Abstract
Environmental pollutants have been increasingly detected and quantified in each sphere-air, water and soil, and hence there are growing concerns about their potentially harmful effects on the biosphere. Heavy metals and dyes are of prime concern owing to their large-scale anthropogenic consumption, and emission, either untreated or improperly, into the open environment. Recently, nanomaterials have been explored for their potential against environmental pollution and have been proved to be effective against a wide range of dyes and heavy metals. But their traditional method of synthesis isn’t economic, energy-efficient, and ecofriendly. In this direction, Phyconanoremediation is a recent approach that is green and sustainable. It provides remediation of pollutants using novel nanomaterials synthesised using algal biomass. This review aims to present an account of the application of algae in nanomaterials synthesis followed by their application in the remediation of heavy metals and dyes. It commences with an introduction to environmental pollution, associated public health risks, and remediation strategies. Synthesis mechanism of nanoparticles and their characterisation methods followed by role of algal bioactive compounds in nanomaterials synthesis as reducing, capping or stabilising agent has been discussed. The article concludes by highlighting outlook and importance of this interesting field for environmental remediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years industrialization and urbanization have improved our way of living but on the other hand, it has polluted the environment to a great extent. The activities such as mining, textile dyeing, use of harmful metals for various purposes such as in paints and electronics has resulted in an environment contaminated with heavy metals and toxic dyes. These toxic and recalcitrant compounds lead to dangerous impacts on both the environment and human beings. The use of these heavy metals and dyes in one or another way leads to their accumulation beyond the permissible limits and causes pollution of water, air, and soil. The heavy metals even at low concentrations are harmful to the environment as well as to humans because they are highly toxic in nature; they are proven to be carcinogenic and mutagenic in nature(Kumar and Bharadvaja 2020). The dyes are xenobiotic in nature and thus cannot be degraded easily. They have also been proved to be carcinogenic in nature (El-Sheekh et al. 2009). There have been ways developed to reduce or eliminate the accumulated pollutants including heavy metals and dyes from the environment. The strategies developed falls under mainly three broad categories namely physical, chemical, and biological ways of remediation. Amongst the three strategies, the biological remediation is most promising strategies as both the physical and chemical strategies often require high input of energy. The latter two methods are highly expensive to operate and are less efficient in removing the pollutants from the targeted site such as polluted water(Pereira and Alves 2012; Sharma et al. 2016). The biological remediation techniques employ the ability of microorganisms and plants to bioaccumulate or biosorp the heavy metals and dyes and thus removing them from the contaminated water (Parameswari et al. 2010). The biological remediation strategies are therefore adopted as they are cheap and do not require high energy inputs and produce no or less secondary pollutant materials.
Algae are one of the promising biological agents for environmental remediation as they can accumulate the heavy metals and degrade dyes to an extent from the wastewater. They possess large surface area for absorption/adsorption and possesses enzymes that can remediate the dyes(Parameswari et al. 2010; Pereira and Alves 2012). The process involving algae to clean up the environment is termed as phycoremediation. The absorbing activities of algae are attributed to the metabolic diversities of algae which enable them to use these inorganic compounds in absence of organic carbon compounds. The most commonly used algae are blue-green algae as it has the capability of absorbing/adsorbing metal ions from the water bodies even if the algae cells are not alive. The remediation using blue-green algae are preferred because it does not produce any chemical sludge. Microalgae such as Spirulina acts as a potential biosorbent due to its large surface area, its ability to grow both in fresh and marine water and a relatively high binding affinity for metals ions and dyes (Pathak et al. 2014). The phycoremediation faces some challenges such as the entire concentration of heavy metals and dyes are not removed, but only the bioavailable amounts are removed from the contaminated water (Kumar and Gopinath 2016).
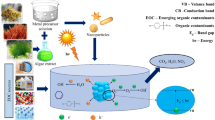
To overcome the challenges faced in phycoremediation, we discuss the concept of “phyco-nano-remediation” strategy in this review. Phyco-nano-remediation refers to the process of bioremediation which is carried out using nanoparticles derived from the algal biomass. Recent research conducted on applicability of nanomaterials to fight environmental pollutants has revealed the potential of nanomaterials to transform the harmful pollutants into comparatively safer compounds (Kumar and Gopinath 2016). Due to their distinctive properties at nanoscale, nanomaterials show remarkable degradable activity towards many environmental pollutants(Rauta et al. 2019). Many nanoparticles like gold, silver, copper etc., have been investigated for remediation of various environmental pollutants. Iron and zinc nanoparticles are commonly used for this purpose. The iron nanoparticles are used for cleaning the groundwater. The zinc nanoparticles are known for degrading dyes and other organic acids from the wastes. The use of nanomaterials-based remediation techniques enhances the rate of removal along with reduction in the time taken for the cleaning purposes. The only concern of application of nanomaterials in environmental remediation is the way nanomaterials are produced. Conventional nanomaterials synthesis routes are not considered as eco-friendly and economically benign.
The physicochemical techniques of production of nanoparticles produce waste materials which again pollute the environment and need special disposal methods. The requirement of high energy input and the generation of toxic by-products limit the use of nanomaterials synthesized by traditional method. Using biological systems for nanomaterials synthesis do not leave behind any toxic compounds which are generally produced as by-products when physical or chemical methods are employed for the synthesis of materials. Phyco-nanoremediation, a green way of synthesising nanomaterials, can be used to overcome this issue. By adopting algae for the synthesis of nanoparticles the toxic by-products and high energy consumption is eliminated to a certain extent. Various nanoparticles such as ZnO-nanoparticles, Ag-nanoparticles, Au-nanoparticles, CdS-nanoparticles have been synthesized using algae (Gour and Jain 2019). There have been a lot of studies indicating the green synthesis nanoparticles from algae such as Sargassum sp., Fucus vesiculosis and other algae are known for their ability to produce gold nanoparticles(Khanna et al. 2019). Codium capitatum, Cystophoramoniliformis have been studied for their ability to synthesize silver nanoparticle (Dhavale et al. 2020). There have also been a lot of research on the bioremediation properties of various nanoparticles such as the adsorption of Hg (II) on citrate coated gold nanoparticles, TiO2 nanoparticles are known for the adsorption of Cd (II) (Yang et al. 2019). Various nanoparticles are known for the degradation of dyes such as Zinc nanoparticles are known to degrade Malachite green (Rauta et al. 2019). In this review, current status, issues and developments in the field of phyconanoremediation for heavy metals and dyes have been presented.
