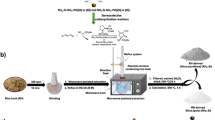
Abstract
Here, we report an inexpensive, versatile, and environmentally benign biomass waste rice husk derived amorphous silica-supported palladium (PdNPs/RH-SiO2) nano-catalyst for Suzuki–Miyaura and Heck–Mizoroki cross-coupling reactions. The proposed catalyst was prepared in two steps. In the first step, amorphous SiO2 obtained by calcination of rice husk and the following deposition of palladium nanoparticles by using the chemical reduction method in the second step. The physico-chemical properties of the catalysts were investigated by N2 adsorption–desorption, XRD, XPS, 29Si CP-MAS NMR, and TEM analysis. The palladium content in the catalysts has been determined by ICP-OES analysis. The 1% PdNPs/RH-SiO2 exhibited excellent catalytic performance for Suzuki–Miyaura and Heck–Mizoroki C–C cross-coupling reactions to produce biaryl and stilbene compounds with >99% selectivity and excellent TON values (529 and 524). Furthermore, the catalyst is repeatedly used for four consecutive cycles without a significant drop in the yield.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Suzuki and Heck coupling reactions are the most powerful one-step methods for carbon–carbon bond formation in organic synthesis, especially these reactions are employed in the preparation of a large variety of complex organic molecules, having prevalent applications in pharmaceutical, crop-protection, polymers, materials, and fine chemical applications [1,2,3,4,5,6,7,8,9,10,11,12]. The current state-of-the-art process relies on precious homogeneous palladium complexes with a combination of sophisticated and sensitive ligands [13,14,15]. Despite their excellent activity, selectivity, and tolerant to broad substrate scope, the catalyst separation and recycling of Pd are the major obstacles for large scale applications [16]. Moreover, these catalytic processes require a tedious workup procedure for the isolation of the product, resulting in large amounts of waste, leading to the expensive manufacturing process [17]. More importantly, the contamination of Pd and ligand residues in the end product limits their application in pharmaceutical industries [9, 18]. In contrast to homogeneous catalysts, solid catalysts are often preferred due to their easy separation and reusability [12, 19]. In this regard, a commercially available Pd/C catalyst was employed for Suzuki and Heck coupling reactions and achieved up to 36,000 ton [20]. But, functional group tolerance and too much leaching of Pd/C are the major problems [21]. Therefore, the research has been directed towards the development of green and eco-friendly heterogeneous Pd catalysts for C–C coupling reactions. In this sense, several methods have been developed for the immobilization of Pd complexes on solid supports/polymers to combine the benefits of both homogeneous and heterogeneous catalysts [12, 22]. However, these catalysts are also encountered with several issues pertaining to activity/selectivity and leaching of palladium into the reaction medium [22]. In addition, several chemists have reported heterogeneous palladium catalysts on various supports such as metal oxides [22,23,24,25,26,27], silica [28,29,30,31,32], zeolites [33,34,35], carbon materials [36,37,38], polymers [39,40,41,42,43], MOFs [44,45,46] and others [47,48,49,50,51,52,53] to facilitate the recovery and reuse of precious Pd catalysts. But, the issues related to the use and cost of catalyst supporter stabilizers, reaction conditions, and reproducibility are questioning the practical applicability in the industrial point of view.
On the other hand, the replacement of fossil resources by renewable materials has been given the top priority in fuel/chemical industries. In recent years, the use of renewable feedstocks emerges as a promising strategy for the production of fuels, chemicals, and materials in a more sustainable approach [54,55,56]. In this regard, highly abundant and low-cost agricultural waste by-product rice husk has received particular attention in academia and industry. In general, rice husk is produced from rice, as 1-ton rice produces 0.2 ton rice husk. Hence, the mass production of rice in the world results a huge volume of rice husk, nearly 148 million metric tons of rice husk from 740 million metric tons of rice annually, and treating as a low-value fuel for bio-refineries [57]. Rice husk is mainly composed of cellulose (30–35%), hemicelluloses (20–25%), lignin (15–20%), and ash (10–15%). Interestingly, more than 90% of rice husk ash is in the form of hydrated amorphous silica. Since the high content of amorphous silica, rice husk is being considered as an excellent precursor for the preparation of nano and mesoporous silica materials [57,58,59]. Recently, rice husk derived carbon-supported catalysts have been developed and applied successfully for C–C coupling reactions [60, 61]. Therefore, the preparation of rice husk derived amorphous SiO2 supported Pd catalysts is considered to be a green and low-cost approach for C–C coupling reactions.
Inspiring from the aforementioned works, herein, we demonstrate a simple and facile method for the preparation of inexpensive and environmentally benign biomass waste rice husk derived amorphous silica-supported palladium nano-catalyst (PdNPs/RH-SiO2). These RH-SiO2 supported Pd nanoparticles to create a stable and reusable catalyst for Suzuki–Miyaura and Heck–Mizoroki C–C cross-coupling reactions under mild reaction conditions.
