Abstract
Elevating the selenium concentration in fruit has the potential to enhance the average dietary selenium intake in humans. The application of selenium fertilizer through a spraying method has been found to be an effective approach for producing selenium-enriched fruit. While kiwifruit (Actinidia chinensis) is known for its ability to accumulate selenium, the ‘Hongyang’ variety grown in Sichuan, China has been observed to have low selenium content. The tree was treated by spraying the leaves with water solution containing 10, 25, 50 and 100 mg per litre in the form of sodium selenite (Na2SeO3) in the flowering period. The total Se and organic Se content, and the effects of different concentrations of Na2SeO3 on antioxidant activity in peels, pulps and seeds of fruits were investigated. The findings indicated that kiwifruit has the ability to transform exogenously absorbed inorganic selenium into organic selenium. The organic selenium content in the pulps treated with 50 mg L−1 sodium selenite was 9.04 times higher than in the control treatment. Furthermore, protein-Se was identified as the main component of organic selenium, comprising 48.04–51.15% of the pulps. The protein-Se is the primary component of organic Se, which the proportion in pulps was 48.04–51.15%. The application of 50 and 100 mg L−1 sodium selenite via foliar spraying resulted in a notable enhancement of ferric reducing/antioxidant power (FRAP) and oxygen radical absorbance capacity (ORAC) values across all tissues. A significant positive correlation between FRAP value and organic Se content in pulps, and a significant relationship between ORAC values and protein-Se and polysaccharide-Se content of kiwifruit tissue showed regression equation. In general, the optimum Se application is 50 mg L−1, and some areas with a severe selenium deficiency can apply 100 mg L−1 selenite. It might serve as a source of selenium in dietary supplements or as an ingredient for the formulation of nutraceuticals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is a trace element beneficial to the health of humans. The World Health Organization (WHO) and the Food and Agriculture Organization of the United Nations (FAO) recommended that Se supplements be approximately 55 µg d−1 Se for healthy adults, with maximum intake not exceeding 400 µg d−1 Se. The human demand for selenium is attributed its antioxidant properties (Alfthan et al. 2015; Luo et al. 2021; Xie et al. 2021). The may way plants acquire selenium and introduce the food chain is absorption from the soil. However, vegetables and fruits contribute only 8% of the total Se in human diets (Combs 2001). Therefore, inadequate selenium supplementation can have negative health effects. Keshan and Kaschin-Beck disease is caused by severe Se deficiency in humans, and has been reported especially in rural parts of both China and Russia (Li et al. 2013; Abrahams 2006). Se is mainly present in inorganic and organic species (Dong et al. 2021). Inorganic Se is usually toxic, while organic Se is generally beneficial to human health (Yin et al. 2019). The form in which Se is provided also matters: organic Se is better absorbed and retained than inorganic Se (Shi et al. 2010). This makes organic Se preferable to inorganic Se as a nutraceutical or food additive. It has been reported that an organic Se supplement with immunostimulatory, antioxidant, and antidiabetic activity can be derived from some fungi and bacteria (Jin et al. 2012; Malinowska et al. 2009).
It has previously been suggested that the biological activity of protein and polysaccharides may be enhanced by selenylation and other structural modifications (Lei et al. 2021). Supplementation of Se from natural food sources containing organic selenium is safe and effective compared to supplementation with of inorganic Se (Huang et al. 2007; Ren et al. 2022). Finally, Ip et al. (2000) reported that the specific bioactivity of Se is highly dependent on its chemical forms and that organic Se has the highest bioactivity. Protein-Se is redox activate and a free-radical scavenger (Lu and Holmgren 2009). As has already been shown, Se status can reduce the incidence of cancer (Wallace et al. 2009; Reid et al. 2008). However, biosynthesis, and antioxidant and immunomodulatory activity of polysaccharides containing Se have not been extensively studied (Malinowska et al. 2009).
The concentration of Se in plants depends on both the abundance and availability of Se in the soil. Regional variations in soil properties therefore influence Se content of entire communities (Fan et al. 2015; He et al. 2018). Both selenate (Se VI) and selenite (Se IV) are generally able to transverse the cuticle of the plants and are assimilated by by the sulphur metabolic pathway (Li et al. 2018; Wen 2021). However, Se (IV) is more susceptible to transform to seleno-amino acids than Se (VI). Although Se is an essential element in animals, but not essential in plants (Liu et al. 2016). The cellular effects of Se are concentration dependent. When Se is abundant, it promotes oxidation leading to cellular damage and reduced yield and even pose a threat to the environment (Wu 2004). On the other hand, at low concentrations of Se, it can improve the nutritional quality of plants, such as total amino acids, vitamin C and flavonoids (Wen 2021), stimulate plant growth and act as an antioxidant (Hartikainen et al. 2000; Jiang et al. 2021). The threshold between toxic and beneficial concentrations of Se depends on the plant species and the form in which Se is provided. As a result, it is necessary to find safe and reasonable concentration to increase Se content in plant foods.