Environmental pollution and concerns
The word pollution refers to the introduction of hazardous/ unwanted substances beyond the permissible limits by humans into the environment at a specific location. These substances are not only hazardous to environment but in turn cause harms to humans as well (Imani et al. 2011). The environment pollution is also referred as ‘environmental crisis’, which addresses both the environmental as well as ecological changes, caused by both human and natural interference. Humans played a part in environmental pollution and degradation by deletion and over-exploitation of resources. Urbanization and industrialization have substantiated the rate of release of untreated effluent in the environment, transfer of natural resources from one place to other near or distant places, use of biocides including pesticides and herbicides for crop productivity and soil improvement and so on. Nature can also cause pollution by phenomenon like forest fires, and volcanic eruptions. Environmental degradation does not affect the environment only but it has started to effect mankind also (Appannagari 2017). Environmental degradation has reached such a proportion that, if strict measures are not taken for its restoration, it will not be able to support any living organism after few decades. The most serious problems leading to such a critical situation can be attributed to greenhouse gases, water pollution, acid and heavy metal deposition along with waste mis-management (Kelishadi 2012). There is a complex effect of human activities on the environment which creates a number of changes in both biotic and abiotic components of the environment. These effects/impacts on the environment can be direct or indirect. The direct impacts are the immediate impact whereas the indirect impact is seen after long period and having long lasting effects. The main reason for the deteriorating environmental condition is the industrial development. Industries most often dump enormous amount of pollutants which contains heavy metals like chromium, lead, arsenic etc., and many dyes directly into the water bodies which leads to contamination of water (Appannagari 2017). The presence of these compounds in the water bodies affect the marine life and thus affects the whole ecosystem (Sall et al. 2020). Environmental pollution causes various adverse effects on the health starting from the very early stage of life. The environmental pollution may lead to infant mortality, allergies, perinatal disorders, respiratory disorders, malignancies, endothelial dysfunction, mental disorders, and various such diseases which are often life threatening. Studies have also reported that there is an increased risk of morbidity and mortality with an increase in the exposure to particulate matter from the environment in comparison to any other diseases (Kelishadi 2012). Sources of environmental pollutants and their effects on environment and human health have been illustrated in Fig. 1.
Environmental impact of heavy metals
By the set notion, heavy metals are defined as the metals having density greater than or equal to 5 (and atomic number greater than 11) who have proven risks to the environment and the mankind. The pollution caused due to the presence of heavy metals are of the major concern because they are non-biodegradable and are extremely toxic for both, first, for their immediate environment of discharge and second, to the entire biosphere. Even exposure to very low concentrations of heavy metals can lead to serious diseases such as kidney failure, respiratory problems and even cancer. Chromium being a carcinogen leads to the formation of skin lesions and can also cause respiratory problems. Beyond the threshold quantity, metal trace elements (MTEs) are demonstrated to cause negative physiological effects on the mammals, reptiles, avian population, amphibians, and fishes. MTEs are also reported to cause disturbances in the seed germination, metabolic activities of plants, and their growth. The plants bioconcentrate these metals, thus the heavy metals are found in higher concentrations as compared to the concentration of these metals in the surroundings. Studies indicate that MTEs are able to induce oxidative stress in algae. These heavy metals and MTEs not only harms the environment but also poses serious health concern for humans as they affect the nervous system, kidneys, respiratory functions, and liver. MTEs have carcinogenic properties, delays the overall growth and development, and may also lead to psychological disorders. The intoxication caused by certain heavy metals like mercury is even capable of inducing auto-immune like circumstances (Sall et al. 2020).
Environmental impact of dyes
A substance that binds to a substrate and provides it a colour due to the absorption of light at a certain wavelength in the visible range is known as dye. Conjugately bonded electron delocalization system called chromophore serves as the centre for light absorption in the dyes. Dyes contain electron donors which enhances the intensity of the colour and the affinity for the substrate binding known as auxochromes (Pereira and Alves 2012). Based on the source, dyes can be synthetic which are produced by chemical synthesis or natural which are extracted from the natural sources like plant, lichens, minerals, clay, metal salt, insects, semi-precious stones, or shellfish. Chemically synthesised dyes are classified as derivatives of azo, xanthene, anthraquinone, aryl-methane, indigoid, or phthalocyanine. Most widely produced and versatile among them is the azo dye. According to their chemical properties and solubility dyes are classified as anionic and water-soluble acidic dye, reactive dye and direct dye, cationic and water-soluble basic dye, or non-ionic solvent dye, disperse dye and pigment dye, vat dyes and mordant dyes. Apart from textile industry, dyes are used in paper production, cosmetics, food, pharmaceuticals, leather tanning, photography, and photoelectrical cell (Kuhad et al. 2004; Pereira and Alves 2012). As a discharge from the textile and other industries, dyes can change the solubility of oxygen and aesthetic merit of the water. Dyes also hinder with the amount and wavelength of the sunlight entering to the water bodies, and thus challenge the survival of the aquatic life. The extreme conditions of pH and temperature cause physical and chemical changes to the water due the presence of phenols, solid and oils which increases the BOD and COD of the water. This again disturbs the aquatic life. Degradation of the dyes without removal may also increase the toxicity of the dye at the polluted site. For example, upon degradation azo dyes produces aromatic amines which shows carcinogenic properties. These aromatic amines can then cause bladder cancer, hepatocarcinoma, chromosomal abbreviations and nuclear abnormality in humans (Pereira and Alves 2012) .
