Abstract
Sol–gel synthesis of mesoporous titania–silicas with the structure related to Type MCM-41 is carried out. The method of nitrogen adsorption has established influence of fluoride-anion on the adsorptive and catalytic properties of titania–silica. The pore structure in titania–silica is in the form of an hexagonal array of uniform tubular channels. Asymptotic increase in a specific surface area of titania–silica catalyst with growth of F/Si ratio is shown. Both silicas and titano-silicas showed catalytic activity in the reaction of isomerization of α-pinene epoxide. The largest activity (100% conversion) and selectivity for campholenic aldehyde (57%) was shown by titanosilicate synthesized at Ti/Si = 25/75 and F/Si = 0.4.
Graphic abstract
Isotherms of nitrogen low-temperature adsorption-desorption and PSD curves for titania-silicas (7, 12) and silica (6) received at presence of fluoride-anion in ratio of F/Si equal: 7–0, 6–0.4, 12–0.4 mol/mol.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
From the moment of opening mesoporous molecular sieves of MCM-41 and MCM-48 and creation of titanium-containig zeolites of the TS-1 and TS-2 types [1] there was the significant amount of the works devoted to studying of influence of synthesis conditions on properties of these materials. The separate group was assembled by researches on use of fluoride anions at synthesis [2].
In the present work the adsorptive and textural properties of titania–silicas obtained by using fluoride anions and cetylpyridinium template were measured from the isotherms of nitrogen low-temperature (77 K) physical adsorption–desorption. The activity and selectivity of mesoporous titania–silicas were estimated in reaction of α-pinene epoxide isomerization. Oxygen containing derivatives of terpene hydrocarbons, isolated from turpentine and essential oils, are widely used in the synthesis of various fragrant and biologically active compounds. Thus, α-pinene oxide (2,3-epoxypinane) is a highly labile terpenoid and can be converted to various valuable products [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19] (Fig. 1). In industry, isomerization of the α-pinene oxide is carried out in the presence of zinc halides as homogeneous catalysts to form campholenic aldehyde(I). Campholenic aldehyde is an important intermediate in the synthesis of several sandalwood fragrances which constitutes an important growth area in perfumery chemicals (sandalor, bramanol, bogdanol, etc.). [3,4,5,6]. This process has several drawbacks, such as the formation of toxic waste, and the difficulty of separating the catalyst from the reaction mixture [4, 5]. Recently, a number of studies to develop new heterogeneous catalytic systems for 2,3-epoxypinane isomerization to campholenic aldehyde, as well as other valuable products (trans-carveol, pinocarveol, isopinocamphone, etc.) have been performed. A special attention was given to investigations of influence of the nature and strength of the acid sites on the yield of reaction products [8,9,10,11,12,13,14,15,16,17,18,19]. Relatively high selectivity to campholenic aldehyde was exhibited B2O3/SiO2 (69%), HY (66.0%) and Al-MSU-S (86%) zeolites, which is explained by a large amount of Lewis acid sites [11]. The physicochemical and catalytic properties of iron-modified Beta-75 and ZSM-5 zeolites, MCM-41 silicate, as well as Al2O3 and SiO2 were studied in [8]. In the presence of toluene as a solvent, Fe-MCM-41 was the most selective towards campholenic aldehyde (66%), which can be explained by the predominance of Lewis acid sites in the catalyst. A high concentration of weak and medium Brønsted acid sites in Fe-Beta-75 afforded formation of up to 23% of iso-campholenic (fencholenic) aldehyde (III). When N,N-dimethylacetamide was used as a solvent, the α-pinene oxide isomerization on H- and Fe- forms of Beta zeolites proceeded to form 31–43% trans-carveol (VI), which finds application in perfume compositions [9].