2 Experimental section
2.1 Materials
Unless otherwise noted, all chemicals were purchased from M/s. Sigma–Aldrich and used as received. Gel-Silica (G-SiO2) with 60–120 mesh purchased from M/s. SD Fine Chemicals Pvt. Ltd. Rice-Husk was obtained from the local rice mill, Telangana. 35.4% HCl purchased from M/s. Rankem Pvt. Ltd. Phenylboronic acid (98%) purchased from M/s. Alfa Aesar, sodium carbonate (99.5%), Hydrazine (99%) purchased from M/s. SD Fine Chemicals Pvt. Ltd.
2.2 Preparation of catalysts
2.2.1 Preparation of rice-husk derived silica support (RH-SiO2)
RH-SiO2 support was prepared according to the reported procedure with modifications and details are shown in Scheme 1 [57, 60]. In a typical procedure, a required amount of raw rice husk was added to 0.1 M HCl solution and stirred at 100 °C for 6 h. Then, the mixture was cooled down to room temperature, and the solids were filtered and washed with distilled water to reach the pH ~ 7. The resultant solids were oven-dried at 100 °C for 12 h. The dried solids were calcined at 600 °C for 3 h under airflow with a 90 ml/min flow rate and yielded the RH-SiO2 support. The obtained silica was symbolized as RH-SiO2.
2.2.2 Preparation of RH-SiO2-supported palladium nanoparticles (PdNPs/RH-SiO2)
The PdNPs/RH-SiO2 catalysts with 0.5%, 1%, and 1.25% Pd on RH-SiO2 support were prepared as per the reported method [62]. Typically, the required amount of PdCl2 was dissolved in distilled water, and 2–3 drops of HCl were added. Then, the requisite amount of RH-SiO2 support was added to the above solution and stirred at room temperature for 30 min. Subsequently, hydrazine hydrate was added drop wise under vigorous stirring for 1 h. The resulted reaction mixture was left for stirring for another 2 h, and the reaction mixture turns into black. The dark solids were filtered and washed several times with water followed by methanol and diethyl ether, respectively. The black solids were dried under vacuum at 100 °C for 12 h. In a similar procedure, 1% Pd on G-SiO2 (1% Pd/G-SiO2) and F-SiO2 (1% Pd/FM-SiO2) catalysts were prepared.
2.3 Catalytic activity
2.3.1 Procedure for Suzuki cross-coupling reaction
All reactions were performed in a 25 ml round bottom flask. In a typical experiment, Bromobenzene (1 mmol), phenylboronic acid (1.25 mmol), and Na3PO4 (1 mmol) were added to 2 ml EtOH:H2O (1:1 v/v) solvent. Then, 20 mg of the respective catalyst was added to the reaction mixture and stirred at 80 °C for the required time. After the reaction, the catalyst was recovered by simple filtration and washed numerous times with ethyl acetate followed by drying.
2.3.2 Procedure for Heck–Mizoroki coupling reaction
A mixture of iodobenzene (1 mmol), styrene (1.2 mmol), Na2CO3 (1 mmol), and 20 mg of catalyst in 2 ml of DMF solvent was taken into 25 ml round bottom flask and stirred at 120 °C for the required time. After completion of the reaction, the reaction mixture cooled down to room temperature and diluted with ethyl acetate solvent. The catalyst was recovered by filtration and washed with ethyl acetate and dried.
The products of all the above reactions were analyzed and identified by HP-GC and GC–MS (QP-2010 model, M/s. Shimadzu Instruments, Japan) equipped with EB–5 MS capillary column (30 m × 0.25 mm × 0.25 μm).
3 Result and discussions
Pd/RH-SiO2 nano-catalyst were prepared by a simple two-step method, (1) in the first step; rice husk biomass-derived SiO2 support was obtained by calcination of rice husk biomass under airflow at 600 °C, (2) secondly, the palladium nanoparticles (PdNPs) were deposited on RH-SiO2 support by chemical reduction with hydrazine hydrate.
3.1 Catalyst characterization
In order to understand the structure-activity of the 1% Pd/RH-SiO2 nano-catalyst for selective C–C coupling reactions, the catalyst was extensively characterized with comprehensive analytical techniques such as XRD, XPS, BET, TEM, solid-state 29Si CP-MAS NMR, and ICP-OES analysis.