Phycoremediation approach
Algae has the ability to accumulate the pollutants from the water as they have large surface area of absorption/adsorption (Parameswari et al. 2010). Application of algae for remediation is termed as Phycoremediation. It is eco-friendly and is more promising than any other remediation technique as it is inexpensive and is in accordance with sustainable development. Algae can bind up to 10% of its biomass to metals. The metal removal is based on the principle of adsorption of metal on the surface of the algal cell surface and is independent of the metabolism while the absorption of metals by algal cells depends on metabolic activities. The efficiency of biosorption depends upon the bioavailability of the metals, surface area/volume ratio, presence of metal binding groups on algal cells, metal uptake and storage efficiency of the storage of metal (Ahmad et al. 2020).
There have been studies reporting the capability of various algae for the adsorption/absorption of heavy metals from the wastewater. Anabaena variabilis has been studied for its capability of removing Pb, Cr, Ni and Cd from the sewage water with an efficiency of removing 100% bioavailable concentration of these metals within 28 days and not only they eliminate these metals but also reduces the offensive odour from the treated water (Parameswari et al. 2010). In another set of studies, it was reported that Oscillatoria anguistissima has the capability of effective adsorption of Cu2+ from the mine water (Ahuja et al. 1997). Porphyra leucosticte has been reported to remove 95% of Pb(II) from waste water (Ye et al. 2015). Chlorella marina has been reported to remove 89% Cr and 87% Pb from the targeted source (Dinesh Kumar et al. 2015). There have also been attempts to immobilize algal cells of Anabaena doliolum and Chlorella vulgaris on chitosan, alginate, agar and carrageenan to improve the efficiency of remediation of heavy metals such as Ni and Cr (Mallick and Rai 1994). Dunaliella alga has been demonstrated for the removal of heavy metals such as Cd, Hg, Pb present at higher concentrations (Imani et al. 2011).
Algae are also the promising agents for remediation of dyes. The surface of the algal cell wall surface contains many functional groups which help in chelation or electrostatic binding of the dyes. This kind of removal is known as biosorption. The algae Caulerpa scalpelliformis removes basic yellow by biosorption (Aravindhan et al. 2007). Algae secrete extracellular biopolymers which coagulates the dye on their surface. The removal of pollutant by this method is termed as bio-coagulation. Algae Spirogyra Rhizopus exhibited potential for removal acid red 274 from the environment via bio-coagulation as reported in the study (Özer et al. 2006). In another approach, termed as biodegradation, algal enzymatic system works on the pollutants and degrades them. For example, degradation of methyl red dye by the enzyme azo-dye reductase produced by Nostoc linckia(El-Sheekh et al. 2009).
The phycoremediation being eco-friendly and efficient also faces some challenges. Such as not the entire concentration of heavy metals is removed, but only the bioavailable amounts are only removed from the contaminated water. Also, surrounding conditions or algal growth conditions substantially control the phycoremediation efficiency. Moreover, the remediation of pollutant materials varies with strain used. So, it is imperative to prospect highly efficient algal species for remediation of each of pollutant material. To overcome this problem, the focus of research community has been now shifted to nano-bioremediation approach. It addresses well the problems of pollution created during nanomaterials synthesis and growth dependent algal cells-based remediation of pollutants under diverse climatic conditions.
Phyco-nanotechnological approach for remediation of environmental pollutants
Synthesis of nanomaterials and their characterization
Nanomaterials fall in the range of 1-100 nm and can be synthesized using two approaches- either bottom-up or top-down (Fig. 2). In top-down method, nanoparticles are created by deconstructing a bulk material into nano sized particles by performing microfabrication cutting, grinding, crushing, and milling etc., on bulk materials. Top-down methods often do not provide very small and uniform nanomaterial even after using high energy. It also alters the surface chemistry and physical properties of the nanomaterials. Crystallographic structure is also lost to an extend due to the processing in top-down method (Khanna et al. 2019). In bottom-up method, nanomaterials are created by self- assembling atom by atom or molecule by molecule. Bottom-up method controls the chemical synthesis process thus can produce very small and uniform nanomaterials. There are various ways of bottom-up methods like combustion synthesis, hydrothermal method, sol-gel processing, gas-phase method, and microwave synthesis. A colour change in the solution is an indicator for the synthesis of the nanomaterials(Khanna et al. 2019).
Synthesis of nanomaterials via both top-down and bottom-up follows a specific kinetic procedure which helps to determine the shape and size of nanomaterials. So, characterization techniques talk about the size, surface morphology, shape, and surface area. Spectroscopic and microscopic techniques are used to characterize nanomaterials. Most of nanomaterials form a colloidal solution and thus exhibit tyndall effect. Commonly used microscopic techniques are scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM) which are the direct method of characterization. UV-vis spectroscopy; 190-380 nm for UV and 380-800 nm for visible range, Raman spectroscopy, Fourier transform infrared spectroscopy (FT-IR), dynamic light scattering (DLS), X-Ray diffraction (XRD) are the spectroscopy-based characterization methods. To characterize the thermal stability of nanomaterials, thermal gravimetric analysis (TGA) is performed (Khanna et al. 2019).
