Abstract
The biomechanical effects of acetabular revision with jumbo cups are unclear. This study aimed to compare the biomechanical effects of bionic trabecular metal vs. titanium jumbo cups for the revision of acetabular bone defects. We designed and reconstructed American Academy of Orthopaedic Surgeons (AAOS) type I–III acetabular bone defect models using computed tomography scans of a man without acetabular bone defects. The implantation of titanium and trabecular metal jumbo cups was simulated. Stress distribution and relative micromotion between the cup and host bone were assessed using finite element analysis. Contact stress on the screws fixing the cups was also analyzed. The contact stress analysis showed that the peak contact stress between the titanium jumbo cup and the host bone was 21.7, 20.1, and 23.8 MPa in the AAOS I–III models, respectively; the corresponding values for bionic tantalum jumbo cups decreased to 4.7, 6.7, and 11.1 MPa. Analysis of the relative micromotion showed that the peak relative micromotion between the host bone and the titanium metal cup was 10.2, 9.1, and 11.5 μm in the AAOS I–III models, respectively; the corresponding values for bionic trabecular metal cups were 17.2, 18.2, and 31.3 μm. The peak contact stress on the screws was similar for the 2 cup types, and was concentrated on the screw rods. Hence, acetabular reconstruction with jumbo cups is biomechanically feasible. We recommend trabecular metal cups due to their superior stress distribution and higher relative micromotion, which is within the threshold for adequate bone ingrowth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the rapid increase in the number of Total Hip Arthroplasties (THAs) in recent times, the need for revision THAs is gradually increasing [1]. Osteolysis and prosthesis loosening are the main indications for revision THA, and these are often accompanied by acetabular bone defects, which present a challenge for orthopedic surgeons [2]. Several surgical techniques are available for the reconstruction of acetabular bone defects, such as impaction allografting with a mesh [3, 4], structural bone grafting [5], and reinforcing rings [6, 7], but these procedures are complicated, time-consuming, and associated with a high risk of postoperative infection. In contrast, acetabular revision with jumbo cups is a common and effective technique for the treatment of extensive acetabular defects [8] that greatly simplifies revision surgery procedures, avoids extensive bone grafting, and increases the Cup Coverage (CC) between the cup and host bone [9].
Compared with primary THA, acetabular revision surgery may carry a greater risk of adverse events, such as periprosthetic osteolysis, loosening, and dislocation. Furthermore, as acetabular defect reconstruction with jumbo cups changes the position of the natural Hip Center of Rotation (HCOR), the issue of adverse effects deserves more attention [10]. The use of jumbo cups has been reported to quantitatively change the HCOR and to yield a CC of more than 70% in patients with acetabular bone defects classified as American Academy of Orthopaedic Surgeons (AAOS) types I–III [11]. Good initial stability and stress distribution are known to be essential for bone growth into the cup [12, 13]. Nevertheless, no study has yet reported the biomechanical effects of acetabular revision surgery using jumbo cups for acetabular defects of varying severity.
Conventional titanium jumbo cups can be difficult to use in complex surgical procedures, and lead to an increased risk of failure [14]. Encouragingly, tantalum metal has particular advantages in acetabular revision surgery, and is a promising solution to avoid postoperative complications [15]. Tantalum has been fabricated into a three-dimensional (3D) macroporous bionic structure very similar to cancellous bone, known as trabecular metal. The mechanical properties of bionic structures made from trabecular metal include a Young’s modulus similar to that of natural cancellous bone, a unique combination of high elasticity and a high coefficient of friction, and a high porosity of 75–85%, which can promote bone ingrowth [16, 17].
The aim of this study was to comparatively analyze the biomechanical effects of conventional titanium cups vs. bionic trabecular metal cups for the reconstruction of acetabular defects of varying severity. For this purpose, we established several models of acetabular bone defects of AAOS types I–III and simulated acetabular revision surgery with jumbo cups. The bone defect model is a universal model that summarizes the characteristics of multiple acetabular bone defects based on a previous study [11]. We then used Finite Element Analysis (FEA) to compare the biomechanical effects of conventional titanium cups and bionic trabecular metal cups.
