Abstract
Biosynthesis of gold nanoparticles has been accomplished via reduction of an aqueous chloroauric acid solution with the dried biomass of an edible freshwater epilithic red alga, Lemanea fluviatilis (L.) C.Ag., as both reductant and stabilizer. The synthesized nanoparticles were characterized by UV–visible, powder X-ray diffraction (XRD), transmission electron microscopy (TEM), Fourier transform infrared (FT-IR), and dynamic light scattering (DLS) studies. The UV–visible spectrum of the synthesized gold nanoparticles showed the surface plasmon resonance (SPR) at around 530 nm. The powder XRD pattern furnished evidence for the formation of face-centered cubic structure of gold having average crystallite size 5.9 nm. The TEM images showed the nanoparticles to be polydispersed, nearly spherical in shape and have sizes in the range 5–15 nm. The photoluminescence spectrum of the gold nanoparticles excited at 300 nm showed blue emission at around 440 nm. Gold nanoparticles loaded within the biomatrix studied using a modified 2,2-diphenyl-1-picrylhydrazyl (DPPH) method exhibited pronounced antioxidant activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past several years research on developing efficient methods for the large-scale synthesis of gold nanoparticles (Au NPs) are being intensely pursued. Wide variety of synthetic strategies, including both physical and chemical methods, have been employed to synthesize gold nanoparticles with controllable size, shape, and size distribution [1–10]. Synthesis of gold nanoparticles by physical methods requires sophisticated equipments and is thus quite expensive. Gold nanoparticles synthesized by chemical methods often require toxic reducing and stabilizing agents. These toxic substances adsorbed on the surfaces of the gold nanoparticles limit its applications in biomedical fields. Therefore, there is an overwhelming need to develop environmentally benign processes for the synthesis of gold nanoparticles without using any toxic chemicals. Biosynthesis of gold nanoparticles thus became an eco-friendly alternative to the currently available physical and chemical methods. Gold nanoparticles have amazing importance due to their unique optical and electronic properties and find applications in optoelectronic devices, drug delivery, biosensing and catalysis [11–14]. Besides, surface funtionalized gold nanoparticles are also known to show excellent antioxidant and antibacterial properties [15, 16]. Biomaterials like bacteria, fungi, different plant parts, and algae have been considered as eco-friendly nano-factories [17–21]. Proteins, peptides, and natural biotemplates like eggshell membrane have been efficiently used for the synthesis of fluorescent gold nanoparticles [22–24]. Recently, poly-cytosine DNA and poly-adenine DNA were utilized as protecting agents for the preparation of blue emitting gold nanoparticles [25]. Proteins were considered as green templates in the synthesis of fluorescent gold nanoparticles. Xie et al. [26] fabricated fluorescent gold nanoparticles using bovine serum albumin as both capping and reducing agents. An insulin-directed synthesis of red emissive gold nanoparticles is documented where the bioactivity and biocompatibility of insulin were retained [27]. Algae mediated synthesis of fluorescent gold nanoparticles did not appear to have been reported earlier. Recently, protein capped silver and gold nanoparticles synthesized using Escherichia coli were found to show potent antibacterial and antioxidant properties [28]. Silver and gold nanoparticles were accessed using aqueous extract of Solanum torvum fruit and their antibacterial and antioxidant properties have also been documented [29]. Comprehensive review on the biosynthesis of nanoparticles by various marine organisms has appeared recently [30]. Algae are the naturally occurring plant organisms that are the important source of phytochemicals responsible for the production of metal nanoparticles. Cyanobacteria and green algae have recently been demonstrated to yield gold nanoparticles [31]. Biosynthesis of gold nanoparticles using brown seaweed, Sargassum wightii is on record [32]. Another brown seaweed, Fucus vesiculosus was found to be capable of gold(III) biosorption and bioreduction [33]. Seaweeds apart, microalgae such as diatoms (Navicula atomus, Diadesmis gallica) are also known to afford gold nanoparticles, and gold–silica nanocomposites [34]. As a part of our ongoing studies on algal diversity of Manipur, a remotely located north-eastern state of India, we came across a rare freshwater edible red alga, L. fluviatilis [35]. Use of the red alga, L. fluviatilis, in the biosynthesis of noble metal nanoparticles did not appear to have been accomplished earlier. Gold ashes are traditionally known to be used as therapeutic agents in the Indian Ayurveda medicine to combat many clinical disorders [36–38]. Accordingly, we report herein the biosynthesis of highly stable, fluorescent gold nanoparticles using the freshwater red alga, L. fluviatilis and antioxidant activity of the biomatrix loaded nanoparticles.