3.1.1 N2 adsorption–desorption analysis
The textural characteristics of various silica-based catalysts are determined by N2 adsorption–desorption analysis. The BET surface area, pore-volume, and pore diameter and are depicted in Table 1. The commercially available Fumed-silica (F-SiO2) and G-SiO2 have the BET surface area of 552 and 318 m2/g with 0.3, 0.5 cm3/g pore volume and 3.2, 3.1 nm pore diameters (Table 1, entry 2 and 3). However, the rice husk derived SiO2 support has a low BET surface area (127 m2/g) and pore volume (0.1 cm3/g), but a large pore diameter (11.8 nm) was noticed with respect to commercial SiO2 supports (Table 1, entry 1). Further, a decrease in the surface area of all SiO2 supports has been observed after the introduction of PdNPs. A similar trend has been monitored for pore volume and pore diameter, respectively (Table 1, entry 4–8). These results suggest the pore blockage by the deposition of PdNPs. The Pd content in the catalysts was determined by ICP-OES analysis, and the results are close to the theoretical values (Table 1, entry 4–8).
3.1.2 XRD analysis
The wide-angle XRD pattern of 1% Pd/RH-SiO2, 1% Pd/G-SiO2, and 1% Pd/F-SiO2 nano-catalysts are shown in Fig. 1. The 1% Pd/RH-SiO2 nanocatalyst exhibits a broad peak between 20° and 30° on the 2θ scale, which is a characteristic peak of amorphous silica. The 1% Pd/RH-SiO2 nanocatalyst displays a diffracted peak at 2θ = 40.1°, 2θ = 44.8° are referred to (111) (200) planes of palladium in zero oxidation state (JCPDS #05-0681). However, the peak corresponding to (220) plane is not observed, indicating the fine and homogeneous distribution of PdNPs throughout the SiO2 matrix. The similar XRD patterns observed with G-SiO2 and F-SiO2 supported Pd nanocatalysts, respectively (Fig. 1).
3.1.3 XPS analysis
XPS analysis was performed to ascertain the oxidation state of palladium and metal-support interactions in the different SiO2 supported Pd catalysts, and the corresponding spectrum is shown in Fig. 2. As shown in Fig. 2, the Pd 3d spectra of 1% PdNPs/RH-SiO2 catalyst display two peaks, the binding energies centered at 343.2 and 337.8 eV are attributed to 3d5/2 and 3d3/2 of palladium in zero oxidation state, which is consistent with the reported values for palladium in zero-valent [60]. In the case of 1% PdNPs/FM-SiO2 and 1% PdNPs/G-SiO2 catalysts, the Pd 3d peaks are shifted to lower binding energies (342.9, 337.1 eV and 341, 336 eV). These results are indicating that the 1% PdNPs/RH-SiO2 nanocatalyst has strong metal-support (Pd–SiO2) interactions.
3.1.4 Solid-state 29Si CP-MAS NMR
The 29Si CP-MAS NMR analysis provides information regarding the structural environment of silicon atoms in the SiO2 matrix. The 29Si CP-MAS NMR spectra of the different SiO2 samples are shown in Fig. 3. The spectrum of RH-SiO2 can be fitted into two peaks at −100 and −110 ppm ascribed to silanol groups [Q3, Si(Si–O)3(OH)] and (Si–O)4 of the silica framework [Q4, Si(Si–O)4] [63]. FM-SiO2 and G-SiO2 also exhibited the same NMR pattern (Fig. 3) but, the lines are with much larger line widths.
3.1.5 TEM analysis
The size and morphology of Pd nanoparticles in 1% PdNPs/RH-SiO2, 1% PdNPs/FM-SiO2, and 1% PdNPs/G-SiO2 catalysts were investigated by using transmission electron microscopic (TEM) analysis, and the resultant TEM image is displayed in Fig. 4a. The Pd particles in 1% PdNPs/RH-SiO2 catalysts are spherical with an average particle diameter in the range of 3–5 nm and uniformly dispersed throughout the RH-SiO2 matrix. In the case of 1% PdNPs/G-SiO2 and 1% PdNPs/FM-SiO2 catalysts, the aggregation of Pd particles was observed in (Fig. 4b, c).