Foliar sprays and soil supplementation are two methods that can be used in to enrich Se in crops. However, Hartfiel and Bahners (1988) reported that soil supplementation can be less reliable due to variations in soil properties including pH, redox potentials, and microbial activities. On the other hand, foliar application of Se (IV or VI) has been shown to increase the Se content of many crops including potato (Poggi et al. 2000), rice (Hu et al. 2002), soybean (Yang et al. 2003), cabbage, onion, garlic, radish (Slejkovec and Goessler 2005), buckwheat and pumpkin (Smrkolj et al. 2005, 2006). However, only a few studies have systematically characterized a dose-response to foliar Se application. Furthermore, little is known about the bioavailability of Se and its health impacts in humans. In the present study, we detected the accumulation of organic Se in kiwifruits by different concentration range of sodium selenite (Na2SeO3) to the leaves.
Materials and Methods
Experimental Design and Plants Cultivation
The experiment was carried out under greenhouse conditions at the demonstration orchard in Cangxi County, Sichuan Province (China), using a cement trough (length: width: depth = 1.5:1.5:1) containing samples in a 20–40 cm layer of a sandy clay loam. The organic matter content was 5.41 g/kg and effective [Se] is 4.57 µg/kg. ‘Hongyang’ kiwifruit (Actinidia chinesis cv ‘Hongyang’) trees (6 years old) were tested and divided into five groups (A, B, C, D, E) containing eight trees each, with three replications. The location and soil were the same for all replicates. Plants were separated to avoid contamination between treatments solution. Leaves were sprayed with solutions of sodium selenite at different concentrations during flowering, three times at an interval of two days between each application. Group A was sprayed with distilled water without sodium selenite (Se0). Simultaneously, group B, C, D, and E were sprayed with distilled water containing Se in the form of sodium selenite at 10 mg Se/L (Se10), 25 mg Se/L (Se25), 50 mg Se/L (Se50) and 100 mg Se/L (Se100), respectively. During each treatment, 50 ml of sodium selenite solution (pH 7.0–7.5) was applied by spraying to on the leaves of each group of plants, with care to ensure that the treatments were evenly distributed. Fruits were assayed at physiological maturity.
Extraction and Measurement of Total Selenium
Total Se was measured using HG-AFS (hydride generation atomic absorption spectrometry) according to Smrkolj and Stibilj (2004). Each sample was aliquoted in closed polyfluoroethylene tubes to which 1.5 mL HNO3 and 0.5 mL H2SO4 and 0.2 g Na2MoO4 were added for digestion. The closed tubes were heated for 60 min at 130 ℃ in an aluminum block. After cooling, 2 mL of H2O2 was added followed by heating at 115 ℃ for 10 min. Next 0.1 mL 40% hydrofluoric acid (HF) was added and heated at 115 ℃ for 10 min, followed by a second addition of H2O2 (2 mL). At the end of this digestion, the contents were cooled to room temperature and 0.1 mL of V2O5 in H2SO4 was added. Tubes were subsequently heated at 115 ℃ for 20 min. Reduction of Se (VI) to Se (IV) with 2.5 mL of concentrated HCl was carried out at 100 ℃ for 10 min. Finally, each sample was diluted and Se was quantified using HG-AFS, by comparing to a series of matrix-matched standards, which were prepared fresh daily. The linear ranges of total selenium were 0–125 µg/L, r2 = 0.9979. The limit of detection (LOD) is three times the Signal-to-noise ratio (SNR) and the limit of quantification (LOQ) is ten times the SNR.
Analysis of Organic Se
Organic Se:
30.0 g of fruit tissue (pulp, peel, or seed) were homogenized with a solution of 1.0 mL mercaptoethanol in 20 mL Tris-HCl-glycerol buffer (pH 7.8). Pulp, peel, and seed residues were observed with an electron microscope to ensure cells had been fully disrupted and then the residue was put into a pre-treatment dialysis bag was tied and put it into distilled water (4 ℃) to dialyze 120 h. The distilled water was changed every 12 h. The organic-selenium was analyzed by HG-AFS.
Protein-Se
The Protein of kiwifruit tissues protein was conducted as described by Tomotake et al. (2006). In brief, freeze-dried kiwifruit tissues powder (20.0 g) was firstly defatted by soaking in petroleum ether (w/v, 1:10) for 12 h. The residual solvent was eliminated by air-drying (fume hood for 24 h). Then, the defatted samples were stirred with distilled water at a ratio of 1:10 (w/v) and the pH was adjusted to 9.0 using 1 mol/L NaOH. After stirring for 2 h, the mixture was centrifuged at 1000 × g for 10 min. The supernatants were collected and the pH was adjusted to 4.5 using 1 mol/L HCl, followed by centrifuging at 1000 × g for 10 min repeatedly. The precipitate was lyophilized for further analysis. The selenium in the protein (protein-Se) was subsequently analyzed by HG-AFS.
