The conditions for isomerization of α-pinene epoxide (2,3-epoxypinane) on modified clays that gave comparatively high contents (33.0%) of fencholenic (iso-campholenic) aldehyde in the product mixture were determined. An effective method for isolating it was proposed. The structure of iso-campholenic aldehyde was confirmed by analyzing 2D NOESY and HMBC NMR spectra.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
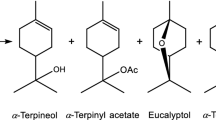
Epoxides are accessible reactive compounds that are converted by Lewis and Bronsted acids into aldehydes, ketones, or alcohols [1,2,3]. Thus, α-pinene epoxide (2,3-epoxypinane) is produced by chemical processing of turpentine and acts as a source for trans-carveol [4], pinocarveol [5], and campholenic aldehyde, which are used to synthesize fragrances and formulate perfumes. Campholenic aldehyde is used to produce compounds with sandalwood scents (sandalore, brahmanol, bogdanol, etc.) and can be obtained in 85.0% yield from isomerization of 2,3-epoxypinane in the presence of acidic catalysts, e.g., ZnCl2 [6, 7].
Fencholenic (iso-campholenic) aldehyde was first reported in 1961 [8] and is also of interest for preparing new fragrances. However, its fraction is usually small in the product mixture from isomerization of 2,3-epoxypinane. For example, isomerization of 2,3-epoxypinane in the presence of montmorillonite clays K-10, Filtrol-105, and Tonsil LF-80 gave fencholenic aldehyde in 11–19% yield in a mixture with campholenic aldehyde (27–40%) and other isomerization products [9]. Pinocarveol, a difficultly accessible starting compound, was used to synthesize fencholenic aldehyde [10].

The goals of the present work were to optimize the conditions for isomerizing α-pinene epoxide into iso-campholenic aldehyde and to develop a method for isolating it from the reaction mixture. α-Pinene epoxide (1) was isomerized at 30°C in the presence of clay (3.0%) as a catalyst. Illite clays, sepiolite, and glauconite were treated beforehand with HCl solution (10%), rinsed to remove traces of HCl, and dried at 50–200°C to increase their activity [11]. The solvents were cyclohexane, decane, toluene, benzene, and CH2Cl2. Compositions of reaction mixtures were determined by GC. A complicated reaction-product mixture was formed over all studied clays for isomerization of 1. The main products were campholenic (4, up to 41%) and iso-campholenic aldehydes (5, up to 28.0%) (Table 1). Besides them, the product mixture included trans-carveol (2, 13.1–15.0%), trans-sorberol (6, 7.3–11.2%), and small amounts of pinocarveol (3) and iso-pinocamphone (7). p-Cymene (8) and pinol (9) were secondary isomerization products of 2,3-epoxypinane and were formed by dehydration of hydroxylated products [4].
Isomerization of α-pinene epoxide (1) was complete (99.9% conversion) after 10–30 min over montmorillonites K-10 and K-30 and illites L-1, IL-RS, and halloysite (Table 1). The conversion was incomplete even after reacting for 4 h for montmorillonite KSF, sepiolite, and glauconite. The contents of campholenic aldehyde (4) in the isomerization products were about equal. However, the greatest amounts of iso-campholenic aldehyde formed over clays L-1 and IL-RS (Table 1).
Isomerization over clay L-1 of α-pinene epoxide in benzene, toluene, and CH2Cl2 gave significantly lower amounts of iso-campholenic aldehyde (5) in the product mixture than if cyclohexane was used (Table 2). The ratio of the amounts of campholenic–iso-campholenic aldehydes (1.5:1) in n-decane was close to that in cyclohexane (1.4:1). Thus, a nonpolar solvent enhanced the formation of iso-campholenic aldehyde.
Increasing the preliminary drying temperature of clay L-1 increased the content of 4 and decreased that of 5 in the reaction mixture. Thus, practically equal amounts of the aldehydes (33–35%) formed on catalyst dried at 50°C. The content of iso-campholenic aldehyde decreased to 25% if the drying temperature was increased to 200°C. The yield of fencholenic aldehyde on illite IL-RS heat-treated at 50°C was 32%.