Experimental
Materials and physical measurements
The red alga, L. fluviatilis was collected from the Thoubal district (93°45′–94°15′ East longitude and 23°43′–24°45′ North latitude) of the state of Manipur, North East India. The alga grows luxuriantly during winter (November to January) in the rocks and boulders of river Chakpi situated at an elevation of ~790 m from sea level. The specimen was brought to the laboratory cleaned thoroughly by fresh water followed by distilled water and then shade dried for a week. The algal morphology was examined under an optical microscope and using standard keys the specimen was identified to be L. fluviatilis [39]. The herbarium is deposited in the department of Ecology and Environmental Sciences, Assam University, Silchar, India. The dried thalli were ground to powder in a glass mortar. Chloroauric acid was purchased from Sigma-Aldrich. Double distilled water was used throughout the experiment. Absorption spectrum was taken on a Shimadzu 1601 PC UV–visible scanning spectrophotometer. X-ray diffraction (XRD) measurement was carried out on a Bruker AXS D8-Advance powder X-ray diffractometer with Cu–Kα radiation (λ = 1.5418 Å) with a scan speed of 2°/min. Transmission electron microscopy (TEM) images were obtained on a JEOL, JEM2100 equipment. TEM grids were prepared using a few drops of the nanoparticles followed by drying. The particle size distribution was determined by dynamic light scattering (DLS) technique using a particle size analyser (Delsa Nano S, Beckman Coulter, USA). FT-IR spectrum was recorded on a Shimadzu Varian 4300 spectrometer on KBr pellets. Photoluminescence (PL) spectrum was recorded on a Shimadzu RF-5301 PC spectrofluorophotometer.
Biosynthesis of gold nanoparticles
1 g of dry algal powder was added to 100 ml (10−3 M) aqueous solution of chloroauric acid and was stirred at room temperature for 12 h. The progress of the reaction was routinely monitored by observing color change as well as recording UV–visible spectrum. The initial light yellow color of chloroauric acid solution turned to red indicating formation of colloidal gold. The supernatant containing gold nanoparticles were collected by centrifugation at 10,000 rpm and used for further characterization. The purple colored biomass loaded with gold nanoparticles obtained after centrifugation was washed repeatedly with double distilled water to remove any unreacted chloroauric acid and dried.
Assessment of antioxidant activity
The antioxidant activity of the gold nanoparticles loaded within the biomatrix was assessed using a modified DPPH method [40]. At first 20 mg of the powdered sample was taken in a test tube and to it 3 mL of 100 µM methanolic solution of DPPH was added. To enhance the surface reaction between the sample and the DPPH reagent, the mixture was further sonicated and kept in the dark. After centrifugation at 10,000 rpm, the supernatant was collected and absorbance was measured at 515 nm. Methanol was kept as blank. A DPPH control was also measured as reference. The aforementioned procedure was followed to examine the time-dependent DPPH scavenging at an interval of 5, 15, 30, 45 and 60 min. The percentage scavenging was calculated using the formula
where A C and A S are absorbances of the control DPPH and DPPH with the biomatrix loaded gold nanoparticles at 515 nm, respectively. For evaluating SC50 (the amount of samples required to scavenge 50 % of DPPH) a similar procedure is adopted with 10, 20, 30, 50, 70, and 100 mg of the samples and absorbances were recorded after 30 min in each case.
Results and discussion
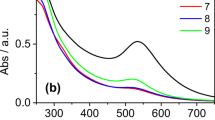
Gold nanoparticles were accessed via bioreduction of aqueous chloroauric acid solution with the dried biomass of an edible freshwater red alga, L. fluviatilis. The protein molecules present in the alga served both as reductant and stabilizer. The as-synthesized nanoparticles were characterized by UV–visible, XRD, TEM, DLS, and FT-IR studies. Gold nanoparticles show surface plasmon resonance (SPR) at around 510–560 nm [41]. Fig. 1 shows the UV–visible spectra of the aqueous chloroauric acid-L. fluviatilis reaction medium as a function of reaction time. Gold nanoparticles synthesized in the present study showed SPR at around 530 nm with a gradual increase in intensity of absorption with reaction time. The bioreduction of the aqueous chloroaurate ions was completed in 12 h. The inset of Fig. 1 shows the change in solution color (light yellow to red). The synthesized nanoparticles being capped with biomolecules are fairly stable (up to 3 months) as indicated by retention of red color of the solution and lack of any significant change in SPR band position.