Polysaccharide-Se
Triplicate fruit tissue (20.0 g) was placed in the test tubes and selenium amylase was extracted with 200 ml 1.0 mol/L NaOH at 60 ℃ for 4 h. The liquid was drawn out and residuum was extracted twice by same method. Then the extraction was collected to remove protein by the Sevag method (Whistler 1965). The supernatant (fluid) was added to 75% ethanol to precipitate polysaccharide at 4 ℃ for 12 h, which was subsequently centrifuged 20 min at 4 ℃ at 3000 g. The precipitate was dried in vacuum and stored at −70 ℃. The selenium in the polysaccharide (polysaccharide-Se) was analyzed by HG-AFS.
Nucleic Acid-Se
Triplicate fruit tissue (20.0 g) was placed in the test tubes with 50 ml 2% NaCl to extract selenium nucleic acid at 90 ℃ for 2 h. The liquid was removed and residuum was extracted once by same method. The filtrate collected and protein removed by the Sevag method (Whistler 1965). To the extract, 6 mol/L HCl was added to pH 2.5 and held at 4 ℃ for 12 h. The solution was centrifuged at 3000 g for 10 min at 4 ℃ for nucleic acid precipitation. The precipitate was rinsed with 95% ethanol three times and was stored at −70 ℃ or assays after it was dried in vacuum. The selenium in the nucleic acid (nucleic acid-Se) was analyzed by HG-AFS.
Antioxidant Activity
Antioxidants in tissues can be measured as: 1,1-diphenyl-2-picrylhydrazyl (DDHP); oxygen radical absorbance capacity (ORAC); total reactive antioxidant capacity (TEAC); and ferric reducing/antioxidant power (FRAP). In the present study, FRAP and ORAC were chosen to evaluate the antioxidant activities of kiwifruit pulp, peels and seeds.
FRAP was determined essentially as described by Benzie and Strain (1996). The FRAP reagent was prepared freshly and warmed at 37 ℃ and comprised 2.5 mL 10 mM TPTZ (2,4,6- tripyridy-s-triazine, Sigma) in 40 mM HCl plus 2.5 mL of 20 mM FeCl3 and 2.5 mL of 0.3 mmol/L acetate buffer, pH 3.6. A volume of supernatant of 40 µL from each sample was mixed with 1.8 ml FRAP reagent and water (0.2 mL, distilled). The mixture was incubated at 37 ℃ for 10 min, and a spectrophotometer was used to measure the absorbance at 593 nm. A 1.0 mM FeSO4 solution was used as a standard. The result was expressed in terms of the concentration of antioxidants a ferric reducing ability equivalent to 1.0 mmol/L FeSO4.
ORAC was measured according to Wang and Lin, and modified from the original method of Cao et al. (1993). Accordingly, 1.7 mL phosphate buffer (75 mM, pH 7.0), 100 µL of 3.4 mg L−1 R‑PE (R-Phycoerythrin, Sigma), 100 µL of 320 nmol/L AAPH [2, 2’-azobis(2-amidinopropane) dihydrochloride, Sigma], and 100 µL of sample were mixed in each reaction. Trolox was used as a standard. The fluorescence was recorded (540 nm) excitation, 570 nm emission; Shimadzu RF-Mini 150 recording fluorometer (Columbia, MD). This continued at 5 min intervals until the fluorescence measured was < 5% of the previous measurement. The final value was calculated using the differences of areas under the quenching curves of R‑PE, between a blank and a sample, and was expressed as micromoles of TE (Trolox equivalents) per gram of fresh weight.
Statistical Analysis
All data were presented as the mean of three replicates, with the standard error of the means. For ANOVA, least significance difference (LSD) test was used to compare means (SAS Institute, Cary, USA). Differences at p < 0.05 were significant.
Results
Exogenous Na 2 SeO 3 Treatments Induced Organic Se Accumulation
As shown in Table 1, in all fruit parts the concentration of total Se and organic Se increased following spraying. The value of total Se was from 7.61 to 124.18 µg kg−1 FW and the lowest concentration of total Se was found in the peels following foliar spraying with 10 mg L−1 sodium selenite. The organic Se was in the range of 6.44 to 76.28 µg kg−1 FW, and the highest concentration of organic Se, 76.28 µg kg−1 FW, was found in the pulp following 100 mg L−1 sodium selenite spraying.