Bronsted and Lewis acids are known to catalyze isomerization of α-pinene epoxide [4]. Acidic hydroxyls, coordinatively unsaturated and exchangeable catalyst cations, and polarized water molecules in the clay can act as such centers [12]. Recently, the yield of iso-campholenic aldehyde was shown by us to increase if the acidity was decreased and the ratio of Lewis–Bronsted centers in the clays was increased [11]. The proton-donating properties of water molecules associated with exchangeable cations (primarily Al3+) and Al3+ cations on faces of tetrahedral layers of the clay minerals increased with decreasing degree of hydration, i.e., with increased drying temperature [12]. However, the amount of iso-campholenic aldehyde (5) decreased with increasing drying temperature for the illite clays. It could be proposed that α-pinene epoxide isomerized into iso-campholenic aldehyde in the presence of polarized water molecules located on the surface of the illite clay, i.e., weak Bronsted centers.
Obviously, the first step of the isomerization was reaction of a proton with the α-pinene epoxide oxirane ring to open it and form carbocation A. Rearrangement of A on the clays could occur via migration of the C1–C7 bond to cationic C2 to form ion B. Cleavage of the C2–C3 bond in cation B and subsequent loss of a proton formed campholenic aldehyde 4. An alternative pathway for conversion of A is shifting of the C1–C6 bond to C2 to form carbocation C, which rearranged similarly to B to give iso-campholenic aldehyde 5. The A→B,C conversion in the presence of illite clay dried at 50°C occurred with the same rate. Pathway A→B began to dominate if the drying temperature was increased to 200°C and the strength of the acidic center increased because the strong Bronsted acidic centers enhanced formation of 4 [11].

Thus, isomerization of 1 into 5 was more effective in cyclohexane over weakly acidic illite clay catalysts. The product yields were up to 33%.
A method for separating 5 from the reaction mixture was developed. An aldehyde fraction containing 90% iso-campholenic aldehyde and 2% campholenic aldehyde could be separated from the other products.
The structures of the isomeric aldehydes were established using convergent synthesis of 4 according to the literature method of Arbuzov [7]. The compound had physicochemical properties (IR, PMR, and mass spectra) that agreed with the literature so it was used as a reference for spectra of 4 and 5 isolated from the isomerization reaction products. Also, a convergent synthesis of 5 via isomerization of 2,3-epoxypinane (1) in the presence of BF3·Et2O according to the literature was attempted [13]. However, 4 was formed in 46% yield in this instance and had physicochemical properties identical to those of campholenic aldehyde synthesized by the literature method [7].
PMR spectra of isomeric aldehydes 4 and 5 differed considerably with respect to the chemical shift of the H atom on the ring double bond. Thus, H-3 of iso-campholenic aldehyde (5) was shielded by three methyls and had chemical shift δ 5.13 ppm in the PMR spectrum. This differed by 0.1 ppm from the chemical shift δ 5.23 ppm of the less shielded H atom of 4. The chemical shifts of the aldehyde H atoms were the same for the isomers. The methyl resonances differed by 0.03–0.05 ppm; geminal protons H-5 and the chain, by 0.01–0.05 ppm. The vicinal SSCC J3 of H-4 with the neighboring H-5 ring protons in 4 and the J4 constant of H-3 in 5 were <1 Hz.
13C NMR spectra of 4 and 5 showed considerably different resonances for all three methyls and C-3, C-4, and C-5 of the cyclopentane ring. However, this did not allow any definite conclusions about the structures of the isomers to be drawn.
Two-dimensional NMR spectroscopy was used to establish unambiguously the spectral and structural correlations of the isomeric aldehydes. Final conclusions were made based on an analysis of NOESY and HMBC spectra because COSY spectra gave ambiguous assignments. Cross peaks of a proton and methyl on the double bond with the C-5 protons were observed for both isomers without coupling to the C-1 proton. The most informative difference between the isomers in the HMBC spectra was a cross peak between the C-2 methyl protons and the methine C atom (135.77 ppm) in 5. These protons in 4 coupled with quaternary C-3 (148.12 ppm). C-3 in the NOESY spectrum of 5 had cross peaks with all methyl protons whereas the C-4 proton in 4 coupled with the C-3 methyl protons and the C-5 methylene protons. Based on these results, it was considered proved that the methyl of 4 was situated on C-3; of 5, on C-4.
Thus, isomerization of 2,3-epoxypinane (1) in the presence of BF3·Et2O according to the literature method [13] gave campholenic aldehyde (4). As a result, the method described by us for preparing iso-campholenic aldehyde was more effective.