The XRD pattern (Fig. 2) of the purple colored biomass showed five diffraction peaks at 2θ = 38.47°, 44.57°, 64.73°, 77.68°, and 81.75° which corresponds to (111), (200), (220), (311), and (222) planes of the face-centered cubic gold (space group Fm3m, JCPDS File No.89-3697). No peaks attributable to impurities were observed. The broad diffraction peaks clearly suggested reduced crystallite size. The average crystallite size of the gold nanoparticles estimated by the Debye–Scherrer formula, using a Gaussian fit was found to be 5.9 nm.
The TEM image (Fig. 3) showed that the synthesized gold nanoparticles were polydispersed. The particles were nearly spherical in shape with sizes in the range of 5–15 nm. The low magnification TEM image also revealed that the nanoparticles have a strong tendency to assemble together to form chain-like structure. The HRTEM image showed the lattice fringes between the two adjacent planes to be 0.231 nm apart which corresponds to the interplanar separation of the (111) plane of face-centered cubic gold. The electron diffraction (ED) pattern indicated polycrystalline nature of the synthesized material.
The differential intensity related to particle size distributions of the as-synthesized gold nanoparticles was obtained from DLS study (Fig. 4a). The average particle diameter was found to be 47.6 ± 61.3 nm. The cumulative mean diameter was found to be 35.8 nm. The larger particle size and more polydispersity observed by DLS as compared to TEM are due to the fact that the measured size also included the biomaterials covering the surface of gold nanoparticles. FT-IR spectrum was recorded to discern the possible biomolecules responsible for effective capping and stabilization of the gold nanoparticles. The FT-IR spectrum of the dried gold loaded purple colored biomass (Fig. 4b) showed bands at 3,314 and 1,158 cm−1 due to N–H and C–N stretching vibrations of primary amines, respectively [42]. The band at 2,944 cm−1 arose due to C–H stretching, those at 1,670 and 1,453 cm−1 corresponds to the amide I of the polypeptides and symmetric stretching of the carboxylate groups in the amino acid residues of the protein molecules [43]. This gives a clear indication of the presence of proteins and other organic molecules in the material which might have been produced by L. fluviatilis extracellularly.
Gold nanoparticles have been reported to exhibit photoluminescence in the wavelength 500–590 nm and 410–430 nm [44, 45]. The emissions in the range 500–590 nm occur due to the electronic transitions between the sp band just below the Fermi level and the first d band and those in the range 410–430 nm due to the recombination of electrons with holes in the lower energy band. The photoluminescence spectrum (Fig. 5) of the gold nanoparticles excited at 300 nm showed blue emission at around 440 nm. The blue emission has been observed earlier in case of nanosize gold clusters [46]. The presence of Au8 magic cluster has been found responsible for blue emission from gold nanodots [47, 48]. Blue emission at 411 nm has also been observed earlier for proteins/biotins functionalized gold nanoparticles [49]. Our present study clearly reveals that bioreduction of aqueous chloroaurate ions with the red alga, L. fluviatilis could in fact produce fluorescent gold nanoparticles.
