Abstract
Remediation of heavy metal-contaminated soils has been drawing our attention toward it for quite some time now and a need for developing new methods toward reclamation has come up as the need of the hour. Conventional methods of heavy metal-contaminated soil remediation have been in use for decades and have shown great results, but they have their own setbacks. The chemical and physical techniques when used singularly generally generate by-products (toxic sludge or pollutants) and are not cost-effective, while the biological process is very slow and time-consuming. Hence to overcome them, an amalgamation of two or more techniques is being used. In view of the facts, new methods of biosorption, nanoremediation as well as microbial fuel cell techniques have been developed, which utilize the metabolic activities of microorganisms for bioremediation purpose. These are cost-effective and efficient methods of remediation, which are now becoming an integral part of all environmental and bioresource technology. In this contribution, we have highlighted various augmentations in physical, chemical, and biological methods for the remediation of heavy metal-contaminated soils, weighing up their pros and cons. Further, we have discussed the amalgamation of the above techniques such as physiochemical and physiobiological methods with recent literature for the removal of heavy metals from the contaminated soils. These combinations have showed synergetic effects with a many fold increase in removal efficiency of heavy metals along with economic feasibility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the beginning of the industrial modernization, anthropogenic factors are the main cause of the release of heavy metals and organic pollutants into the atmosphere. Soils, being the ultimate part of the ecological system, are profoundly polluted (Wu et al. 2010). Processes like pollution, salinization, and erosion are weakening soil’s physical, chemical, and biological properties. Due to pollution, many toxic materials have been released and accumulated in the soils. Contaminated soils have clear consequences on the social, economic, and environmental fronts.
Heavy metals are categorized as high density- and high atomic weight-based metallic elements which are toxic even at a very low concentration (< 1 ppb) (Olaniran et al. 2013). These metals are an indispensable part of all biological systems and must be present in optimal concentration ranges. At very low concentrations, they tend to decrease the metabolic functions of the body, while at higher concentrations they hinder the metabolic reactions of chemical functional groups and other metal ions present in our body. Sometimes, these heavy metals can alter the active sites of the biological molecules and can be toxic for both microorganisms and macro-organisms (Garbisu and Alkorta 2003). In the worst scenarios, some metals can even mount their way up the food chain and hoard in the human body causing bioaccumulation (Wu et al. 2010). Table 1 summarizes the problems caused by heavy metals in plants and humans with their origins and permissible limits in soils (Dixit et al. 2015; Chibuike and Obiora 2014).
The real problem of this decade is the presence of heavy metals in contaminated soils along with various other industrial pollutants termed as co-contamination. According to the EPA record, 40% of the hazardous waste-polluted areas, presented in national priority list, are co-contaminated with heavy metals and organic pollutants (Olaniran et al. 2013). Bioremediation of heavy metals is more difficult than that of organic contaminants, as the latter can be mineralized into carbon dioxide and water, but heavy metals cannot be mineralized and can only can be converted to less toxic form or immobilized to reduce their bioavailability. Besides, heavy metals impede the biodegradation of organic and inorganic contaminants making co-contaminated soils difficult to remediate.
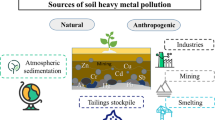
Pollution due to heavy metals has become the foremost issue because of its pervasive practices in industries and untreated dispersal of wastes containing these metals. Also, their toxicity to human beings, plants, and animals is becoming a major medical/health concern. Major heavy metal contaminations arise from industrial effluents and agricultural activities. In this review, we will go through various conventional and advanced methods for the remediation of contaminated soils and weigh their pros and cons. Figure 1 describes different methods of heavy metal (co-contaminant) remediation, categorized as physical, chemical, biological, physiochemical, and physiobiological methods.
Physical methods
Physical methods of heavy metal remediation in contaminated soils have a wide range of spectrum for different waste products. Almost all of the pollutants can be removed through physical removal methods. However, there are disadvantages too. Generally, the pollutants removed using the physical techniques require further processing and have a relatively high cost of application as compared to other techniques. Physical separation methods are mostly based on particle size distribution of pollutants. Few such physical treatments of heavy metal-contaminated soil are described below:
Heat treatment
In this method, the substrate (like soil or sludge) is heated to 300–400 °C. During this process, hydrocarbons and heavy metals, are exposed to very high temperature, making them evaporate and later are recovered by condensation. The advantages of this process are shorter treatment time and complete degradation or removal of metals like Cd and Cu (94 and 97%, respectively) (Shi et al. 2013). Also, the heavy metals can be recovered from ash generated by thermochemical methods such as treatment with KCl and MgCl2 at a high temperature of 900–1000 °C. However, the disadvantage of this technique is the need of very high temperature for operations. Such high temperatures lead to more leaching of the metals and also greater loss of humus (Shi et al. 2013).
Electroremediation
Electroremediation works on the principle of electrokinesis. This process involves application of low electric current to the contaminated substrate for the confinement of the pollutants near the vicinity of electrodes from where they can be recovered later. During the process, contaminants are destroyed, mineralized, and mobilized (Elicker et al. 2014). The advantage of this process is lesser or shorter time interval. It is reported that pre-acidification of substrate improved the remediation results as it increased the dissolution of heavy metals (Wang et al. 2005a).
Electrokinetic remediation basically works on three principles:
-
1.
Electromigration of charged pollutants: it includes metals getting ionized in the applied electric field, leading positively charged (cations) heavy metals to move toward the cathode.
-
2.
Electro-osmosis: it includes movement of electrolytic ions due to viscous drag caused by charged ions’ electromigration.
-
3.
Electrophoresis: it includes migration of colloidal charged ion particles (Gao et al. 2013a, b).
During the process, the H+ ions produced during hydrolysis at the anode are drifted toward the cathode by an electric field and exchange with cationic metals on the surface (Kim et al. 2009; Peng and Tian 2010). The remediation of heavy metals can be achieved by ion exchange, electrodeposition, or precipitation upon their migration to the electrodes.
Poor extraction of heavy metals from soil is the major setback of this technique. Recent reports revealed that the use of enhancing agent aided the solubility of cations and their migration toward the cathode. Pietro et al. investigated an enhanced EK (electrokinetic) treatment of marine sediments co-contaminated with Hg and PAHs (polyaromatic hydrocarbons). MGDA (methylglycinediacetic acid) and non-ionic surfactant were used as novel enhancing agents (EA). The results revealed a synergic action of EK and EA, which led to high Hg and PAH removals (> 60% mobilization of metals occurred). Hence, the use of enhancing agents facilitates more cost-effective and efficient removal of contaminants (Falciglia et al. 2017).
Soil replacement method
Soil replacement method is based on the dilution of pollutant’s concentration in the soils via partially or completely replacing the contaminated soils. In this method, the entire biome of the contaminated soil is isolated with its surrounding to arrest it from affecting the natural and normal environment. Three major modus operandi techniques that sum up the soil replacement method are as follows:
-
1.
The first is the replacement of soil by itself, briefly; the new soil replaces the contaminated soil by complete removal. The major challenge with this technique is the necessity to treat the removed soils feasibly to avoid incurred secondary pollution, if any. Also, this technique is limited to applications at very small areas.
-
2.
The second technique includes the deep burrowing of the soils or soils spading from the contaminated area. By this method, the pollutants get degraded due to lowering of their concentrations in the contaminated soils.
-
3.
In the third technique, namely soil importing, the clean soils are brought from other areas and mixed with the contaminated soils. This decreases the concentration of the pollutants automatically at the contaminated site.
Though the aforementioned soil replacement methods are effective, but being costlier these methods are generally applied when a small area of soil is heavily contaminated (Yao et al. 2012).
Vitrification technology
Vitrification is the process of heating contaminated soils (substrate) to a very high temperature until they liquidize (melting) and then rapidly freeze, forming solids through glass transition. This glass-like solid formation, also called as vitrified product, entraps and immobilizes the contaminant, hence isolating them from the environment. It has low porosity and leaching activity. Vitrification is thus capable of treating co-contaminated soils.
In this method, electric current is used on heat-contaminated soil to a very high temperature (1700–2000 °C), which melts the metals to the vitrified form (Navarro et al. 2013). In Japan, this method was used to reduce the radioactive wastes produced from its nuclear plants and also after the nuclear attack on Hiroshima and Nagasaki. Moreover, few new amendments in this technique outlined the increased immobilization/stabilization efficiency to almost 96% by treatment with fly ash, activated carbon, and nanometallic additives at 1200 °C (Mallampati et al. 2015).
Chemical methods
By using the chemical methods for soil remediation, we aim at remediating the contaminants that have been hoarded in the soils or in making such kind of changes to their chemical characteristics that decrease their hazardous properties (Leštan et al. 2008). Although chemical treatments are highly effective at field scale, they have the drawback of by-product formations, which increases further downstream processing steps. Below are few such chemical treatment techniques for co-contaminated soils.
Precipitation
It is the most conventional method used for effective elimination of heavy metals from contaminated sources. The mechanism involved is as follows:
where M2+ stands for dissolved metal ions, OH− stands for the precipitant, and M(OH)2 stands for insoluble metal hydroxide.
