Abstract
This study investigated the effect of selenium (Se) biofortification on the mineral composition, phenolic content and antioxidant properties of Jute leaf (Corchorus olitorius). Jute seeds were cultivated in four groups containing 0%, 0.01%, 0.05% and 0.1% Se-fortified organic fertilizer. The leaves were harvested at maturity and the mineral content, total phenol and flavonoid contents were determined. In vitro antioxidant properties of the leaves shown by their free radical scavenging abilities, reducing property, Fe2+ chelating ability, inhibition of Fenton reaction and lipid peroxidation were also assayed. Se content significantly increased from 0.18 ± 0.01 mg/100 g in 0% fortification group to 0.4 ± 0.03 mg/100 g in 0.1% fortification. Total phenol and flavonoid contents with the antioxidants properties increased at 0.05% fortification but reduced at 0.1% fortification. The result suggests that Jute leaf bioaccumulation of Se at 0.05% Se biofortification optimally influenced its mineral, phenolic contents and antioxidant properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium is an essential micronutrient which is vital for human health (Wu et al. 2015). Initially, the focus on selenium research was on its toxicity (Smith et al. 1936), after reports of diseased animals raised in seleniferous areas. However, more research on the element led to the discovery that it exists as a micronutrient and possesses anti- carcinogenic and antioxidant properties (Shamberger and Rudolph 1966). Studies also showed that enzymes such as glutathione peroxidase, thioredoxin reductase and other selenoproteins which include deiodinases, selenoproteins P, K and S possess this element in their active sites (Allan et al. 1999; Rayman 2000; Tinggi 2003). Moreover, the outbreak of Keshan disease in China also ushered in more research on the beneficial roles of this essential micronutrient in the body (Chen 2000). Selenium was then observed to be involved in processes in the body such as anti-oxidation, proper functioning of the immune system, fertility, especially in males and preservation of the integrity of the DNA, among others (Finley et al 2001; Ip et al 2000; Kotrebai et al. 2000). Hence, selenium deficiency has been linked with oxidative stress, rapid degeneration of diseases such as HIV/AIDS, cancer, Alzheimer's disease, cardiovascular diseases and diabetes (Fairweather et al. 2011). Enzymes which also possess the micronutrient in their active site could also be inhibited, affecting their proper function (Guo and Wu 1998).
The exposure of humans to selenium depends largely on the levels of selenium in the environment as it is mainly derived from diet. Plants absorb selenium from the soil and biotransform it into the organic form. However, the level of selenium varies widely in the environment. Research has shown that environments with sedimentary rocks tend to have a higher level of selenium in the soil compared to environments encompassed with igneous rocks (Neal 1995). As a result, crops cultivated on the latter will have little or no level of selenium, predisposing the people in such area to selenium deficiency and also increasing the risk and prevalence of certain diseases such as Keshan disease, Kashin-Beck disease, white muscle disease etc. (Fordyce 2013). Selenium deficiency is sometimes mitigated by the use of oral selenium supplements such as barium selenate, sodium selenate, sodium selenite and potassium selenate in some parts of the world (Timbo et al. 2006; Graham 1991), but over supplementing could occur, leading to selenosis with symptoms such as dizziness, stomach upset, brittle hair and nails, diarrhea, nausea (Reid et al. 2004; Morris and Crane 2013). In selenium deficient areas, a major strategy used for ameliorating low levels of selenium in the soil is the fortification of the soil with selenium fortified fertilizer. Food crops with the ability to absorb and bioaccumulate selenium are then planted on this soil. This ultimately leads to an increase in the selenium content of various parts of these crops. However, a food crop must be able to effectively accumulate selenium in its edible parts before it can be considered to be used to ameliorate selenium deficiency in populations.
Jute (Corchorus olitorius) is a leafy vegetable consumed in countries such as Egypt, Southern Asia, Japan, India, China, Lebanon, Palestine, Syria, Jordan, Tunisia and Nigeria. It is a leading vegetable in Nigeria, Sudan, Uganda, Zimbabwe and Cameroon and is cultivated for its leaves as food (Loumerem and Alercia 2016). The leaves are good sources of Vitamin A and C, protein, ash, fibre, calcium, iron, potassium, thiamine and riboflavin (Loumerem and Alercia 2016; Islam 2013). The leaves were also shown to possess anti-inflammatory, anti-nociceptive, anti- pyretic and antioxidant properties (Oboh et al. 2009; Islam 2013; Zakaira et al. 2006; Yakoub et al. 2018). It is used in the treatment of chronic cystitis, dysuria, gonorrhea, toothache, liver disorders and dysentery (Islam 2013; Hillocks 1998). More research also revealed that the leaves are rich in phenolic compounds such as chlorogenic acid, isorhamnetin and caffeic acid (Oboh et al. 2012). Considering the widespread acceptability of Jute leaf across some West African and Sub- Saharan countries (Duke 1983) and the role of selenium in immune boosting (Riaz and Mehmood 2012; Brigelius-Flohé 2018), selenium bioaccumulation in Jute leaf is expected to increase dietary intake of Se with relatively high geographical spread with the aim of offering a Se rich vegetable for potential immune boosting.