Metabolism of Organic Se in Kiwifruit with Na 2 SeO 3 Foliar Spraying
The most abundant organic Se species present in kiwifruit was protein-Se (Table 2). The concentration of protein-Se in pulp (in the range 4.18–36.64 µg kg−1 FW) was much higher than what was observed in peels (2.95–4.18 µg kg−1 FW) and in seeds (3.57–23.20 µg kg−1 FW). The highest concentration of protein-Se, 36.64 µg kg−1 FW, was found in the pulp of fruits treated with 100 mg L−1 sodium selenite. The lowest level of protein-Se, 2.95 µg kg−1 FW, was shown in peels of fruits sprayed with 10 mg L−1 sodium selenite.
The concentration of polysaccharide-Se was measured in peels, pulp and seeds and was expressed on the basis of fresh weight (Table 3). As the concentration of sodium selenite in the foliar spray increased, an incremental increased in the percentage of polysaccharide-Se in organic Se and in total Se was found in peels and pulp. All the parts of fruit in foliar spraying with sodium selenite accumulated more polysaccharide-Se than they did following spraying with water. In peels, the lowest concentration of polysaccharide-Se was observed, with values between 1.06 and 3.32 µg kg−1 FW. The max value, 17.71 µg kg−1 FW, was observed in pulp of plants that had been foliar sprayed with 100 mg L−1 sodium selenite.
As shown in Table 4, for all treatments, the lowest concentration of nucleic acid-Se was found in pulps, and the values were between 0.04 and 0.13 µg kg−1 FW. Peels and seeds accumulated more nucleic acid-Se, and the values were in the range of 0.49–0.67 µg kg−1 FW. The percentage of nucleic acid-Se in organic selenium decreased from 7.54 to 6.22% in peels and from 1.47 to 0.75% in pulps, respectively, as the applied concentration of sodium selenate increased.
Antioxidant Activities of Different Kiwifruit Part with Na2SeO3 Foliarly Spraying
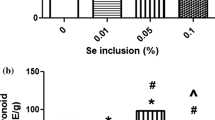
The value of FRAP in peels, expressed as 39.03–59.39 µmol FeSO4 g−1 FW, was much lower in comparison to that in pulps (91.08–119.46 µmol FeSO4 g−1 FW) and seeds (82.59–173.12 µmol FeSO4 g−1 FW) (Fig. 1). Treatments with 50 and 100 mg L−1 sodium selenite foliar spraying caused a significant increase in FRAP value in all tissues. As compared to the control, the FRAP values in the seed, peel and pulp increased by 109.62%, 51.37% and 27.68%, respectively. Low concentration (10 mg L−1) sodium selenite foliar spraying could not increase the FRAP value of peels and pulps.
In addition, the results presented in Fig. 2 show that the seeds contain higher value of ORAC, in the range of 96.48–111.71 µmol TE g−1 FW, in comparison with the corresponding peels (9.70–35.38 µmol TE g−1 FW) and pulps (41.80–74.65 µmol TE g−1 FW). The ORAC in peels increased linearly as the concentration of sodium selenite in the applied solution increased such that the ORAC in peels of plants sprayed with 100 mg L−1 sodium selenite was 3.65 times higher than plants sprayed with water. In pulp, the ORAC increased following sodium selenite foliar spraying (50 and 100 mg L−1), but the effect of treatments with 10 and 25 mg L−1 sodium selenite foliar spraying was not obvious. The ORAC in seeds of plants sprayed with 25 mg L−1 sodium selenite was significantly lower than what was observed following water treatment (0 mg L−1 sodium selenite) decreasing by 11.18%.
Discussion
Evaluation of dietary needs for Se depends on knowledge of both the total Se abundance and the availability of Se in relation to the molecular forms and species present. Long et al. (2016) reported that the application of exogenous Se in both irrigation water and organic fertilizer could increase Se content in kiwifruit. Our observations are consistent with previous reports by Liu et al. (2014) of kiwifruit, which reported that foliar fertilization with the dose of 0.5 kg was optimal, whose selenium content of kiwifruits increased by 103.5%. This tested the hypothesis that exogenous sodium selenite, could induce Se accumulation in kiwifruit. Accurate knowledge of the speciation of Se in plant tissues is important to understanding metabolic pathways and human nutrition (Zhu et al. 2009). It was observed that the amount of protein-Se in pulps increased linearly with the increase of concentration of sodium selenite foliar spraying. The same relation was also displayed in seeds. Previously, Zhang et al. (2009) reported that selenomethionine is the major form of Se within the protein. Smrkolj et al. (2006) concluded that most of the Se present as selenomethionine in buckwheat seeds, representing an average of 93 ± 5% of the Se content. Since selenium and sulfur are elements of the same main group and have similar properties, selenium is absorbed by plants and transported along with the sulfur transport family. At the same time, selenium will participate in the metabolism and assimilation of sulfur, mainly replacing the sulfur in the protein cysteine and methionine, forming seleno-amino acids (Sors et al. 2005). In peels or pulps, with increasing concentration of sodium selenite, the percentage of protein-Se in organic Se and in total Se decreased. While, the opposite relation was discovered in seeds. This suggests that after spraying selenium on the leaf surface, selenium may first accumulate on the fruit surface, and then slowly transfer to the seeds.