Chemical precipitation occurs at basic pH in the range of 9–11 (Wang et al. 2005b). During this treatment, the associated organic contaminants also undergo alkaline hydrolysis (Emmrich 1999; Bhogle and Pandit 2017; Albers et al. 2017). The benefits of using precipitation comprise its simple, cost-effective, and nontoxic operations. Still, this process requires ample amount of chemical reagents to reduce cation charged metals to a permissible limit of discharge. Other drawbacks are the secondary waste generation, slow and poor settling of precipitate, the aggregation of different metal precipitates, and slow degradation rate of sludge (Aziz et al. 2008).
Ion exchange
The ion exchange process is used to exchange cations or anions from the contaminants and is used effectively in industrial sectors to decrease the concentration of heavy metals in the effluent stream. In this method, heavy metal (undesirable) ions are swapped by other cations which are usually non-polluting in nature (Dabrowski et al. 2004). Co-contaminated soil can also be remediated by using suitable substrate (soil) solution and cation exchange matrix. The heavy metal cations in the soil get exchanged with the cation present in the matrices, maintaining the balance of charge transfer (Kim et al. 2005). In various research studies, natural zeolite has been extensively exploited for the exchange of heavy metal cations such as Cu, Co, Zn, and Mn from co-contaminated soils (Li et al. 2018; Wen et al. 2018; Boros-Lajszner et al. 2018). Matrices generally used are synthetic organic ion exchange resins. However, membrane fouling, pH sensitivity, and non-selectivity of the membrane are few of the drawbacks of this technique.
Chemical extraction and oxidation
Chelating agents are organic compounds which have metal ions binding sites in their structure (Xue et al. 2009). Chelating agents such as EDTA (ethylenediaminetetraacetic acid), NTA (nitrilotriacetic acid), and a chelating resin produced by Diaion named CR11 have been used to treat heavy metal-contaminated sludge. EDTA is an effective reagent in removing metals and has the advantage of being recoverable post-reaction. It has high affinity for metals and forms metal–EDTA complexes. One such study was done using EDTA and hydrogen peroxide to extract organic and heavy metal pollutants. 3% hydrogen peroxide (H2O2) and 0.1 M EDTA removed approximately 70% of the petroleum and 60% of the Cu and Pb in the soils, respectively. Oxidation and extraction together, regardless of the sequence, were effective in bioremediation of TPH (total petroleum hydrocarbon) and heavy metals (Yoo et al. 2017). Nowadays, biodegradable chelants made via green chemistry are being used such as GLDA (diacetic glutamic acid). It is used for the removal of different metals such as Ni, Cd, and Cu-contaminated sludge at acidic pH of 4. When used in the ratio of 3:1 (chelator to sample) the removal efficiency was observed as 89% for Cd, 82% for Ni, and 84% for Cu (Wu et al. 2015). In a recent study, citric acid (167.6 mM) was used as a chelating agent for the effective removal of Zn from contaminated soils (about 92.8%) at acidic pH 4.43 within 30 min (Asadzadeh et al. 2018).
Chemical leaching
This process involves dissolving heavy metal ions into the leaching liquid and extracting them out. The leaching solution is generally acidic in nature to enhance the solubility of the metal ions. This acidification is generally achieved by using inorganic acid such as H2SO4, HCl, or HNO3, maintained at a pH of 1.5–2.0 (Dermont et al. 2008). Chemical leaching is preferred when the concentration of heavy metals is significant and the area of action is huge (Alghanmi et al. 2015). The standpoint of this technique is the need of hefty amount of acid to maintain the pH for suitable solubilization, and later neutralization of such acidified sludge in the downstream requires large operational cost.
Soil amendments (chemical fixation)
In this technique, the solubility and mobility of heavy metal contaminants are decreased using chemical agents such as fly ash, cement, silica, and lime. This process of using chemical agents for immobilization of toxic metals is termed as chemical fixation and the chemicals used for this process are termed as amendments. Metals such as As, Pb, and Cr are the best suited for this process, while metals like Cd, Cu, and Zn can also be stabilized. In a study, calcined cockle shell (CCS) comprising lime showed effective immobilization of Cd, Pb, and Zn in mine tailing soils in 28 days of incubation period. Upon extraction using acid leaching, Cd, Pb, and Zn were reduced up to 85, 85, and 91%, respectively, suggesting CCS to be low-cost soil amendment (Islam et al. 2017).
Similarly, silica treatments were found to immobilize inorganics by the formation of hydroxides, metal silicates, or polymerized silicates. These silicates encapsulate metals or provide active sites for the adsorption of metals (Camenzuli et al. 2017). A study done by Wolfgang Friesl-Hanl showed the heavy metals’ (Cd, Zn, and Pb) immobilizing effect of gravel sludge, red mud, and their combinations (in situ). The high immobilizing efficacies (91, 94, and 83% of Cd, Zn, and Pb, respectively) were attributed to red mud’s high alkalinity and high contents of iron oxides, clay minerals, and calcium carbonate (Friesl et al. 2004).
Recently, an organic polymer chelator potassium dipropyl dithiophosphate (PDD) (5%) in combination with humic acid (7%), generated very stable chelating precipitate by reacting with the heavy metals present in contaminated soil sediment. The obtained stabilization efficiencies for heavy metal were reported as Cu 99.98%, Zn 90.66%, Pb 99.38%, and Cd 92.83% in the sediment (Xu 2017). Likewise, in a study, FeHP (iron hydroxyl phosphate) was used as a chemical fixation agent to simultaneously immobilize Pb, Cd, and As in the soil contaminated with a mixture of heavy metals from the wastewater irrigation area. The immobilization efficiency of Pb, Cd, and As was observed as 59, 44, and 69%, respectively, with only 10% usage of FeHP proving it can be used as a broad-range soil amendment (Yuan et al. 2017).
Nanoremediation
Nanoscale zero-valent iron particles (nZVI) have been most widely used in nanoremediation and are ideal candidates to remediate heavy metals from industrial waste. nZVI has a metallic iron core and iron oxide shell. Its metallic iron core possesses the well-characterized reducing or electron-donating power, while the surface iron hydroxides offer the coordinative and electrostatic functions to attract and adsorb charged ions of heavy metals. Hence, nZVI has two nanocomponents with distinct and complementary functions for the removal of oxyanions (e.g., As(V), Cr(VI)) and cations (e.g., Cu(II), Zn(II), Cd(II), Pd(II), Ni(II)). Nanomaterials are also explored as adsorbents. A recent study evaluated the adsorption of heavy metals (Cd (II), Pb(II), and As (II)) from soil and aqueous samples by zeolite-aided zero-valent iron nanoparticles. The mechanism involved the complex formation with modified nanoparticle, followed by co-precipitation, and finally removal (Li et al. 2018). The advantage of nanoremediation comprises eco-friendly end products, which are produced in very less quantity (Li et al. 2017a). In a study, nano-ZnO particles were investigated for bioremediation of Cd- and Pb-contaminated site. Heavy metal-contaminated sites cause low CAT (catalase coding gene) activity in plants growing in that region, leading to their poor defense mechanism. The presence of ZnO nanoparticles (ZnONPs) in the contaminated soil led to improved CAT and POX (peroxidase coding gene) activity in plants and thereby protected the plants against oxidative stresses. Decreased heavy metal accumulation in plants showed the decrease in the presence of contaminants in the soils (Venkatachalam et al. 2017). Similarly, nanomagnetite coated by silica and MgO (MTM) showed excellent removal capacities toward heavy metals (Pb, Cd, Cu). The heavy metal removal mechanism involved was mainly substitution, followed by precipitation (Nagarajah et al. 2017).
Biological methods
The use of microorganisms, plants, and animals for bioremediation has its own advantages over the conventional methods. These are economically feasible, practical, and moreover do not create any secondary pollution. The contaminant does not require any other treatment and also the natural flora and fauna of the contaminated land can be restored to the original form (Abbas et al. 2014). Below are few techniques being used for remediation using biological agents.
Bioleaching
In this technique, microbes such as iron-oxidizing bacteria (Acidithiobacillus ferrooxidans) or sulfur-oxidizing bacteria are used for leaching purpose instead of chemical reagents. There are two methods by which the microbe aids in metal leaching, as discussed below.
Direct surface attachment
In this technique, bacteria directly interact with heavy metal-contaminated sites by attaching to the metal salts and cause dissolution of metals as explained by the following equation:
where MeS2 is an insoluble metal sulfide and Me2+ is a free metal ion.
Indirect bacterial oxidation
Bacteria such as iron-oxidizing bacteria lead to the formation of Fe3+. This Fe3+ then reacts with the metals and metal solubilization occurs.
According to a recent discovery of an active strain, Hyhel-1, identified as Bacillus sp., was utilized to bioleach copper from heavy metal-contaminated e-wastes. Interestingly, this strain does not need an acidic environment and works better at neutral pH and moderate temperature. Thus, Hyhel-1 strain offers a new cost-effective alternative to recover metals from e-waste without using solvents or acidification (Rozas et al. 2017).
Biological stabilization
In this technique, immobilized bacteria act as solubilizing agents. In a study, nutrient beads of immobilized sulfur-reducing bacteria (SRB) were used for effective transformation of heavy metals into the more stable bound phases. Polyvinyl alcohol (PVA) trapped SRB, provided a protection to bacteria from metal toxicity, and at the same time allowed increased surface area for transmission of matter between cells and sediments. The removal efficiencies of Cu, Zn, Pb, and Cd were obtained as 76.3, 95.6, 100, and 91.2%, respectively. The advantages of this process are that it is recyclable and has no secondary waste generation (Li et al. 2017b).