Algal biomass for synthesis of nanomaterials: an alternative to physicochemical methods of synthesis of nanomaterials
Even being more efficient, bottom-up methods require a lot of energy and cost. They can also lead to secondary pollution due to the by-products formed during the nanomaterial synthesis (Khanna et al. 2019). Algae can accumulate large amount of metal ions which are stabilized by the bioactive compounds present in the algae. Algal biomass extracts along with protein, carbohydrates and fatty acids contain a variety of numerous other bioactive compounds such as the pigments, chlorophylls, carotenoids, phycobilins, and antioxidants like terpenoids or polyphenol. These bioactive compounds also oxidized the metal ions to convert them into a malleable form. The synthesis of nanomaterials from algae requires algal extract and a molar solution of metal precursor. These two components are then mixed into a fixed proportion to initiate the synthesis of nanomaterials which can be identified by a change in colour of the mixture (Fig. 3). The bioactive compounds present in the extract facilitate nucleation of the metal and the nucleonic particle self-assemble to form a thermodynamically stable nanomaterial (Sharma et al. 2016; Khanna et al. 2019). Algal biomass is a promising source of synthesis of nanomaterials as (a) they are abundantly available, (b) have a low cost of large-scale production with easy to harvest, (c) they have a short doubling time, (d) they have a simple yet well-developed systems, (e) they have a negative charge on the surface which enhances the process of nucleation and crystallization (Khanna et al. 2019). Table 1 gives the list of nanomaterials synthesized by using algal biomass and their diverse applicability in the field of medicine, and remediation etc. There are several factors which effect algal nanomaterials synthesis. For example, concentration of the extract, pH, temperature, and contact time have a significant role in nanomaterials synthesis using algal biomass (Lengke et al. 2007).
Intracellular mode of synthesis of nanomaterials
Synthesis of nanomaterials occurs inside of the algal cell undergoing through various metabolic pathways of the algae. NADPH or NADPH dependent reductase bound to membrane or ETS serve as reducing agent and it does not require any pre-treatment of microalgae cells. When treated with chloroauric acid at 20 °C, Ulva intestinalis and Rhizocluniumfortinalae fabricated gold nanoparticle which was identified by a change of chloroplast colour to purple from green after 72 h (Sicard et al. 2010; Sharma et al. 2016).
Extracellular mode of synthesis of nanomaterials
Synthesis of nanomaterials occurs outside of the algal cell by using the metabolites produced by the cell. Before the synthesis, algal cell is washed and blended as pre-treatment (Dahoumane et al. 2016). For the extracellular synthesis of nanomaterials, the optimum concentration of the metal precursor and algal extract is mixed in a certain ratio and maintained till the reaction is complete. Gold uptake by the algae takes place in two phases (a) rapid phase- active biomolecule facilitates the quick uptake of the gold ions, (b) slow phase- Transport of gold ion across the membrane, suggesting its time dependence. In Spirulina platensis, an absorption maxima at 530 nm is observed for the gold indicating that the surface enzymes and active biomolecule facilitated the extracellular nanomaterial synthesis (Khanna et al. 2019).
Mechanism of algal nanoparticle synthesis
To synthesize algal nanoparticles, metal concentrate is mixed with the algal biomass. To initiate this process, metal ions adsorbs and accumulate on the algal surface. Then algae use different enzymes and metabolites like chlorophyll, phycobilin, polyunsaturated fatty acids, carbohydrates, polysaccharides, and minerals to remodel the metal ions into malleable form by reducing them. For example, brown algae use the hydroxyl group in their cell wall polysaccharide to synthesize Ag-nanoparticles. Reduction is the process which algae implies to covert the metal ions into precursors of nano-scaled particles. The functional group present in the metabolites produced by the algae helps in the reduction of metal ions as illustrated in Table 2. The step of reduction is marked by the change in colour of the solution (Ramakrishna et al. 2016; Gour and Jain 2019).
After reduction, the nanoparticles undergo nucleation, where they self organizes to achieve a thermodynamically stable appearance. The process of reduction and nucleation together marks the first phase of algal nanoparticle synthesis, called the activation phase. The second phase is called the growth phase, where the reduced and nucleated metal ions start to amalgamate with each other forming variable sized nanoparticles. During the growth phase to prevent the nucleated metal ions from forming large amalgamates and to provides them stability, capping reagents are used. Capping reagents (Table 2) are amphiphilic substances like membrane polysaccharides which covalently binds to the nanoparticles. This binding induces stearic hindrance into the structure and thus provides stability. In the final stage or termination phase, the stabilized nanoparticles undergo conditioning through temperature, pH, and physical shearing with due course of time to attain a final refined shape and size (Ramakrishna et al. 2016; Gour and Jain 2019; Javed et al. 2020).
Application of phyco-nanomaterials in remediation of heavy metals
The presence of heavy metals and their salts impart detrimental effects to the micro-organisms involved during the biological waste-water treatment. Most of the salts of the heavy metals are highly soluble in aqueous medium and thus cannot be efficiently removed by the commonly employed physical methods but these methods are highly expensive so cannot be used on large scale. For making the process of removal of heavy metals efficient and inexpensive, biological methods are employed on large scale which includes exploiting the ability of organism to bioaccumulate or biosorp the heavy metals and thus removing them from the contaminated water.
In phyconanoremediation, the nanoparticles produced using the algal biomass is used for the remediation purposes. The biological methods of nanoparticles production are eco-friendly, cost-effective and does not produce any hazardous by-products and is a sustainable approach (Saxena and Harish 2019). Several studies have been conducted so far for assessment of application of algal biomass-based nanomaterials in remediation of heavy metals. Table 3 presents a list of studies where algal biomass was used for synthesis of several metal nanoparticles for the purpose of heavy metal removal from different polluted sites.
Application of phyco-nanomaterials in remediation of dyes
Although the physiochemical methods are highly effective, but they are very expensive in terms of instruments and power required. Bioremediation uses the metabolic reaction of the living cell like bacteria, plant, algae, fungi, and animals to remove the dyes from the environment. Biological methods degrade dyes aerobically or anaerobically or by using the enzymes of the living system (Pereira and Alves 2012). Just like in the case of heavy metal remediation, phycoremediation of dyes have some challenges. Phycoremediation depends on many factors such as pH, temperature, dye structure, size and concentration, concentration of algal biomass, contact time, and the pre-treatment used. All these factors either effect the functioning of the enzyme, surface functional group activity or binding of the dye to the algae. Also, dyes are not the part of the natural environment, so they do not serve as a prime source of nutrient. Thus, additional source of energy and carbon is required as well (Mandal et al. 2016; Aragaw and Asmare 2018).