In the presence of Fe2O3–TiO2 mixed oxides, synthesized by the sol gel method, the main product of 2,3-epoxypinane conversion was campholenic aldehyde (selectivity 42%), which is explained the presence of strong Lewis acid sites in the solids, whereas over TiO2 a predominant (40%) formation of pinocarveol (IV) was observed [12]. An increase in the yield of aldehyde (II) from 25 to 38% was caused by gold deposition on TiO2 (> 70% anatase). In smaller amounts, the formation of compounds (IV) and (V) from Fig. 1 occurred on this catalyst [13]. The grafting of Fe3+ ions on SiO2 surface resulted in a catalyst with Lewis acidity, allowing selectivity to compound (II) of 56–72% [14].
The texture of the catalysts can have a significant effect on selectivity. Thus, isomerization of α-pinene oxide was studied at 30 °C in the presence of porous metal-benzenetricarboxylates, such as MIL100 (Al, Fe and Cr), MIL-110 (Al) and MIL-96 (Al). The highest selectivity for campholenic aldehyde (51–61%) at the substrate conversion of 96–98% was achieved on MIL-100 series, which is presumably due to their unique structure [16]. In the presence of US-Y zeolites having a three-dimensional large pore system (7.4 Å) with supercages of 12 Å and a large amount of mesopores, selectivity to campholenic aldehyde was 75% at 0 °C [17]. A very high selectivity to this product (89–94%) was observed in the presence of Ti-Beta zeolites, which may be in result of comparable sizes of pores, reagents and intermediates [18]. However the influence of type and features of texture, just as the nature and strength of the acid sites, of ordered mesoporous titania–silicas on the composition of α-pinene epoxide isomerization products remains not studied. Therefore the establishment of such quantitative regularities and receiving new catalytic materials for α-pinene epoxide isomerization is represented very relevant task.
2 Experimental
In the present work the procedure of the selective synthesis of titania–silica was carried out by analogy with synthesis of alumina–silica catalyst by neutralization of mix of solutions of the corresponding salts at presence of fluoride anion. Titanyl sulfate dihydrate and sodium metasilicate with the module of 1:1 were used as sources of Ti(IV) and Si(IV). For sodium hydroxide neutralization sulfuric acid (35%) was added into the solution and the mixture was kept at pH = 1 at 313 K within half an hour. Then water solution of titanyl sulfate dehydrate was added to the reaction mixture. Its hydrolysis at pH value of 6.0 was carried out in the presence of ammonia hydrate solution (1: 1). The sediment was filtered and then dried up on air. For the purpose of cationic exchange xerogel was suspended in water solution of ammonium salt (3.0%) and then at value pH 9.0—twice in ammonia hydrate solution then twice washed with the distilled water heated to 333 K. At last, the filtered xerogel again was dried on air and thermally processed at first at 393 K and at 923 K, both times during 2 h. The precipitation of titania–silica with molar ratio of Ti/Si = 25/75 was carried out in a periodic regime by template method on cetylpyridinium matrix. The amount of fluoride at synthesis corresponded to F/Si ratio in work [2].
The adsorption and textural properties of the samples were estimated from isotherms of low-temperature nitrogen physical adsorption–desorption obtained via volumetry on an ASAP 2020 MP surface area and porosity analyzer (Micromeritics, United States). The pore surface area per mass unit of each solid (the specific surface area) was determined using the single-point (Asp), BET (ABET), and Langmuir (AL) methods. The single-point method was also used in calculating the adsorption and desorption pore volumes (Vsp ads and Vsp des). The cumulative adsorption and desorption volumes (VBJH ads and VBJH des) of pores of diameters in the range of 1.7–300 nm were calculated by the BJH (Barrett–Joyner–Halenda) method. The mean pore diameter, D, of a group of mesopores, is defined as (4 V/A = D) where (4 V/A) is the ratio of the volume to the area of walls of the group. The VHK and DHK were pore volume and pore diameter by Horvath-Kawazoe method. The DFT (density functional theory) model was used to describe the real structure of pore distribution in samples with pores in a broad range of sizes.