In recent decades, it has been commonly reported that inorganic selenite can be bound to polysaccharides in various organisms, as part of the process of transformation to organic forms. These organic forms are generally considered to be effective and safer than inorganic selenium as a dietary supplement (Fang et al. 2003; Sanmartín et al. 2012; Xia et al. 2022). Polysaccharides-Se have higher bioactivities compared with selenium-free polysaccharide. These activities include immunomodulation, hypoglycaemic, hypolipidemic, antitumor and antibacterial effects (Fan et al. 2006). Foliar application of Se has been reported to increases Se content in kiwifruit (Wu et al. 2024; Deng et al. 2018). It is worth noting that selenium was efficiently transported from treated leaves to other tissues and organs, following foliar treatment. It has been reported selenium is efficiently transferred to other parts of the plant when foliarly applied in kiwifruit (Ghafouri et al. 2022) and other plants (Germ and Stibilj 2007; Smrkolj et al. 2006). However, sodium selenite foliar spraying caused a decrease in the nucleic acid-Se content in all the parts of fruit compared to plants that had been foliarly sprayed with water in this study. The percentage of polysaccharide-Se on organic selenium and total selenium in the seeds with foliarly sprayed sodium selinite was remarkably lower than following water spraying.
Selenium is considered to be an antioxidant, which can scavenge free radicals, thereby limiting the chain reaction induced by lipid peroxidation that normally results in membrane damage (Tinggi 2008). Ghafouri et al. (2022) sprayed selenium on the leaves of kiwifruit significantly increased the selenium content of fruit. Selenium content significantly delayed the aging of kiwi fruit. The mechanism is to preserve ascorbic acid and enhance antioxidant enzymes such as superoxide dismutase, catalase and ascorbate peroxidase. These protect the cell from severe oxidation by free radicals (El-Sayed et al. 2006; Zapletal et al. 2008). In addition, the antioxidant capacity and phenolic content of selenium-rich kiwifruit were increased, which was due to the higher phenylalanine ammoniase of selenium-rich kiwifruit. It was observed that the value of FRAP in seeds increased with concentration of sodium selenite, and that the correlation between FRAP value and organic selenium content of the peel, pulp and seed was very high at r = 0.9087, 8380 and 0.8408, respectively. In conclusion, the improvement of the antioxidant activity of organic Se quantitatively depended on its protein-Se content.
We also found that ORAC in the peels and pulp were significantly increased with increasing concentration of sodium selenite in the foliar spraying. This observation was consistent with previous reports which found that antioxidant activity of green tea increased as Se incorporation increased, and scavenging free radicals also increased as Se levels increased (Xu et al. 2003). In the present study, a significant relationship between ORAC values and protein-Se and polysaccharide-Se content of kiwifruit tissue could be described by the regression equation of y = 1.8730 × 1 + 45.5263 × 2 + 14.3172 (x1 and x3 was protein-Se and polysaccharide-Se), which provided further evidence that the main antioxidants in kiwifruit are protein-Se and polysaccharide-Se.
Conclusion
The results presented in this study showed that the antioxidant properties of organic selenium on kiwifruits (measured by FRAP and ORAC) could be significantly enhanced by foliar application of selenite. The mechanism might involve organic Se as a redox-active free-radical scavenger. The primary component of organic selenium was protein-Se, which was mainly present in the pulp and its amount increased linearly with the increased concentration of sodium selenite in the foliar spray. These results show potential for increasing bioactivity of kiwifruits by agronomic measures. Moreover, this could also be a method of selenium enrichment for nutraceutical or dietary supplements.
Data availability
No data was used for the research described in the article.
