 L). The pH of Hoagland nutrient solution was adjusted at 6.5 by adding NaOH/HCl solution.
After 5 days of transplanting, the nutrient solution was upgraded to 50 % along with the following five different vanadium treatments that were performed: control (0 vanadium), 15, 30, 60, and 120 mg V L−1 by using ammonium metavanadate (NH4VO3). The nutrient solution was renewed after every 2 days and aerated continuously. In total, the seedlings were remained for 12 days in nutrient solution/or plus vanadium. Then, plants were harvested and washed thoroughly with distilled water, and harvested shoots were put in the liquid nitrogen and stored at −80 °C for future analyses.
DNA extraction and gel electrophoresis
The frozen leaves of chickpea genotypes, C-44 and Balkasar, exposed to different vanadium treatments were grounded in liquid nitrogen to fine powder. DNA was isolated by means of cetyl trimethylammonium bromide (CTAB) method (Thompson 1980). Extracted DNAs from all the samples were treated with 25 μg mL−1 DNase-free RNase A (Sigma, Missouri, USA) for 1 h at 37 °C before gel electrophoresis. Finally, the breakdown of DNA was observed by standard agarose gel electrophoresis and stained with ethidium bromide and visualized under ultraviolet (UV) light.
Preparation of enzyme extracts
To examine the enzyme activities in aerial parts of the treated and control plants, washed fresh aboveground part (0.5 g) was ground in liquid nitrogen and homogenized in 5 mL of 0.2 mol/L sodium phosphate buffer (pH 7.8). The homogenate was centrifuged at 12,000 rpm for 15 min at 4 °C, and the supernatant was collected in another falcon tube for the determination of enzyme assays.
Enzyme assay
Superoxide dismutase (SOD) activity was checked by measuring the ability to reduce the photochemical reduction of nitroblue tetrazolium as illustrated by Beauchamp and Fridovich (1971). Catalase (CAT) activity was determined using the method demonstrated by Aebi and Bergmeyer (1983). Peroxidase (POD) activity was assayed using the method by Putter (1974).
Determination of chlorophyll and protein
Protein contents were determined with the method used by Bradford (1976). The final concentration of soluble protein was calculated by using bovine serum albumin (BSA) standard curve. The photosynthetic pigments, i.e., chlorophyll (a + b) and total carotene (C x + c ), were measured according to the method explained by Lichtenthaler and Wellburn (Lichtenthaler and Wellburn 1985).
The amount of photosynthetic pigments was calculated by using the following formula:
where C a = chlorophyll a, C b = chlorophyll b, C x + c = total carotene.
Determination of electrolyte leakage
Electrolyte leakage was estimated by using the method of Dionisio-Sese and Tobit (Dionisio-Sese and Tobit 1998).
The electrolyte leakage (EL) was expressed by using the following formula:
where EC = electric conductivity, EC1 = electric conductivity before boiling, EC2 = electric conductivity after boiling.
Vanadium determination
The vanadium concentration in shoots and roots of chickpea plants of both chickpea genotypes was determined according to Hou et al. (2013). The vanadium concentration was determined by using a graphite furnace atomic absorption spectrophotometer (GFAAS-GTA 120).
The experimental design was laid out and analyzed based on a complete randomized design (CRD) with four replications, and data means ± SD are presented. All the collected data were subjected to analyze using SAS software. Firstly, analyzed analysis of variance (ANOVA) to find out the significant (P ≤ 0.05) vanadium treatment and cultivar effect mean comparison was evaluated by Duncan’s multiple-range test at 1 %.
Results
Effects of vanadium on antioxidant enzymes
The obtained findings indicate the positive correlation between antioxidant enzyme activities and V treatments in both genotypes of chickpea. The activities of SOD, CAT, and POD in both genotypes were significantly (P ≤ 0.05) more in vanadium treated as compared to control; however, C-44 showed more activities than Balkasar in response to V stress (Figs. 1 and 2). The results of analysis of variance indicated that the activity of SOD was increased about 200 and 303 %, CAT exhibited about 331 and 846 %, and POD was also increased about 340 and 757 % for Balkasar and C-44, respectively, from 0 to 120 mg L−1.