3.2 Catalyst screening
The catalytic activity of as-prepared 1% Pd/RH-SiO2 nanocatalyst was systematically examined for the Suzuki–Miyaura cross-coupling reaction with bromobenzene (BB) and phenylboronic acids as model substrates and the corresponding results are shown in Table 2. Control experimental data shows that the reaction did not proceed in the absence of a catalyst (Table 2, entry 1). Similarly, no reaction was observed when SiO2 supports were directly used as catalysts (Table 2, entry 2–4), indicating the need for active metal species to perform the reaction. The yield of biphenyl (BP) was only 42% with 1% PdNPs/G-SiO2 catalyst, while the biphenyl yield was increased to 57% with 1% PdNPs/FM-SiO2 catalyst (Table 2, entry 5 and 6). To our delight, the reaction with 1% PdNPs/RH-SiO2 at 80 °C for 6 h using Na3PO4 base in H2O: Ethanol solvent mixture, the yield was significantly increased to >99% and without the formation of any undesired side products (Table 2, entry 7). The remarkable catalytic activity of 1% PdNPs/RH-SiO2 catalyst can be attributed to the formation of small-sized nanoparticles (3–5 nm) with uniform distribution on the RH-SiO2 surface and strong metal-support interactions. In order to optimize the reaction parameters, the reaction has been performed in different solvents (Table 2, entry 8–10). In non-polar toluene solvent, only 41% yield obtained (Table 2, entry 8), while the yields were improved to 68–78% in polar DMF and ethanol solvents (Table 2, entry 9 and 10). The maximum biphenyl yield (>99%) was achieved in H2O:Ethanol solvent mixture within the 6 h of reaction time (Table 2, entry 7). Next, we evaluated the effect of the base on the product yields. In general, the base plays a crucial role in the product yields and selectivity in Suzuki–Miyaura C–C coupling reactions [64]. From Table 2, we found that the reaction in inorganic bases proceeded smoothly than the organic base and obtained good to excellent desired product yields (Table 2, entry 11–14). Furthermore, the selectivity and yields depend on the strength of the base, as the yield was increased with decreasing the strength of the base. Further, we studied the effect of Pd loading on RH-SiO2 support for the Suzuki cross-coupling reaction of bromobenzene (BB) and phenylboronic acid (Table 2, entry 15 and 16). With an increase, the Pd loading on the RH-SiO2 support, the desired biphenyl product yields were also increased and obtained a maximum of >99% yield with 1% Pd loading (Table 2, entry 7). A further increase in the Pd loading, from 1 to 1.5 wt%, there is a significant drop in the biphenyl yield observed (Table 2, entry 16), which could be due to the aggregation of Pd nanoparticles on the surface of RH-SiO2 support with increasing Pd loading.

With an optimized reaction condition in hand, Suzuki–Miyaura C–C cross-coupling of aryl halides and phenylboronic acids with a diverse functional group in the presence of 1% PdNPs/RH-SiO2was evaluated, and the results are depicted in Table 3. The reaction with simple iodobenzene proceeds smoothly and obtained 99% conversion and selectivity within 3 h of reaction time, whereas the bromobenzene required 6 h reaction time (Table 3, entry 1 and 6). No reaction was observed with chlorobenzene (Table 3, entry 15). This result suggesting the iodobenzene has more reactivity than the Bromo and chlorobenzene, which is due to the –I is better leaving group than the –Br and –Cl groups. Next, we investigated the electronic effect of substituted aryl halides on the activity and selectivity of 1% PdNPs/RH-SiO2 catalyst. The aryl halides with electron-withdrawing groups at para-position need short reaction times for complete conversion with 99% selectivity (Table 3, entry 4, 7, 10). In contrast, the electron-donating groups require a longer reaction time (Table 3, entry 3, 9, 14). Further, the aryl halides with electron-withdrawing/donating groups at ortho-position are less reactive than the para-position (Table 3, entry 2, 8, 11, 13). This phenomenon could be due to the substitute groups at the ortho position create a steric hindrance, which further restricts the reactivity of aryl halides. Also, the –OCH3 substituted phenylboronic acids were successfully applied for the Suzuki–Miyaura C–C cross-coupling reaction in the presence of 1% PdNPs/RH-SiO2 catalyst (Table 3, entry 5, 12).

In the present context, the exact mechanism is not clear. However, according to the literature and the current results, we have proposed the plausible mechanism, as shown in Scheme 2. Initially, oxidative addition of aryl halide to Pd(0)/RH-SiO2 (I) to form a phenyl Pd halide complex (II). Next, the trans-metalation step involves the transfer of phenyl group from phenylboronic acid to phenyl Pd halide complex (II) to form complex III. Finally, the reductive elimination step leads to the formation of the desired biphenyl product and regeneration of Pd(0)/RH-SiO2 (I) catalyst.
3.2.1 Heck–Mizoroki coupling
Since the remarkable activity and selectivity of 1% PdNPs/RH-SiO2 catalyst for C–C bond formation via Suzuki–Miyaura cross-coupling reaction of aryl halides with phenylboronic acids, we further investigated the activity of 1% PdNPs/RH-SiO2 catalyst for Heck–Mizoroki coupling reaction of iodobenzenes with styrene for the preparation of stilbenes. The reactions were carried out at 120 °C in DMF solvent using Na2CO3 base over 1% PdNPs/RH-SiO2 catalyst for required reaction time, and the results are shown in Table 4. The catalyst is tolerant of a variety of iodobenzene derivatives and afforded good yields of stilbenes with excellent selectivity (Table 4, entry 1–5).