Plant phenols and flavonoids are the secondary metabolites produced naturally in plants via the shikimate or phenylpropanoid pathway. They act as the plant defense system, signaling compounds, protection agents from oxidants and ultraviolet radiation (Lattanzio 2013; Liu 2013). Natural polyphenols have the ability to scavenge free radicals, chelate metal ions and activate antioxidant enzymes (Adefegha and Oboh 2013). They exert health benefits when consumed as a result of their antioxidant activities (Adefegha 2017). Minerals present in the earth crust are absorbed by plants and used for their nutrition. After absorption by the roots, these minerals are translocated to other plant parts where they carry out various biological functions. These minerals are involved in osmoregulation, cell signaling, chlorophyll synthesis, cell turgor and control of cell permeability (Pandey 2015).
Previous studies have observed that selenium biofortification led to an increase in selenium contents in plants and the total phenolic and flavonoid content as well as the mineral composition were affected. Pazurkiewicz-Kocot et al. (2003) observed that selenium altered the accumulation of some minerals in Zea mays. Schiavon et al. (2013) also observed that the chemical composition and antioxidant properties of tomato (Solanum lycopersicon L.) was affected as a result of selenium fertilization. The same applies to mushroom as its antioxidant properties were enhanced as a result of selenium uptake (Bhatia et al. 2014; Gasecka et al. 2016). Considering the role of selenium in immune boosting, and antioxidant augmentations (Huang et al. 2012), Jute leaf Se biofortification is hypothesized to increase dietary source of Se with relatively high geographical spread, with the aim of offering a Se rich vegetable for potential immune boosting and antioxidant properties.
The aim of this study therefore, is to cultivate Se-biofortified Jute leaf as a dietary source of Se. We also investigated the influence of Se-biofortification on the total phenol content and antioxidant properties of the vegetable as previous studies have linked the antioxidant properties of Jute leaves to their constituent phenol content (Oboh et al. 2012).
Materials and methods
Preparation of selenium-fortified fertilizer
Jute leaf (Corchorus olitorius) cultivation on Se-biofortified soil
It has been observed that selenite is the major form of selenium in acidic soils and is readily reduced to organic compounds in plants (John et al. 1991; Kahakachchi et al. 2004). In this experiment, selenium (Se) was sourced as sodium selenite because jute is cultivated mostly in the South- western part of Nigeria where the soils are mostly acidic (Fashina et al. 2015). The selenium fortified fertilizer was produced according to the modified method of Hu et al. (2000) and the organic component of the fertilizer was obtained from the Teaching and Research farm, Federal University of Technology, Akure, Nigeria. Jute leaf was cultivated with the seeds at the botanical garden (Research Section) of the Federal University of Technology, Akure. The seeds were divided into 5 groups, each group per bed (1 m by 5 m): Group 1 is made up of seeds cultivated on vegetable bed containing no fertilizer. Group 2 is seed cultivated on vegetable bed containing 0.01% inclusion of Se-fortified fertilizer to top soil; Group 3 is seeds cultivated on vegetable bed containing 0.05% inclusion of Se-fortified fertilizer to top soil while Group 4 is seeds cultivated on 0.1% inclusion of Se-fortified fertilizer to top soil. The leaves were harvested at maturity (6 weeks after cultivation) and rinsed in distilled water. They were them homogenized in distilled water and stored in the refrigerator at 4 °C for further analysis.
Determination of Se concentration in biofortified Jute leaf
Different concentrations of selenium (0.125, 0.25, 0.50, 1.00 and 2.00 mg/l) were prepared from stock standard solution by serial dilution using 10% Nitric acid. Thereafter, 2 g of samples were weighed into a digesting tube and 10 ml mixture of concentrated nitric acid with concentrated hydrochloric acid (1:3) was added. It was then placed in a microwave digester at a controlled temperature to avoid the volatilization of selenium elements for about 1 h and monitored until the fume of the nitric acid ceased and a clear solution obtained. The digest obtained were made up with distilled deionized water in a 10 ml standard flask which was later transferred into a well cleaned and pre rinsed sample bottled for selenium determination using Hydride generator-Atomic Absorption Spectrophotometer (Ag-AAS) 230ATS installed with hydride generator model 1018 both manufactured by Buck Scientific INC, USA. This method was optimized by spiking sample with a known concentration of selenium solution and the percentage recovery calculated.