The annealed at 923 K samples were trained prior to analysis; i.e., they were held in a vacuum for 2 h at 523 K and a residual pressure of 133.3 × 10–3 Pa. The samples dried in air were held in a vacuum for 2 h at 373 K. Data of gas adsorption are shown in Figs. 1, 2 and Tables 1, 2, 3, 4 and 5. The catalytic activity of samples was studied in α-pinene epoxide (0.5 g per run) isomerization at 353 K in toluene solution during 1 h. The amount of solid was 0.1 g. The reaction products were analyzed by gas chromatography according to the procedure described in [5, 20].
In addition to gas adsorption, the samples were examined by X-ray diffraction on diffractometer Bruker ADVANCE D8, recording reflexes characteristic of ordered structures of type MCM-41 in the small angle region. FT IR spectra were recorded on IR-Fourier spectrometer Tenzor-27 in the region 4000–400 cm−1 using powder tableting with potassium bromide at a sample/KBr ratio of 2/800.
3 Results and discussion
The measured sorption isotherms of silicas and also titania–silicas precipitated with supramolecular template in the presence of fluorides belong to Type IV (a) of sorption isotherms, characteristic for mesoporous adsorbents, according IUPAC classification (Figs. 2, 3). The features of hybrid hysteresis of Type H5 prove existence of the open and partially blocked mesopores (Fig. 2) [12]. The circumstance that the second step on isotherms of titania–silicas is more indistinct (Fig. 2) in comparison with the isotherms measured on silica attracts attention. Porous bodies with isotherms of Type IV (a) and hysteresis of Type H5 can be characterized as uniformly mesoporous adsorbents with the prevailing MCM-41 motive of ordering structure elements. Their texture is described by well defined irreversible isotherm of Type IV (a). At values of relative pressure lower than the beginning of the loop (p/p0 ˂ 0.42) the texture is described as isotherm of Type IV (b). It is characteristic for reversible nitrogen capillary condensation in this p/p0 area and inherent in mesoporous adsorbents with the blocked cylindrical mesopores. The emergence of hysteresis loop of Type H5 and the lack of micropores are connected with special conditions of template synthesis of sorbents therefore a contribution of micropores near contacts of particles and also influence of “alignment” of contact places when aging and drying hydrogels it is necessary to recognize as extremely small. In the presence of fluoride-anions the type of isotherm remains. The hysteresis on nitrogen sorption isotherms of the samples received with fluoride-anion, but in the absence of the template, has Type H2 inherent in porous bodies with expansions and narrowings of mesopores “equivalent” cylindrical on all length and with more wide PSD curves (Fig. 3).
By t-plot method it is shown that micropores in samples are absent (or are absent almost completely), and respectively micropore surface area and micropore volume can not be counted (Tables 1, 2, 4, 5). It should be noted increasing in external surface (Aext) of samples received in the presence of fluorides (Table 1). It can be explained with more correct form of particles. The resulting materials are more correctly considered to be disordered mesoporous molecular sieves. The adsorption isotherms inherent in them are a hybrid of Type I and Type IV isotherms. Both the Langmuir equation and the BET equation model the adsorption behavior of nitrogen on the surface in terms of the monolayer capacity can be used for surface area measurement based on that assumption. The Table 1 shows that Langmuir surface area is always higher than BET surface area The difference (AL − ABET) in the series of samples 1–6 is almost one and a half times greater than in the series of samples 7–12 and is about 26%.
The gas adsorption research of influence of fluorides on properties of titania–silicas allows to establish the dependence of specific characteristics of surface area on concentration of fluoride anion (Table 1). The most significant changes of isotherms and pore size distributions caused by presence of fluoride anion before nucleation stage of synthesis and further in the process of maturing of a mesomorphic phase happen at increase in its concentration to F/Si values of 0.1–0.2. At the same time changing specific surface area with growth of F/Si has an asymptotic current, and changing pore volume—generally extreme (Tables 1, 2).
Change of a mean pore diameter depends on the nature of changing specific surface area and pore volume (Table 3). Pore diameter values average 3–4 nm that will be coordinated with the data obtained for a model mesoporous adsorbent MCM-41 (Table 3) [7].