References
Abrahams PW (2006) Soil, geography and human disease: A critical review of the importance of medical cartography. Prog Phys Geog 30:490–512. https://doi.org/10.1191/0309133306pp493ra
Alfthan G, Eurola M, Ekholm P, Venäläinen ER, Root T, Korkalainen K, Hartikainen H, Salminen P, Hietaniemi V, Aspila P, Aro A (2015) Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J Trace Elem Med Bio 31:142–147. https://doi.org/10.1016/j.jtemb.2014.04.009
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
Cao G, Alessio HM, Culter RG (1993) Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Bio Med 14:303–311. https://doi.org/10.1016/0891-5849(93)90027-R
Combs GFJ (2001) Selenium in global food systems. Br J Nutr 85:517–547. https://doi.org/10.1079/BJN2000280
Deng XF, Lu CH, Huang LQ, Zhang HQ, Zhao ZQ, Liu XW (2018) Effects of spraying selenium in different forms and at different stages on selenium absorption and accumulation and main quality indexes of ‘Jintao’ kiwifruit. J Fruit Sci 35:1385–1392. https://doi.org/10.13925/j.cnki.gsxb.20180110 (In Chinese)
Dong Z, Xiao YQ, Wu H (2021) Selenium accumulation, speciation, and its effect on nutritive value of Flammulina velutipes (Golden needle mushroom). Food Chem 350:128667. https://doi.org/10.1016/j.foodchem.2020.128667
El-Sayed WM, Aboul-Fad T, Lamb JG, Roberts JC, Franklin MR (2006) Effect of selenium-containing compounds on hepatic chemoprotective enzymes in mice. Toxicology 220:179–188. https://doi.org/10.1016/j.tox.2005.12.016
Fan J, Zhang J, Tang Q, Liu Y, Zhang A, Pan Y (2006) Structural elucidation of a neutral fucogalactan from the mycelium of Coprinus comatus. Carbohyd Res 341:1130–1134. https://doi.org/10.1016/j.carres.2006.03.039
Fan J, Wang R, Hu HQ, Huo G, Fu QL, Zhu J (2015) Transformation and bioavailability of selenate and selenite added to a Nicotiana tabacum L. planting soil. Commun Soil Sci Plant 46:1362–1375. https://doi.org/10.1080/00103624.2015.1033544
Fang WX, Wu PW, Hu RZ (2003) Geochemical research of the impact of Se-Cu-Mo-V-bearing coal layers on the environment in Pingli County, Shanxi Province, China. J Geochem Explor 80:105–115. https://doi.org/10.1016/S0375-6742(03)00186-9
Germ M, Stibilj V (2007) Selenium and plants. Acta Agr Slov 89:65–71. https://doi.org/10.2478/v10014-007-0008-8
Ghafouri M, Razavi F, Arghavani M, Gheshlaghi EA (2022) Delaying postharvest senescence and improving antioxidant capacity of kiwifruits cv. Hayward by preharvest selenium appl-ication. J Food Process Preserv. https://doi.org/10.1111/JFPP.17185
Hartfiel W, Bahners N (1998) Selenium deficiency in the Federal Republic of Germany. Biol Trace Elem Res 15:1–12. https://doi.org/10.1007/bf02990123
Hartikainen H, Xue T, Piironen V (2000) Selenium as an antioxidant and prooxidant in ryegrass. Plant Soil 225:193–200. https://doi.org/10.1023/A:1026512921026
He L, Kong J, Li G, Meng G, Chen K (2018) Similar responses in morphology, growth, biomass allocation, and photosynthesis in invasive Wedelia trilobata and native congeners to CO2 enrichment. Plant Ecol 219:145–157. https://doi.org/10.1007/s11258-017-0784-0
Hu Q, Chen L, Xu J, Zhang Y, Pan G (2002) Determination of selenium concentration in rice and the effect of foliar application of Se-enriched fertilizer or sodium selenite on the selenium content of rice. J Sci Food Agr 82:869–872. https://doi.org/10.1002/jsfa.1115
Huang Z, Guo BJ, Wong RNS, Jiang Y (2007) Characterization and antioxidant activity of selenium-containing phycocyanin isolated from Spirulina platensis. Food Chem 100:1137–1143. https://doi.org/10.1016/j.foodchem.2005.11.023
Ip C, Birringer M, Block E, Kotrebai M, Tyson JF, Uden PC, Donald JL (2000) Chemical speciation influences comparative activity of selenium-enriched garlic and yeast in mammary cancer prevention. J Agr Food Chem 48:2062–2070. https://doi.org/10.1021/jf000051f
Jiang Y, Huang R, Jiang L, Chen K, Zhu W (2021) Alleviation of cadmium toxicity to Medicago truncatula by AMF involves the changes of Cd speciation in rhizosphere soil and subcellular distribution. Phyton-int J Exp Bot 90:403–415. https://doi.org/10.32604/phyton.2021.014376
Jin M, Lu Z, Huang M, Wang Y (2012) Effects of Se-enriched polysaccharides produced by Enterobacter cloacae Z0206 on alloxan-induced diabetic mice. Int J Biol Macromol 50:348–352. https://doi.org/10.1016/j.ijbiomac.2011.12.019
Lei YB, Xia HX, Chen K, Plenković-Moraj A, Huang W, Sun G (2021) Photosynthetic regulation in response to fluctuating light conditions under temperature stress in three mosses with different light requirements. Plant Sci. https://doi.org/10.1016/j.plantsci.2021.111020