2 Materials and Methods
2.1 Geometric Model of Acetabular Bone Defects
This study was approved by the Institutional Review Board of our hospital (No: 2020-NSFC-007). A FEA model was derived from a de-identified Computed Tomography (CT) scan of the pelvis of a healthy 66-year-old male volunteer, who had no musculoskeletal disease, no history of hip surgery, and no acetabular bone defect. Prior to the CT study, the subject signed an informed consent form. CT images were obtained using the Philips iCT 256 CT scanner (iCT 256, Philips Healthcare), with a resolution of 512 × 512 pixels and a slice thickness of 0.602 mm at 120 kVp and 156 mA. CT slices of the volunteer’s pelvis were saved in the Digital Imaging and Communications in Medicine format, imported into Mimics v19.0 software (Materialise NV, Leuven, Belgium) to create a 3D pelvic model, and then smoothed. The diameter of the volunteer’s native acetabulum was 48 mm.
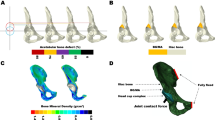
Acetabular bone defect models were simulated using the Boolean operation on the Mimics v19.0 software. Using maps of AAOS type I–III acetabular bone defects reported in a previous study [11], we designed and reconstructed 3 acetabular bone defect models (Fig. 1). The specific steps were as follows: (1) A sphere with a diameter of 60 mm was placed on the native HCOR, and raised upward in the coronal view. (2) The position of the 60-mm sphere was adjusted forward or backward, and the Boolean operation was used to cut the pelvic bone such that the acetabulum would have main segmental or cavity bone defects. (3) Additional bone defects on the acetabular ring were created using the Simulation/Cut orthogonal to Screen procedure to conform to the bone defect map. (4) An egg-shell cup (with negligible thickness) was developed to replace the sphere, and its HCOR and CC were measured and validated.
(a–c) AAOS type I–III acetabular bone defect models established based on a previous study. (d–f) Three-dimensional simulation of acetabular revision surgery with jumbo cup implantation, and visualization of the distribution of bone defects and cup coverage (CC). The blue line represents a 5-mm shift in the center of acetabular rotation, and the red line represents a 10-mm shift in the center of acetabular rotation
2.2 Simulation of Jumbo Cup Implantation
Acetabular component implantation in acetabular revision surgery was simulated in a 3D environment. Acetabular components with a shell thickness of 4 mm and a diameter of 60 mm were developed and imported into Mimics software in the stereolithography format. Cup inclination of 40° and anteversion of 20° relative to the anterior pelvic plane were preset to accommodate the structure of the acetabular bone defect [18]. The jumbo acetabular components consisted of two 60-mm cups, one made of a titanium alloy and the other made of bionic trabecular metal (Fig. 2). To obtain sufficient initial cup stability, 3 screws measuring 30 mm in length and 5 mm in diameter were used to fix the acetabular cups. Solid models of the cups were assembled in Magics v19.0 software (Materialise NV, Leuven, Belgium), and used to simulate the reconstruction of the acetabular bone defect models via acetabular revision surgery.
2.3 Material Properties
The above model of acetabular revision surgery using jumbo cups was imported into Hypermesh 2020 (Altair Engineering, Troy, MI, USA) for mesh creation and definition of material properties based on a previous study [19]. Hounsfield Units (HUs) were automatically acquired from grayscale CT images, and the properties of the pelvic bone were defined based on the relationship between the elastic modulus, density, and CT value of the bone. The specific formula [20] was as follows:
For finite element meshing, sensitivity analysis of the mesh quality was carried out until mesh refinement resulted in a change of < 5% in the maximum principal stress. Finally, an average mesh size of 1 mm was set onto the designed components. The volume mesh was meshed with 4-node tetrahedral elements after two-dimensional meshing, which has been verified in previous studies [19]. We constructed three 3D models of the right pelvis using the aforementioned data, namely, AAOS type-I with 802,701 elements, AAOS type-II with 796,602 elements, and AAOS type-III with 740,022 elements. The material properties used in the model are shown in Table 1 and Fig. 3. Each simulated implanted cup was fixed by 3 cancellous screws with an elastic modulus (E) of 110,000 Mpa and a Poisson ratio (v) of 0.3. The friction coefficient between the titanium cup and the bone was 0.30. To fully imitate the mechanical and biological properties of human trabecular bone, we set the friction coefficient of the trabecular metal cup to 0.88 and the elastic modulus to 2000 MPa, which are equivalent to a porosity of 80% [21, 22].