Vanadium induces DNA degradation
Degradation of genomic DNA during PCD consisted of the two succeeding steps: an early degradation into high-molecular-weight fragments and, after this, an extreme disintegration; commonly, oligonucleosomal fragments are formed after this kind of cleavage (Brotner et al. 1995), which can be examined by gel electrophoresis. Therefore, DNA cleavage patterns were determined via gel electrophoresis against V stress in C-44 (tolerant) and Balkasar (sensitive) genotypes of chickpea. Exposure of chickpea seedlings to V leads to concentration-dependent alterations in DNA integrity (Fig. 3). There was no DNA degradation observed at any level of V-stress in C-44 and at 15 and 30 mg V L−1 in Balakasar; however, it caused DNA breakdown in Balakasar genotypes at concentrations of 60 and 120 mg V L−1 and became more significant at 120 mg V L−1. Our findings show that V stress caused progressive DNA fragmentation in a dose- and genotype-dependent manner.
DNA breakdown in chickpea genotypes, a C-44 (tolerant to V stress) and b Balkasar (sensitive to V stress) grown in hydroponic conditions under vanadium stress. DNA was isolated from control and V-treated seedlings after 27 days of germination and analyzed by agarose gel electrophoresis. Lane 1 control DNA (0 V) and lanes 2 to 5 DNA from V-treated seedlings, lane 2 15 mg L−1 NH3VO4, lane 3 30 mg L−1 NH3VO4, lane 4 60 mg L−1 NH3VO4, lane 5 120 mg L−1 NH3VO4. The arrow indicates the breakdown of DNA
Change of chlorophyll in response to vanadium
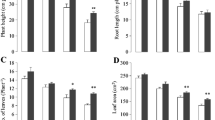
The total photosynthetic pigments, i.e., chlorophyll a, chlorophyll b, and total carotene, significantly decreased (P ≤ 0.05) as the V concentrations increased from 15 to 120 mg V L−1 as compared with control in both genotypes. The plants treated with 0 V showed 17.49, 8.25, and 4.09 chlorophyll a, chlorophyll b, and carotene contents, respectively. The highest decline in chlorophyll a and chlorophyll b were observed at 120 mg V L−1 (7.25 and 3.97) in C-44 and Balkasar. In addition to this, total carotene contents were significantly declined (P ≤ 0.05) from 2. 99 to 1.34 in Balkasar, while C-44 also indicated decrease in total carotene (3.11 to 1.79) but less than Balkasar when exposed to V (15–120 mg V L−1; Fig. 4).
Effects of vanadium on protein contents
Figure 5 presents the results about V-induced changes in protein contents in chickpea genotypes. In general, the protein contents decreased about 11.40–55.50 % in C-44 and 13.81–76.07 % in Balkasar for the application of 15–120 mg V L−1, respectively, whereas C-44 indicated non-significant results for 15 to 60 mg V L−1 treatments. Overall, the protein contents showed decreasing trend (P ≤ 0.05) as the V concentrations increased from 15 to 120 mg V L−1 as compared with 0 V.
Effects of vanadium on electrolyte leakage
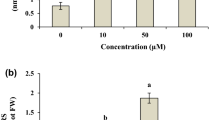
The relative conductivity of leaf leachate was significantly (P ≤ 0.05) affected by V stress. The degree of cell membrane integrity was estimated indirectly by solute leakage from leaf tissues of vanadium-treated chickpea genotypes. Clearly, the results showed that the values of electrical conductivity increased with higher dosed of V (Fig. 6). The results illustrated that addition V caused the disruption of membrane integrity in both genotypes; C-44 showed about 5-fold, and Balkasar exhibited about 6.7-fold higher by the application of 120 mg V L−1 as compared to control. However, there was a non-significant result for control and 15 mg V L−1 in C-44 genotype. In general, the ion leakage showed increasing trend (P ≤ 0.05) as the V concentrations increased from 15 to 120 mg V L−1 as compared to control.
Accumulation of vanadium in chickpea tissues
The accumulation of V in roots and shoots of both chickpea genotypes is shown in Fig. 7. The accumulation of V in aerial parts and roots was proportional with increasing levels of V in Hoagland nutrient solution. In all treatments, roots accumulated significantly (P ≤ 0.05) higher V as compared to shoots in both genotypes of chickpea. Especially, roots and shoots of C-44 plants accumulated higher concentration than Balkasar. At higher stress of V (120 mg L−1), there was about 30-fold in shoots and 64-fold higher in roots of C-44 genotype, whereas 24.4-fold increase in shoots and 47.4-fold increase in roots of Balkasar genotype were examined when compared with their respective controls (Fig. 7). Overall, under V stress, shoots accumulated about 4–30-fold and roots accumulated about 7–64-fold higher V as compared to control in C-44 plants, while in Balkasar, shoots accumulated about 2.7–24.4-fold higher and roots accumulated about 6.2–47.4-fold higher V than untreated plants when treated with 15–120 mg V L−1, respectively. Moreover, results also indicated that root growth was badly affected by V stress and root damage was evident (Fig. 1).
