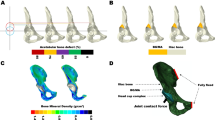
Abstract
Background
This study used finite element analysis (FEA) to compare the biomechanical stability of bispherical metal augment (BA) and wedge-shaped trabecular-metal augment (TA) in different acetabular defect reconstruction models, thereby explaining the application value of this novel bispherical augment in complex hip revision.
Methods
Three different acetabular defect pelvis models originating from three representative patients with different types of severe acetabular defects (Paprosky IIC, IIIA, and IIIB) were constructed and reconstruction with BA and TA technique was simulated. Based on the FEA models, the displacement of reconstruction implants, relative displacement of bone implants, and hemi-pelvic von Mises stress were investigated under static loads.
Results
BA acquired smaller reconstruction system displacement, less relative displacement of bone implants, and lower pelvic von Mises stress than TA in all Paprosky IIC, IIIA, and IIIB defect reconstructions.
Conclusion
The FEA results show that BA could acquire favourable biomechanical stability in severe acetabular defect reconstruction. This technique is a reliable method in complex hip revision.
Similar content being viewed by others
Introduction
Given the high rate of success of total hip arthroplasty (THA), patients with end-stage hip disease treated with THA are increasing [1]. Meanwhile, concomitant revision of a failed THA is becoming more popular [2]. From 2014 to 2030, hip revision incidence is projected to increase by 43–70% [1, 2]. The key to a successful hip revision surgery depends on judgement of failure aetiology, extraction of well-fixed components, and reconstruction of large bone defects [3]. Among these, the most challenging aspect of hip revision surgery during intra-operative management of acetabular bone loss is the selection of appropriate defect filling materials and associated surgical installation [4].
Over the past few decades, several techniques have been proposed to reconstruct acetabular bone defects and achieve initial stability of the acetabular component, such as morselized impaction allograft, structural allograft, jumbo cup, double cup, cup-cage, and custom implants [5,6,7,8,9,10,11]. Disadvantages of these techniques include lack of bone graft incorporation, absence of true biological fixation, and technical difficulty in acquiring intimate contact between bone-implant interface, which was unfavourable for long-term stability [12, 13]. As an alternative, uncemented hemispherical cups with trabecular-metal augments have been used for reconstruction in complex acetabular defects [12, 14,15,16]. However, this wedge-shaped trabecular-metal augment is difficult to orient and place in severe irregular acetabular defects, which in turn affects the initial stability of acetabular reconstruction.
A novel bispherical metal augment with sufficient hemispherical area for surface contact for reconstructing these severe acetabular defects has been developed (Fig. 1), the rationale for the design is based on the philosophy of acetabular defect evolution. With progressive wear and migration of failed acetabular components, the outline of acetabular defects gradually becomes an oblong or oval shape [17], and can be divided into several hemispheric defects. Therefore, a precise fit with each patient’s pelvis could theoretically be achieved with the bispherical augment [18]. The clinical and radiological outcomes of this bispherical metal augment were comparable with those of wedge-shaped trabecular-metal augments in severe acetabular reconstruction at a mean follow-up of three years. At the final clinical evaluations, the mean harris hip score (HHS) was increased from 33.9 to 84.1, and mean lower limb discrepancy was improved from 2.2 cm to 0.8 cm. No evidence of acetabular augment-cup construct migration was observed. Furthermore, this technique also facilitated intra-operative restoration of the hip centre of rotation (HCOR), which was attributed to the gradual size and graded thickness that finally allowed better biomechanical reconstruction of hip revision.
Over the nearly decades, finite element analysis (FEA) has been widely utilized in orthopaedics, especially in implant stability evaluation. Amirouche et al. used finite element model to simulate and evaluate cup insertion and fixation in the context of segmental rim defects and the results indicated cup stability was related to the defect location, superior or inferior defect had a minimal effect while columns defect created cup instability and increased stress [19]. Wang et al. applied FEA to explore the biomechanical effect of different augmented materials for acetabular reconstruction in THA on cup stability, and they concluded that metal augment could achieved better stability in augment-cup interface than autologous bone graft [20]. Although early clinical outcomes of this novel bispherical metal augment seem promising, it is still worthwhile to further illustrate its mechanical advantages in acetabular defect management. The purpose of this paper is to verify the biomechanical rationality of the design of the bispherical metal augment and the surgical plan using finite element comparison.
Materials and methods
Clinical information
To illustrate the internal tissue biomechanical characteristics of BA and TA techniques during virtual loading, three representative patients with different types of severe acetabular defects, Paprosky IIC (female, 78 years), IIIA (female, 70 years), and IIIB (female, 71 years) were selected. The study was approved by the Ethics Committee of our hospital. Written informed consent was obtained from all patients.