2.4 Boundary and Loading Conditions
The friction coefficient of each contact surface was determined from published literature [23,24,25], and all contact surfaces were defined as nonlinear. Without considering the muscles around the hip joint, we applied the resultant equivalent load to the center of the femoral head to simulate the force of the hip joint when a person stands on one leg. The peak unilateral contact load on the hip joint was measured as 1948 N, which is consistent with most of the literature [19, 26]. According to the anatomy of the pelvis, the corresponding nodes at the pubic symphysis and sacroiliac joint were fully immobilized and constrained against translation and rotation (Fig. 3). A quasi-static loading nonlinear analysis was adopted during the simulation process using Optistruct in Hypermesh 2020 (Altair Engineering, Troy, MI, USA), and the iterative method was the Newton–Raphson method until convergence. Contact stress distribution and relative micromotion were used to evaluate the effects of stress shielding and bone ingrowth.
3 Results and Discussion
3.1 Characteristics of Acetabular Bone Defect Models
Our acetabular bone defect models conformed to previously reported characteristics of acetabular reconstruction surgery with 60-mm acetabular cups. The results showed that in the AAOS type-I model, the CC between the host bone and the acetabular cup was 86.8%, and the HCOR was elevated 5 mm. In the AAOS type-II model, the CC was determined to be 85.5%, and the HCOR was elevated 5 mm. In the AAOS type-III model, the CC was determined to be 74.0%, and the HCOR was elevated 10 mm (Fig. 1). These results are consistent with our previous findings (Table 2), confirming that the acetabular bone defect models we designed were appropriate.
3.2 Contact Stress Distribution
The AAOS type I–III acetabular bone defects were reconstructed with titanium jumbo cups, and the distribution of von Mises stress in the 3 FEA models under the applied load is shown in Fig. 4. According to the FEA results, the acetabular components showed varying degrees of stress concentration at the bottom of the acetabulum. Among the 3 models, the peak pelvic von Mises stress was the highest in the AAOS type-I model, reaching 37.6 MPa, followed by the AAOS type-III model (32.3 MPa) and the AAOS type-II model (30.6 MPa). The peak contact stress between the titanium jumbo cups and the host bone was relatively high in all 3 models. The peak contact stress was similar in the AAOS type-I and type-II models (21.7 vs. 20.1 MPa), and both of these were lower than the peak contact stress in the AAOS type-III model (23.8 MPa). Analysis of the distribution of von Mises stress on the acetabular components showed that the contact stress was mainly distributed on the rim of the titanium cups (Fig. 5). The stress on the screws was mainly concentrated on the full lengths of 2 of the 3 screws (Fig. 6).
Compared with the titanium cups, the trabecular metal jumbo cups resulted in slightly higher peak contact stress on the acetabulum in all 3 models. This stress was also mainly distributed at the bottom of the acetabulum. Among the 3 models, the peak contact stress was highest in the AAOS type-I model, reaching 38.6 MPa. However, the peak contact stress of the acetabular component was relatively small, with a maximum reduction of 78% in the AAOS type-I model. The stress on the screws was higher in the case of the trabecular metal jumbo cups than in the case of titanium jumbo cups in the AAOS type-I and type-III models, but not in the AAOS type-II model (Fig. 7).