Discussion
Agricultural soils are being polluted by various soil pollutants arising from wide range of sources throughout the world. Most of the recent studies about agricultural soil pollution has revealed that major share of these pollutant is coming from heavy metals (e.g., V, Cd, Pb, and Ni). Steel industry boom of early 2000 resulted in increased use of V for making non-ferrous alloys. Rapid industrial growth of V consumption units has now raised V as a major environmental pollutant, affecting plant, animals, and human beings. Hence, it is paramount to introspect process and mechanism behind V toxicity.
Plants have evolved several biochemical mechanisms to relieve plant from metal-induced oxidative damage. Enzymatic antioxidants, including SOD, CAT, and POD, showed a strong antioxidant response V-treated plant compared to control.
These antioxidant enzymes are efficient scavenging agent for ROS, and other generated free radicals thus maintain viability and permeability of plant cell membrane under exposed stress. Elevated expressions of SOD, CAT, and POD are usually linked to repair mechanism due to the oxidative damage of plants under stress (Malecka et al. 2001).
SOD activity was significantly higher under V stress. This increment in SOD activity might be attributed to de novo induction of enzyme protein (Lozano et al. 1996), suggesting to keep intact the plant defense apparatus against exposed stress conditions. Additionally, higher SOD activity also helps to maintain plant defense system under oxidative stress. Moreover, higher SOD activity was noticed in C-44 compared to Balkasar. However, lower concentrations (15 and 30 mg L−1) showed comparatively non-significant results with each other as compared to higher concentrations (60 and 120 mg L−1). This is possibly due to the induction of more superoxide free radical and oxidative damage by NH4VO3 in response to higher concentrations.
CAT is present oxidoreductase with highest turnover rate to convert (6 million molecule min−1) H2O2 into water and molecular oxygen backed up by an efficient ROS exclusion in stressed plant (Reddy et al. 2005). The higher activity of CAT in plants contributed for ROS decrease under stressful conditions. The CAT activity in all V-treated plants was significantly higher compared with non-V-treated plant, with more pronounced CAT activity in C-44 than Balkasar genotype. An increase activity of POD highlights the intrinsic involvement of POD to guard plant from V-induced oxidative damage mediated by the removal of toxic peroxides. In addition, these toxic peroxides can also be found in many plant cell components and used as a marker for sub-lethal metal stress in plant (Shah et al. 2001; Pergent-Martini and Pergent 2000).
The POD activities in both chickpea genotypes were significantly increased against V treatments. Highest POD activity (120 mg L−1) was recorded in C-44 compared to Balkasar. POD activity build up a physical barrier in response to a biotic stress through lignin biosynthesis. Our results are in agreement with the previous findings of Shah et al. (2001); Reddy et al. (2005); Patade et al. (2011); Shulan et al. (2010), and Zhang et al. (2009). In addition, these results also suggest that C-44 has more effective scavenging mechanism to repair V-induced oxidative damages in plant as compared to Balkasar.
PCD is essential for the development of multicellular organisms and key phenomenon to negate depressing effect of stressor on plant. In plants, PCD involves active elimination of unwanted and harmful cells in plant species (Gechev et al. 2006; Lam 2004).
The incidence of DNA laddering due to heavy metal toxicity has been well documented in human and animal cells (EI Azzouzi et al. 1994; Habeebu et al. 1998) than plant cells. The kinetics of PCD can be employed to differentiate both plant and animal cells. However, stress differentiation between animal and plant cells in response to metal stress can also be segregated based on following characteristic observation, e.g., occurrence of PCD in tobacco plant 10 times higher than animals cells; cell necrosis in cell culture of tobacco was 20-fold times more than in lymphocyte culture, and DNA laddering appeared upon 90 % cell death (tobacco plant), while oligonucleosomal fragmentation appeared prior to decreasing cell viability (Fojtova and Kovarik 2000).