Animal remediation
When different kinds of wastes which are generally organic in nature are composted, many soil macrofauna and mesofauna help in the remediation process by using such organic wastes in their own metabolism. Moreover, they boost up the metabolic activity of soil microbes. Generally, abiotic conditions are difficult and unfriendly for soil decomposer animals to perform the bioremediation process. The physiochemical conditions of the soil may not always be acceptable by most of the species or animals, due to which they may not be able to colonize in the soil. Furthermore, animals do not generally possess significant metabolic capabilities to reduce the chemicals to completely nontoxic or less toxic states; one may debate that these decomposer animals are incapable of remediation processes.
Recently, a tunicate, Styela plicata, was studied for its bioconcentration capacity in Pb- (40%) and Cd- (13%) polluted environment, and hence its use for the bioremediation activities (Colozza et al. 2017). Vermicomposting has proved to be an efficient and faster method of decomposition of household-related wastes which utilizes earthworms than in the conventional composting process (Haimi 2000). Earthworms by their activities in soil generate –COOH and –CO groups that acidify the soil and in turn activate heavy metals, but because of its slow rate more studies are yet to be done to increase the performance (Wu et al. 2010). According to a study by Suthar et al., Eisenia fetida was fed on paper mill wastewater (PMS) sludge and bioaccumulation of metals was observed. A significant decrease in the level of Cd (32–37%), Cr (47.3–80.9%), Cu (68.8–88.4%), and Pb (95.3–97.5%) was reported, which indicates vermistabilization as a suitable routine for bioremediation of heavy metals (Suthar et al. 2014).
Composting
In this technique, compost is mixed with contaminated soil samples and checked for heavy metal remediation. Compost consists of nutrients for the indigenous microbes present in the contaminated soil. These microbes utilize these nutrients for the remediation of contaminants present. Hence by this method, compost acts as both soil amendment agent and bioremediation technique. In the study of Taiwo et al., Hibiscus cannabinus seeds were used to co-remediate metals (Fe, Mn, Cu, Zn, and Cr). In this technique, compost along with plant technology remediated 72% Mn, 65% Fe, 60% Zn, and 42% Cu respectively (Taiwo et al. 2016). Compost acts as a stabilization agent in the heavy metal-contaminated sites by forming the complexes, or helps in absorption or (co)precipitation of heavy metals (Chen et al. 2015).
Phytoremediation
Phytoremediation involves the application of plants and different plant-associated microbes to partially or completely remove selected contaminants/pollutants from different contaminated sources (Dixit et al. 2015). This process is based on the plant’s ability to take up, store, or degrade pollutants that are present in the vicinity of the plant, irrespective of the soil and water environments (Khan et al. 2004). The efficiency of phytoremediation is dependent on various biological processes such as the interaction between plant and microbes (a rhizospheric process), uptake capacity of plant, translocation and tolerance mechanisms, and plant chelation ability. Recent advancements in various forms of phytotechnology have made our understanding more wide and clear in the fields of plant and soil sciences (Mani and Kumar 2013). There are a number of factors that regulate the efficiency of the plant species to bioremediate heavy metal-contaminated sites, as follows:
-
1.
Accumulation capacity of the candidate plant.
-
2.
Different types of metal ions/contaminants.
-
3.
Plant growth rate at the contaminated site.
-
4.
Planting density.
Other factors that are important in selecting plants for phytoremediation are their fast growth rate, ease in harvesting, high tolerance limits, and efficiency to accumulate different types of metal contaminants. Phytoremediation can be subdivided based on the mode of the action of plants for removing or reducing the toxic contaminants from the soils as follows:
Phytoextraction
In this type of phytoremediation, accumulator plant species are used to extract metals or organic contaminants from soils by accumulating in various plant parts that can be harvested. Generally, plants prefer accumulating essential metals more than heavy metals, where the low accumulation of heavy metals can be explained by competition for binding sites. For instance, lower accumulation of Cd in cowpea plant is reduced in the presence of higher valence cations (Fe) (Akanang and Adamu 2017).
Phytotransformation
It involves the incorporation of partially or completely degraded organic molecules into plant tissues. In this technique, plant uptake soil pollutants which further undergo enzymatic breakdown into simpler forms that can be utilized by plants for metabolic growth processes. Cannas plant was found to detoxify various soil xenobiotics by undergoing phytotransformation (Kvesitadze et al. 2006).
Phytostimulation
It involves the stimulation of the microbial and fungal populations in soils due to secretion of plant exudates, enzymes, or by-products into the root zone which in turn degrades organic pollutants. This technique relies on the symbiotic effect of plant and plant growth-promoting microbes. In a recent study done on Brassica campestris L. (mustard), an endophytic fungi, isolated from heavy metal-contaminated site, Mucor sp. MHR-7, showed reduction in heavy metal toxicity by 94% along with increased phytostimulation (Zahoor et al. 2017).
Phytostabilization
This approach of phytoremediation exploits the ability of the plants to reduce the migration of various forms of contaminants by checking unwanted processes such as erosion, leaching, or runoff. In this way, they are able to reduce the bioavailability of pollutants in the environment, thus inhibiting their mobility to the food chain. Effective phytostabilization of heavy metals especially Ni and Cr was observed in the roots of Nerium oleander (Elloumi et al. 2017).
Studies have also explored Fetuca rubra L., a grass also named as red fescue, for its role in phytostabilization of heavy metal-contaminated soils. Nowadays, research is progressing toward enhanced phytostabilization using various soil additives such as inherent mineral sorbents (Touceda-González et al. 2017; Radziemska et al. 2017). Radziemska studied the decrease in heavy metal (Pb, Zn, and Cd) concentrations in soils by the presence of minerals (dolomite, halloysite, and chalcedonite) through phytostabilization in the roots of Festuca rubra L. (Radziemska 2018). Hence, aiding phytostabilization using soil additives has come up as a promising technique in remediation of multimetal-contaminated soils.
Phytovolatilization
In this process, plants absorb contaminants from the soil and metabolically transform them into volatile by-products and thereby release them into the atmosphere. Few studies have shown the application of this technique for remediation of Se and Hg from the contaminated soil samples (Henry 2000) (Bañuelos et al. 2000). This process is extensively studied for the production of Se gas from organic and inorganic Se compounds (Terry et al. 1992; Brooks 1998). Considerable attempts have also been made in inserting Hg reductase genes in the genome of plants to enable phytovolatilization (Brooks 1998; Bizily et al. 1999). Studies on As phytovolatilization have been carried out on Pteris vittata, where up to 90% phytovolatilization of As was observed at laboratory scale, suggesting the ability of the plant to release Se using secretory glands present on its frond’s edges (Sakakibara et al. 2010). Researchers have also studied the suitability of this process for the remediation of soil contaminated by radioactive substances (Dushenkov 2003). Apart from the advantages, phytovolatilization is still a controversial technique as it releases toxic heavy metals back into the environment.
Rhizofiltration
This process mainly concerns the uptake of pollutants such as heavy metals and organic contaminants from wastewater and water streams contaminated with industrial wastes (Mathew 2005). The total amount of metals removed can be estimated by multiplying the amount of metal concentration in the harvested plant material and the harvested amount of biomass (Salt et al. 1998). In a recent study by Chen et al., plant growth-promoting bacteria (PGPB) were utilized, which helped in increasing the bioavailability of heavy metal Ni for the plant (Chen et al. 2017).
Plants that can accumulate high amount of various heavy metals from soils were termed as ‘hyperaccumulator’ and they translocate them from the roots to the aboveground plant organs such as shoots and leaves at a very high concentration without showing any phytotoxicity. Such hyperaccumulator plants overcome phytotoxicity due to the presence of a versatile property known as hypertolerance, making them as sensitive as non-hyperaccumulators. The amount of hyperaccumulation of different heavy metals can vary significantly in different species or also within the same species based on their ecotype and populations (Rascio and Navari-Izzo 2011). In a study by Stein et al., Arabidopsis halleri, commonly known as Rockcress, were grown on contaminated brownfield sites and were found to be slurping up and bioaccumulating heavy metal ions from soils into leaves. Its bioaccumulation concentration was as high as Zn 5.4 and 0.3% Cd based on dry biomass (Bradley 2017). Table 2 presents a list of plant species being used for phytoremediation. Phytoremediation is a comparatively slower process than existing traditional physical and chemical techniques, because it requires several growing seasons with appropriate soil conditions that can support their growth for site cleanup (McIntyre 2003; Mathew 2005).
Microbial remediation
In such kind of bioremediation processes, microorganisms remediate organic contaminants along with heavy metals. Organic contaminants are converted to products such as CO2, water, or other metabolites which are further directly used by microbes in their metabolism and growth. Microorganisms perform bioremediation by two main methods: either they release contaminant-degradative enzymes or they develop resistance toward these contaminants. In case of heavy metal bioremediation, the uptake of heavy metals by microorganisms takes place either by bioaccumulation which is an active process and/or through adsorption which is a passive process. Microbial cell wall comprises various functional groups such as carboxylate, hydroxyl, amino, and phosphate. The metal ions can easily bind to such groups and be separated from the environment. Metal ions bind to the bacterial cell surface via different interactions such as covalent bonding, and electrostatic and van der Waals forces (Rajendran et al. 2003). Microorganisms which act as metal accumulators possess an inherent property of converting toxic form of metal contaminants to nontoxic or less toxic form. There are various mechanisms by which organisms survive metal toxicity:
-
1.