Li NN, Xie WW, Zhou XB, Chai YR, Xu WH (2018) Comparative effects on nutritional quality and selenium metabolism in two ecotypes of Brassica rapa exposed to selenite stress. Environ Exp Bot 150:222–231. https://doi.org/10.1016/j.envexpbot.2018.03.023
Li Q, Liu MF, Hou J, Jiang CX, Li SC, Wang T (2013) The prevalence of Keshan disease in China. Int J Cardiol 168:1121–1126. https://doi.org/10.1016/j.ijcard.2012.11.046
Liu HQ, Chen Y, Fang Y, Zhang J, Zong LG, Sun HJ, Chen X, Xue M, Zhao FJ, Hu QH (2014) Effects of selenium fertilizers on selenium content and nutrient quality of Kiwifruit. J Agr Res Environ 31:565–569. https://doi.org/10.13254/j.jare.2014.0256 (In Chinese)
Liu XW, Wang QL, Hu CX, Zhao XH, Duan BH, Zhao ZQ (2016) Regulatory effects of sulfur on oilseed rape (Brassica napus L.) response to selenite. Soil Sci Plant Nutr 62:247–253. https://doi.org/10.1080/00380768.2016.1172023
Long YH, Zhang C, Wu XM, Li M, Yao X, Shao S (2016) Application of se-fertilizer affects selenium content, cadmium and lead accumulation and fruit quality in Kiwifruits. Food Sci 37:82–88. https://doi.org/10.7506/spkx1002-6630-201613015 (In Chinese)
Lu J, Holmgren A (2009) Selenoproteins. J Biol Chem 284:723–727. https://doi.org/10.1074/jbc.R800045200
Luo LP, Zhang JP, Zhang KY, Wen QY, Ming K, Xiong H, Ning FJ (2021) Peanut selenium distribution, concentration, speciation, and effects on proteins after exogenous selenium biofortification. Food Chem 354:129515. https://doi.org/10.1016/j.foodchem.2021.129515
Malinowska E, Krzyczkowski W, Herold F, Łapienis G, Ślusarczyk J, Suchocki P, Kuraś M, Turlo J (2009) Biosynthesis of selenium-containing polysaccharides with antioxidant activity in liquid culture of Hericium erinaceus. Enzyme Microb Tech 44:334–343. https://doi.org/10.1016/j.enzmictec.2008.12.003
Poggi V, Arcioni A, Filippini P, Pifferi PG (2000) Foliar application of selenite and selenate to potato (Solanum tuberosum): effect of a ligand agent on selenium content of tubers. J Agr Food Chem 48:4749–4751. https://doi.org/10.1021/jf000368f
Reid ME, Duffield-Lillico JD, Slate E, Natarajan N, Turnbull B, Jacobs E, Combs GF, Alberts DS, Clark LC, Marshall JR (2008) The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutr Cancer 60:155–163. https://doi.org/10.1080/01635580701684856
Ren H, Huang RH, Li W, Zheng L, Plenkovic-Moraj A, Chen K (2022) Photosynthetic regulation in response to strontium stress in moss Racomitrium japonicum L. Environ Sci Pollut Res 1:72–85. https://doi.org/10.1007/s11356-022-23684-4
Sanmartín C, Plano D, Sharma AK, Palop JA (2012) Selenium compounds, apoptosis and other types of cell death: an overview for cancer therapy. Int J Mol Sci 13:9649–9672. https://doi.org/10.3390/ijms13089649
Shi WL, Han H, Chen GZ, Chen X, Hong YK, Chen LK, Chen D, Zhen L (2010) Extraction, characterisation of the polysaccharide extracts from Se-enriched G. lucidum (Se-GLP) and its inhibition against oxidative damage in ischemic reperfusion mice. Carbohyd Polym 80:774–778. https://doi.org/10.1016/j.carbpol.2009.12.027
Slejkovec M, Goessler W (2005) Accumulation of selenium in natural plants and selenium supplemented vegetable and selenium speciation by HPLC-ICPMS. Chem Spec Bioavailab 17:63–73. https://doi.org/10.3184/095422905782774919
Smrkolj P, Stibilj V (2004) Determination of selenium in vegetables by hydride generation atomic fluorescence spectrometry. Anal Chim Acta 512:11–17. https://doi.org/10.1016/j.aca.2004.02.033
Smrkolj P, Stibilj V, Kreft I, Kápolna E (2005) Selenium species determination in selenium-enriched pumpkin (Cucurbita pepo L.) seeds by HPLC-UV-HG-AFS. Anal Sci 21:1–5. https://doi.org/10.2116/analsci.21.1501
Smrkolj P, Stibilj V, Kreft I, Germ M (2006) Selenium species in buckwheat cultivated with foliar addition of Se(VI) and various levels of UV‑B radiation. Food Chem 96:675–681. https://doi.org/10.1016/j.foodchem.2005.05.002
Sors T, Ellis D, Salt D (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86:373–389. https://doi.org/10.1007/s11120-005-5222-9
Tinggi U (2008) Selenium: Its role as antioxidant in human health. Environ Health Prev Med 13:102–108. https://doi.org/10.1007/s12199-007-0019-4
Tomotake H, Shimaoka I, Kayashita J, Nakajoh M, Kata N (2002) Physicochemical and functional properties of buckwheat protein product. J Agric Food Chem 50:2125–2129. https://doi.org/10.1021/jf011248q
Wallace K, Kelsey K, Schned A, Morris JS, Andrew AS, Karrgas MR (2009) Selenium and risk of bladder cancer: a population-based case-control study. Cancer Prev Res 2:70–73. https://doi.org/10.1158/1940-6207.CAPR-08-0046
Wen D (2021) Selenium in horticultural crops. Sci Hortic 289:110441. https://doi.org/10.1016/j.scienta.2021.110441
Whistler LR (1965) Removal of moteln: Sevag medical in carbohydrate chemistry. Academic Press, New York London, p 25.