Our findings revealed that DNA breakdown was not detected in C-44 genotype despite being exposed at higher concentration, followed by Balkasar at lower concentrations. It could be linked to mechanistic efficiency of the cells to counter both higher and lower concentrations of V. Moreover, Ojima and Ohira (1983) showed carrot cells resistant against metal stress with enhanced systematic efficiency of the plant cells to cope metal toxicity. More importantly, these mechanistic findings may not well be suited to Balkasar genotype at higher concentration, owing its sensitivity against vanadium stress. In some previous studies, cadmium induced cell termination in onion root and tobacco and subsequent PCD has been reported (Fojtova and Kovarik 2000; Behboodi and Samadi 2004). Moreover, De Michele et al. (2009) found a concentration-dependent effect of Cd on Arabidopsis thaliana, tolerant at lower concentration of cadmium (50 μM CdCl2) and initiation of PCD at higher levels (100 and 150 μM CdCl2), respectively.
The production of reactive oxygen species (ROS) is considered as one of the most important features of plant PCD against a biotic stress (Lin et al. 2006; Gao et al. 2008). Heavy metals block the functional capabilities of antioxidant-based plant defense system, ultimately increasing the oxidative stress for plant. In our study, DNA laddering was more visualized in Balkasar than C-44, attributed to a substantially higher and lower ROS production for the respective chickpea genotype. Moreover, ROS production highlights tolerance (C-44) and sensitivity (Balkasar) pattern of selected chickpea genotypes for these toxic species. Similar research finding has also been reported earlier by Yakimova et al. (2006).
The lower concentrations of vanadium did not have any apparent effect on DNA in Balakasar genotype, as DNA breakdown at higher concentrations disclosed that both lower and moderate level V may possibly involve in genetic alterations (mutation) that accumulate beyond threshold initiating episode of PCD. These findings confirm the earlier obtained results from Fojtova and Kovarik (2000). Moreover, metal-induced degradation in genomic DNA has been well established in some model plant like in Pisum sativum and A. thaliana (Adamakis et al. Adamakis et al. 2011; Balk et al. 2003).
Previous studies have proved that chlorophyll contents may reflect plant’s sensitivity to abiotic stress. In general, the chlorophyll (a + b) and total carotene contents were decreased with the increasing V levels, compared with their respective control. The chlorophyll a contents were significantly reduced in both genotypes against V stress. Chlorophyll “b” revealed no significant alteration in response to 30–120-mg V L−1 treatments. However, C-44 chlorophyll (a + b) of C-44 genotype also showed non-significant results for 30 and 60 mg V L−1, respectively. In addition, the results obtained in this study also indicate the sensitivity of total carotene of selected chickpea genotype against V stress. Carotene content of C-44 genotype had non-significant response at 15–60 mg V L−1. In case of Balkasar, total carotene contents were significantly decreased against V treatments. It reflects that carotenoid contents of Balkasar were more sensitive to V stress (Fig. 4). Additionally, Marschner and Cakmak (1986) and Chary et al. (Chary et al. 2008) found that induction of ROS is one of the key factors that reduce chlorophyll contents in plants. Similar findings related to decline in chlorophyll (a + b) and carotene contents under metal contamination were reported in Aeluropus littoralis (Rastgoo et al. 2014) and Trigonella (Choudhary et al. 2012).
Protein synthesis n is very sensitive to heavy metal stress (Yurela 2005). In this study, the protein content was much higher in control plant compared to V-stressed plant. Heavy metal toxicity is generally attributed to impair functional potential of different enzymes having functional sulfhydryl group, leading to reduction in protein synthesis (Yurela 2005). In this study, decrease in protein content was much higher in sensitive genotype (Balakasar) than tolerant genotype (C-44) cultivars. Similarly, Andon and Fernando (2011) also reported that lowering of protein synthesis due to Cd stress was much higher in sensitive plant compared to tolerant plant. Our results also matched with the findings of Singh et al. (2007) and Choudhary et al. (2012).