Thus, it is revealed that fluorine ions rather actively influence on synthesis of titania–silica. They catalyze reaction of hydrolysis of silicate ions and it is possible that thereby accelerate formation of a mesophase. Though noticeable influence of fluoride anions on process of the organization of ordered mesostructure on stages of nucleation was not observed, it is quite probable that the surface hydrophobization with fluorine ions in the presence of cetylpyridinium matrix promotes formation of silicate framework with higher rigidity. Maturing of a mesophase further accelerates under influence fluoride-anions. The effect of fluoride anion is obviously not related to the coagulation process under the influence of cations. Theoretically, a change in the texture of titania–silica under the action of fluoride anion can be explained by the fact that the ≡SiOH groups are at least partially replaced by ≡ SiF groups, as a result of which the surface becomes hydrophobic. Such silicas then undergo flocculation by hydrophobic bonds. The decrease of the number of ≡SiO− groups is partially compensated by the incorporation of F− anions into the neutral silica framework I0 (S+[I0F−] mechanism).
Use of fluorides before nucleation stage of titania–silica synthesis can lead to transformation of their adsorptive properties and textural characteristics. Fluoride-anions promote transformation of pore volume, internal and external surface area. Thus, by gas adsorption research of influence of fluorides on properties of titania–silica before nucleation stage and further during maturing mesophase dependences of specific characteristics of surface area, pore volume and average pore diameter on concentration of fluoride anions are established.
Both silicas and titania–silicas showed catalytic activity in the reaction of isomerization of α-pinene epoxide (Table 6). For all experiments, campholenic aldehyde II was the main reaction product (53–57%), which is obviously due to the presence of Lewis acidity in the solids [20]. It is important to note that although the selectivity of the studied materials to (II) was similar, the introduction of titanium atoms led to a sharp increase in the conversion of α-pinene epoxide to 94–100% (Table 6). This fact clearly indicates an increase in the acidity of the catalyst by reason of the presence of titanium. A slight increase in selectivity (from 53 to 57%) with an increase in F/Si from 0 to 0.4 can be explained by an growth in the strength of acid sites on the surface of the titania–silica catalyst happens in the presence of fluoride anions in the course sol–gel synthesis. However, as can be seen from Tables 2 and 3, the diameter and volume of pores does not affect the activity and selectivity of the studied materials.
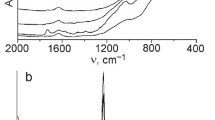
IR spectra confirm the presence of titanium (IV) in the framework of titania–silicas (Fig. 4). Along with the absorption peaks of 1100, 800, 465–475 cm−1, which correspond to the Si–O-Si bond vibrations, the absorption band with a frequency of 960–970 cm−1, which is characteristic of titanium-containing molecular sieves, is localized in the spectra. It is usually referred to as the Si–O–Ti bridge vibrations and is considered responsible for the antisymmetric vibrations of the Si–O–Ti bond in which the titanium atom is in tetrahedral coordination [21]. Antisymmetric vibrations of Ti–O–Ti bond during tetrahedral coordination are absorbed in the area of about 735 cm−1 [22].
Analysis of diffractograms in the region of small Bragg angles, 2θ < 10°, by the most intense reflex d100 = 4.1204 nm [ASTM 049-1712] allows the identification in the silica samples of a defect-free “mesoporous molecular sieve” MCM-41 with hexagonal packaging of cylindrical mesopores (Fig. 5). Inclusion of titanium (IV) is accompanied by their disordering, and d100 reflex is not recorded. The main peaks in the low-angle region characteristic of the MCM-41 disappear. This may be due to the destruction of the far order in the mesoporous structure and the increase in the number of defects in the hexagonal capillary packaging.