Nanoparticles have been used for the remediation of dyes from the environment in recent times. Being in the range of nanometres, nanoparticles provide an efficient adsorption due to the large surface. Nanoparticles can also degrade the pollutants into a simpler form. Silver nanoparticles (Ag-NP) are used to degrade dyes like Methylene blue, Congo Red, and Coomassie Brilliant Blue. Similarly, Cu-NP are found to be promising for degradation of Methylene Orange, Zn-NP for Malachite Green, Brown CGG dye and Congo Red, and Au-NP for Methylene blue (Rauta et al. 2019). However, methods used for synthesis of nanoparticles are expensive, require heavy machinery and energy, may change the surface chemical and physical properties of the nanomaterials, and can cause secondary pollution due to the produced by-products (Khanna et al. 2019). To overcome these problems biosynthesis of nanoparticles from algae is considered to provide an eco-friendly and cost-effective remediation strategy for dyes. Table 4 presents a list of studies where algal biomass was used for synthesis of several metal nanoparticles for the purpose of degradation of different dyes from different polluted sites.
Challenges and future prospects
Though there are numerous advantages of the phyco-nano-remediation, there are certain disadvantages associated with this new emerging remediation technique. The nanoparticles of different metals have different sizes even when prepared from same organism. For the synthesis of nanoparticles, specific and well-studied culture conditions are required. The isolation techniques involved makes the entire process labour intensive and expensive. The size of nanoparticles synthesizes is highly dependent on the culture parameters used for the cultivation of algae such as pH of the medium, light availability, temperature, strength of the used buffer, mixing speed and the enzymes employed by the algae for the accumulation and conversion of metals (Shah et al. 2015). One of the major challenges in the intracellular synthesis of the nanoparticles by some of the algae such as Ulva intestinalis, encapsulated Klebsormidium flaccidum and many more, is the extraction of the synthesized nanoparticles requires cell disruption and other techniques which is expensive and time taking (Khanna et al. 2019). Till date, all the studies related to the phyco-nano-remediations have been conducted in the laboratory. In-situ remediation is a challenging task as large number of nanoparticles is required for such projects. Also, the nanomaterial must be removed from the water bodies after use as these nanoparticles have various adverse effects on the environment and humans. These nanoparticles can cross-contaminate the surroundings, and due to extremely small size may even become aerosols, and travel to distances along with air and water. There have been studies reporting the toxicity of ZnO nanoparticles on the long-term exposure to fresh water microalgae, Scenedesmus rubescens(Aravantinou et al. 2020). The dermal exposure of carbon nanotubes is reported to cause problems in the humans. The nanoparticles used for the remediation may induce the transformation of other organic or inorganic material and may hamper the photochemical reactions occurring in the environment (Guzmán et al. 2006). A lot of waste comes from the discharge from the industries. These discharges contain nutrients that can be used as a system to grow the algae at the site of discharge. The cultivated algae will help in remediation on site. Not only this system will reduce the contaminants, but it will also help to reduce the cost of the operation. Further, this system helps to remove the gap from lab-scale development to the industrial application. This system can also be merged with other systems like an algal fuel cell to achieve maximum output capacity from the system (Sharma et al. 2018).
Conclusions
The environmental pollution caused due to the heavy metals and dyes, produced by textile industries, mining activities, and effluent discharge directly into the environment causes great environmental threats. These chemicals and metals get bio-accumulated in the food chain and prove to be highly toxic for the higher organisms as the toxic chemical and metals gets accumulated in higher concentrations in them due to bioaccumulation. There have been a number of treatment technologies adopted for the clearance of these pollutants which includes physical treatment methods such as ion exchange, precipitation, electrocoagulation, membrane filtration, electrodialysis, chemical treatments which include chemical washing, reduction and chelate flushing, and biological treatment methods such as biofiltration, bioremediation, biosorption, bioaugmentation, phytoremediation, phycoremediation, and microbial reduction of compounds. In addition to these traditional technologies, nanotechnology has also been used for the environment remediation and is gaining popularity due to its relative ease of operation and high efficiency. In spite of numerous advantages, nanotechnology faces some draw backs such as the traditional methods of synthesis of nanoparticles produces toxic by-products and requires lot of energy input. So, a new methodology called ‘phyco-nano-remediation’ is employed which involves the green synthesis of the nanoparticles and its use in the remediation process. By adopting algae for the synthesis of nanoparticles, the generation of the toxic by-products and high energy consumption is almost eliminated. These nanoparticles can be synthesised either by intracellular or extracellular mode using algal biomass. Using algae gold, silver, zinc oxide, iron, and copper and several other metallic nanoparticles have been synthesized. The synthesized nanoparticles were then used to remediate lead, copper, mercury, and other heavy metals along with many dyes including malachite green, methylene blue, congo red, and crystal violet. Phyconanoremediation is advantageous as compared to phycoremediation because it is independent of the growth of algal cells and the prevailing environmental conditions at the site of remediation. The remediation efficiency for phycoremediation is dependent on the growth of the algal species under consideration whereas in case of phyconanoremediation, the remediation efficiency is independent of the environmental as well as algal growth conditions from which these nanoparticles have been derived from.