The DPPH is a stable and well-known free radical for evaluating antioxidant potency of compounds. It is reduced by accepting the hydrogen or electron. The DPPH reducing ability of the biomatrix loaded gold nanoparticles was assessed spectrophotometrically by observing color change from purple to yellow. The control DPPH does not show any change of absorbance with time. However, DPPH with biomatrix loaded gold nanoparticles showed a steady decrease of absorbance at 515 nm with time (Fig. 6a). The DPPH scavenging (%) can be calculated from the decrease of absorbance at 515 nm, which corresponds to the quantity of DPPH in the methanolic solution. The DPPH scavenging (%) values for different weights of the sample were thus determined (vide experimental). The SC50 value, ascertained graphically from the plot of DPPH scavenging (%) vs. different weights of the sample (Fig. 6b) was found to be 18.10 mg. The observed antioxidant activity is attributed to the neutralization of free radical character of DPPH by the transfer of an electron [50]. Liu and co-worker [51] first observed enhanced DPPH radical scavenging activity by the gold nanoparticles coated with trolox, a vitamin E analogue. Very recently, their group put forward the possible mechanism and performed cellular kinetic studies on the enhancement of antioxidant activity by employing surface functionalization of gold nanoparticles with the antioxidant salvianic acid A [52]. Enhancement of antioxidant activity of gold nanoparticle embedded 3,6-dihydroxyflavone has also been reported recently [53]. The strategy described here resulted in biomatrix loaded gold nanoparticles having good antioxidant activity without any additional surface functionalizations.
Conclusion
Fluorescent gold nanoparticles have been successfully synthesized using the dried biomass of an edible freshwater red alga, L. fluviatilis following a green procedure. The methodology adopted herein is simple, low-cost, and non-toxic. The protein molecules present in the alga served a twofold role as reducing and stabilizing agent. No external reducing agent or surfactants were needed for the synthesis of gold nanoparticles. The biomatrix loaded gold nanoparticles has been demonstrated to be a potent antioxidant. The use of red alga, L. fluviatilis for the fabrication of gold nanoparticles, indicated its potential in future production of other valuable nanostructures in the emerging field of nanobiotechnology. Owing to the inherent ability of antioxidants to elicit free radical scavenging activity, their formulation as nanomaterials might enhance their efficiency. Utilization of biomatrix loaded gold nanoparticles in antioxidant activity study might prove to be a suitable alternative to traditional methods, where separation of nanoparticles from a biological system is cumbersome.
References
Zhou X, Wei Q, Sun K, Wang L (2009) Formation of ultrafine uniform gold nanoparticles by sputtering and redeposition. Appl Phys Lett 94:133107
Lucas BD, Kim JS, Chin C, Guo LJ (2008) Nanoimprint lithography based approach for the fabrication of large-area, uniformly-oriented plasmonic arrays. Adv Mater 20:1129–1134
Mishra YK, Avasthi DK, Kulriya PK, Singh F, Kabiraj D, Tripathi A, Pivin JC, Bayer IS, Biswas A (2007) Controlled growth of gold nanoparticles induced by ion irradiation: an in situ X-ray diffraction study. Appl Phys Lett 90:073110
Kabashin AV, Meunier M (2003) Synthesis of colloidal nanoparticles during femtosecond laser ablation of gold in water. J Appl Phys 94:7941–7943
Magnusson MH, Deppert K, Malm JO, Bovin JO, Samuelson L (1999) Gold nanoparticles: production, reshaping, and thermal charging. J Nanopart Res 1:243–251
Turkevich J (1985) Colloidal gold. Part I. Gold Bull 18:86–91
Brust M, Schiffrin DJ, Bethell D, Kiely CJ (1995) Novel gold-dithiol nano-networks with non-metallic electronic properties. Adv Mater 7:795–797
Gittins DI, Caruso F (2001) Spontaneous phase transfer of nanoparticulate metals from organic to aqueous media. Angew Chem Int Ed 40:3001–3004
De G, Bhattacharyya S (2008) Au nanoparticles in alumina sols and coatings. J Mater Chem 18:2816–2824