Determination of the Mineral content of Se-biofortified Jute leaves
1 g of plant sample was digested using a mixture of 12 ml HNO3 and 4 ml HCl. Samples were boiled for 2 h in covered beakers on a hot plate. All solutions with undissolved residual phases are transferred into the 100 ml volumetric flask and filled to the mark with deionized water followed by filtration through medium filters. The obtained digests were stored no longer than 24 h at a temperature of 8 °C prior to Atomic Absorption Spectroscopy (AAS) analysis. Zn an Fe concentrations in respective solutions were determined with the use of AAS. Flame photometer was used to determine Na and K while Ca and Mg concentrations were determined by titration. FAAS (the GBC 932 plus spectrophotometer) with air acetylene flame and hollow lamps (HCl) as light sources.
Total phenol content
The total phenol content was determined according to the method of Singleton et al. (1999). The homogenates were diluted in varying concentrations and then oxidized with 500 ul of 10% Folin- Ciocalteau's reagent (v/v) and then neutralized with 400 µL 7.5% sodium carbonate. The reaction was incubated for 40 min at 45 °C and then read at 765 nm in the spectrophotometer (721-VIS spectrophotometer). The total phenol content was then calculated and expressed as mg GAE/g, where GAE is garlic acid equivalent.
Total flavonoid content
Determination of the total flavonoid content was carried out according to the method of Meda et al. (2005). 500 µl of methanol, 50 µl of AlCl3, 50 µl of 1 M potassium acetate were added to the sample and allowed to incubate at room temperature for 30 min and read in the spectrophotometer at a wavelength of 415 nm. The total flavonoid content was calculated and expressed as mg QAE/g where QAE is quacertin equivalent.
Ferric reducing antioxidant property (FRAP)
The samples´ reducing property were determined according to the method of Oyaizu (1986). 250 uL of the samples were mixed with 250 µL of 0.2 M phosphate buffer pH (6.6) and 250 µl potassium ferric cyanide. The mixture was incubated for 20 min at 50 °C and trichloroacetic acid was added to it. This was now centrifuged at 650 rpm for 10 min. The supernatant was then mixed with 1 ml of distilled water and 200 µl of ferric chloride. The absorbance was read at 700 nm with the use of spectrophotometer and the ferric reducing antioxidant property was then calculated and expressed as mg AAE/g, where AAE is ascorbic acid equivalent.
DPPH free radical scavenging ability
The ability of the samples to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical was assessed as described by Gyamfi et al. (1999) The extracts (1 ml) were mixed with 1 ml of 0.4 mM methanolic solution containing DPPH radicals, the mixture was then allowed to incubate in the dark for 30 min, after which the absorbance was read at 516 nm. The DPPH free- radical scavenging ability was subsequently calculated as percentage of control.
ABTS* scavenging ability
The ABTS* radical scavenging ability was determined according to Re et al.'s method (1999). The ABTS* radical was generated by the reaction of 7 mmol/l ABTS aqueous solution with K2S2O8 (2.45 mmol/l, final concentration) in the dark for 16 h. The absorbance of the solution was further adjusted with ethanol at the wavelength of 743 nm to 0.700. Thereafter, 200 µL of the appropriate dilution of the sample was added to 1.8 mL of the ABTS solution and the absorbance was read at 735 nm following incubation of 15 min. The trolox equivalent antioxidant capacity was then calculated.
Fenton's reaction
The ability of the samples to prevent Fe2+/H2O2 induced degradation of deoxyribose was carried out according to the method of Halliwell and Gutteridge (1981). Appropriate dilutions of the sample were added to 120 µL of 20 mM deoxyribose. 400 µl of 0.1 M phosphate buffer was then added alongside 40 µl of 21% H2O2, 40 µl Fe2+ solution, and 800 µl of distilled water. The mixture was then allowed to incubate at 37 °C for 30 min. 500 µl of 2.8% of TCA was then added with 400 µl of 0.6% TBA. The mixture was further incubated in boiling water for 20 min. The absorbance was read at 532 nm in the spectrophotometer. The OH− radical scavenging ability was subsequently calculated and expressed as percentage of control.
Fe2+ chelating ability
The Fe2+ chelating ability of the samples was determined according to the method of Minotti and Aust (1987) and modification by Puntel et al. (2005). Freshly prepared 500 µM FeSO4 (150 µl) was added to the reaction mixture containing 168 µl of 0.1 M Tris-HCl (pH 7.4), 218 µl saline and the samples. The mixture was then incubated for 5 min, before the addition of 13 µl of 0.25% 1, 10-orthophenanthroline (w/v). The absorbance was subsequently measured at 510 nm in a spectrophotometer. The Fe (II) chelating ability was then calculated and expressed as percentage of control.