3.3 Relative Micromotion
The distribution of the relative micromotion between the host bone and titanium jumbo cups is presented in Fig. 8. The relative micromotion in the AAOS type I–III models was 10.2, 9.1, and 11.5 μm, respectively. Compared to titanium cups, trabecular metal cups were associated with greater relative micromotion between the host bone and the acetabular cup in all models, and this micromotion gradually increased with the severity of the acetabular bone defect. The peak relative micromotion between the host bone and the bionic trabecular metal cups was 17.2, 18.2, and 31.3 μm in AAOS type I–III models, respectively (Fig. 7).
3.4 Clinical Relevance
It is well known that acetabular revision surgery with a bone defect is a challenge for orthopedic surgeons [27, 28]. The jumbo cup technique is widely used for the reconstruction of acetabular bone defects because of its advantages of simplifying the surgery and providing sufficient initial cup stability. However, surgeons should be concerned about potential complications. The use of the jumbo component in acetabular revision surgery will lead to an upward shift of the HCOR, leading to biomechanical changes, which may increase the risk of muscle imbalance and implant dislocation [29, 30]. Studies have reported that upshifting the HCOR will increase the stress on the hip joint, which was validated in our FEA model of acetabular bone defects. In our study, when a titanium cup was used for the reconstruction of AAOS type-I and type-II acetabular defects, the HCOR elevation was 5 mm, and the cup stress was average 20.9 MPa. In contrast, for AAOS type-III acetabular defects, we noted a 10-mm elevation in HCOR and a cup stress of 23.8 MPa. The von Mises stress markedly increases due to the upward movement of the HCOR. This increase may be attributed to a decrease in the contact area caused by the acetabular bone defect. Moreover, the stress was concentrated mainly at the edge of the cup assembly, which also conforms to the hoop stress of the fixation of the jumbo cup [31, 32]. Thus, orthopedic surgeons should attempt to reduce stress shielding and surrounding bone resorption when reconstructing acetabular bone defects with titanium jumbo cups [33].
When bionic trabecular metal cups were used to reconstruct the acetabular bone defects, the peak stress on the cup gradually increased with the severity of the defect, but remained lower than the stress observed with the titanium jumbo cups for all models. This shows that the tantalum jumbo cups have superior and well-distributed contact stress, which can better avoid stress shielding and reduce bone resorption. We speculated that this finding was mainly attributable to the porosity of trabecular metal and its similar elastic modulus to cancellous bone, which may alleviate stress-increasing effects [34]. In addition, compared with titanium jumbo cups, trabecular metal jumbo cups resulted in increased stress distribution on the hip bone in all models. This suggested that when the material characteristics were changed, the load could be transferred to the host bone. Notably, the peak stress remained lower than the fatigue strength of the cortical bone (93.4 MPa).
Acetabular cups are often fixed with screws to enhance initial cup stability, but screw fracture is a common complication after acetabular reconstruction [35,36,37]. In our study, we found that the stress on the screws was mainly concentrated on the screw rods, especially in the AAOS type-3 model, and the peak stress was 25.0 MPa and 30.5 MPa which is much lower than the yield strength of titanium. This shows that the screw strength was sufficient to withstand the applied load when acetabular revision is performed with a titanium or tantalum jumbo cup.
Excessive early micromotion between the acetabular cup and the host bone can impede bone ingrowth or bone growth. After the acetabular component is implanted, it can osseointegrate with the host bone to achieve sufficient stability and long-term survival. Relative micromotion is an important biomechanical factor affecting bone ingrowth at the cup–bone interface [38, 39]. Studies have confirmed that relative micromotion of 20–40 μm can significantly promote bone ingrowth, while micromotion beyond 75–150 μm induces fibrous tissue ingrowth, which is unfavorable [40, 41]. The results of our study showed that the von Mises stress on the AAOS type I–III acetabular defect models was higher after revision with titanium jumbo cups than after revision with tantalum jumbo cups. We speculated that this was mainly due to the porosity and lower elastic modulus of trabecular metal. However, the relative micromotion for both cups was less than 40 μm, indicating that both materials can provide initial stability for bone ingrowth to ensure good long-term outcomes. Jumbo cups also have the advantages providing considerable CC for severe acetabular bone defects and creating favorable conditions for long-term biological fixation. This finding was verified in a clinical study by Gustke et al. [42], who found that the application of tantalum metal cups in acetabular reconstruction surgery was effective, and no prosthesis loosening occurred 2 years after the surgery.