Experimental
The isomerization catalysts for α-pinene epoxide (Sigma-Aldrich, 98%) were commercial montmorillonites (K-10, K-30, Al-Pillared Mont., KSF), sepiolite and halloysite (Sigma-Aldrich), natural illites L-1 (Belarus) and IL-RS (Russia), and Belarusian glauconite from Loev deposit. The catalytic activity of the clays, except for K10, K-30, and Al-Pillared Mont., was increased by treatment with HCl (10%, 5 mL/g) at 90°C for 3 h, rinsing thoroughly to remove acid, and drying at 50–200°C.
The quantitative composition of the reaction mixture was determined by GC using a Khromos GKh-1000 chromatograph with a flame-ionization detector and Zebron ZB-5 capillary column (30 m × 0.25 mm 0.25 μm) and the literature method [11].
IR spectra were taken from films on a Bruker Tensor 27 FT-IR spectrometer in the range 400–4000 cm–1. PMR and 13C NMR spectra were recorded on an Avance 500 spectrometer (Bruker BioSpin) at operating frequency 500.0 and 125.7 MHz for 1H and 13C, respectively, using a Z-gradient 5-mm broad-band observe (BBO) probe. Spectra were recorded from CDCl3 solutions with residual solvent resonances [δ 7.26 (1H, CDCl3); 77.16 (13CDCl3)] as internal standards. Correlation spectra (HSQC, COSY, HMBC, NOESY) were recorded and processed using standard software (Bruker BioSpin). Spin–spin coupling constants (SSCCs) were given in Hz; chemical shifts, in ppm on the δ scale. Chromatographic analysis and mass spectral measurements used a Thermo Scientific Trace GC Ultra/DSQ II quadrupole GC-MS with direct sample introduction. Elemental analysis was performed on a vario MICRO element (CHNS) analyzer.
Isomerization of α-Pinene Epoxide (1). Compound 1 (2.0 g) was treated with solvent (total mixture volume of 20 mL), heated to 30°C, treated with catalyst (0.06 g, 3.0%), and stirred. The composition of the reaction mixture was monitored using GC. The solvents were dry cyclohexane, n-decane, toluene, benzene, and CH2Cl2.
Isolation of iso-Campholenic Aldehyde (5). The mixture (20 mL) of α-pinene epoxide isomerization products containing 35% campholenic aldehyde (4) and 33% iso-campholenic aldehyde (5) according to GC data was filtered to remove clay. The solvent was evaporated to produce a transparent oil (2 g) that was treated with H2O (3 mL) and dropwise with an aqueous solution of sodium metabisulfite (Na2S2O5, 2 g, 0.01 mol). The suspension was stirred for 2 h. Side products from the isomerization were extracted by petroleum ether (2 × 10 mL) and EtOAc (3 × 10 mL) and discarded. The aqueous layer was made basic using saturated aqueous Na2CO3 solution to pH 8 and stirred for 1 h. Products were extracted by petroleum ether (2 × 10 mL) and EtOAc (2 × 10 mL). The solvent was evaporated to afford a transparent oil (0.48 g, 24% yield) with a pine scent that contained 89–90% iso-campholenic and ~2% campholenic aldehyde according to GC data.
2-(2,2,3-Trimethylcyclopent-3-en-1-yl)acetaldehyde (campholenic aldehyde) (4) was prepared by the literature method [7]. α-Pinene epoxide (2 g) in anhydrous benzene (5 mL) was treated with ZnCl2 (0.2 g), heated on an oil bath at 80°C for 20 min, and evaporated to give a transparent colorless oil (2 g) containing 80–84% campholenic aldehyde according to GC data. The reaction product was purified by fractional vacuum distillation [bp 120°C (10 mm)] to afford a colorless oil (1.4 g, 70% yield) that contained 92% campholenic aldehyde. IR spectrum (KBr, ν, cm–1): 3441, 2957–2928, 1725 (C=O), 1629 (C=C), 1464, 1362. 1H NMR spectrum (500 MHz, CDCl3, δ, ppm, J/Hz): 0.79 (3H, s, CH3-2), 1.01 (3H, s, CH3-2), 1.62 (3H, s, CH3-3), 1.88 (1H, m, H-5), 2.28 (1H, dd, J = 4.3, 8.8, H-1), 2.38 (2H, m, H-5, H2C-CHO), 2.52 (1H, dd, J = 2.4, 15.6, H2C-CHO), 5.23 (1H, s, H-4), 9.80 (1H, s, CHO). 13C NMR spectrum (125 MHz, CDCl3, δ, ppm): 12.75 (CH3, C-3), 20.16 (CH3, C-2), 25.75 (CH3, C-2), 35.65 (CH2, C-5), 44.33 (CH, C-1), 45.24 (CH2, C-CHO), 47.05 (C, C-2), 121.69 (CH, C-4), 148.12 (C, C-3), 203.21 (CH, CHO). Mass spectrum, m/z (Irel, %): [M]+ 152.18 (4), 119.07 (6), 108.06 (100), 93.04 (87), 81.03 (15), 67.02 (20), 41.02 (18). C10H16O.