References
Abboud Y, Saffaj T, Chagraoui A et al (2014) Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata). Appl Nanosci 4:571–576. https://doi.org/10.1007/s13204-013-0233-x
Abdel-Raouf N, Al-Enazi NM, Ibraheem IBM (2017) Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab J Chem 10:S3029–S3039. https://doi.org/10.1016/j.arabjc.2013.11.044
Aboelfetoh EF, El-Shenody RA, Ghobara MM (2017) Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): reaction optimization, catalytic and antibacterial activities. Environ Monit Assess 189. https://doi.org/10.1007/s10661-017-6033-0
Ahmad S, Ahmad A, Khan S et al (2018) Algal extracts based biogenic synthesis of reduced graphene oxides (rGO) with enhanced heavy metals adsorption capability. J Ind Eng Chem 72:117–124. https://doi.org/10.1016/j.jiec.2018.12.009
Ahmad S, Pandey A, Pathak VV et al (2020) Phycoremediation: algae as eco-friendly tools for the removal of heavy metals from wastewaters. Bioremediat Ind Waste Environ Saf 53–76. https://doi.org/10.1007/978-981-13-3426-9_3
Ahuja P, Gupta R, Saxena RK (1997) Oscillatoria anguistissima: a promising Cu2 + biosorbent. Curr Microbiol 35:151–154. https://doi.org/10.1007/s002849900229
Appannagari RR (2017) Environmental pollution causes and consequences: a study. North Asian Int Res J Soc Sci Humanit 3:2454–9827. https://doi.org/10.3389/fpubh.2020.00014
Aragaw TA, Asmare AM (2018) Phycoremediation of textile wastewater using indigenous microalgae. Water Pract Technol 13:274–284. https://doi.org/10.2166/wpt.2018.037
Aravantinou AF, Andreou F, Manariotis ID (2020) Long-term toxicity of zno nanoparticles on scenedesmus rubescens cultivated in semi-batch mode. Nanomaterials 10:1–14. https://doi.org/10.3390/nano10112262
Aravindhan R, Rao JR, Nair BU (2007) Kinetic and equilibrium studies on biosorption of basic blue dye by green macro algae Caulerpa scalpelliformis. J Environ Sci Heal - Part A Toxic/Hazardous Subst Environ Eng 42:621–631. https://doi.org/10.1080/10934520701244383
Aziz N, Faraz M, Pandey R et al (2015) Facile agae-derived route to biogenic silver nanoparticles: synthesis, antibacterial, and photocatalytic properties. Langmuir 31:11605–11612. https://doi.org/10.1021/acs.langmuir.5b03081
Azizi S, Namvar F, Mahdavi M et al (2013) Biosynthesis of silver nanoparticles using brown marine macroalga, Sargassum muticum aqueous extract. Mater (Basel) 6:5942–5950. https://doi.org/10.3390/ma6125942
Barwal I, Ranjan P, Kateriya S, Yadav SC (2011) Cellular oxido-reductive proteins of Chlamydomonas reinhardtii control the biosynthesis of silver nanoparticles. J Nanobiotechnol 9:1–12. https://doi.org/10.1186/1477-3155-9-56
Castro L, Blázquez ML, Muñoz JA et al (2013) Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol 7:109–116. https://doi.org/10.1049/iet-nbt.2012.0041
Chaudhary R, Nawaz K, Khan AK et al (2020) An overview of the algae-mediated biosynthesis of nanoparticles and their biomedical applications. Biomolecules 10:1–35. https://doi.org/10.3390/biom10111498
Dahoumane SA, Wujcik EK, Jeffryes C (2016) Noble metal, oxide and chalcogenide-based nanomaterials from scalable phototrophic culture systems. Enzyme Microb Technol 95:13–27. https://doi.org/10.1016/j.enzmictec.2016.06.008
Dhavale R, Jadhav S, Sibi G (2020) Microalgae mediated silver nanoparticles (Ag-NPs) synthesis and their biological activities. J Crit Rev 7:15–20. https://doi.org/10.31838/jcr.07.02.04
Dinesh Kumar S, Santhanam P, Jayalakshmi T et al (2015) Excessive nutrients and heavy metals removal from diverse wastewaters using marine microalga Chlorella marina (Butcher). Indian J Geo-Marine Sci 44:97–103
Dotto GL, Cadaval TRS, Pinto LAA (2012) Preparation of bionanoparticles derived from Spirulina platensis and its application for Cr (VI) removal from aqueous solutions. J Ind Eng Chem 18:1925–1930. https://doi.org/10.1016/j.jiec.2012.05.005
Edison TNJI, Atchudan R, Kamal C, Lee YR (2016) Caulerpa racemosa: a marine green alga for eco-friendly synthesis of silver nanoparticles and its catalytic degradation of methylene blue. Bioprocess Biosyst Eng 39:1401–1408. https://doi.org/10.1007/s00449-016-1616-7
El-Kassas HY, El-Sheekh MM (2014) Cytotoxic activity of biosynthesized gold nanoparticles with an extract of the red seaweed Corallina officinalis on the MCF-7 human breast cancer cell line. Asian Pac J Cancer Prev 15:4311–4317. https://doi.org/10.7314/APJCP.2014.15.10.4311
El-Kassas HY, Aly-Eldeen MA, Gharib SM (2016) Green synthesis of iron oxide (Fe3O4) nanoparticles using two selected brown seaweeds: Characterization and application for lead bioremediation. Acta Oceanol Sin 35:89–98. https://doi.org/10.1007/s13131-016-0880-3
El-Rafie HM, El-Rafie MH, Zahran MK (2013) Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr Polym 96:403–410. https://doi.org/10.1016/j.carbpol.2013.03.071
El-Sheekh MM, Gharieb MM, Abou-El-Souod GW (2009) Biodegradation of dyes by some green algae and cyanobacteria. Int Biodeterior Biodegrad 63:699–704. https://doi.org/10.1016/j.ibiod.2009.04.010
El-Sheekh MM, Shabaan MT, Hassan L, Morsi HH (2020) Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int J Environ Health Res 1–12. https://doi.org/10.1080/09603123.2020.1789946