Our study has limitations that need to be acknowledged. First, the acetabular bone defect models in this study were designed on the basis of a previous study [11], and may not exactly replicate the real situation in the clinic. However, the acetabular bone defect models based on the proposed acetabular bone defect map can be more generalized. Second, the cup surface coating is only one of many factors that affect initial cup stability, and we did not consider pathological conditions such as osteoporosis, which may seriously affect the mechanical properties of the host bone. Third, our results confirmed mechanically that the bionic trabecular metal cup is superior to the titanium cup for reconstructing acetabular defects, but biological factors were not considered, which should be evaluated in future studies.
4 Conclusion
In this study, 3D FEA models of AAOS type I–III acetabular bone defects were successfully constructed, and the effects of acetabular revision with jumbo cups on cup stability were quantitatively compared between titanium cups and trabecular metal cups. The present study revealed 2 principal findings: First, we explored the biomechanics (contact stress and relative micromotion) during the revision surgery of AAOS type I–III acetabular bone defects using jumbo cups. Second, compared with conventional titanium acetabular cups, bionic trabecular metal acetabular cups showed better biomechanical characteristics during the reconstruction of acetabular bone defects. In conclusion, the reconstruction of acetabular bone defects using titanium or tantalum jumbo cups are both feasible approaches. Compared to the titanium cup, the tantalum jumbo cup resulted in higher relative micromotion in our model, but this motion was still within the threshold of adequate bone ingrowth. In addition, the tantalum cup had superior stress distribution, which was biomechanically supported. The results gained from this study contribute to the optimization of surgical techniques and implant selection, ultimately enhancing patient outcomes in acetabular revision surgeries. Further prospective clinical studies are necessary to validate these findings and translate them into clinical practice.
Data availability
All materials and data generated from this study are available upon request to the corresponding author.
References
Roth, A., Khlopas, A., George, J., Churchill, J. L., Molloy, R., Mont, M. A., Piuzzi, N. S., & Higuera, C. A. (2019). The effect of body mass index on 30-day complications after revision total hip and knee arthroplasty. Journal of Arthroplasty, 34, S242–S248. https://doi.org/10.1016/j.arth.2019.02.005
Prock-Gibbs, H., Pumilia, C. A., Meckmongkol, T., Lovejoy, J., Mumith, A., & Coathup, M. (2021). Incidence of osteolysis and aseptic loosening following metal-on-highly cross-linked polyethylene hip arthroplasty: A systematic review of studies with up to 15-year follow-up. Journal of Bone and Joint Surgery-American, 103, 728–740. https://doi.org/10.2106/JBJS.20.01086
Garcia-Rey, E., Madero, R., & Garcia-Cimbrelo, E. (2015). THA revisions using impaction allografting with mesh is durable for medial but not lateral acetabular defects. Clinical Orthopaedics and Related Research, 473, 3882–3891. https://doi.org/10.1007/s11999-015-4483-7
Waddell, B. S., & Della Valle, A. G. (2017). Reconstruction of non-contained acetabular defects with impaction grafting, a reinforcement mesh and a cemented polyethylene acetabular component. Bone & Joint Journal, 99, 25–30. https://doi.org/10.1302/0301-620X.99B1.BJJ-2016-0322.R1