Extrusion system: metals are pushed out through the cells using mechanisms such as chromosomal or plasmid-mediated events.
-
2.
Biotransformation: this is a process by which microorganisms convert the toxic metal to nontoxic forms.
-
3.
Using enzymes like oxidases and reductases: microbes produce these enzymes to convert pollutants to metabolic products.
-
4.
By producing exopolysaccharide (EPS): microorganisms get adapted to the contaminated surrounding by secreting EPS, which develops as an outer hydrophobic cell membrane comprising efflux pumps against the cell membrane disrupting contaminants (e.g., solvents) (Dixit et al. 2015; Garbisu and Alkorta 2003; Singh and Ward 2004; Wu et al. 2010).
-
5.
Synthesizing metallothioneins: these are the metal-binding proteins to which metals form a complex.
Any organism possessing all of these mechanisms working together will be extremely metal-resistant bacteria, but generally microorganisms lack such complete machinery of resistance against metals. This brings forth the need of mutual interaction between microbes, where they complement each other and is named as the mixed culture.
Actinobacteria are excellent candidates for these mixed cultures because of their proven versatility and abundance in the environment. Recent works highlighted that actinobacteria strains are able to remove heavy metals and pesticides simultaneously (Alvarez et al. 2017).
Several works reported the ability of actinobacteria to bioremediate organic compounds or heavy metals. Streptomyces, Rhodococcus, and Amycolatopsis are among the most studied genera.
Few theories regarding such interactions between microbes in mixed culture works are as follows:
-
1.
Conversion of metals into less toxic form, excreting them out, and then immobilization onto the exopolysaccharide of another organism.
-
2.
Extracellular reduction or oxidation of metals by one bacteria, followed by their absorption and intracellular immobilization by metallothionein of another bacteria.
-
3.
Biomineralization of metals by capable bacteria where the rest of the bacteria nutritionally help the bacteria in the process.
A study on marine living bacteria reported various other strategies (apart from above listed) to resist high concentrations of Pb/Hg. These strategies include extracellular sequestration, alteration in cell morphology, altered permeability, or intracellular accumulation, precipitation of heavy metals, biosorption of heavy metals, or their volatilization, demethylation (Naik and Dubey 2017). Table 3 lists different microorganisms that are used for bioaccumulation of heavy metals. Few sulfur-reducing bacteria utilize heavy metals as their electron acceptor for respiration and precipitate them as their metal sulfides and hence help in bioremediation of heavy metals. These bacteria produce hydrogen sulfide by using sulfate as electron acceptor, which helps in oxidizing the organic matter.
Fungi are also being utilized as a contrivance for remediation of heavy metal-contaminated areas because of their ability to accumulate toxic metals. Coprinopsis atramentaria is studied for its bioaccumulation capacity of 76% of Cd2+, when the concentration of Cd2+ was 1 mg L−1, and 94.7% of Pb2+, when the concentration of Pb2+ was 800 mg L−1. Hence, it has been recognized as an able accumulator of heavy metal ions and a very important tool for mycoremediation (Lakkireddy and Kües 2017).
Antarctic-inhabiting psychrotolerant yeasts are also studied for their heavy metal tolerance and are found to remediate 1 mM of Cr(VI), Cd(II), and Cu(II) by 55, 68, and 80%, respectively (Fernández et al. 2017).
Algae also perform well in the field of bioremediation. The term ‘phycoremediation’ is used to denote the remediation which includes either removal, or degradation and assimilation, using various types of algae and cyanobacteria (Chabukdhara et al. 2017). Similar to bacteria, algae have various chemical moieties on their surface such as hydroxyl, carboxyl, phosphate, and amide, which act as metal-binding sites (Abbas et al. 2014; He and Chen 2014). Phaeophyta macroalgae (brown algae) act as a sink for heavy metals. It has a large amount of carboxylic groups on its cell wall leading to high level of heavy metal accumulation due to electrostatic attraction between metals and cell wall. In a study, different species of brown algae were examined for their metal uptake activity. Among them, Padina sp. and Cystoseira sp. were reported to have effective metal uptake capabilities (Ali et al. 2017).
Metal removal using biofilm
The biofilm acts as an efficient bioremediation tool and biological stabilization agent. Biofilms have very high tolerance against toxic compounds even when the concentration is lethal for planktonic cultures. In a study done on Rhodotorula mucilaginosa, metal removal efficiency was in the range from 4.79 to 10.25% for planktonic cells and 91.71–95.39% for biofilm form (Grujić et al. 2017). Biofilms perform bioremediation in two ways:
-
1.
They act as biosorbent and adsorb heavy metals onto surfaces.
-
2.
Exopolymeric substances present in biofilms contain molecules with surfactant or emulsifier properties, which enhance the bioavailability of contaminants (El-Masry et al. 2004).
Integrated phytoremediation system (IPS)
This method involves the presence of plant and microorganism working hand in hand, in a symbiont relationship. In a recent study, IPS comprising plant, fungi, and bacteria was used for As, Cd, Pb, and Zn bioremediation. In the study Acacia saligna along with rhizosphere bacteria aided the phytostabilization of heavy metals in the roots of Eucalyptus camaldulensis (Guarino and Sciarrillo 2017). Plant growth-promoting bacteria (PGPB) are those that constitute the rhizosphere which facilitates the growth of plants in severely heavy metal-contaminated soils. They have been used intensively as adjuncts in heavy metal phytoremediation (Glick 2010; Gamalero and Glick 2011; Kong et al. 2015). These bacteria decrease the bioavailability of heavy metals by binding them with their anionic functional groups and chelating them by secreting extracellular metabolites such as EPS, siderophores (chelator), and organic acids (Ledin et al. 1999; Chen and Cutright 2003; Ma et al. 2011; Park et al. 2011; Madhaiyan et al. 2007).
Another experiment demonstrated that Magnaporthe oryzae CBMB20 and Burkholderia sp. CBMB40 reduced Ni and Cd availability in soils and decreased their accumulation in tomato roots and shoots by immobilizing them (Kuffner et al. 2010; Ahmad et al. 2014; Dourado et al. 2013). In a study conducted on Solanum nigrum growing in Pb-contaminated soils inoculated with Mucor circinelloides, the efficiencies of removal was in the order of: microbial + phytoremediation (58.6%) > phytoremediation (47.2%) > microbial remediation (40.2%) > control. Soil fertility was increased after bioremediation due to change in enzyme activities (Sun et al. 2017).
Genetic engineering
With the advanced genetic engineering tools, it is possible now to engineer microbes with desired characteristics like ability to tolerate metal stress, overexpression of metal-chelating proteins and peptides, and ability of metal accumulation. The design of smart strains can bind to specific metal ions, precipitate, and transform them to less harmful groups (Valls and De Lorenzo 2002).
Corynebacterium glutamicum, the workhorse of biotechnological research, has been used as an As biocontainer and genetically modified using overexpression of ars operons (ars1 and ars2) to sanitize As-contaminated sites (Mateos et al. 2017). Likewise, overexpression of CrMTP4, coding metal tolerance protein (MTP) responsible for Cd tolerance and uptake, was reported. Engineered Chlamydomonas reinhardtii yielded a significant increase in tolerance to Cd toxicity and its accumulation (Ibuot et al. 2017).
Physiochemical methods
Physiochemical methods are developed as an amalgamation of both physical separation and the chemical extraction methods of soil remediation, as they work better together rather than being singularly used (Dermont et al. 2008). Various physiochemical methods for the remediation of contaminated soils are discussed below.
Soil washing
Soil washing involves removal of those soil particles, which host most of the pollutants, from the bulk soil fraction using aqueous chemical extraction on a solid substrate (Wuana and Okieimen 2011). It is an ex situ remediation technique in which violent mixing and scrubbing of soil particles with a washing liquid cause desorption of heavy metal pollutants from the contaminated soils. Recently, application of low-frequency ultrasonic waves aided desorption of pollutants due to microscale sonophysical effects and macroscale mixing (Park and Son 2017). The advantages of this process are working under low acidic conditions and less requirement of washing liquids. Soil washing becomes highly unsuitable due to various drawbacks such as highly bound metal ions on soil particles, surface morphology, and density of metal-contaminated soil particles, different chemical forms of metals, contamination of metal ions in all particle size fractions of soils, and high amount of humic content at the contaminated site (Dermont et al. 2008).
Chemically activated adsorption
Adsorption is enhanced by chemically modifying the adsorbate to increase its adsorptive potential such as activated carbon. A microporous activated carbon, i.e., citric acid-treated sawdust impregnated with ZnCl2, revealed the highest potential (0.23 mol/g and 0.33 mmol/g for Cd2+ and Ni2+ respectively) at a dose of 0.5 g/L within a contact time of 150 min (Nayak et al. 2017).
Ultrasonic leaching
This technique involves the extraction of heavy metal from soils in highly acidic solvent under ultrasonic treatment which enhances the rate of extraction. During sonication, fragmentation of soil particles leads to diffusion of heavy metals from soils to the acidic solvent, augmenting the overall extraction process. In a study, ultrasonication was performed after acidification of heavy metals Cu, Zn, and Pb at pH of 0.75 maintained using conc. HNO3. Solubilization of Cu by 95%, Zn by 82.2%, and Pb by 87.3% occurred (Deng, Feng et al. 2009).
Physiobiological methods
Physiobiological methods are developed as an amalgamation of both physical and biological methods of soils remediation. Various physiobiological approaches for the remediation of contaminated soils are discussed below.