The results generated from this study suggest that V stress induces membrane damages in both genotypes of chickpea. A significant positive correlation was observed between V and ion leakage from leaves of chickpea plants. Ion leakage caused by V is mainly due to loss of cell membrane integrity. Furthermore, plant membrane disruption might have occurred due to deposited V, resulting in the production of ROS that may have distorted the lipid bilayer through cyclic cascade of reaction (Fenton reaction), accelerating the membrane disruption under V stress. Additionally, metal toxicity usually involves in protein structural interruption by defecting S and N groups of protein, leading to wholesome disruption in ionic channels of the cell membrane, thus favoring ionic out floe from leaf disk cells (Gonzalez-Mendozaa et al. 2009). Similar results were also reported by Xiong and Wang (2005) and Tamas et al. (2006), who illustrated that V and other metals caused electrolyte leakage in Brassica pekinensis and Hordeum vulgare, respectively.
The results obtained in this study showed that V accumulation was much higher in roots compared to shoots of both chickpea genotypes exposed to V stress. Among genotypes, roots and shoots of C-44 had higher V accumulation than Balakasar. Additionally, shoots and roots of both genotypes also showed positive correlation towards exposed level of V stress. These results confirm the earlier findings by Xiao et al. (2012), higher V accumulation in root biomass compared to shoot biomass of Chinese cabbage treated with V stress. Our results are also consistent with the findings of Imtiaz et al. (2015), Narumol et al. (Narumol et al. 2011), and Panichev et al. (2006), which found negative effects of V on plants growth at higher concentrations along with higher accumulation pattern of V in roots than shoots.
Conclusion
The findings of this study revealed that soluble protein and photosynthetic pigments were significantly declined. However, antioxidant enzyme activities and ion leakage were increased with increasing V concentrations. Root growth inhibition due to V stress was noticed in selected genotypes of chickpea. The results presented in this study also indicate the potential involvement of V in PCD of chickpea plants. However, DNA degradation was observed only in V-sensitive genotype (Balkasar) at higher concentrations. Further experimentation is required in the future to underpin the mechanistic processes and possible pathways involved in plant PCD exposed to V stress.
References
Adamakis IDS, Panteris E, Eleftheriou EP (2011) The fatal effect of tungsten on Pisum sativum L. root cells: indications for endoplasmic reticulum stress-induced programmed cell death. Planta 234:21–34
Aebi HE, Bergmeyer HO (1983) Catalase, methods enzymology. Academic, New York, p. 2
Agency for Toxic Substances and Disease Registry (ATSDR), Public Health Service, U.S. Department of Health and Human services (2012) Toxicological profile for vanadium. Georgia, Atlanta. http://www.atsdr.cdc.gov/toxprofiles/tp58.pdf
Andon V, Fernando L (2011) Cd-induced membrane damages and changes in soluble protein and free amino acid contents in young barley plants. Emirates J Food Agri 23(2):130–136
Anke M (2004) Vanadium—an element both essential and toxic to plants, animals and humans? Anales de la Real Academia Nacional de Farmacia Madrid 70(4):961–999
Balk J, Chew SK, Leaver CJ, McCabe PF (2003) The intermembrane space of plant mitochondria contains a DNase activity that may be involved in programmed cell death. Plant J 34:573–583
Banu NA, Hoque MA, Watanabe-Sugimoto M, Matsuoka K, Nakamura Y, Shimoishi Y, Murata Y (2009) Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J Plant Physiol 166:146–156
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Behboodi BS, Samadi L (2004) Detection of apoptotic bodies and oligonucleosomal DNA fragments in cadmium-treated root apical cells of Allium cepa Linnaeus. Plant Sci 167:411–416
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dyes binding. Anal Biochem 72:248–254
Brotner CD, Oldenburg NB, Cidlowski JA (1995) The roleof DNA fragmentation in apoptosis. Trends Cell Biol 5:21–26
Chary NS, Kamala CT, Raj DS (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol Environ Saf 69:513–524