Undoubtedly, the mesoporous titanosilicate MCM-41 catalyst with a structure ordered by the Type of MCM-41, which changes the course and direction of reactions in terpene conversions, opens up new prospects for renewable raw materials in the fields of fine organic synthesis, including in the production of medicines and aromatic substances, organic products of small tonnage chemistry, etc. Compared with the works on the synthesis and structural characterization of micromesoporous materials, mesoporous zeolites, materials with pores of 1–2 nm, containing d- metals, and hierarchically organized porous systems with stable cohesion of micropores, mesopores and macropores, providing satisfactory transport of reagents [23,24,25,26,27], the present work is a study of the relatively simple low-temperature synthesis of materials ordered by type MCM-41, and the expansion of their use in catalysis as a catalyst for the reaction of isomerization of α-pinene epoxide. In theory and in the applied field, an adequate reflection of the progress achieved is the optimization of the physicochemical properties of nanoporous materials by comprehensively characterizing their texture (porous structure), including analysis of pore sizes, surface area, porosity, and pore size distribution [21, 28,29,30,31].
4 Conclusion
According to adsorption–desorption N2, the texture of titanosilicates has the shape of a hexagonal matrix of homogeneous tubular channels. Isotherms measured correspond to Type IV(a) and hybrid hysteresis of Type H5, according to the IUPAC classification, and confirm the presence of both open and partially blocked mesopores. Such isotherms are observed on the model mesoporous adsorbent MCM-41 and confirm that capillary condensation-evaporation can occur in open space with open ends about 3–4 nm wide. The obtained titania–silicas exhibit catalytic activity in the reactions of isomerization of α-pinene epoxide. The main reaction product is campholenic aldehyde, which is associated with the presence of Lewis acidity. Although the reliability of the investigated materials for α-pinene is about 94–100%, which proves the increase in the acidity of the catalyst due to the presence of titanium. A slight increase in the selectivity of the titania–silica catalyst with increasing F/Si ratio is associated with an increase in the strength of acid sites on its surface under constant conditions in the presence of fluoride anion. Moreover, the pore diameter and pore volume are satisfactory transport agents and do not affect the activity and selectivity of the studied materials.
References
Corma A (1994) Synthesis of an ultralarge pore titanium silicate isomorphous to MCM-41 and its application as a catalyst for selective oxidation of hydrocarbons. J Chem Soc Chem Commun 2:147–148
Kruk M, Cao L (2007) Synthesis of an ultralarge pore titanium silicate isomorphous to MCM-41 and its application as a catalyst for selective oxidation of hydrocarbons. Langmuir 23:7247–7254
Surburg H, Panten J (2006) Common fragrance and flavor materials: preparation, properties and uses, 5th edn. Wiley, Weinheim
Monteiro JLF, Veloso CO (2004) Catalytic conversion of terpenes into fine chemicals. Top Catal 27:169–180
Mäki-Arvela P, Holmbom B, Salmi T et al (2007) Recent progress in synthesis of fine and specialty chemicals from wood and other biomass by heterogeneous catalytic processes. Catal Rev 49:197–340
Golets M, Ajaikumar S, Mikkola JP (2015) Catalytic upgrading of extractives to chemicals: monoterpenes to “EXICALS”. Chem Rev 115:3141–3169
Kaminska J, Schwegler MA, Hoefnagel AJ, van Bekkum H et al (1992) The izomerization of α–pinene oxide with Bronsted and Lewis acids. Recl Trav Chim Pays Bas 11:432–437
Da Silva Rocha KA, Kozhevnikov IV, Gusevskaya EV (2005) Isomerisation of α-pinene oxide over silica supported heteropoly acid H3PW12O40. Appl Catal A Gen 294:106–110