Wu L (2004) Review of 15 years of research on ecotoxicology and remediation of land contaminated by agricultural drainage sediment rich in selenium. Ecotoxicol Environ Saf 57:257–269. https://doi.org/10.1016/S0147-6513(03)00064-2
Wu D, Zhang TL, Li T, Wang HH, Li C, Ding L (2024) Effects of selenium fertilizer application on fruit of ‘Hongyang’ Kiwifruit. J Fruit Res 5:60–62+66. https://doi.org/10.16010/j.cnki.14-1127/s.2024.03.024 (In Chinese)
Xia H, Cheng X, Zheng L, Ren H, Li W, Lei Y, Plenković-Moraj A, Chen K (2022) Sex-specific physiological responses of Populus cathayana to uranium stress. Forests 13:1123
Xie YD, Su LH, He ZQ, Zhang JW, Tang Y (2021) Selenium inhibits cadmium absorption and improves yield and quality of cherry tomato (lycopersicon esculentum) under cadmium stress. J Soil Sci Plant Nutr 21:1125–1133. https://doi.org/10.1007/s42729-021-00427-x
Xu J, Yang F, Chen L, Hu Q (2003) Effect of selenium on increasing the antioxidant activity of tea leaves harvested during the early spring tea producing season. J Agr Food Chem 51:1081–1084. https://doi.org/10.1021/jf020940y
Yang F, Chen L, Hu Q, Pan G (2003) Effect of the application of selenium on selenium content of soybean and its products. Biol Trace Elem Res 93:249–256. https://doi.org/10.1385/BTER:93:1-3:249
Yin HQ, Qi ZY, Li MQ, Ahammed GJ, Chu XY, Zhou J (2019) Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol Environ Saf 169:911–917. https://doi.org/10.1016/j.ecoenv.2018.11.080
Zapletal C, Heyne S, Breitkreutz R, Gebhard MM, Golling M (2008) The influence of selenium substitution on microcirculation and glutathione metabolism after warm liver ischemia/reperfusion in a rat model. Microvasc Res 76:104–109. https://doi.org/10.1016/j.mvr.2008.04.005
Zhang B, Zhou K, Zhang J, Chen Q, Liu G, Shang N, Qin W, Li PL, Lin FX (2009) Accumulation and species distribution of selenium in Se-enriched bacterial cells of the Bifidobacterium animalis 01. Food Chem 115:727–734. https://doi.org/10.1016/j.foodchem.2008.12.006
Zhu YG, Pilon-Smits EAH, Zhao FJ, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14:436–442. https://doi.org/10.1016/j.tplants.2009.06.006
Acknowledgements
This research was supported by Jingmen Key Science and Technology Plan Projects(2023YFZD014), Sichuan Science and Technology Department Program (2020YFS0344), the Doctoral Fund of Jingchu Institute of Technology (YY202201) and the Opening Project of Hubei Engineering Research Center for Specialty Flowers Biological Breeding (2022ZD003).
Author information
Authors and Affiliations
Contributions
Conceptualization, Yun-mei Lu and Ren-hua Huang; methodology, Yun-mei Lu, Mao Mu; software, Mao Mu and Li-ai Wang; validation, Yun-mei Lu, Mao Mu, Ren-hua Huang; formal analysis, Ren-hua Huang; investigation, Yun-mei Lu, Mao Mu and Li-ai Wang; resources, Ren-hua Huang; data curation, Yun-mei Lu; writing-original draft preparation, Yun-mei Lu; writing-review and editing, Ren-hua Huang; supervision, Ren-hua Huang; project administration, Ren-hua Huang. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Y.-m. Lu, M. Mu, L.-a. Wang and R.-h. Huang declare that they have no competing interests.
Ethical standards
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, Ym., Mu, M., Wang, La. et al. Effect of Foliar Application of Selenite On Organic Se and Antioxidant Activity in Kiwifruit. Applied Fruit Science (2024). https://doi.org/10.1007/s10341-024-01167-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10341-024-01167-9