Bio-electrokinetic approach
In a study, heavy metals in the phosphate-associated contaminated soils were detoxified using microbial pretreatment (BIO) and electrokinetic remediation (EKR) in combination. This study provided compelling evidence that the sequential usage of the bioleaching and electrokinetics is superior to the individual methods for the detoxification of heavy metals from the contaminated soils. The detoxification efficiencies of heavy metals in a sequential system were higher than those using the single BIO and EKR technique except for As, and the detoxification efficiency of Zn was found to be the highest. Bioleaching, generation of passivation, and migration direction of the ions are reported to be the attributed factors to the final results (Huang et al. 2017).
Sediment microbial fuel cells (SMFCs)
SMFCs are novel technology for the simultaneous production of renewable energy and bioremediation of heavy metals. SMFCs lack any membrane and are completely anoxic as compared to conventional microbial fuel cells. SMFC utilizes exoelectrogens, which have the ability to transfer electrons to the electrodes by using natural electron shuttles, and electrotrophs, which have ability to accept electrons from electrodes hence reducing the metal ions. These elctrotrophs help in reducing metals present in the sediments. This powering by microbes is an emerging technique for the remediation of heavy metals from sediments, which are used as inoculum for these fuel cells (Abbas et al. 2017).
Immobilized biosorption
Biosorption is a fast method of passive metal removal from the contaminated samples using biological biomass/adsorbents. It has many advantages compared with the conventional techniques. When we encapsulate the biomass it improves the biosorption performance of the biomass as well as increases its physical and chemical stability and reusability. In our previous work, the Agrobacterium biomass was encapsulated in alginate with iron oxide nanoparticles and showed an adsorption capacity of 197.02 mg g−1 for Pb, and was seen to be effective for five consecutive cycles (Tiwari et al. 2017). Large-scale application in industries was made possible due to the reusability property of such adsorption techniques. The other offered advantages of immobilizing the microbial biomass in polymeric matrixes are the improvement in the biomass rigidity and heat resistivity with optimum porosity for practical applications. Hence, it became popular for implementing immobilization techniques for heavy metal treatment from industrial effluents with improved efficiencies (Aryal and Liakopoulou-Kyriakides 2015; Wang and Chen 2009).
Tables 4 and 5 list out various biosorbents used for the bioremediation of heavy metals. Several biosorbent materials have high affinity and selectivity toward heavy metals. Research studies have explored several biosorbents for their metal-binding efficiency by varying various experimental parameters. Biosorbents are alive or dead biomasses which have chemical groups on their surface for selective biosorption to occur. These biomasses are commonly bacteria, yeast, fungi, and algae obtained from activated sludge or fermented wastes. Various other fast-growing organisms, e.g., crustaceans (particularly their shells), moss, and seaweeds are also being used as biosorbents. Agricultural waste products such as tea waste, whey, exhausted coffee, straw, and defatted rice bran are also used for biosorption.
In general, biosorption efficiency of any biosorbent irrespective of its chemical nature depends upon a number of external factors such as the solution chemistry, i.e., its pH, metal ion concentration, and the concentration of the biomass as well as physiochemical parameters such as temperature, contact time, and the nature of the aqueous environment (Rani et al. 2010; Das et al. 2008; Abbas et al. 2014). Free or unbound microbial biomass for heavy metal adsorption was not found to be efficient enough and suffered from various cons such as low-density particle sizes, low mechanical stability, and difficulty in post-operation recovery.
Conclusions
In this review, we have reviewed most of the methods used for remediation of soils starting from conventional physical methods to recent advances in biosorption, microbial fuel cell techniques, and various other bioleaching modifications, which are now being followed worldwide. Conventional methods being expensive and inefficient have slowly paved the way for new methods. Biosorption- and microbial fuel cells-based techniques have come up as strong contenders in recent years. Biosorption is a technique where the biomass exhibits a property like that of a chemical sorbent or ion exchanger, but of biological origin. This characteristic of adsorption and concentration of heavy metals is observed in most inactive dead biomass. Exploiting this property and enhancing it by adding with other popularly known adsorbents have made it even better over the years. The attractive features of biosorption, being its low cost, high efficiency and reusability, have made it an easy approach for remediating soils and also in helping us now to replace many conventional methods. Likewise, the new electrotroph and exoelectrogen studies have opened the gate to the use of contaminated samples for generating electricity. Many other modifications in various aspects of conventional techniques have made heavy metal remediation a cost-effective, efficient, and recyclable technique with negligible secondary waste generation.
References
Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH (2014) Biosorption of heavy metals: a review. J Chem Sci Tech 3(4):74–102
Abbas SZ, Rafatullah M, Ismail N, Syakir MI (2017) A review on sediment microbial fuel cells as a new source of sustainable energy and heavy metal remediation: mechanisms and future prospective. Int J Energy Res 41(9):1242–1264
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Akanang H, Adamu H (2017) The potential of cowpea (Vigna Unguiculata) as bioremediation tool of heavy metals contaminated soil. Journal of Chemical Society of Nigeria 40 (1)
Albers CN, Jacobsen OS, Flores EM, Johnsen AR (2017) Arctic and subarctic natural soils emit chloroform and brominated analogues by alkaline hydrolysis of trihaloacetyl compounds. Environ Sci Technol 51(11):6131–6138
Alghanmi SI, Al Sulami AF, El-Zayat TA, Alhogbi BG, Salam MA (2015) Acid leaching of heavy metals from contaminated soil collected from Jeddah, Saudi Arabia: kinetic and thermodynamics studies. Int Soil Water Conserv Res 3(3):196–208
Ali AY, Idris AM, Ebrahim AM, Eltayeb MA (2017) Brown algae (Phaeophyta) for monitoring heavy metals at the Sudanese Red Sea coast. Appl Water Sci 1–8
Alvarez A, Saez JM, Costa JSD, Colin VL, Fuentes MS, Cuozzo SA, Benimeli CS, Polti MA, Amoroso MJ (2017) Actinobacteria: current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 166:41–62
Aryal M, Liakopoulou-Kyriakides M (2015) Bioremoval of heavy metals by bacterial biomass. Environ Monit Assess 187(1):4173. https://doi.org/10.1007/s10661-014-4173-z
Asadzadeh F, Maleki-Kaklar M, Soiltanalinejad N, Shabani F (2018) Central composite design optimization of zinc removal from contaminated soil, using citric acid as biodegradable chelant. Sci Rep 8(1):2633
Aziz HA, Adlan MN, Ariffin KS (2008) Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: Post treatment by high quality limestone. Bioresour Technol 99(6):1578–1583
Banerjee S, Gothalwal R, Sahu PK, Sao S (2015) Microbial observation in bioaccumulation of heavy metals from the Ash dyke of thermal power plants of Chhattisgarh, India. Adv Biosci Biotechnol 06(02):131–138. https://doi.org/10.4236/abb.2015.62013
Banerjee A, Sarkar P, Banerjee S (2016) Application of statistical design of experiments for optimization of As (V) biosorption by immobilized bacterial biomass. Ecol Eng 86:13–23
Bang J, Kamala-Kannan S, Lee KJ, Cho M, Kim CH, Kim YJ, Bae JH, Kim KH, Myung H, Oh BT (2015) Phytoremediation of heavy metals in contaminated water and soil using Miscanthus sp. Goedae-Uksae 1. Int J Phytoremediation 17(1–6):515–520. https://doi.org/10.1080/15226514.2013.862209
Bañuelos G, Zambrzuski S, Mackey B (2000) Phytoextraction of selenium from soils irrigated with selenium-laden effluent. Plant Soil 224(2):251–258
Bhogle CS, Pandit AB (2017) Ultrasound-assisted alkaline hydrolysis of waste poly (ethylene terephthalate) in aqueous and non-aqueous media at low temperature. Indian Chemical Engineer 1–19
Bizily SP, Rugh CL, Summers AO, Meagher RB (1999) Phytoremediation of methylmercury pollution: merB expression in Arabidopsis thaliana confers resistance to organomercurials. Proc Natl Acad Sci 96(12):6808–6813