3.3 Reusability
To assess the stability and activity of the 1% PdNPs/RH-SiO2 for Suzuki–Miyaura C–C cross coupling reaction of aryl bromide with phenylboronic acid, the catalyst was separated by centrifugation and washed several times with ethyl acetate followed by acetone solvents. The catalyst was vacuum dried at 60 °C, reused four times under the optimized reaction conditions (Fig. 5). Within repeated cycles, there is a little drop in the bromobenzene (Bb) conversion observed, but the selectivity of biphenyl (Bp) remains constant up to four successive cycles. Palladium leaching test was conducted by hot filtration method, in which the reaction was carried out for half of the reaction time (after 3 h) and separated the catalyst from the product mixture by centrifugation and continued the reaction for 6 h, but no conversion or product formation was observed. Further, the reaction solution was analyzed by ICP-OES and found no trace of Pd suggesting the no leaching of Pd nanoparticles during the reaction. From these results, it is concluded that this catalyst involves in a complex heterogeneous mechanism [52].
4 Conclusions
In summary, we have developed a green and environmentally benign biomass waste rice husk derived amorphous silica-supported palladium (1% PdNPs/RH-SiO2) nanocatalyst via the deposition of PdNPs on rice husk derived amorphous silica obtained by chemical reduction method with an average particle size of 3–5 nm. This novel catalyst exhibits an excellent activity for Suzuki–Miyaura and Heck–Mizoroki C–C cross-coupling reactions for the selective preparation of biaryl and stilbene compounds under mild reaction conditions. Furthermore, the catalyst is tolerant of various functional groups under the optimized reaction conditions and reused four times for the Suzuki–Miyaura C–C coupling reaction of bromobenzene and phenylboronic acid. However, a little drop in the conversion was monitored, but the selectivity remains constant in all four cycles.
References
Miyaura N, Suzuki A (1979) Stereo selective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. Chem Commun 19:866–867. https://doi.org/10.1039/C39790000866
Blaser HU, Indolese A, Naud F, Nettekoven U, Schnyder A (2004) Industrial r&d on catalytic c-c and c-n coupling reactions: a personal account on goals, approaches and results. Adv Synth Catal 346:1583–1598. https://doi.org/10.1002/adsc.200404156
Heravi MM, Hashemi E (2012) Recent applications of the Suzuki reaction in total synthesis. Tetrahedron 68:9145–9178. https://doi.org/10.1016/j.tet.2012.08.058
Kanchana U, Diana EJ, Mathew TV, Anilkumar G (2020) Cyclodextrin based palladium catalysts for Suzuki reaction: an overview. Carbohydr Res 489:107954. https://doi.org/10.1016/j.carres.2020.107954
Hong K, Sajjadi M, Suh JM, Zhang K, Nasrollahzadeh M, Jang HW, Varma RS, Shokouhimehr M (2020) Palladium nanoparticles on assorted nanostructured supports: applications for suzuki, heck, and sonogashira cross-coupling reactions. ACS Appl Nano Mater 3(3):2070–2103. https://doi.org/10.1021/acsanm.9b02017
Kostas ID (2017) Suzuki-Miyaura cross-coupling reaction and potential applications. MDPI AG-Multidisciplinary Digital Publishing Institute, Basel
Nishihara Y (2013) Applied cross-coupling reactions. Springer, Berlin
Chinchilla R, Nájera C (2007) The Sonogashira reaction: a booming methodology in synthetic organic chemistry. Chem Rev 107:874–922. https://doi.org/10.1021/cr050992x
Torborg C, Beller M (2009) Recent applications of palladium-catalyzed coupling reactions in the pharmaceutical, agrochemical, and fine chemical industries. Adv Synth Catal 351:3027–3043. https://doi.org/10.1002/adsc.200900587
Bolm C, Beller M (2004) Transition metals for organic synthesis. Wiley, Weinheim
Miyaura N, Suzuki AA (1995) Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem Rev 95:2457–2483. https://doi.org/10.1021/cr00039a007
Pagliaro M, Pandarus V, Ciriminna R, Béland F, DemmaCarà P (2012) Heterogeneous versus homogeneous palladium catalysts for cross-coupling reactions. ChemCatChem 4:432–445. https://doi.org/10.1002/cctc.201100422