Polavarapu L, Xu QH (2009) A simple method for large scale synthesis of highly monodisperse gold nanoparticles at room temperature and their electron relaxation properties. Nanotechnology 20:185606–185612
Hu MS, Chen HL, Shen CH, Hong LS, Huang BR, Chen KH, Chen LC (2006) Photosensitive gold- nanoparticle-embedded dielectric nanowires. Nat Mater 5:102–106
Ghosh P, Han G, De M, Kim CK, Rotello VM (2008) Gold nanoparticles in delivery applications. Adv Drug Deliv Rev 60:1307–1315
Pingarron JM, Sedeno PY, Cortes AG (2008) Gold nanoparticle-based electrochemical biosensors. Electrochim Acta 53:5848–5866
Mikami Y, Dhakshinamoorthy A, Alvaroa M, García H (2013) Catalytic activity of unsupported gold Nanoparticles. Catal Sci Technol 3:58–69
Singh DK, Jagannathan R, Khandelwal P, Abraham PM, Poddar P (2013) In situ synthesis and surface functionalization of gold nanoparticles with curcumin and their antioxidant properties: an experimental and density functional theory investigation. Nanoscale 5:1882–1893
Zhao Y, Tian Y, Cui Y, Liu W, Ma W, Jiang X (2010) Small molecule-capped gold nanoparticles as potent antibacterial agents that target gram-negative bacteria. J Am Chem Soc 132:12349–12356
Nair B, Pradeep T (2002) Coalescence of nanoclusters and formation of submicron crystallites assisted by lactobacillus strains. Cryst Growth Des 2:293–298
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Ramani R, Parischa R, Ajayakumar PV, Alam M, Sastry M, Kumar R (2001) Bioreduction of AuCl4 − ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew Chem Int Ed 40:3585–3588
Gardea-Torresdey JL, Parsons JG, Gomez E, Peralta-Videa J, Troiani HE, Santiago P, Jose Yacaman M (2002) Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett 2:397–401
Shankar SS, Ahmad A, Pasricha R, Sastry M (2003) Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem 13:1822–1826
Nayak D, Nag M, Banerjee S, Pal R, Laskar S, Lahiri S (2006) Preconcentration of 198Au in a green alga, Rhizoclonium. J Radioanal Nucl Chem 268:337–340
Yan L, Cai Y, Zheng B, Yuan H, Guo Y, Xiao D, Choi MMF (2012) Microwave-assisted synthesis of BSA- stabilized and HSA-protected gold nanoclusters with red emission. J Mater Chem 22:1000–1005
Roy S, Pauli G, Banerjee A (2012) The as-prepared gold cluster-based fluorescent sensor for the selective detection of AsIII ions in aqueous solution. Nanoscale 4:2734–2740
Devi PS, Banerjee S, Chowdhury SR, Kumar GS (2012) Eggshell membrane: a natural biotemplate to synthesize fluorescent gold nanoparticles. RSC Adv 2:11578–11585
Kennedy TAC, MacLean JL, Liu J (2012) Blue emitting gold nanoclusters templated by poly-cytosine DNA at low pH and poly-adenine DNA at neutral pH. Chem Commun 48:6845–6847
Xie J, Zheng Y, Ying JY (2009) Protein-directed synthesis of highly fluorescent gold nanoclusters. J Am Chem Soc 131:888–889
Liu CL, Wu HT, Hsiao YH, Lai CW, Shih CW, Peng YK, Tang KC, Chang HW, Chien YC, Hsiao JK, Cheng JT, Chou PT (2011) Insulin-directed synthesis of fluorescent gold nanoclusters: preservation of insulin bioactivity and versatility in cell imaging. Angew Chem Int Ed 50:7056–7060
Veeraapandian S, Sawant SN, Doble M (2012) Antibacterial and antioxidant activity of protein capped silver and gold nanoparticles synthesized with Escherichia coli. J Biomed Nanotechnol 8:140–148
Ramamurthy CH, Padma M, Daisy mariya samadanam I, Mareeswaran R, Suyavaran A, Suresh Kumar M, Premkumar K, Thirunavukkarasu C (2013) The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloids Surf B 102:808–815
Asmathunisha N, Kathiresan K (2013) A review on biosynthesis of nanoparticles by marine organisms. Colloids Surf B 103:283–287
Parial D, Patra HK, Dasgupta AK, Pal R (2012) Screening of different algae for green synthesis of gold nanoparticles. Eur J Phycol 47:22–29
Singaravelu G, Arockiamary JS, Kumar VG, Govindaraju K (2007) A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B 57:97–101
Mata YN, Torres E, Blazquez ML, Ballester A, Gonzalez F, Munoz JA (2009) Gold(III) biosorption and bioreduction with the brown alga Fucus vesiculosus. J Hazard Mater 166:612–618