Stekrova MA, Kumar N, Aho A, Sinev I, Grünert W, Dahl J, Roine J, Arzumanov S, Mäki-Arvela P, Murzin DYu (2014) Isomerization of α-pinene oxide using Fe-supported catalysts: Selective synthesis of campholenic aldehyde. Appl Catal A Gen 470:162–176
Da Silva Rocha KA, Hoehne JL, Gusevskaya EV (2008) Phosphotungstic acid as a versatile catalyst for the synthesis of fragrance compounds by α-pinene oxide isomerization: solvent-induced chemoselectivity. J Chem Eur 14:6166–6172
Ravindra DB, Nie YT, Jaenicke S, Chuah GK (2004) Isomerisation of α-pinene oxide over B2O3/SiO2 and Al-MSU catalysts. Catal Today 96:147–153
Neri G, Rizzo G, Galvagno S et al (2004) Sol-gel synthesis, characterization and catalytic properties of Fe–Ti mixed oxides. Appl Catal A Gen 274:243–251
Demidova SYu, Ardashov OA, Simakova OA, Volcho KP, Salakhutdinov NF, Murzin DYu (2014) Isomerization of bicyclic terpene epoxides into allylic alcohols without changing of the initial structure. J Mol Catal A Chem 388–389:162–166
Ravasio N, Zaccheria F, Gervasini A, Messi C (2008) Nanodispersed Fe oxide supported catalysts with tuned properties. Catal Commun 9:1125–1127
Anikeev VI, Il’ina IV, Volcho KP, Yermakova A, Salakhutdinov NF (2010) Reactivity of α-pinene epoxide in supercritical solvents. J Supercr Fluids 52:71–75
Panchenko VN, Timofeeva MN, Jhung SH (2016) Acid-base properties and catalytic activity of metal-organic frameworks: a view from spectroscopic and semiempirical methods. Catal Rev Sci Eng 58:209–307
Hölderich WF, Röselar J, Heitmann JG, Liebens AT (1997) The use of zeolites in the synthesis of fine and intermediate chemicals. Catal Today 37:353–366
Kunkeler PJ, van der Waal JC, Bremmer J, Zuurdeeg BJ, Downing RS, van Bekkum H (1998) Application of zeolite titanium Beta in the rearrangement of α-pinene oxide to campholenic aldehyde. Catal Lett 53:135–138
Sidorenko AYu, Ignatovich ZhV, Ermolinskaya AL, Kravtsova AV, Baranovskii AV, Koroleva EV, Agabekov VE (2018) Synthesis of fencholenic aldehyde from α-pinene epoxide on modified clays. Chem Nat Compd 54:893–897
Sidorenko AYu, Kravtsova AV, Aho A, Heinma I, Kuznetsova TF, Murzin DYu, Agabekov VE (2018) Catalytic isomerization of a-pinene oxide in the presence of acid-modified clays. Mol Catal 448:18–29
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl Chem 87:1051–1069
Boccuti MR, Rao KM, Zecchina A et al (1989) Spectroscopic characterization of silicalite and titanium-silicalite. Stud Surf Sci Catal 48:133–144
Young DA, Linda Y (1967) Crystalline Tinanosilicate Zeolites. US Patent 3 329 481
Scheffler F, Schwieger W, Freude D, Liu H, Heyer W (2002) Transformation of porous glass beads into MFI-type containing beads. Microporous Mesoporous Mater 55:181–191
Serrano D, Aguado J, Morales G et al (2009) Molecular and meso- and macroscopic properties of hierarchical nanocrystalline ZSM-5 zeolite prepared by seed silanization. Chem Mater 21:641–654
Shpeizer BG, Bakhmutov VI, Clearfield A (2006) Supermicroporous alumina–silica zinc oxides. Microporous Mesoporous Mater 90:81–86
Shpheizer BG, Bakhmoutov VI, Zhang P, Prosvirin AV, Clierfield A (2010) Transition metal–alumina/silica supermicroporous composites with tunable porosity. Coll Surf A Physicochem Eng Asp 357:105–115
Thommes M (2010) Physical adsorption characterization of nanoporous materials. Chem Ing Technol 82:1059–1073
Gregg SJ, Sing KSW (1982) Adsorption, surface area and porosity, 2nd edn. Academic Press, London
Neimark A, Sing KSW, Thommes M (2008) Surface area and porosity. Wiley, Weinheim
Ravikovitch PI, Neimark AV (2006) Density functional theory model of adsorption on amorphous and microporous silica materials. Langmuir 22:11171–11179
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kouznetsova, T.F., Sidorenko, A.Y., Ivanets, A.I. et al. Sol–gel synthesis, texture and catalytic activity of titania–silica sorbents. SN Appl. Sci. 1, 1734 (2019). https://doi.org/10.1007/s42452-019-1781-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1781-9