Boros-Lajszner E, Wyszkowska J, Kucharski J (2018) Use of zeolite to neutralise nickel in a soil environment. Environ Monit Assess 190(1):54
Bradley D (2017) Rockcress against heavy metal. Mater Today 1(20):6–7
Brooks RR (1998) Plants that hyperaccumulate heavy metals: their role in phytoremediation, microbiology, archaeology, mineral exploration, and phytomining
Camenzuli D, Wise LE, Stokes AJ, Gore DB (2017) Treatment of soil co-contaminated with inorganics and petroleum hydrocarbons using silica: implications for remediation in cold regions. Cold Reg Sci Technol 135:8–15
Chabukdhara M, Gupta SK, Gogoi M (2017) Phycoremediation of heavy metals coupled with generation of bioenergy. In: Algal Biofuels. Springer, pp 163–188
Chaudhary A, Shirodkar S, Sharma A (2017) Characterization of nickel tolerant bacteria isolated from heavy metal polluted glass industry for its potential role in bioremediation. Soil Sediment Contam: Int J 26(2):184–194
Chen H, Cutright TJ (2003) Preliminary evaluation of microbially mediated precipitation of cadmium, chromium, and nickel by rhizosphere consortium. J Environ Eng 129(1):4–9
Chen M, Xu P, Zeng G, Yang C, Huang D, Zhang J (2015) Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: applications, microbes and future research needs. Biotechnol Adv 33(6):745–755
Chen X, Liu X, Zhang X, Cao L, Hu X (2017) Phytoremediation effect of Scirpus triqueter inoculated plant-growth-promoting bacteria (PGPB) on different fractions of pyrene and Ni in co-contaminated soils. J Hazard Mater 325:319–326
Chibuike G, Obiora S (2014) Heavy metal polluted soils: effect on plants and bioremediation methods. Applied and Environmental Soil Science 2014
Çolak F, Atar N, Yazıcıoğlu D, Olgun A (2011) Biosorption of lead from aqueous solutions by Bacillus strains possessing heavy-metal resistance. Chem Eng J 173(2):422–428. https://doi.org/10.1016/j.cej.2011.07.084
Colozza N, Gravina MF, Amendola L, Rosati M, Akretche DE, Moscone D, Arduini F (2017) A miniaturized bismuth-based sensor to evaluate the marine organism Styela plicata bioremediation capacity toward heavy metal polluted seawater. Sci Total Environ 584:692–700
Congeevaram S, Dhanarani S, Park J, Dexilin M, Thamaraiselvi K (2007) Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J Hazard Mater 146(1):270–277
Dabrowski A, Hubicki Z, Podkościelny P, Robens E (2004) Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 56(2):91–106
Das N, Vimala R, Karthika P (2008) Biosorption of heavy metals—an overview. Indian J Biotechnol 7(2):159–169
de la Rosa G, Peralta-Videa JR, Montes M, Parsons JG, Cano-Aguilera I, Gardea-Torresdey JL (2004) Cadmium uptake and translocation in tumbleweed (Salsola kali), a potential Cd-hyperaccumulator desert plant species: ICP/OES and XAS studies. Chemosphere 55(9):1159–1168. https://doi.org/10.1016/j.chemosphere.2004.01.028
Dermont G, Bergeron M, Mercier G, Richer-Laflèche M (2008) Soil washing for metal removal: a review of physical/chemical technologies and field applications. J Hazard Mater 152(1):1–31
Dil EA, Ghaedi M, Ghezelbash GR, Asfaram A, Purkait MK (2017) Highly efficient simultaneous biosorption of Hg2+, Pb2+ and Cu2+ by Live yeast Yarrowia lipolytica 70562 following response surface methodology optimization: Kinetic and isotherm study. J Ind Eng Chem 48:162–172
Dixit R, Wasiullah EY, Malaviya D, Pandiyan K, Singh U, Sahu A, Shukla R, Singh B, Rai J, Sharma P, Lade H, Paul D (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7(2):2189–2212. https://doi.org/10.3390/su7022189
Dourado M, Martins P, Quecine M, Piotto F, Souza L, Franco M, Tezotto T, Azevedo R (2013) Burkholderia sp. SCMS54 reduces cadmium toxicity and promotes growth in tomato. Ann Appl Biol 163(3):494–507
Duda-Chodak A, Wajda L, Tarko T (2013) The immobilization of Arthrospira platensis biomass in different matrices—a practical application for lead biosorption. J Environ Sci Health A Tox Hazard Subst Environ Eng 48(5):509–517. https://doi.org/10.1080/10934529.2013.730425
Dushenkov S (2003) Trends in phytoremediation of radionuclides. Plant Soil 249(1):167–175
Elicker C, Sanches Filho P, Castagno K (2014) Electroremediation of heavy metals in sewage sludge. Braz J Chem Eng 31(2):365–371
Elloumi N, Belhaj D, Mseddi S, Zouari M, Abdallah FB, Woodward S, Kallel M (2017) Response of Nerium oleander to phosphogypsum amendment and its potential use for phytoremediation. Ecol Eng 99:164–171
El-Masry MH, El-Bestawy E, El-Adl NI (2004) Bioremediation of vegetable oil and grease from polluted wastewater using a sand biofilm system. World J Microbiol Biotechnol 20(6):551–557
Emmrich M (1999) Kinetics of the alkaline hydrolysis of 2, 4, 6-trinitrotoluene in aqueous solution and highly contaminated soils. Environ Sci Technol 33(21):3802–3805
Esmaeili A, Saremnia B, Kalantari M (2015) Removal of mercury (II) from aqueous solutions by biosorption on the biomass of Sargassum glaucescens and Gracilaria corticata. Arabian J Chem 8(4):506–511
Espinosa-Ortiz EJ, Shakya M, Jain R, Rene ER, van Hullebusch ED, Lens PN (2016) Sorption of zinc onto elemental selenium nanoparticles immobilized in Phanerochaete chrysosporium pellets. Environ Sci Pollut Res 23(21):21619–21630
Falciglia PP, Malarbì D, Greco V, Vagliasindi FG (2017) Surfactant and MGDA enhanced-electrokinetic treatment for the simultaneous removal of mercury and PAHs from marine sediments. Sep Purif Technol 175:330–339
Friesl W, Horak O, Wenzel WW (2004) Immobilization of heavy metals in soils by the application of bauxite residues: pot experiments under field conditions. J Plant Nutr Soil Sci 167(1):54–59
Gabr RM, Hassan SHA, Shoreit AAM (2008) Biosorption of lead and nickel by living and non-living cells of Pseudomonas aeruginosa ASU 6a. Int Biodeterior Biodegrad 62(2):195–203. https://doi.org/10.1016/j.ibiod.2008.01.008
Gamalero E, Glick BR (2011) Mechanisms used by plant growth-promoting bacteria. In: Bacteria in agrobiology: plant nutrient management. Springer, pp 17–46
Gao J, Luo Q-S, Zhu J, Zhang C-B, Li B-Z (2013a) Effects of electrokinetic treatment of contaminated sludge on migration and transformation of Cd, Ni and Zn in various bonding states. Chemosphere 93(11):2869–2876
Gao J, Luo Q, Zhang C, Li B, Meng L (2013b) Enhanced electrokinetic removal of cadmium from sludge using a coupled catholyte circulation system with multilayer of anion exchange resin. Chem Eng J 234:1–8
Garbisu C, Alkorta I (2003) Basic concepts on heavy metal soil bioremediation. ejmp & ep (European Journal of Mineral Processing and Environmental Protection) 3 (1):58–66
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28(3):367–374
Grujić S, Vasić S, Radojević I, Čomić L, Ostojić A (2017) Comparison of the Rhodotorula mucilaginosa biofilm and planktonic culture on heavy metal susceptibility and removal potential. Water Air Soil Pollut 228(2):73
Guarino C, Sciarrillo R (2017) Effectiveness of in situ application of an integrated phytoremediation system (IPS) by adding a selected blend of rhizosphere microbes to heavily multi-contaminated soils. Ecol Eng 99:70–82
Haimi J (2000) Decomposer animals and bioremediation of soils. Environ Pollut 107(2):233–238
Han X, Gong YF, Wong YS, Tam NF (2014) Cr(III) removal by a microalgal isolate, Chlorella miniata: effects of nitrate, chloride and sulfate. Ecotoxicology 23(4):742–748. https://doi.org/10.1007/s10646-014-1178-x
He J, Chen JP (2014) A comprehensive review on biosorption of heavy metals by algal biomass: materials, performances, chemistry, and modeling simulation tools. Bioresour Technol 160:67–78. https://doi.org/10.1016/j.biortech.2014.01.068
Henry JR (2000) Overview of the Phytoremediation of lead and mercury. In: Overview of the phytoremediation of lead and mercury. EPA
Huang W, Liu ZM (2013) Biosorption of Cd(II)/Pb(II) from aqueous solution by biosurfactant-producing bacteria: isotherm kinetic characteristic and mechanism studies. Colloids Surf B Biointerfaces 105:113–119. https://doi.org/10.1016/j.colsurfb.2012.12.040
Huang T, Peng Q, Yu L, Li D (2017) The detoxification of heavy metals in the phosphate tailing contaminated soil through sequential microbial pretreatment and electrokinetic remediation. Soil Sediment Contam: Int J 26(3):308–322
Ibuot A, Dean AP, McIntosh OA, Pittman JK (2017) Metal bioremediation by CrMTP4 over-expressing Chlamydomonas reinhardtii in comparison to natural wastewater-tolerant microalgae strains. Algal Res 24:89–96
Iram S, Abrar S (2015) Biosorption of copper and lead by heavy metal resistant fungal isolates. Int J Sci Res Publ 5(1):1–5