Lipid peroxidation
Induction of lipid peroxidation in an isolated rat kidney was carried out according to the method of Ohkawa et al. (1979) 100 µl of kidney homogenate was added to a mixture containing 30 µl of 0.1 M Tris-HCl buffer (pH 7.4), 0–300 µl of the samples and distilled water. This was followed by incubation at 37 °C for 1 h and the reaction was allowed to cool. 300 µl of 8.1% sodium dodecyl sulfate (SDS) and 500 µl of acetic acid/HCl (pH 3.4) solution were added. 500 µl of 0.8% thiobarbituric acid (TBA) was also added and this mixture was boiled for 1 h. The absorbance of thiobarbituric acid reactive species (TBARS) was measured at 5332 nm and calculated using malondialdehyde as the control.
Statistical analysis
Results were expressed as mean ± standard deviation (SD) of all experiments. All data were appropriately analyzed using one-way ANOVA coupled with Tukey's post hoc test (significant level of mean difference was accepted at p < 0.05) via Graph pad PRISM (V.5.0). The Pearson correlation coefficients (r) were carried out with Statistical Program for Social Science (SPSS version 21.0.Armonk, NY: IBM Corp).
Results and discussion
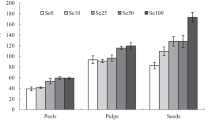
Table 1 shows the Se concentration, as well as calcium (Ca), magnessium (Mg), potassium (K), sodium (Na), iron (Fe) and zinc (Zn) levels of Se biofortified Jute leaf (Corchorus olitorius) samples. The result showed a significant increase (p < 0.05) in Se concentration of Se biofortified leaves with increase in Se concentration. This could be associated with bioaccumulation of Se in the aerial parts of the vegetable. Studies have shown that the accumulation of selenium occurs as a result of modifications in the enzyme pathways that play a role in sulfur metabolism (Brown and Shift, 1982; Giessel- Nielsen et al. 1984). Our result therefore suggests that the plant absorbs selenium from the soil, and the leaves which are the edible portion of the plant successfully bioacccumulates the element.
Furthermore, we observed that bioaccumulation of Se in Jute leaf modulates the levels of other minerals in the leaf. There was a significant increase (p < 0.05) in Zn, Fe and Na levels with increase in Se biofortification (0.01–0.1%) when compared to control. A significant decrease in Ca level was observed at 0.01 and 0.05 selenium percentage inclusion, while no significant changes were observed for K level across the groups. Pazurkiewicz-Kocot et al. (2003) reported an increase in Na concentration in the leaves of Zea mays treated with sodium selenite. Rios et al. 2013 also reported a decrease in the Ca levels in the leaves of tomato plants treated with sodium selenite. Although they also reported an increase in K levels in the tomato leaves, we observed that there was no significant difference in K levels between the groups treated with the selenium fortified fertilizer and the control. The Pearson correlation coefficient (Table 2) revealed a significant positive correlation (p < 0.05) between Se and Zn, Fe as well as Na levels. However, there is a significant negative correlation (p < 0.05) between Selenium and Calcium.
The presence of selenium in plants could affect the absorption and accumulation of other elements in plants. This observation is most likely one of the first signs of an increase in selenium concentration (Pazurkiewicz-Kocot et al. 2003). Na+, K+ and Ca+ are responsible for the regulation of cell membrane potential and turgor in plants and organisms. They may also be involved in some physiological processes in plants such as phloem uploading, elongation growth, water uptake, activation of proteinase inhibitor genes and gas exchange (Allan et al. 1999; Bandurski and Krekule 1988; Loneragan and Webb 1993). Zn and Fe are responsible for protein synthesis, maintenance of cell membrane integrity, enhancement of chlorophyll in plant tissues (Hussain et al. 2015; Rout and Sahoo 2015). The changes in the concentration of these elements could be a result of the changes in the absorption pathway or permeability coefficient of cell membranes (Pazurkiewicz-Kocot et al. 2003) which could be affected by selenium accumulation.
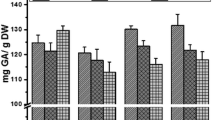
Figure 1 shows the total phenol and total flavonoid contents in each group of Se biofortified Jute leaves. The result showed that the total phenol concentration at 0.05% and 0.1% Se biofortification were significantly higher (p < 0.05) than no Se biofortification. It also showed that the total phenol and total flavonoid contents at 0.1% Se biofortification significantly reduced when compared to 0.05% biofortification. In addition, a strong positive correlation was observed between Se and total phenolic contents in the samples.