Schrofel A, Kratosova G, Bohunicka M, Dobrocka E, Vavra I (2011) Biosynthesis of gold nanoparticles using diatoms-silica-gold and EPS-gold bionanocomposite formation. J Nanopart Res 13:3207–3216
Bhosale R, Rout J, Chaugule B (2012) The ethnobotanical study of an edible freshwater red alga, Lemanea fluviatilis (L.) C.Ag. from Manipur, India. Ethnobot Res Appl 10:69–76
Bajaj S, Vohora SB (2000) Anti-cataleptic, anti-anxiety and anti-depressant activity of gold preparations used in Indian systems of medicine. Indian J Pharmacol 32:339–346
Shah ZA, Vohora SB (2002) Antioxidant/restorative effects of calcined gold preparations used in Indian systems of medicine against global and focal models of ischaemia. Pharmacol Toxicol 90:254–259
Shah ZA, Gilani RA, Sharma P, Vohora SB (2005) Attenuation of stress-elicited brain catecholamines, serotonin and plasma corticosterone levels by calcined gold preparations used in Indian system of medicine. Basic Clin Pharmacol Toxicol 96:469–474
Edmonson WT (1992) Freshwater Biology. International Books and Periodicals Supply Service, New Delhi
Serpen A, Capuano E, Fogliano V, Gokmen V (2007) A new procedure to measure the antioxidant activity of insoluble food components. J Agric Food Chem 55:7676–7681
Richard JP (1978) The Chemistry of Gold. Elsevier, Amsterdam
Correa-Llanten DN, Munoz-Ibacache SA, Castro ME, Munoz PA, Blamey JM (2013) Gold nanoparticles synthesized by Geobacillus sp. strain ID17 a thermophilic bacterium isolated from Deception Island, Antarctica. Microb Cell Fact 12:75
Sarkar J, Roy SK, Laskar A, Chattopadhyay D, Acharya K (2013) Bioreduction of chloroaurate ions to gold nanoparticles by culture filtrate of Pleurotus sapidus Quel. Mater Lett 92:313–316
Boyd GT, Yu ZH, Shen YR (1986) Photoinduced luminescence from the noble metals and its enhancement on roughened surfaces. Phys Rev B 33:7923–7936
Dulkeith E, Niedereichholz T, Klar TA, Feldmann J, Von Plessen G, Gittins DI, Mayya KS, Caruso F (2004) Plasmon emission in photoexcited gold nanoparticles. Phys Rev B 70:205424
Wilcoxon JP, Martin JE, Parsapour F, Wiedenman B, Kelley DF (1998) Photoluminescence from nanosize gold clusters. J Chem Phys 108:9137–9143
Zheng J, Petty JT, Dickson RM (2003) High quantum yield blue emission from water-soluble Au8 nanodots. J Am Chem Soc 125:7780–7781
Zheng J, Zhang CW, Dickson RM (2004) Highly fluorescent, water-soluble, size-tunable gold quantum dots. Phys Rev Lett 93:077402
Philip D (2009) Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Spectrochim Acta A 73:374–381
Naik GH, Priyadarsini KI, Satav JG, Banabalikar MM, Sohoni PP, Biyani MK, Mohan H (2003) Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry 63:97–104
Nie Z, Liu KJ, Zhong CJ, Wang LF, Yang Y, Tian Q, Liu Y (2007) Enhanced radical scavenging activity by antioxidant-functionalized gold nanoparticles: a novel inspiration for development of new artificial antioxidants. Free Radical Biol Med 43:1243–1254
Du L, Suo S, Wang G, Jia H, Liu KJ, Zhao B, Liu Y (2013) Mechanism and cellular kinetic studies of the enhancement of antioxidant activity by using surface-functionalized gold nanoparticles. Chem Eur J 19:1281–1287
Medhe S, Bansal P, Srivastava MM (2014) Enhanced antioxidant activity of gold nanoparticle embedded 3,6-dihydroxyflavone: a combinational study. Appl Nanosci 4:153–161
Acknowledgments
Authors thank SAIF, NEHU, Shillong, for TEM facilities. BS thanks University Grants Commission and MT thanks Department of Biotechnology, Government of India for Research Fellowships. We thank DBT e-Library Consortium (DeLCON) of Bioinformatics Centre, Assam University, Silchar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, B., Purkayastha, D.D., Hazra, S. et al. Biosynthesis of fluorescent gold nanoparticles using an edible freshwater red alga, Lemanea fluviatilis (L.) C.Ag. and antioxidant activity of biomatrix loaded nanoparticles. Bioprocess Biosyst Eng 37, 2559–2565 (2014). https://doi.org/10.1007/s00449-014-1233-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1233-2