2-(2,2,4-Trimethylcyclopent-3-en-1-yl)acetaldehyde ( iso -campholenic aldehyde) (5), colorless oil. IR spectrum (KBr, ν, cm–1): 3432, 2956–3025, 1725 (C=O), 1657 (C=C), 1447, 1361. 1H NMR spectrum (500 MHz, CDCl3, δ, ppm, J/Hz): 0.80 (3H, s, CH3-2), 1.02 (3H, s, CH3-2), 1.65 (3H, s, CH3-4), 1.98 (1H, dd, J = 15.4, 8.5, H-5), 2.31 (1H, m, H-1), 2.35 (1H, m, H2C-CHO), 2.39 (1H, m, H-5), 2.51 (1H, d, J = 14.9, H2C-CHO), 5.13 (1H, s, H-3), 9.78 (1H, s, CHO). 13C NMR spectrum (125 MHz, CDCl3, δ, ppm): 16.73 (CH3, C-4), 22.56 (CH3, C-2), 28.13 (CH3, C-2), 42.16 (CH2, C-5), 43.72 (HC, C-1), 45.20 (CH2, C-CHO), 46.35 (C, C-2), 135.77 (HC, C-3), 136.79 (C, C-4), 203.17 (CH, HCO). Mass spectrum, m/z (Irel, %): [M]+ 152.06 (28), 137.04 (31), 119.05 (12), 108.07 (100), 95.06 (92), 93.02 (96), 90.99 (32), 81.02 (18), 76.97 (18), 67.01 (38), 40.98 (22). C10H16O.
References
A. S. Rao, S. K. Paknikar, and J. G. Kirtane, Tetrahedron, 39, 2323 (1983).
J. G. Smith, Synthesis, 8, 629 (1984).
K. Maruoka, N. Murase, R. Bureau, T. Ooi, and H. Yamamoto, Tetrahedron, 50, 3663 (1994).
M. Stekrova, N. Kumar, S. F. Diaz, P. Maki-Arvela, and D. Yu. Murzin, Catal. Today, 241, Part B., 237 (2015).
G. Neri, G. Rizzo, S. Galvagno, G. Loiacono, A. Donato, M. G. Musolino, R. Pietropaolo, and E. Rombi, Appl. Catal., A, 274, 243 (2004).
M. Golets, S. Ajaikumar, and J. P. Mikkola, Chem. Rev., 115, 3141 (2015).
B. A. Arbuzov and E. G. Isaeva, Zh. Obshch. Khim., 24, 1250 (1954).
L. C. King and H. Farber, J. Org. Chem., 26, 326 (1961).
J. Kaminska, M. A. Schwegler, A. J. Hoefnagel, and H. van Bekkum, Recl. Trav. Chim. Pays-Bas, 111, 432 (1992).
K. Schulze and H. Uhlig, Monatsh. Chem., 120, 547 (1989).
A. Yu. Sidorenko, A. V. Kravtsova, A. Aho, I. Heinmaa, T. F. Kuznetsova, D. Yu. Murzin, and V. E. Agabekov, Mol. Catal., 448, 18 (2018).
F. Bergaya and G. Lagaly, Handbook of Clay Science, Part A: Fundamental, Elsevier, Amsterdam, 2013, 1752 pp.
G. Carr, G. Dosanjh, A. P. Millar, and D. Whittaker, J. Chem. Soc., Perkin Trans. 2, 1419 (1994).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2018, pp. 758–761.
Rights and permissions
About this article
Cite this article
Sidorenko, A.Y., Ignatovich, Z.V., Ermolinskaya, A.L. et al. Synthesis of Fencholenic Aldehyde from α-pinene Epoxide on Modified Clays. Chem Nat Compd 54, 893–897 (2018). https://doi.org/10.1007/s10600-018-2506-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-018-2506-9