Ganapathy Selvam G, Sivakumar K (2015) Phycosynthesis of silver nanoparticles and photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Hypnea musciformis (Wulfen) J.V. Lamouroux. Appl Nanosci 5:617–622. https://doi.org/10.1007/s13204-014-0356-8
García FE, Plaza-Cazón J, Montesinos VN et al (2018) Combined strategy for removal of Reactive Black 5 by biomass sorption on Macrocystis pyrifera and zerovalent iron nanoparticles. J Environ Manage 207:70–79. https://doi.org/10.1016/j.jenvman.2017.11.002
González-Ballesteros N, Prado-López S, Rodríguez-González JB et al (2017) Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: Its activity in colon cancer cells. Colloids Surf B Biointerfaces 153:190–198. https://doi.org/10.1016/j.colsurfb.2017.02.020
Gour A, Jain NK (2019) Advances in green synthesis of nanoparticles. Artif Cells Nanomedicine Biotechnol 47:844–851. https://doi.org/10.1080/21691401.2019.1577878
Guzmán KAD, Taylor MR, Banfield JF (2006) Environmental risks of nanotechnology: National nanotechnology initiative funding, 2000–2004. Environ Sci Technol 40:1401–1407. https://doi.org/10.1021/es0515708
Husain S, Afreen S, Hemlata et al (2019) Cyanobacteria as a bioreactor for synthesis of silver nanoparticles-an effect of different reaction conditions on the size of nanoparticles and their dye decolorization ability. J Microbiol Methods 162:77–82. https://doi.org/10.1016/j.mimet.2019.05.011
Imani S, Rezaei-Zarchi S, Hashemi M et al (eds) (2011) Hg, Cd and Pb heavy metal bioremediation by Dunaliella alga. J Med Plants Res 5:2275–2780
Javed R, Zia M, Naz S et al (2020) Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: recent trends and future prospects. J Nanobiotechnol 18:1–15. https://doi.org/10.1186/s12951-020-00704-4
Jena J, Pradhan N, Aishvarya V et al (2015) Biological sequestration and retention of cadmium as CdS nanoparticles by the microalga Scenedesmus-24. J Appl Phycol 27:2251–2260. https://doi.org/10.1007/s10811-014-0499-8
Kelishadi R (2012) Environmental pollution: Health effects and operational implications for pollutants removal. J Environ Public Health 2012:. https://doi.org/10.1155/2012/341637
Khaleelullah MMSI, Murugan M, Radha KV et al (2017) Synthesis of super-paramagnetic iron oxide nanoparticles assisted by brown seaweed Turbinaria decurrens for removal of reactive navy blue dye. Mater Res Express 4. https://doi.org/10.1088/2053-1591/aa9131
Khanna P, Kaur A, Goyal D (2019) Algae-based metallic nanoparticles: Synthesis, characterization and applications. J Microbiol Methods 163:105656. https://doi.org/10.1016/j.mimet.2019.105656
Kuhad RC, Sood N, Tripathi KK et al (2004) Developments in microbial methods for the treatment of dye effluents. Adv Appl Microbiol 56:185–213. https://doi.org/10.1016/S0065-2164(04)56006-9
Kumar L, Bharadvaja N (2020) Microbial remediation of heavy metals. Microb Bioremediat Biodegrad 49–72. https://doi.org/10.1007/978-981-15-1812-6_2
Kumar SR, Gopinath P (2016) Chap. 2 Nano-Bioremediation Applications of Nanotechnology for Bioremediation. 27–48. https://doi.org/10.1201/9781315374536-3
Kumar P, Govindaraju M, Senthamilselvi S, Premkumar K (2013a) Photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Ulva lactuca. Colloids Surf B Biointerfaces 103:658–661. https://doi.org/10.1016/j.colsurfb.2012.11.022
Kumar P, Senthamil Selvi S, Govindaraju M (2013b) Seaweed-mediated biosynthesis of silver nanoparticles using Gracilaria corticata for its antifungal activity against Candida spp. Appl Nanosci 3:495–500. https://doi.org/10.1007/s13204-012-0151-3
Lengke MF, Fleet ME, Southam G (2007) Synthesis of palladium nanoparticles by reaction of filamentous cyanobacterial Biomass with a palladium(II) chloride complex. Langmuir 23:8982–8987. https://doi.org/10.1021/la7012446
Mallick N, Rai LC (1994) Removal of inorganic ions from wastewaters by immobilized microalgae. World J Microbiol Biotechnol 10:439–443. https://doi.org/10.1007/BF00144469
Mandal RP, Sekh S, Sarkar N, Sen et al (2016) Algae mediated synthesis of cadmium sulphide nanoparticles and their application in bioremediation. Mater Res Express 3:1–11. https://doi.org/10.1088/2053-1591/3/5/055007
MubarakAli D, Arunkumar J, Nag KH et al (2013) Gold nanoparticles from Pro and eukaryotic photosynthetic microorganisms-Comparative studies on synthesis and its application on biolabelling. Colloids Surf B Biointerfaces 103:166–173. https://doi.org/10.1016/j.colsurfb.2012.10.014
Nikić J, Tubić A, Watson M et al (2019) Arsenic removal from water by green synthesized magnetic nanoparticles. Water (Switzerland) 11. https://doi.org/10.3390/w11122520
Özer A, Akkaya G, Turabik M (2006) The removal of Acid Red 274 from wastewater: combined biosorption and biocoagulation with Spirogyra rhizopus. Dye Pigment 71:83–89. https://doi.org/10.1016/j.dyepig.2005.06.004
Parameswari E, Lakshmanan A, Thilagavathi T (2010) Phycoremediation of heavy metals in polluted water bodies. Electron J Environ Agric Food Chem 9:808–814
Parial D, Patra HK, Dasgupta AKR, Pal R (2012) Screening of different algae for green synthesis of gold nanoparticles. Eur J Phycol 47:22–29. https://doi.org/10.1080/09670262.2011.653406
Patel A, Enman J, Gulkova A et al (2021) Integrating biometallurgical recovery of metals with biogenic synthesis of nanoparticles. https://doi.org/10.1016/j.chemosphere.2020.128306. Chemosphere 263:
Pathak VV, Singh DP, Kothari R, Chopra AK (2014) Phycoremediation of textile wastewater by unicellular microalga Chlorella pyrenoidosa. Cell Mol Biol 60:35–40. https://doi.org/10.14715/cmb/2014.60.5.7
Pereira L, Alves M (2012) Dyes-environmental impact and remediation. Environ Prot Strateg Sustain Dev 111–162. https://doi.org/10.1007/978-94-007-1591-2_4
Priyadharshini RI, Prasannaraj G, Geetha N, Venkatachalam P (2014) Microwave-mediated extracellular synthesis of metallic silver and zinc oxide nanoparticles using macro-Algae (Gracilaria edulis) extracts and its anticancer activity against human PC3 Cell Lines. Appl Biochem Biotechnol 174:2777–2790. https://doi.org/10.1007/s12010-014-1225-3
Ramakrishna M, Rajesh Babu D, Gengan RM et al (2016) Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J Nanostructure Chem 6:1–13. https://doi.org/10.1007/s40097-015-0173-y
Rao MD, Pennathur G (2017) Green synthesis and characterization of cadmium sulphide nanoparticles from Chlamydomonas reinhardtii and their application as photocatalysts. Mater Res Bull 85:64–73. https://doi.org/10.1016/j.materresbull.2016.08.049
Rauta PR, Mohanta YK, Nayak D et al (2019) Nanobioremediation. Nanotechnol Biol Med 245–257. https://doi.org/10.1201/9780429259333-21
Rösken LM, Cappel F, Körsten S et al (2016) Time-dependent growth of crystalline Au0-nanoparticles in cyanobacteria as self-reproducing bioreactors: 2. Anabaena cylindrica. Beilstein J Nanotechnol 7:312–327. https://doi.org/10.3762/bjnano.7.30
Sall ML, Diaw AKD, Gningue-Sall D et al (2020) Toxic heavy metals: impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ Sci Pollut Res 27:29927–29942. https://doi.org/10.1007/s11356-020-09354-3
Saxena P, Harish (2019) Phyco-Nanotechnology: New Horizons of Gold Nano-Factories. Proc Natl Acad Sci India Sect B - Biol Sci. https://doi.org/10.1007/s40011-016-0813-0
Shah M, Fawcett D, Sharma S et al (2015) Green synthesis of metallic nanoparticles via biological entities. Materials 8(11):7278–7308. https://doi.org/10.3390/ma8115377
Sharma B, Purkayastha DD, Hazra S et al (2014) Biosynthesis of fluorescent gold nanoparticles using an edible freshwater red alga, Lemanea fluviatilis (L.) C.Ag. and antioxidant activity of biomatrix loaded nanoparticles. Bioprocess Biosyst Eng 37:2559–2565. https://doi.org/10.1007/s00449-014-1233-2
Sharma A, Sharma S, Sharma K et al (2016) Algae as crucial organisms in advancing nanotechnology: a systematic review. J Appl Phycol 28:1759–1774. https://doi.org/10.1007/s10811-015-0715-1
Sharma S, Tiwari S, Hasan A et al (2018) Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 8:1–18. https://doi.org/10.1007/s13205-018-1237-8
Shukla MK, Singh RP, Reddy CRK, Jha B (2012) Synthesis and characterization of agar-based silver nanoparticles and nanocomposite film with antibacterial applications. Bioresour Technol 107:295–300. https://doi.org/10.1016/j.biortech.2011.11.092
Sicard C, Brayner R, Margueritat J et al (2010) Nano-gold biosynthesis by silica-encapsulated micro-algae: A “living” bio-hybrid material. J Mater Chem 20:9342–9347. https://doi.org/10.1039/c0jm01735c
Singh M, Kalaivani R, Manikandan S et al (2013) Facile green synthesis of variable metallic gold nanoparticle using Padina gymnospora, a brown marine macroalga. Appl Nanosci 3:145–151. https://doi.org/10.1007/s13204-012-0115-7
Subramaniyam V, Subashchandrabose SR, Thavamani P et al (2015) Chlorococcum sp. MM11—a novel phyco-nanofactory for the synthesis of iron nanoparticles. J Appl Phycol 27:1861–1869. https://doi.org/10.1007/s10811-014-0492-2
Uzair B, Liaqat A, Iqbal H et al (2020) Green and cost-effective synthesis of metallic nanoparticles by algae: Safe methods for translational medicine. Bioengineering 7:1–22. https://doi.org/10.3390/bioengineering7040129
Venkatpurwar V, Pokharkar V (2011) Green synthesis of silver nanoparticles using marine polysaccharide: Study of in-vitro antibacterial activity. Mater Lett 65:999–1002. https://doi.org/10.1016/j.matlet.2010.12.057
Vivek M, Kumar PS, Steffi S, Sudha S (2011) Biogenic silver nanoparticles by gelidiella acerosa extract and their antifungal effects. Avicenna J Med Biotechnol 3:143–148
Yang J, Hou B, Wang J et al (2019) Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials 9. https://doi.org/10.3390/nano9030424
Ye J, Xiao H, Xiao B et al (2015) Bioremediation of heavy metal contaminated aqueous solution by using red algae Porphyra leucosticta. Water Sci Technol 72:1662–1666. https://doi.org/10.2166/wst.2015.386
Zahoor M, Irshad M, Rahman H et al (2017) Alleviation of heavy metal toxicity and phytostimulation of Brassica campestris L. by endophytic Mucor sp. MHR-7. Ecotoxicol Environ Saf 142:139–149. https://doi.org/10.1016/j.ecoenv.2017.04.005
Acknowledgements
The authors would like to acknowledge constant support received by Delhi Technological University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, L., Mohan, L., Anand, S. et al. Phyconanoremediation: a sustainable approach to deal with environmental pollutants heavy metals and dyes. Vegetos 36, 332–347 (2023). https://doi.org/10.1007/s42535-022-00399-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-022-00399-y