Choudhary S, Ansari MYK, Aslam R (2012) Sequential effects of cadmium on plant growth, biochemical and cytophysiological aspects, antioxidant activity, and molecular screening of protein by SDS–PAGE in Trigonella. Toxicol Environ Chem 94(8):1557–1570
De Michele R, Vurro E, Rigo C, Costa A, Elviri L, Di Valentin M, Careri M, Zottini M, Sanita` di Toppi L, Lo Schiavo F (2009) Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol 150(1):217–228
Dionisio-Sese ML, Tobit S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
EI Azzouzi B, Tsangaris GT, Pellegrini O, Manuel Y, Benveniste J, Thomas Y (1994) Cadmium induces apoptosis in a human T cell line. Toxicology 88:127–139
Fojtova MA, Kovarik A (2000) Genotoxic effect of cadmium is associated with apoptotic changes in tobacco cells. Plant Cell Environ 23:531–537
Gadjev I, Stone JM, Gechev TS (2008) Programmed cell death in plants: new insights into redox regulation and the role of hydrogen peroxide. In International Review of Cell and Molecular Biology; the Netherland: Elsevier Inc.: Amsterdam, Volume 270:87–144
Gao CJ, Xing D, Li LL, Zhang LR (2008) Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta 227:755–767
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 28:1091–1101
Goc A (2006) Biological activity of vanadium compounds. Cent Eur J Biol 1(3):314–332
Gonzalez-Mendozaa D, Quiroz-Moreno A, Gracia Medrano RE, Grimaldo-Juarez O, Zapata-Perez O (2009) Cell viability and leakage of electrolytes in Avicennia germinans exposed to heavy metals. Z Naturforsch 64c:391–394
Greenberg JT, Ailan G, Klessig DF, Ausubel FM (1994) Programmed cell death in plants: a pathogen-triggered response activated coordinately with multiple defense functions. Cell 77:551–563
Habeebu SMS, Liu J, Klaassen CD (1998) Cadmium-induced apoptosis in mouse liver. Toxicol Appl Pharmacol 149:203–209
Harmanescu M, Alda LM, Bordean DM, Gogoasa I, Gergen I (2011) Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania. Chem Cent J 5:64
Havel L, Durzan DJ (1996) Apoptosis in plants. Botan Acta 109:203–209
Hou M, Hu C, Xiong L, Lu C (2013) Tissue accumulation and subcellular distribution of vanadium in Brassica juncea and Brassica chinensis. Microchem J 110:575–578
Imtiaz M, Tu S, Xie Z, Han D, Ashraf M, Rizwan MS (2015) Growth, V uptake, and antioxidant enzymes responses of chickpea (Cicer arietinum L.) genotypes under vanadium stress. Plant Soil 390:17–27
Koukalova B, Kovarik A, Fajkus J, Siroky J (1997) Chromatin fragmentation associated with apoptotic changes in tobacco cells exposed to cold stress. FEBS Lett 414:289–292
Lam E (2004) Controlled cell death, plant survival and development. Nat Rev Mol Cell Biol 5:305–315
Lichtenthaler HK, Wellburn AR (1985) Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem Soc T 11:591–592
Lin J, Wang Y, Wang G (2006) Salt stress-induced programmed cell death in tobacco protoplasts is mediated by reactive oxygen species and mitochondrial permeability transition pore status. J Plant Physiol 163:731–739
Lohmann RD, Beyersmann D (1993) Cadmium and zinc mediated changes of the Ca2+-dependent endonuclease in apoptosis. Biochem Bioph Res Co 190:1097–1103
Lozano R, Azcon R, Palma JM (1996) SOD and drought stress in Lactua sativa. New Phytol 136:329–331
Malecka A, Jarmuszkiewicz W, Tomaszewska B (2001) Antioxidative defence to lead stress in subcellular compartments of pea root cells. Acta Biochim Pol 48:687–698
Małuszynski, MJ (2007) Vanadium in environment, Ochrona Srodowiska i Zasobów Naturalnych, Ochr´ Sr Zasobów Nat 31:475–478
Marschner H, Cakmak I (1986) Mechanism of phosphorus-induced zinc deficiency in cotton. II. Evidence for impaired shoot control of phosphorus uptake and translocation under zinc deficiency. Physiol Plant 68:491–496
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Narumol V, Yaowapha J, Greg D, Ashley TT, Paul RH (2011) Effect of vanadium on plant growth and its accumulation in plant tissues. Songklanakarin. J Sci Technol 33(3):255–261
Ojima K, Ohira K (1983) Characterization of aluminium and manganese tolerant cell lines selected from carrot cell cultures. Plant Cell Physiol 24:789–797c
Panichev N, Mandiwana KL, Moema D, Molatlhegi R, Ngobeni P (2006) Distribution of vanadium (V) species between soil and plants in the vicinity of vanadium mine. J Hazard Mater 137(2):649–653