Martin R, Buchwald SL (2008) Palladium-catalyzed suzuki−miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc Chem Res 41:1461–1473. https://doi.org/10.1021/ar800036s
Bruno NC, Tudge MT, Buchwald SL (2013) Design and preparation of new palladium precatalysts for C–C and C–N cross-coupling reactions. Chem Sci 4:916–920. https://doi.org/10.1039/C2SC20903A
Wu XF, Anbarasan P, Neumann H, Beller M (2010) From noble metal to nobel prize: palladium-catalyzed coupling reactions as key methods in organic synthesis. Angew Chem 122:9231–9234. https://doi.org/10.1002/ange.201006374
Polshettiwar V, Len C, Fihri A (2009) Silica-supported palladium: sustainable catalysts for cross-coupling reactions. Coord Chem Rev 253:2599–2626. https://doi.org/10.1016/j.ccr.2009.06.001
Das P, Linert W (2016) Schiff base-derived homogeneous and heterogeneous palladium catalysts for the Suzuki–Miyaura reaction. Coord Chem Rev 311:1–23. https://doi.org/10.1016/j.ccr.2015.11.010
Crevoisier M, Barle EL, Flueckiger A, Dolan DG, Ader A, Walsh A (2016) Cleaning limits-why the 10-ppm criterion should be abandoned. Pharm Technol 40:52–56
Benaglia M (2009) Recoverable and recyclable catalysts. Wiley, Hoboken, NJ
Heidenreich RG, Koehler K, Krauter JG, Pietsch J (2002) Pd/C as a highly active catalyst for Heck, Suzuki and Sonogashira reactions. Synlett 2002:1118–1122. https://doi.org/10.1055/s-2002-32589
Akiyama R, Kobayashi S (2003) the polymer incarcerated method for the preparation of highly active heterogeneous palladium catalysts. J Am Chem Soc 125:3412–3413. https://doi.org/10.1021/ja029146j
Nasrollahzadeh M, Sajjadi M, Shokouhimehr M, Varma RS (2019) Recent developments in palladium (nano) catalysts supported on polymers for selective and sustainable oxidation processes. Coord Chem Rev 397:54–75. https://doi.org/10.1016/j.ccr.2019.06.010
Hajipour AR, Kalantari Tarrari M, Jajarmi S (2018) Synthesis and characterization of 4-AMTT-Pd(II) complex over Fe3O4@SiO2 as supported nanocatalyst for Suzuki-Miyaura and Mizoroki-heck cross-coupling reactions in water. Appl Organomet Chem 32:4171. https://doi.org/10.1002/aoc.4171
Karimi B, Mansouri F, Vali H (2014) A highly water-dispersible/magnetically separable palladium catalyst based on a Fe3O4@ SiO2 anchored TEG-imidazolium ionic liquid for the Suzuki-Miyaura. Green Chem 16:2587–2596. https://doi.org/10.1039/C3GC42311E
Nasrollahzadeh M, Bidgoli NSS, Issaabadi Z, Ghavamifar Z, Baran T, Luque R (2020) Hibiscus Rosasinensis L. aqueous extract-assisted valorization of lignin: Preparation of magnetically reusable Pd NPs@ Fe3O4-lignin for Cr (VI) reduction and Suzuki. Int J BiolMacromol 148:265–275. https://doi.org/10.1016/j.ijbiomac.2020.01.107
Zhang S, Chang C, Huang Z, Ma Y, Gao W, Li J, Qu Y (2015) Visible-light-activated suzuki–miyaura coupling reactions of aryl chlorides over the multifunctional pd/au/porous nanorods of CeO2 catalysts. ACS Catal 5:6481–6488. https://doi.org/10.1021/acscatal.5b01173
Nasrollahzadeh M, Sajadi SM (2016) Green synthesis, characterization and catalytic activity of the Pd/TiO2 nanoparticles for the ligand-free Suzuki–Miyaura coupling reaction. J Colloid Interface Sci 465:121–127. https://doi.org/10.1016/j.jcis.2015.11.038
Veerakumar P, Thanasekaran P, Lu K-L, Liu S-B, Rajagopal S (2017) Functionalized silica matrices and palladium: a versatile heterogeneous catalyst for suzuki, heck, and sonogashira reactions. ACS Sustain Chem Eng 8:6357–6376. https://doi.org/10.1021/acssuschemeng.7b00921
El Hankari S, El Kadib A, Finiels A, Bouhaouss A, Moreau JJ, Crudden CM, Brunel D, Hesemann P (2011) SBA-15 type organosilica with 4-mercapto-n, n-bis-(3-is-propyl) butanamide for palladium scavenging and cross-coupling catalysis. Chem Eur J 17:8984–8994. https://doi.org/10.1002/chem.201002190
Kim A, Rafiaei SM, Abolhosseini S, Shokouhimehr M (2015) Palladium nanocatalysts confined in mesoporous silica for heterogeneous reduction of nitroaromatics. Energy Environ Focus 4:18–23. https://doi.org/10.1166/eef.2015.1133
Opanasenko M, Stepnicka P, Cejka J (2014) Heterogeneous Pd catalysts supported on silica matrices. RSC Adv 4:65137–65162. https://doi.org/10.1039/C4RA11963K