Sporer, S. M., O’Rourke, M., Chong, P., & Paprosky, W. G. (2006). The use of structural distal femoral allografts for acetabular reconstruction. surgical technique. Journal of Bone and Joint Surgery-American, 88, 92–99. https://doi.org/10.2106/JBJS.E.00903
Marongiu, G., Podda, D., Mastio, M., & Capone, A. (2019). Long-term results of isolated acetabular revisions with reinforcement rings: A 10- to 15-year follow-up. Hip International, 29, 385–392. https://doi.org/10.1177/1120700018802750
Bruggemann, A., Fredlund, E., Mallmin, H., & Hailer, N. P. (2017). Are porous tantalum cups superior to conventional reinforcement rings? Acta Orthopaedica, 88, 35–40. https://doi.org/10.1080/17453674.2016.1248315
Moon, J. K., Ryu, J., Kim, Y., Yang, J. H., Hwang, K. T., & Kim, Y. H. (2019). Acetabular revision arthroplasty using press-fitted jumbo cups: An average 10-year follow-up study. Archives of Orthopaedic and Trauma Surgery, 139, 1149–1160. https://doi.org/10.1007/s00402-019-03214-7
Wedemeyer, C., Neuerburg, C., Heep, H., von Knoch, F., von Knoch, M., Loer, F., & Saxler, G. (2008). Jumbo cups for revision of acetabular defects after total hip arthroplasty: A retrospective review of a case series. Archives of Orthopaedic and Trauma Surgery, 128, 545–550. https://doi.org/10.1007/s00402-007-0501-x
Nwankwo, C., Dong, N. N., Heffernan, C. D., & Ries, M. D. (2014). Do jumbo cups cause hip center elevation in revision THA? a computer simulation. Clinical Orthopaedics and Related Research, 472, 572–576. https://doi.org/10.1007/s11999-013-3169-2
Shen, X. Y., Tian, H., Li, Y., Zuo, J. L., Gao, Z. L., & Xiao, J. L. (2022). Acetabular revision arthroplasty based on 3-Dimensional reconstruction technology using jumbo cups. Frontiers in Bioengineering and Biotechnology, 10, 799443. https://doi.org/10.3389/fbioe.2022.799443
Alkhatib, S. E., Mehboob, H., & Tarlochan, F. (2019). Finite element analysis of porous titanium alloy hip stem to evaluate the biomechanical performance during walking and stair climbing. Journal of Bionic Engineering, 16, 1103–1115. https://doi.org/10.1007/s42235-019-0122-4
Cilingir, A. C. (2010). Finite element analysis of the contact mechanics of ceramic-on-ceramic hip resurfacing prostheses. Journal of Bionic Engineering, 7, 244–253. https://doi.org/10.1016/S1672-6529(10)60247-8
Migaud, H., Common, H., Girard, J., Huten, D., & Putman, S. (2019). Acetabular reconstruction using porous metallic material in complex revision total hip arthroplasty: A systematic review. Orthopaedics & Traumatology: Surgery & Research, 105, S53–S61. https://doi.org/10.1016/j.otsr.2018.04.030
Shen, X. Y., Qin, Y. G., Li, Y., Tang, X. F., & Xiao, J. L. (2022). Trabecular metal versus non-trabecular metal acetabular components for acetabular revision surgery: a systematic review and meta-analysis. International Journal of Surgery, 100, 106597. https://doi.org/10.1016/j.ijsu.2022.106597
Han, Q., Wang, C. Y., Chen, H., Zhao, X., & Wang, J. C. (2019). Porous tantalum and titanium in orthopedics: A review. ACS Biomaterials Science & Engineering, 5, 5798–5824. https://doi.org/10.1021/acsbiomaterials.9b00493
Akhtar, R., Eichhorn, S. J., & Mummery, P. M. (2006). Microstructure-based finite element modelling and characterisation of bovine trabecular bone. Journal of Bionic Engineering, 3, 3–9. https://doi.org/10.1016/s1672-6529(06)60001-2
Yang, Y. H., Zuo, J. L., Liu, T., Xiao, J. L., & Liu, S. L. (2017). Morphological analysis of true acetabulum in hip dysplasia (crowe classes I–IV) via 3-D implantation simulation. Journal of Bone and Joint Surgery-American, 99(17), e92. https://doi.org/10.2106/JBJS.16.00729