a Total phenol content in the different groups of selenium fortified Jute leaves (Corchorus olitorius). *Mean values are significantly different compared to 0% selenium content at p < 0.05. #Mean values are significantly different compared to 0.01% selenium content at p < 0.05. ^Mean values are significantly different compared to 0.05% selenium content at p < 0.05. b Total flavonoid content in the different groups of selenium fortified Jute leaves (Corchorus olitorius). *Mean values are significantly different compared to 0% selenium content at p < 0.05. #Mean values are significantly different compared to 0.01% selenium content at p < 0.05. ^Mean values are significantly different compared to 0.05% selenium content at p < 0.05
Previous studies have reported that jute leaves is rich in total phenol and flavonoid contents which contributed significantly to their antioxidant and other therapeutic properties (Islam 2013; Oboh et al. 2009, 2012). This study shows that total phenolic content increases with increase in selenium biofortification, although the highest total phenol level was observed at 0.05%. The total flavonoid content however was only significantly higher than control at 0.05% Se inclusion. To the best of our knowledge, this is the first report on Se biofortification and its effect on the phenolic content in Jute leaf. Other studies however also observed that selenium fortification increases phenolic content at low concentrations in plants (Groth et al. 2020; Schiavon et al. 2013) and mushroom (Gasecka et al. 2016). Studies have shown that selenium does has some influence on the total phenol and flavonoid contents in plants and mushrooms (Groth et al. 2020; Schiavon et al. 2013; Gupta and Gupta 2017). Attention on phenolic compounds has increased over due the years due to the health benefits associated with them. As a result of their chemical structures, they have the ability to act as antioxidants, mitigating the reactions which can lead to oxidative stress. Selenium induces a change in sulfur assimilation in plants which greatly affects nitrogen metabolism, and consequently the synthesis of amino acids and proteins (Malagoli et al. 2015; Gupta and Gupta 2017).The amino acid phenylalanine is a precursor for phenol synthesis. Hence, an increase in phenolic concentration in the Se biofortified Jute leaves may be as a result of the direct effect of Se with amino acid phenylalanine in the plant. Furthermore, Table 3 shows a positive correlation between selenium and total phenol content, suggesting that selenium has a direct effect on the total phenol content.
Figure 2a shows the ferric reducing antioxidant property of the samples. At 0.05% Se fortification, the reducing ability was significantly higher than that of the 0% and 0.01% Se fortifications. However, the ferric reducing ability at 0.1% fortification was significantly lower, when compared to 0.05% Se fortification.
a Ferric reducing antioxidant property of the different groups of selenium fortified Jute leaves (Corchorus olitorius). *Mean values are significantly different compared to 0% selenium content at p < 0.05. #Mean values are significantly different compared to 0.01% selenium content at p < 0.05. ^Mean values are significantly different compared to 0.05% selenium content at p < 0.05. b DPPH scavenging abilities of the different groups of selenium fortified Jute leaves (Corchorus olitorius). *Mean values are significantly different compared to 0% selenium content at p < 0.05. #Mean values are significantly different compared to 0.01% selenium content at p < 0.05. ^Mean values are significantly different compared to 0.05% selenium content at p < 0.05. c ABTS scavenging ability of the different groups of selenium fortified Jute leaves (Corchorus olitorius). *Mean values are significantly different compared to 0% selenium content at p < 0.05. #Mean values are significantly different compared to 0.01% selenium content at p < 0.05. ^Mean values are significantly different compared to 0.05% selenium content at p < 0.05
1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging ability of the samples is presented in Fig. 2b. The scavenging ability significantly increased across all groups of Se biofortification (0.01–0.1%), when compared to non-biofortified leaves; however, the scavenging ability of 0.1% Se fortification sample was significantly lower, compared to 0.05% Se biofortified leaves. In addition, a strong positive correlation was observed between Se content and DPPH scavenging abilities of the samples (Table 3). Similarly, the ABTS radical scavenging ability at 0.05% Se fortification (Fig. 2c) is shown to be significantly higher (p < 0.05), when compared to the 0% Se fortified group; however, the scavenging ability is shown to be significantly lower at 0.1% Se fortification, when compared to the 0.05% Se fortified group.
One of the properties of antioxidants is the ability to scavenge free radicals which are generated during some metabolic processes. The Se-biofortified vegetables exhibited significant free radical scavenging properties as observed from their DPPH and ABTS scavenging abilities. Both the DPPH and ABTS scavenging abilitiees of the Se-biofortified jute leaves increased with increasing concentration of Se, except the highest concentration (0.1% Se), which is not significantly different (p > 0.05) from the control group. An increase in the in vitro free radical scavenging properties at Se biofortification levels may be due to an increase in the total phenol content of the leaves at the levels of biofortification. Phenols are known to possess free radical scavenging properties. Therefore, we suggest that an increase in the total phenol might be responsible for the increase in the free radical scavenging properties when compared to the control. The decrease in the phenolic content at the highest level (0.1%) of Se biofortification can also be associated with the reduced raical scavenging abilities of 0.1% Se biofortification when compared to the 0.05% Se biofortification.