Chen Z, Cui ZM, Li P, Cao CY, Hong YL, Wu ZY, Song WG (2012) Diffusion induced reactant shape selectivity inside mesoporous pores of pd@meso-sio2 nanoreactor in suzuki coupling reactions. J Phys Chem C 116:14986–14991. https://doi.org/10.1021/jp303992g
Okumura K, Mushiake T, Matsui Y, Ishii A (2015) suzuki coupling reactions catalyzed by PdO dispersed on dealuminated Y-zeolite in air under ambient conditions. ChemPhysChem 16:1719–1726. https://doi.org/10.1002/cphc.201500118
Kumbhar A, Kamble S, Mane A, Jha R, Salunkhe R (2013) Modified zeolite immobilized palladium for ligand-free Suzuki–Miyaura cross-coupling reaction. J Organomet Chem 738:29–34. https://doi.org/10.1016/j.jorganchem.2013.03.031
Xue S, Jiang H, Zhong Z, Low Z-X, Chen R, Xing W (2016) Palladium nanoparticles supported on a two-dimensional layered zeoliticimidazolate framework-L as an efficient size-selective catalyst. Micropor Mesopor Mater 221:220–227. https://doi.org/10.1016/j.micromeso.2015.09.053
Bai C, Zhao Q, Li Y, Zhang G, Zhang F, Fan X (2014) Palladium complex immobilized on graphene oxide as an efficient and recyclable catalyst for Suzuki coupling reaction. Catal Lett 144:1617–1623. https://doi.org/10.1007/s10562-014-1299-0
Kwon TH, Cho KY, Baek K-Y, Yoon HG, Kim BM (2017) Recyclable palladium–grapheme nanocomposite catalysts containing ionic polymers: efficient Suzuki coupling reactions. RSC Adv 7:11684–11690. https://doi.org/10.1039/C6RA26998B
Liu Y, Bai X (2017) Argon glow discharge plasma-reduced palladium nanoparticles supported on activated carbon for Suzuki and Heck coupling reactions. Appl Organomet Chem 31:3561. https://doi.org/10.1002/aoc.3561
Chen J, Zhang J, Sun W, Song K, Zhu D, Li T (2018) Pd immobilized on polyamide based on melamine and terephalic acid as an efficient and recyclable catalyst for Suzuki-Miyaura coupling reaction. Appl Organomet Chem 32:4135. https://doi.org/10.1002/aoc.4135
Dhakshinamoorthy A, Asiri AM, Garcia H (2015) Metal–organic frameworks catalyzed C–C and C–heteroatom coupling reactions. Chem Soc Rev 44:1922–1947. https://doi.org/10.1039/C4CS00254G
Hong MC, Ahn H, Choi MC, Lee Y, Kim J, Rhee H (2014) Pd nanoparticles immobilized on PNIPAM–halloysite: highly active and reusable catalyst for Suzuki–Miyaura coupling reactions in water. Appl Organomet Chem 28:156–161. https://doi.org/10.1016/j.molcata.2011.11.004
Magdesieva TV, Nikitin OM, Levitsky OA, Zinovyeva VA, Bezverkhyy I, Zolotukhina EV, Vorotyntsev MA (2012) Polypyrrole–palladium nanoparticles composite as efficient catalyst for Suzuki-Miyaura coupling. J Mol Catal A Chem 353:50–57. https://doi.org/10.1016/j.molcata.2011.11.004
Uberman PM, Pérez LA, Lacconi GI, Martín SE (2012) PVP-stabilized palladium nanoparticles electrochemically obtained as effective catalysts in aqueous medium Suzuki–Miyaura reaction. J Mol Catal A Chem 363:245–253. https://doi.org/10.1016/j.molcata.2012.06.016
Pascanu V, Hansen PR, Bermejo Gómez A, Ayats C, Platero-Prats AE, Johansson MJ, Pericàs MÀ, Martín-Matute B (2015) Highly functionalized biaryls via suzuki–miyaura cross-coupling catalyzed by Pd@MOF under batch and continuous flow regimes. ChemSusChem 8:123–130. https://doi.org/10.1002/cssc.201402858
Carson F, Pascanu V, Bermejo Gómez A, Zhang Y, Platero-Prats AE, Zou X, Martín-Matute B (2015) Influence of the base on Pd@MIL-101-NH2(Cr) as catalyst for the Suzuki-Miyaura cross-coupling reaction. Chem Eur J 21:10896–10902. https://doi.org/10.1002/chem.201500843
Xu W, Thapa KB, Ju Q, Fang Z, Huang W (2018) Heterogeneous catalysts based on mesoporous metal–organic frameworks. Coord Chem Rev 373:199–232. https://doi.org/10.1016/j.ccr.2017.10.014
Baran T, Baran NY, Menteş A (2018) An easily recoverable and highly reproducible agar-supported palladium catalyst for Suzuki-Miyaura coupling reactions and reduction of o-nitroaniline. Int J Biol Macromol 115:249256. https://doi.org/10.1016/j.ijbiomac.2018.04.057
Baran T (2018) Pd(0) nanocatalyst stabilized on a novel agar/pectin composite and its catalytic activity in the synthesis of biphenyl compounds by Suzuki-Miyaura cross coupling reaction and reduction of o-nitroaniline. CarbohydrPolym 195:45–52. https://doi.org/10.1016/j.carbpol.2018.04.064