Islam MN, Taki G, Nguyen XP, Jo YT, Kim J, Park JH (2017) Heavy metal stabilization in contaminated soil by treatment with calcined cockle shell. Environ Sci Pollut Res 24(8):7177–7183
Iyer A, Mody K, Jha B (2005) Biosorption of heavy metals by a marine bacterium. Mar Pollut Bull 50(3):340–343. https://doi.org/10.1016/j.marpolbul.2004.11.012
Khan FI, Husain T, Hejazi R (2004) An overview and analysis of site remediation technologies. J Environ Manage 71(2):95–122
Kim W-S, Kim S-O, Kim K-W (2005) Enhanced electrokinetic extraction of heavy metals from soils assisted by ion exchange membranes. J Hazard Mater 118(1–3):93–102
Kim D-H, Ryu B-G, Park S-W, Seo C-I, Baek K (2009) Electrokinetic remediation of Zn and Ni-contaminated soil. J Hazard Mater 165(1):501–505
Kong Z, Mohamad OA, Deng Z, Liu X, Glick BR, Wei G (2015) Rhizobial symbiosis effect on the growth, metal uptake, and antioxidant responses of Medicago lupulina under copper stress. Environ Sci Pollut Res 22(16):12479–12489
Kuffner M, De Maria S, Puschenreiter M, Fallmann K, Wieshammer G, Gorfer M, Strauss J, Rivelli A, Sessitsch A (2010) Culturable bacteria from Zn-and Cd-accumulating Salix caprea with differential effects on plant growth and heavy metal availability. J Appl Microbiol 108(4):1471–1484
Kulakovskaya T, Ryazanova L, Zvonarev A, Khokhlova G, Ostroumov V, Vainshtein M (2018) The biosorption of cadmium and cobalt and iron ions by yeast Cryptococcus humicola at nitrogen starvation. Folia Microbiol 1–4
Kvesitadze G, Khatisashvili G, Sadunishvili T, Ramsden JJ (2006) Biochemical mechanisms of detoxification in higher plants: basis of phytoremediation. Springer Science & Business Media
Kwak HW, Kim MK, Lee JY, Yun H, Kim MH, Park YH, Lee KH (2015) Preparation of bead-type biosorbent from water-soluble Spirulina platensis extracts for chromium (VI) removal. Algal Res 7:92–99
Lakkireddy K, Kües U (2017) Bulk isolation of basidiospores from wild mushrooms by electrostatic attraction with low risk of microbial contaminations. AMB Express 7(1):28
Ledin M, Krantz-Rülcker C, Allard B (1999) Microorganisms as metal sorbents: comparison with other soil constituents in multi-compartment systems. Soil Biol Biochem 31(12):1639–1648
Leštan D, C-l Luo, X-d Li (2008) The use of chelating agents in the remediation of metal-contaminated soils: a review. Environ Pollut 153(1):3–13
Li S, Wang W, Liang F, W-x Zhang (2017a) Heavy metal removal using nanoscale zero-valent iron (nZVI): theory and application. J Hazard Mater 322:163–171
Li X, Dai L, Zhang C, Zeng G, Liu Y, Zhou C, Xu W, Wu Y, Tang X, Liu W (2017b) Enhanced biological stabilization of heavy metals in sediment using immobilized sulfate reducing bacteria beads with inner cohesive nutrient. J Hazard Mater 324:340–347
Li Z, Wang L, Meng J, Liu X, Xu J, Wang F, Brookes P (2018) Zeolite-supported nanoscale zero-valent iron: new findings on simultaneous adsorption of Cd (II), Pb(II), and As (III) in aqueous solution and soil. J Hazard Mater 344:1–11
Ma Y, Prasad M, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29(2):248–258
Madhaiyan M, Poonguzhali S, Sa T (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69(2):220–228
Mahmoud ME, Yakout AA, Abdel-Aal H, Osman MM (2012) High performance SiO2-nanoparticles-immobilized-Penicillium funiculosum for bioaccumulation and solid phase extraction of lead. Bioresour Technol 106:125–132. https://doi.org/10.1016/j.biortech.2011.11.081
Mallampati SR, Mitoma Y, Okuda T, Simion C, Lee BK (2015) Dynamic immobilization of simulated radionuclide 133 Cs in soil by thermal treatment/vitrification with nanometallic Ca/CaO composites. J Environ Radioact 139:118–124
Manasi Rajesh V, Rajesh N (2014) Adsorption isotherms, kinetics and thermodynamic studies towards understanding the interaction between a microbe immobilized polysaccharide matrix and lead. Chem Eng J 248:342–351. https://doi.org/10.1016/j.cej.2014.03.022
Mani D, Kumar C (2013) Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: an overview with special reference to phytoremediation. Int J Environ Sci Technol (Tehran) 11(3):843–872. https://doi.org/10.1007/s13762-013-0299-8
Manikandan R, Sahi SV, Venkatachalam P (2015) Impact assessment of mercury accumulation and biochemical and molecular response of Mentha arvensis: a potential hyperaccumulator plant. Sci World J 2015:715217. https://doi.org/10.1155/2015/715217
Mateos LM, Villadangos AF, Alfonso G, Mourenza A, Marcos-Pascual L, Letek M, Pedre B, Messens J, Gil JA (2017) The arsenic detoxification system in corynebacteria: basis and application for bioremediation and redox control. Adv Appl Microbiol 99:103–137
Mathew AM (2005) Phytoremediation of heavy metal contaminated soil. Oklahoma State University
McIntyre T (2003) Phytoremediation of heavy metals from soils. In: Phytoremediation. Springer, pp 97–123
Nagarajah R, Wong KT, Lee G, Chu KH, Yoon Y, Kim NC, Jang M (2017) Synthesis of a unique nanostructured magnesium oxide coated magnetite cluster composite and its application for the removal of selected heavy metals. Sep Purif Technol 174:290–300
Naik MM, Dubey S (2017) Lead-and mercury-resistant marine bacteria and their application in lead and mercury bioremediation. in: marine pollution and microbial remediation. Springer, pp 29–40
Navarro A, Cardellach E, Cañadas I, Rodríguez J (2013) Solar thermal vitrification of mining contaminated soils. Int J Miner Process 119:65–74
Nayak A, Bhushan B, Gupta V, Sharma P (2017) Chemically activated carbon from lignocellulosic wastes for heavy metal wastewater remediation: effect of activation conditions. J Colloid Interface Sci 493:228–240
Olaniran AO, Balgobind A, Pillay B (2013) Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. Int J Mol Sci 14(5):10197–10228
Oves M, Khan MS, Zaidi A (2013) Biosorption of heavy metals by Bacillus thuringiensis strain OSM29 originating from industrial effluent contaminated north Indian soil. Saudi J Biol Sci 20(2):121–129. https://doi.org/10.1016/j.sjbs.2012.11.006
Özdemir S, Kilinc E, Poli A, Nicolaus B, Güven K (2012) Cd, Cu, Ni, Mn and Zn resistance and bioaccumulation by thermophilic bacteria, Geobacillus toebii subsp. decanicus and Geobacillus thermoleovorans subsp. stromboliensis. World J Microbiol Biotechnol 28(1):155–163. https://doi.org/10.1007/s11274-011-0804-5
Özdemir S, Kılınç E, Poli A, Nicolaus B (2013) Biosorption of heavy metals (Cd2+, Cu2+, Co2+, and Mn2+) by thermophilic bacteria, Geobacillus thermantarcticus and Anoxybacillus amylolyticus: equilibrium and kinetic studies. Biorem J 17(2):86–96. https://doi.org/10.1080/10889868.2012.751961
Park B, Son Y (2017) Ultrasonic and mechanical soil washing processes for the removal of heavy metals from soils. Ultrason Sonochem 35:640–645
Park JH, Bolan N, Megharaj M, Naidu R (2011) Isolation of phosphate solubilizing bacteria and their potential for lead immobilization in soil. J Hazard Mater 185(2):829–836
Patel PC, Goulhen F, Boothman C, Gault AG, Charnock JM, Kalia K, Lloyd JR (2007) Arsenate detoxification in a Pseudomonad hypertolerant to arsenic. Arch Microbiol 187(3):171–183
Peng G, Tian G (2010) Using electrode electrolytes to enhance electrokinetic removal of heavy metals from electroplating sludge. Chem Eng J 165(2):388–394
Puyen ZM, Villagrasa E, Maldonado J, Diestra E, Esteve I, Sole A (2012) Biosorption of lead and copper by heavy-metal tolerant Micrococcus luteus DE2008. Bioresour Technol 126:233–237. https://doi.org/10.1016/j.biortech.2012.09.036
Radziemska M (2018) Study of applying naturally occurring mineral sorbents of Poland (dolomite halloysite, chalcedonite) for aided phytostabilization of soil polluted with heavy metals. CATENA 163:123–129
Radziemska M, Gusiatin Z, Bilgin A (2017) Potential of using immobilizing agents in aided phytostabilization on simulated contamination of soil with lead. Ecol Eng 102:490–500
Rajamohan N, Rajasimman M, Dilipkumar M (2014) Parametric and kinetic studies on biosorption of mercury using modified Phoenix dactylifera biomass. J Taiwan Inst Chem Eng 45(5):2622–2627
Rajendran P, Muthukrishnan J, Gunasekaran P (2003) Microbes in heavy metal remediation. Indian J Exp Biol 41(9):935–944
Rani MJ, Hemambika B, Hemapriya J, Kannan VR (2010) Comparative assessment of heavy metal removal by immobilized and dead bacterial cells: a biosorption approach. Afr J Environ Sci Technol 4(2):077–083
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180(2):169–181. https://doi.org/10.1016/j.plantsci.2010.08.016
Rees F, Germain C, Sterckeman T, Morel J-L (2015) Plant growth and metal uptake by a non-hyperaccumulating species (Lolium perenne) and a Cd-Zn hyperaccumulator (Noccaea caerulescens) in contaminated soils amended with biochar. Plant Soil. https://doi.org/10.1007/s11104-015-2384-x