Papini A, Mosti S, Milocani E, Tani G, Di Falco P, Brighigna L (2011) Megasporogenesis and programmed cell death in tillandsia (Bromeliaceae). Protoplasma 248(8):651–662
Patade VY, Bhargava S, Suprasanna P (2011) Salt and drought tolerance of sugarcane under iso-osmotic salt and water stress: growth and osmolyte accumulation and antioxidant defense. J Plant Interact 6:275–282
Pergent-Martini C, Pergent G (2000) Marine phanerogams as a tool in the evaluation of marine trace-metal contamination: an example from the Mediterranean. Int J Environ Pollut 13(1–6):126–147
Poor P, Kovacs J, Szopko D, Tari I (2013) Ethylene signaling in salt stress- and salicylic acid-induced programmed cell death in tomato suspension cells. Protoplasma 250:273–284
Putter J (1974) In: Bergmeyer HU (ed) Peroxidases. Methods of enzymatic analysis: II. Academic Press, New York, pp. 685–690
Rastgoo L, Alemzadeh A, Tale AM, Tazangi SE, Eslamzadeh T (2014) Effects of copper, nickel and zinc on biochemical parameters and metal accumulation in gouan, Aeluropus littoralis. Plant Knowledge J 3(1):31–38
Reddy AM, Kumar SG, Jyonthsnakumari G, Thimmanaik S, Sudhakar C (2005) Pb induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (lam.) verdc.) and bengalgram (Cicer arietinum L.). Chemosphere 60:97–104
Reijonen I, Metzler M, Hartikainen H (2016) Impact of soil pH and organic matter on the chemical bioavailability of vanadium species: the underlying basis for risk assessment. Environ Pollut 210:371–379
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, super oxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Shulan Z, Qing L, Yanting Q, Lian D (2010) Responses of root growth and protective enzymes to copper stress in turfgrass. ACTA Biol Cracov Bot (Series Botanica) 52(2):7–11
Singh D, Nath K, Sharma YK (2007) Response of wheat seed germination and seedling growth under copper stress. J Environ Biol 28:409–414
Tamas L, Budikova S, Simonovicova M, Huttova J, Siroka B, Mistrik I (2006) Rapid and simple method for Al-toxicity analysis in emerging barley roots during germination. Biol Plant 50:87–93
Walker PR, Sikorska M (1994) Endonuclease activities, chromatin structure, and DNA degradation in apoptosis—review. Biochem Cell Biol 72:615–623
Wang GP, Zhang ZH, Kong DJ, Liu QX, Zhao GL (2012) Programmed cell death is responsible for replaceable bud senescence in chestnut (Castanea mollissima BL.). Plant Cell Rep 31(9):1603–1610
Wazalwar SS, Bhave NS, Dikundwar AG, Ali P (2011) Microwave assisted synthesis and antimicrobial study of Schiff base vanadium (IV) complexes of phenyl esters of amino acids. Synth React Inorg M 41(5):459–464
Wyllie AH (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284:555–556
Xiao X, Yang M, Guo Z, Luo Y, Bi J (2012) Permissible value for vanadium in allitic udic ferrisols based on physiological responses of green Chinese cabbage and soil microbes. Biol Trace Elem Res 145:225–232
Xiong ZT, Wang H (2005) Copper toxicity and bioaccumulation in Chinese cabbage (Brassica pekinensis Rupr.). Environ Toxicol 20:188–194
Yakimova ET, Kapchina-Toteva VM, Laarhoven LJ, Harren FM, Woltering EJ (2006) Involvement of ethylene and lipid signalling in cadmium-induced programmed cell death in tomato suspension cells. Plant Physiol Biochem 44:581–589
Yurela I (2005) Copper in plants. Braz J Plant Physiol 17:145–156
Zhang S, Zhang H, Qin R, Jiang W, Liu D (2009) Cadmium induction of lipid per oxidation and effects on root tip cells and antioxidant enzyme activities in Vicia faba L. Ecotoxicology 18:814–823
Acknowledgments
This research was supported by a grant from the National Science Foundation (grant number 41071309), research grants from Sino Hydropower Group (grant number GW-KJ-2012-10-01), and Special Fund for Agro-scientific Research in the Public Interest (201303106, 201103007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Imtiaz, M., Mushtaq, M.A., Rizwan, M.S. et al. Comparison of antioxidant enzyme activities and DNA damage in chickpea (Cicer arietinum L.) genotypes exposed to vanadium. Environ Sci Pollut Res 23, 19787–19796 (2016). https://doi.org/10.1007/s11356-016-7192-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7192-1