Baran T (2017) Practical, economical, and eco-friendly starch-supported palladium catalyst for Suzuki coupling reactions. J Colloid Interface Sci 496:446–455. https://doi.org/10.1016/j.jcis.2017.02.047
Elavarasan S, Baskar B, Senthil C, Bhanja P, Bhaumik A, Selvam P, Sasidharan M (2016) An efficient mesoporous carbon nitride (g-C3N4) functionalized Pd catalyst for carbon–carbon bond formation reactions. RSC Adv 6:49376–49386. https://doi.org/10.1039/C6RA04170A
Chen Z, Vorobyeva E, Mitchell S, Fako E, Ortuño MA, López N, Collins SM, Midgley PA, Richard S, Vilé GA (2018) Heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling. Nat Nanotechnol 13:702–707. https://doi.org/10.1038/s41565-018-0167-2
Velpula VRK, Ketike T, Paleti G, Kamaraju SRR, Burri DR (2020) A facile synthesis of Pd–C3N4@ Titanate nanotube catalyst: highly efficient in Mizoroki–Heck, Suzuki–Miyaura C–C couplings. Cat Lett 150:95–105. https://doi.org/10.1007/s10562-019-02955-9
Xinran Z, Hongsheng F, Jinlong Z, Sibin D, Yunxia H, Yimin C, Rongming W (2018) Pd–Zn nanocrystals for highly efficient formic acid oxidation. Catal Sci Technol 8:4757–4765. https://doi.org/10.1039/C8CY01503A
Shylesh S, Gokhale AA, Ho CR, Bell AT (2017) Novel strategies for the production of fuels, lubricants, and chemicals from biomass. Acc Chem Res 50:2589–2597. https://doi.org/10.1021/acs.accounts.7b00354
Lin CSK, Pfaltzgraff LA, Herrero-Davila L, Mubofu EB, Abderrahim S, Clark JH, Koutinas AA, Kopsahelis N, Stamatelatou K, Dickson F (2013) Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ Sci 6:426–464. https://doi.org/10.1039/C2EE23440H
Brun N, Hesemann P, Esposito D (2017) Expanding the biomass derived chemical space. Chem Sci 8:4724–4738. https://doi.org/10.1039/C7SC00936D
Liu N, Huo K, McDowell MT, Zhao J, Cui Y (2013) Rice husks as a sustainable source of nanostructured silicon for high performance Li-ion battery anodes. Sci Rep 3:1919. https://doi.org/10.1038/srep01919
Kamari S, Ghorbani F (2020) Extraction of highly pure silica from rice husk as an agricultural by-product and its application in the production of magnetic mesoporous silica MCM–41. Biomass Conv Bioref 219. https://doi.org/10.1007/s13399-020-00637-w
Umeda J, Kondoh K (2010) High-purification of amorphous silica originated from rice husks by combination of polysaccharide hydrolysis and metallic impurities removal. Ind Crop Prod 32:539–544. https://doi.org/10.1016/j.indcrop.2010.07.002
Thirupathaiah K, Venkata Ramana KV, Venkata RM, Seetha Rama RK, Burri DR (2018) Carbonylative Suzuki-Miyaura cross-coupling over Pd NPs/Rice-Husk carbon-silica solid catalyst: effect of 1, 4-dioxane solvent. Chem Select 3:7164–7169. https://doi.org/10.1002/slct.201801100
Patel AR, Asatkar A, Patel G, Banerjee S Synthesis of rice husk derived activated mesoporous carbon immobilized palladium hybrid nano-catalyst for ligand-free mizoroki-heck/suzuki/sonogashira. Chem Select 4:5577–5584. https://doi.org/10.1002/slct.201900384
Dang TT, Zhu Y, Ngiam JS, Ghosh SC, Chen A, Seayad AM (2013) Palladium nanoparticles supported on ZIF-8 as an efficient heterogeneous catalyst for aminocarbonylation. ACS Catal 3:1406–1410. https://doi.org/10.1021/cs400232b
Hamdan H, Muhid MNM, Endud S, Listiorini E, Ramli Z (1997) 29Si MAS NMR, XRD and FESEM studies of rice husk silica for the synthesis of zeolites. J Non Cryst Solids 211:126–131
Amatore C, Jutand A, Le Duc G (2011) Kinetic data for the transmetalation/reductive elimination in Palladium-catalyzed suzuki–miyaura reactions: unexpected triple role of hydroxide ions used as base. Chem Eur J 17:2492–2503. https://doi.org/10.1002/chem.201001911
Acknowledgements
The author TK thanks UGC-New Delhi, India, for the award of the fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Velpula, V.R.K., Peesapati, S., Enumula, S.S. et al. Biomass waste rice husk derived silica supported palladium nanoparticles: an efficient catalyst for Suzuki–Miyaura and Heck–Mizoroki cross-coupling reactions. SN Appl. Sci. 2, 2177 (2020). https://doi.org/10.1007/s42452-020-03920-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03920-7