Rozas EE, Mendes MA, Nascimento CA, Espinosa DC, Oliveira R, Oliveira G, Custodio MR (2017) Bioleaching of electronic waste using bacteria isolated from the marine sponge Hymeniacidon heliophila (Porifera). J Hazard Mater 329:120–130
Sakakibara M, Watanabe A, Inoue M, Sano S, Kaise T (2010) Phytoextraction and phytovolatilization of arsenic from As-contaminated soils by Pteris vittata. In: Proceedings of the annual international conference on soils, sediments, water and energy. vol 1. p 26
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49(1):643–668. https://doi.org/10.1146/annurev.arplant.49.1.643
Selvankumar T, Radhika R, Mythili R, Arunprakash S, Srinivasan P, Govarthanan M, Kim H (2017) Isolation, identification and characterization of arsenic transforming exogenous endophytic Citrobacter sp. RPT from roots of Pteris vittata. 3. Biotech 7(4):264
Senoro DB, Godezano JB, Wan M-W, Tayo LL, Sauli Z, Aris H (2017) Effects of pH and concentration on the capability of E. coli and S. epidermidis with bentonite clay as biosorbent for the removal of copper, nickel and lead from polluted water. In: EPJ Web of Conferences. EDP Sciences, p 01081
Shen Y, Li H, Zhu W, Ho S-H, Yuan W, Chen J, Xie Y (2017) Microalgal-biochar immobilized complex: a novel efficient biosorbent for cadmium removal from aqueous solution. Bioresour Technol 244:1031–1038
Shi W, Liu C, Ding D, Lei Z, Yang Y, Feng C, Zhang Z (2013) Immobilization of heavy metals in sewage sludge by using subcritical water technology. Bioresour Technol 137:18–24
Singh A, Ward OP (2004) Biotechnology and bioremediation—an overview. In: Biodegradation and Bioremediation. Springer, pp 1–17
Srinath T, Verma T, Ramteke P, Garg S (2002) Chromium (VI) biosorption and bioaccumulation by chromate resistant bacteria. Chemosphere 48(4):427–435
Strouhal M, Kizek R, Vacek J, Trnková L, Němec M (2003) Electrochemical study of heavy metals and metallothionein in yeast Yarrowia lipolytica. Bioelectrochemistry 60(1–2):29–36. https://doi.org/10.1016/s1567-5394(03)00043-4
Sun L, Cao X, Li M, Zhang X, Li X, Cui Z (2017) Enhanced bioremediation of lead-contaminated soil by Solanum nigrum L. with Mucor circinelloides. Environ Sci Pollut Res 1–9
Suthar S, Sajwan P, Kumar K (2014) Vermiremediation of heavy metals in wastewater sludge from paper and pulp industry using earthworm Eisenia fetida. Ecotoxicol Environ Saf 109:177–184
Taiwo A, Gbadebo A, Oyedepo J, Ojekunle Z, Alo O, Oyeniran A, Onalaja O, Ogunjimi D, Taiwo O (2016) Bioremediation of industrially contaminated soil using compost and plant technology. J Hazard Mater 304:166–172
Terry N, Carlson C, Raab T, Zayed AM (1992) Rates of selenium volatilization among crop species. J Environ Qual 21(3):341–344
Tian S, Lu L, Labavitch J, Yang X, He Z, Hu H, Sarangi R, Newville M, Commisso J, Brown P (2011) Cellular sequestration of cadmium in the hyperaccumulator plant species Sedum alfredii. Plant Physiol 157(4):1914–1925. https://doi.org/10.1104/pp.111.183947
Tiwari S, Hasan A, Pandey LM (2017) A novel bio-sorbent comprising encapsulated Agrobacterium fabrum (SLAJ731) and iron oxide nanoparticles for removal of crude oil co-contaminant, lead Pb(II). J Environ Chem Eng 5(1):442–452
Touceda-González M, Álvarez-López V, Prieto-Fernández Á, Rodríguez-Garrido B, Trasar-Cepeda C, Mench M, Puschenreiter M, Quintela-Sabarís C, Macías-García F, Kidd P (2017) Aided phytostabilisation reduces metal toxicity, improves soil fertility and enhances microbial activity in Cu-rich mine tailings. J Environ Manage 186:301–313
Tsekova K, Kaimaktchiev A, Tzekova A (2014) Bioaccumulation of heavy metals by microorganisms. Biotechnol Biotechnol Equip 12(2):94–96. https://doi.org/10.1080/13102818.1998.10818998
Valenzuela C, Campos V, Yañez J, Zaror C, Mondaca M (2009) Isolation of arsenite-oxidizing bacteria from arsenic-enriched sediments from Camarones River, Northern Chile. Bull Environ Contam Toxicol 82(5):593–596
Valls M, De Lorenzo V (2002) Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol Rev 26(4):327–338
Velasquez L, Dussan J (2009) Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J Hazard Mater 167(1–3):713–716. https://doi.org/10.1016/j.jhazmat.2009.01.044
Venkatachalam P, Jayaraj M, Manikandan R, Geetha N, Rene ER, Sharma N, Sahi S (2017) Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol Biochem 110:59–69
Visoottiviseth P, Francesconi K, Sridokchan W (2002) The potential of Thai indigenous plant species for the phytoremediation of arsenic contaminated land. Environ Pollut 118(3):453–461
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27(2):195–226. https://doi.org/10.1016/j.biotechadv.2008.11.002
Wang J-Y, Zhang D-S, Stabnikova O, Tay J-H (2005a) Evaluation of electrokinetic removal of heavy metals from sewage sludge. J Hazard Mater 124(1):139–146
Wang LK, Hung Y-T, Shammas NK (2005b) Physicochemical treatment processes, vol 3. Springer, Humana Press
Wang T, Sun H, Mao H, Zhang Y, Wang C, Zhang Z, Wang B, Sun L (2014) The immobilization of heavy metals in soil by bioaugmentation of a UV-mutant Bacillus subtilis 38 assisted by NovoGro biostimulation and changes of soil microbial community. J Hazard Mater 278:483–490. https://doi.org/10.1016/j.jhazmat.2014.06.028
Wen J, Peng Z, Liu Y, Fang Y, Zeng G, Zhang S (2018) A case study of evaluating zeolite, CaCO 3, and MnO 2 for Cd-contaminated sediment reuse in soil. J Soils Sed 18(1):323–332
Wu G, Kang H, Zhang X, Shao H, Chu L, Ruan C (2010) A critical review on the bio-removal of hazardous heavy metals from contaminated soils: issues, progress, eco-environmental concerns and opportunities. J Hazard Mater 174(1–3):1–8. https://doi.org/10.1016/j.jhazmat.2009.09.113
Wu Q, Cui Y, Li Q, Sun J (2015) Effective removal of heavy metals from industrial sludge with the aid of a biodegradable chelating ligand GLDA. J Hazard Mater 283:748–754
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology 2011:1–20. https://doi.org/10.5402/2011/402647
Xu Y (2017) Stabilization of heavy metal-contaminated sediment with a chelator and humic acid mixture. Water Air Soil Pollut 228(1):20
Xu P, Zeng GM, Huang DL, Lai C, Zhao MH, Wei Z, Li NJ, Huang C, Xie GX (2012) Adsorption of Pb(II) by iron oxide nanoparticles immobilized Phanerochaete chrysosporium: equilibrium, kinetic, thermodynamic and mechanisms analysis. Chem Eng J 203:423–431. https://doi.org/10.1016/j.cej.2012.07.048
Xue S, Chen Y, Reeves RD, Baker AJ, Lin Q, Fernando DR (2004) Manganese uptake and accumulation by the hyperaccumulator plant Phytolacca acinosa Roxb. (Phytolaccaceae). Environ Pollut 131(3):393–399
Xue X, Hanna K, Despas C, Wu F, Deng N (2009) Effect of chelating agent on the oxidation rate of PCP in the magnetite/H2O2 system at neutral pH. J Mol Catal A: Chem 311(1):29–35
Yao Z, Li J, Xie H, Yu C (2012) Review on remediation technologies of soil contaminated by heavy metals. Procedia Environmental Sciences 16:722–729. https://doi.org/10.1016/j.proenv.2012.10.099
Yoo J-C, Lee C, Lee J-S, Baek K (2017) Simultaneous application of chemical oxidation and extraction processes is effective at remediating soil co-contaminated with petroleum and heavy metals. J Environ Manage 186:314–319
Yuan Y, Chai L, Yang Z, Yang W (2017) Simultaneous immobilization of lead, cadmium, and arsenic in combined contaminated soil with iron hydroxyl phosphate. J Soils Sed 17(2):432–439
Zahoor M, Irshad M, Rahman H, Qasim M, Afridi SG, Qadir M, Hussain A (2017) Alleviation of heavy metal toxicity and phytostimulation of Brassica campestris L. by endophytic Mucor sp. MHR-7. Ecotoxicol Environ Saf 142:139–149
Zhu H, Fu Y, Jiang R, Yao J, Xiao L, Zeng G (2014) Optimization of copper(II) adsorption onto novel magnetic calcium alginate/maghemite hydrogel beads using response surface methodology. Ind Eng Chem Res 53(10):4059–4066. https://doi.org/10.1021/ie4031677
Zouboulis AI, Loukidou MX, Matis KA (2004) Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process Biochem 39(8):909–916. https://doi.org/10.1016/s0032-9592(03)00200-0
Acknowledgements
The authors would like to thank the Department of Science and Technology, Government of India, for financial supports (Sanction nos: DST/INSPIRE/04/2014/002020, ECR/2016/001027).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in the publication of this article.
Rights and permissions
About this article
Cite this article
Sharma, S., Tiwari, S., Hasan, A. et al. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 8, 216 (2018). https://doi.org/10.1007/s13205-018-1237-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1237-8