Zhao, X., Xue, H. W., Sun, Y., Zhang, A. B., Liu, Y., Chen, H., Wan, Q., Zhang, J. B., Xiao, J. L., Wang, C. Y., Han, Q., & Wang, J. C. (2021). Application of novel design bone grafting for treatment of segmental acetabular rim defects during revision total hip arthroplasty. Journal of Bionic Engineering, 18, 1369–1377. https://doi.org/10.1007/s42235-021-00097-6
Hao, Z. X., Wan, C., Gao, X. F., & Ji, T. (2011). The effect of boundary condition on the biomechanics of a human pelvic joint under an axial compressive load: a three-dimensional finite element model. Journal Biomechanical Engineering, 133, 101006. https://doi.org/10.1115/1.4005223
De Paolis, M., Zucchini, R., Romagnoli, C., Romantini, M., Mariotti, F., & Donati, D. M. (2019). Middle term results of tantalum acetabular cups in total hip arthroplasty following pelvic irradiation. Acta Orthopaedica et Traumatologica Turcica, 53, 165–169. https://doi.org/10.1016/j.aott.2019.03.007
Migaud, H., Common, H., Girard, J., Huten, D., & Putman, S. (2019). Acetabular reconstruction using porous metallic material in complex revision total hip arthroplasty: A systematic review. Orthopaedics & Traumatology-Surgery & Research, 105, S53–S61. https://doi.org/10.1016/j.otsr.2018.04.030
Zhang, Y. D., Ahn, P. B., Fitzpatrick, D. C., Heiner, A. D., Poggie, R. A., & Brown, T. D. (1999). Interfacial frictional behavior: Cancellous bone, cortical bone, and a novel porous tantalum biomaterial. Journal of Musculoskeletal Research, 3(04), 245–251.
Jiang, H. B. (2007). Static and dynamic mechanics analysis on artificial hip joints with different interface designs by the finite element method. Journal of Bionic Engineering, 4, 123–131. https://doi.org/10.1016/s1672-6529(07)60024-9
Dong, E. C., Wang, L., Iqbal, T., Li, D. C., Liu, Y. X., He, J. K., Zhao, B. H., & Li, Y. (2018). Finite element analysis of the pelvis after customized prosthesis reconstruction. Journal of Bionic Engineering, 15, 443–451. https://doi.org/10.1007/s42235-018-0035-7
Zuo, J. L., Xu, M., Zhao, X., Shen, X. Y., Gao, Z. L., & Xiao, J. L. (2021). Effects of the depth of the acetabular component during simulated acetabulum reaming in total hip arthroplasty. Scientific Reports, 11, 9836. https://doi.org/10.1038/s41598-021-89292-3
Chiarlone, F., Zanirato, A., Cavagnaro, L., Alessio-Mazzola, M., Felli, L., & Burastero, G. (2020). Acetabular custom-made implants for severe acetabular bone defect in revision total hip arthroplasty: A systematic review of the literature. Archives of Orthopaedic and Trauma Surgery, 140, 415–424. https://doi.org/10.1007/s00402-020-03334-5
Li, H. W., Qu, X. H., Mao, Y. Q., Dai, K. R., & Zhu, Z. A. (2016). Custom acetabular cages offer stable fixation and improved hip scores for revision THA with severe bone defects. Clinical Orthopaedics and Related Research, 474, 731–740. https://doi.org/10.1007/s11999-015-4587-0
Hu, X. J., Zheng, N., Chen, Y. S., Dai, K. R., Dimitriou, D., Li, H., & Tsai, T. Y. (2021). Optimizing the femoral offset for restoring physiological hip muscle function in patients with total hip arthroplasty. Frontiers in Bioengineering and Biotechnology, 9, 645019. https://doi.org/10.3389/fbioe.2021.645019
Renkawitz, T., Weber, T., Dullien, S., Woerner, M., Dendorfer, S., Grifka, J., & Weber, M. (2016). Leg length and offset differences above 5mm after total hip arthroplasty are associated with altered gait kinematics. Gait & Posture, 49, 196–201. https://doi.org/10.1016/j.gaitpost.2016.07.011
Manley, M. T., Capello, W. N., D’Antonio, J. A., Edidin, A. A., & Geesink, R. G. (1998). Fixation of acetabular cups without cement in total hip arthroplasty. a comparison of three different implant surfaces at a minimum duration of follow-up of five years. Journal of Bone and Joint Surgery-American, 80, 1175–1185.