Selenium biofortified Jute leaves at 0.05% and 0.1% exhibited significantly higher inhibition of Fenton's reaction, when compared to 0% and 0.01% Se biofortified samples (Fig. 3a). In addition, a strong positive correlation was observed between Se content and inhibition of Fenton reaction by the samples. The Fe (II) chelating ability of samples biofortified with 0.01% and 0.1% was observed to be significantly lower, when compared to control (Fig. 3b). However, at 0.05% biofortification, the sample exhibited significantly higher chelating ability when compared to 0.01% biofortification. Figure 3c shows the result for lipid peroxidation reaction in the different sample groups. The ability of the samples to control lipid peroxidation at 0.05% biofortification when compared to the control group and 0.01% biofortified samples is noteworthy. However, at 0.01% biofortification the lipid peroxidation ability is significantly low when compared to 0.05% biofortification.
a Hydroxyl radical scavenging property of the different groups selenium fortified Jute leaves (Corchorus olitorius). *Mean values are significantly different compared to 0% selenium content at p < 0.05. #Mean values are significantly different compared to 0.01% selenium content at p < 0.05. ^Mean values are significantly different compared to 0.05% selenium content at p < 0.05. b Iron chelating ability of the different groups of selenium fortified Jute leaves (Corchorus olitorius). *Mean values are significantly different compared to 0% selenium content at p < 0.05. #Mean values are significantly different compared to 0.01% selenium content at p < 0.05. ^Mean values are significantly different compared to 0.05% selenium content at p < 0.05. c Lipid peroxidation inhibitory ability of the different groups of selenium fortified Jute leaves (Corchorus olitorius). *Mean values are significantly different compared to 0% selenium content at p < 0.05. #Mean values are significantly different compared to 0.01% selenium content at p < 0.05. ^Mean values are significantly different compared to 0.05% selenium content at p < 0.05
Ions such as the ferrous ion when free in the system, that is, not sequestered to a protein have the ability to react with hydrogen peroxide in a reaction known as Fenton's reaction to produce hydroxyl radical (Valko et al. 2007). The hydroxyl radical is a short lived radical which acts at the site of its production. It has the ability to oxidize lipids, proteins and DNA. The cellular membranes of cells are composed of phospho- and sphingo-lipids which contain polyunsaturated fatty acids (PUFA). These PUFAs are targets of oxidation leading to the generation of peroxyl radicals which undergo rearrangement to form endoperoxides, finally forming malondialdehyde as one of the final products of lipid peroxidation (Valko et al. 2007). Our result shows that increase in Se fortification brought about an increase in the inhibition of Fenton' reaction, which could be associated with the increased inhibition of Fe2+-induced lipid peroxidation and Fe2+ chelating abilities. Nevertheless, 0.05% Se biofortification presents the optimum inhibition of lipid peroxidation.
In conclusion, application of selenium fortified fertilizer to the soil used for the cultivation of Jute leaves led to the accumulation of this nutrient in the leaves. The concentration of selenium was observed to increase as the level of biofortification increased. This led to changes in the mineral composition, total phenol content and the antioxidant parameters of the leaves. However, the leaves at 0.05% Se-fortification shows more promise for functional and therapeutic use as the antioxidant status at this level was maxima. However, while the highest Se-bioaccumulation was observed at 0.1% Se-fortification, nevertheless, the total phenol content and some antioxidant properties were reduced, suggesting some negative modulatory effects at this level of Se-fortification.
References
Adefegha SA (2017) Functional foods and nutraceuticals as dietary intervention in chronic diseases; novel perspectives for health promotion and disease prevention. J Diet Suppl. https://doi.org/10.1080/19390211.2017.1401573
Adefegha SA, Oboh G (2013) Phytochemistry and mode of action of some tropical spices in the management of type-2 diabetes and hypertension. Afr J Pharm Pharmacol 7(7):332–346
Allan CB, Lacourciere GM, Stadman TC (1999) Responsiveness of selenoproteins to dietary selenium. Annu Rev Nutr 19:1–16
Bandurski RS, Krekule J (1988) Physiology and biochemistry of auxins in plants. Backhuys Publishers
Bhatia P, Prakash R (2014) Enhance antioxidant properties as a function of selenium uptake by edible mushrooms cultivated on a selenium accumulated waste post-harvested wheat and paddy residues. Int J Recycl Org Waste Agric 3:127–132