Doyle, R., van Arkel, R. J., & Jeffers, J. R. T. (2019). Effect of impaction energy on dynamic bone strains, fixation strength, and seating of cementless acetabular cups. Journal of Orthopaedic Research, 37, 2367–2375. https://doi.org/10.1002/jor.24418
Woo, S. H., Sung, M. J., Park, K. S., & Yoon, T. R. (2020). Three-dimensional-printing technology in hip and pelvic surgery: Current landscape. Hip & Pelvis, 32, 1–10. https://doi.org/10.5371/hp.2020.32.1.1
Jafari, S. M., Bender, B., Coyle, C., Parvizi, J., Sharkey, P. F., & Hozack, W. J. (2010). Do tantalum and titanium cups show similar results in revision hip arthroplasty? Clinical Orthopaedics and Related Research, 468, 459–465. https://doi.org/10.1007/s11999-009-1090-5
Benazzo, F., Formagnana, M., Bargagliotti, M., & Perticarini, L. (2015). Periprosthetic acetabular fractures. International Orthopaedics, 39, 1959–1963. https://doi.org/10.1007/s00264-015-2971-8
Tabata, T., Kaku, N., Hara, K., & Tsumura, H. (2015). Initial stability of cementless acetabular cups: Press-fit and screw fixation interaction—an in vitro biomechanical study. European Journal of Orthopaedic Surgery & Traumatology, 25, 497–502. https://doi.org/10.1007/s00590-014-1571-4
Pulido, L., Rachala, S. R., & Cabanela, M. E. (2011). Cementless acetabular revision: Past, present, and future. Revision total hip arthroplasty: The acetabular side using cementless implants. International Orthopaedics, 35, 289–298. https://doi.org/10.1007/s00264-010-1198-y
Spears, I. R., Pfleiderer, M., Schneider, E., Hille, E., & Morlock, M. M. (2001). The effect of interfacial parameters on cup–bone relative micromotions: A finite element investigation. Journal of Biomechanics, 34(1), 113–120.
Wang, Y., Wang, M., Li, C., Nakamura, Y., Deng, L., Yamako, G., Chosa, E., & Pan, C. (2022). Biomechanical effect of metal augment and bone graft on cup stability for acetabular reconstruction of total hip arthroplasty in hip dysplasia: A finite element analysis. BMC Musculoskeletal Disorders, 23, 277. https://doi.org/10.1186/s12891-022-05168-1
Gee, E. C., Jordan, R., Hunt, J. A., & Saithna, A. (2016). Current evidence and future directions for research into the use of tantalum in soft tissue re-attachment surgery. Journal of Materials Chemistry B, 4, 1020–1034.
Wik, T. S. (2012). Experimental evaluation of new concepts in hip arthroplasty. Acta Orthopaedica, 83, 1–26. https://doi.org/10.3109/17453674.2012.678804
Gustke, K. A., Levering, M. F., & Miranda, M. A. (2014). Use of jumbo cups for revision of acetabulae with large bony defects. Journal of Arthroplasty, 29, 199–203. https://doi.org/10.1016/j.arth.2012.11.010
Acknowledgements
Not applicable.
Funding
This work was supported by funding from China Postdoctoral Science Foundation (No: 2020M670863) and Jilin Scientific and Technological Development Program (No: 20230203089SF).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Ethics approval
This study was approved by the Institutional Review Board, Ethics Committee of our institution (No: 2020-NSFC-007).
Informed consent
Informed consent was waived because materials of included patients were anonymized and desensitized.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, J., Xue, H., Qian, Z. et al. Jumbo Bionic Trabecular Metal Acetabular Cups Improve Cup Stability During Acetabular Bone Defect Reconstruction: A Finite Element Analysis Study. J Bionic Eng 20, 2814–2825 (2023). https://doi.org/10.1007/s42235-023-00413-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42235-023-00413-2