Brigelius-Flohé R (2018) Selenium in human health and disease: an overview. Selenium. Springer, Berlin/Heidelberg, pp 3–26
Brown TA, Shift A (1982) Selenium toxicity and tolerance in higher plants. Biol Rev 57:59–84
Chen J (2000) An original discovery: selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac J Clin Nutr 21(3):320–326
Duke JA (1983) Corchorus olitorius L. Handbook of energy crops. http://www.hort.purdue.edu/newcrop/duke_energy/Corchorus_olitorius.html. Accessed 17 Aug 2007
Fairweather- Tait S, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, Hurst R (2011) Selenium in human health and disease. Antioxid Redox Signal 14(7):1337–1383
Fashina AS, Raji A, Oluwatosin GA, Omoju OJ, Oluwadare DA (2015) Properties, genesis, classification, capability and sustainable management of soils from south- western Nigeria. International Journal of Soil Science 10(3):142–152
Finley JW, Ip C, Lisk DJ, Davis CD, Hintze KJ, Whanger PD (2001) Cancer-protective properties of high-selenium broccoli. J Agric Food Chem 49(5):2679–2683
Fordyce FM (2013) Selenium deficiency and toxicity in the environment. Essentials of medical geology. Springer, Dordrecht, pp 375–416
Gasecka M, Mleczek M, Siwulski M, Niendielski P (2016) Phenolic composition and antioxidant properties of Pleurotus ostreatus and Pleurotus eryngii enriched with selenium and zinc. Eur Food Res Technol 242:723–732
Giessel- Nielsen G, Gupta UC, Lamand M, Westermarck T (1984) Selenium in soils and plants. Adv Agron 37:397–460
Graham TW (1991) Trace element deficiencies in cattle. Vet Clin N Am Food Anim Pract 7:153–215
Groth S, Budke C, Neugart S, Ackermann S, Kappenstein FS, Daum D, Rohn S (2020) Influence of selenium biofortification on antioxidants properties and phenolic compounds of apples (Malus domestica). Antioxidants 9:187
Guo X, Wu L (1998) Distribution of free seleno-amino acids in plant tissue of Melilotus indica L. Grown in selenium-laden soils. Ecotoxicol Environ Saf 39(3):207–214
Gupta M, Gupta S (2017) An overview of selenium uptake, metabolism and toxicity in plants. Front Plant Sci. https://doi.org/10.3389/fpls.2016.02074
Gyamfi MA, Yonamine M, Aniya Y (1999) Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. Gen Pharmacol 32:661–667
Halliwell B, Gutteridge JMC (1981) Formation of a thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett 128:347–352
Hillocks RJ (1998) The potential benefit of weeds with reference to small holder agriculture in Africa. Integr Pest Manag Rev 3:155–167
Hu Q, Pan G, Zhu J (2000) Effect of fertilization on selenium content of tea and the nutritional function of Se-enriched tea in rats. Plant Soil 238(1):91–95
Huang Z, Rose AH, Hoffman PR (2012) The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 16(7):706–743
Hussain A, Arshad M, Zahir ZA, Asghar M (2015) Prospects of zinc solubilizing bacteria for enhancing growth of maize. Pak J Agric Sci 52(4):915–922
Ip C, Thompson HJ, Zhu Z, Ganther HE (2000) In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Can Res 60(11):2882–2886
Islam MM (2013) Biochemistry, medicinal and food values of jute (Corchorus capsularis L. and C. olitorius L.) leaf: a review. Int J Enhanc Res Sci Technol Eng. 2(11):35–44
John LF, Roger F, Deverel SJ (1991) Selenium mobility and distribution in irrigated and non irrigated alluvial soils. Soil Sci Soc Am 55:1313–1320
Kahakachchi C, Boakye HT, Uden PC, Tyson JF (2004) Chromatographic speciation of anionic andneutral selenium compounds in Se accumulating Brassica juncea (Indian mustard) and selenized yeast. J Chromatogr A 1054:303–312
Kotrebai M, Birringer M, Tyson JF, Block E, Uden PC (2000) Selenium speciation in enriched and natural samples by HPLC-ICP-MS and HPLC-ESI-MS with perfluorinated carboxylic acid ion-pairing agents. Analyst 125(1):71–78
Lattanzio V (2013) Phenolic compounds: introduction. In: Ramawat KG, Merillon JM (eds) Natural products. https://doi.org/10.1007/978-3642-22144-6_52
Liu RH (2013) Dietary bioactive compounds and their health implications. J Food Sci 78(1):18–25
Loneragan IF, Webb MJ (1993) Interactions between zinc and other nutrients affecting the growth of plants. Soil Sci Soc Am J 46:345–352
Loumerem M, Alercia A (2016) Descriptors for jute (Corchorus olitorius L.). Genet Resourc Crop Evol 63:1103–1111
Malagoli M, Schiavon M, Dall’Acqua S, Pilon-Smits EAH (2015) Effects of selenium biofortification on crop nutritional quality. Front Plant Sci 6:280. https://doi.org/10.33389/fpls.2015.00280
Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG (2005) Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 91:571–577
Minotti G, Aust SD (1987) An investigation into the mechanism of citrate-Fe2_-dependent lipid peroxidation. Free Radic Biol Med 3:379–387
Morris JS, Crane SB (2013) Selenium toxicity from a misformulated dietary supplement, adverse health effects, and the temporal response in the nail biologic monitor. Nutrients 5:1024–1057. https://doi.org/10.3390/nu5041024
Neal RH (1995) Selenium. In: Alloway BJ (ed) Heavy metals in soils. Blackie Academic & Professional, London, pp 260–283
Oboh G, Raddatz H, Henle T (2009) Characterization of the antioxidant properties of hydrophilic and lipophilic extracts of Jute (Corchorus Olitorus) leaf. Int J Food Sci Nutr 60(S2):124–134
Oboh G, Ademiluyi A, Akinyemi A, Henle T, Saliu J, Schwarenbolz U (2012) Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. J Funct Foods 4:450–458
Ohkawa H, Ohisi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Oyaizu M (1986) Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Japan Soc Nutr Food Sci 44:307–315
Pandey R (2015) Mineral nutrition of plants. In: Bahadur B et al. (eds) Plant biology and biotechnology, vol 1. https://doi.org/10.1007/98-81322-22866_20
Pazurkiewicz-Kocot K, Galas W, Kita A (2003) The effect of selenium on the accumulation of some metals in Zea mays L. plants treated with indole-3-acetic acid. Cell Mol Biol Lett 8:97–104
Puntel RL, Nogueira CW, Rocha JBT (2005) Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem Res 30:225–235
Rayman M (2000) The importance of selenium to human health. Lancet J 356(9225):233–241
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radic Biol Med 26:1231–1237
Reid ME, Stratton MS, Lillico AJ, Fakih M, Natarajan R, Clark LC, Marshall JR (2004) A report of high-dose selenium supplementation: response and toxicities. J Trace Elem in Med Biol 18:69–74
Riaz M, Mehmood KT (2012) Selenium in human health and disease: A review. Journal of Postgraduate Medical Institute 26:120–133
Rios JJ, Blasco B, Leyva R, Sanchez-Rodriguez E, Rubio-Wilhelmi MM, Romero L, Ruiz JM (2013) Nutritional balance changes in lettuce plant grown under different doses and forms of selenium. J Plant Nutr 36:1344–1354. https://doi.org/10.1080/01904167.2013.790427
Rout GR, Sahoo S (2015) Role of iron in plant growth and metabolism. Rev Agric Sci 3:1–24. https://doi.org/10.78331/ras3.1
Schiavon M, Dallaqua S, Mietto A, Pilon-Smits E, Sambo P, Masi A, Malagoli M (2013) Selenium fertilization alters the cchemical composition and antioxidant constituents of Tomato (Solanum lycoppersiccon L.). J Agric Food Chem 61:10542–10554
Shamberger RJ, Rudolph G (1966) Protection against carcinogenesis by antioxidants. Experienta 22:116
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin_Ciocalteu reagent. Methods in enzymology 299 (oxidants and antioxidants, part A). Academic Press, San Diego, pp 152–178
Smith MI, Franke KW, Westfall BB (1936) Problem in relation to public health: a preliminary survey to determine the possibility to selenium intoxification in the rural population living on seleniferous soil. Public Health Rep 51(44):1496–1505
Timbo BB, Ross MP, McCarthy PV, Lin CTJ (2006) Dietary supplements in a national survey: prevalence of use and reports of adverse effects. J Am Diet Assoc 106:1966–1974
Tinggi U (2003) Essentiality and toxicity of selenium and its status in Australia: a review. Toxicol Lett 137(1–2):103–110
Valko M, Leibtritz D, Moncol J, Cronin M, Mazura M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Wu Z, Bañuelos GS, Lin ZQ, Liu Y, Yuan L, Yin X, Li M (2015) Biofortification and phytoremediation of selenium in China. Front Plant Sci 6:136. https://doi.org/10.3389/fpls.2015.00136
Yakoub AR, Abdehedi O, Jridi M, Elfalleh W, Nasiri M, Ferchichi A (2018) Flavonoids, phenols, antioxidants and antimicrobial activities in various extracts from Tossia jute leaves (Corchorus olitorus). Ind Crops Prod 118:206–213
Zakaira ZA, Sulaiman MR, Arifah AK, Mat Jais AM, Somchit MN, Kirissnaveni K, Punnitharrani D, Safarul M, Fatimah CA, Johari R (2006) The anti inflammatory and antipyretic activities of Corchorus oliotorus in rats. J Pharmacol Toxicol 1:39–46
Acknowledgements
The authors wish to appreciate the Tertiary Education Trust Fund (TETFUND) of the Nigeria Federal Government under the National Research Fund (NRF) scheme for financially supporting this research work (TETFund/DR &D/CE/NRF/CC/11/VOL1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akindoyeni, I.A., Ogunsuyi, O.B., Aletor, V.A. et al. Effect of selenium biofortification on phenolic content and antioxidant properties of Jute leaf (Corchorus olitorius). Vegetos 35, 94–103 (2022). https://doi.org/10.1007/s42535-021-00288-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-021-00288-w