Geometric model
3D reconstruction STL models of the defective pelvis were generated using Mimics Research 24.0 (Materialise NV, Leuven, Belgium) using DICOM CT images of three patients, and smoothed using the Geomagic 2013 (Geomagic, Morrisville, NC, USA) software while maintaining overall model fidelity. The bispherical and wedge-shaped augments were designed and assembled using Solidworks (Dassault Systèmes Inc., Vélizy-Villacoublay, France) based on the instructions. The structure of a bispherical augment was obtained by Boolean subtraction of two balls, and the structure of a wedge-shaped augment was cut out on a sphere with a plane. The prothesis implantation methods were suggested in a previous study [18]. The cup was inserted at a reasonable position with reference to the contralateral hip rotation centre, and during cup size selection we tried to reserve as much host bone as possible instead of sacrificing bone stock at the anterior and posterior column. The cup orientation follows the principle of the Lewinnek safe zone [21]. The interface of augment and cup was designed with a bone cement layer for connection.
In the Paprosky IIC defect model, a 40-48-10 mm bispherical metal augment and a 50 mm cup were used as shown in Fig. 2c, and a 50–15 mm trabecular-metal augment and a 50 mm cup were used as shown in Fig. 2d. In the Paprosky IIIA defect model, one bispherical metal augment (52-56-15 mm) with a 64 mm cup was inserted in the BA group (Fig. 2g), while in the TA group, two schemes were designed according to the circumstances of different patients, with two installation directions of the trabecular-metal augment (54–15 mm) with a 64 mm cup shown in Fig. 2h and i. In the Paprosky IIIB defect model, one bispherical metal augment (56-60-15 mm) with a 66 mm cup was inserted in BA group as shown in Fig. 2l, and in the TA group, considering the large extent of the defect, we inserted one and two tantalum augments (58–15 mm), respectively, with a 66 mm cup as shown in Fig. 2m and n.
Assembled acetabular defects reconstruction models with BA and TA technique. X-ray images (a, e, j) and CT 3D images (b, f, k) of Paprosky IIC, IIIA and IIIB defects, respectively. Defects reconstructed with BA (c, g, l) and TA (d, h, i, m, n). The convex surface of wedge-shaped augment direct acetabular medial wall in (h) while the convex surface toward acetabular opening in (i). In (m), a single augment was used, while in (n), two augments were employed as a “footing” to allow support for the cup
FEA model
Finite element models were built using ABAQUS 2016 (Dassault Systèmes). Solid element type was chosen as C3D10M, the mesh size was set as 1–2 mm. Mesh convergence verification was carried out using the IIC-BA model by setting the mesh sizes to 2.5, 2.0, 1.5, 1.0, and 0.5 mm respectively, and recording the results of the maximum value of the stress on the bone, and the resultant error was controlled to be within 5% as shown in Suppl. Figure 2. 1 mm-thick shell elements were generated on the pelvic volume to represent the shell of cortical bone, as validated in previous studies [22]. Given the acetabular defect and the surgical revision, cartilage or cortical bone would not be present in the acetabular socket; therefore, the shell elements were not present in the acetabular socket. A total of eight FEA models were established for three patients using the BA and TA techniques. The total number of elements and nodes generated is shown in Table 1. Material properties used in the models are presented in Table 2 [23].
Fixed constraint was assumed at the sacroiliac joint and pubic symphysis. The loading condition was assessed at the geometric centre of the cup, by analysing the hip reaction force, which was set to the maximum value observed during gait (Fx = 277.85 N, Fy = 287.12 N, Fz = 2120.95 N ) [24]. Tied contact was assumed at the interfaces between bone cement and augment, as well as between bone cement and acetabular cup, since the study focused on analysing the results pertaining to the pelvic bone. The bone-augment and bone-cup interface were set as a small sliding contact with normal hard contact and tangential friction coefficient of 0.1 [22]. The mesh model with boundary conditions included force and displacement applied at the FE model was shown in Suppl. Figure 2. The comparison index includes system displacement, bone-implants interface motion, and von Mise stress of the pelvic bone.
Results
The results of system displacement for eight reconstruction models are shown in Fig. 3. The maximum values occurred around the roof rim of the cup in all eight models; these were 0.7752, 1.0004, 0.6343, 0.8502, 0.6592, 0.6637, 0.8010, and 0.6658 mm as shown in Table 3. In all three acetabular defect reconstruction models, the maximum displacement value of BA group was lower than that of TA group. The results of augment displacement were shown in Suppl. Figure 3. The maximum value of augment displacement in BA group was also lower than of TA group as shown in Suppl. Table 1.
The results of bone-implant interface motion are shown in Fig. 4. The maximum value in BA group corresponding to Paprosky IIC, IIIA, and IIIB defects models were 105.1, 114.6, and 167.8 μm, respectively. The lowest maximum value in TA group was 182.1 μm, as shown in Fig. 4h, using two TA augments for Paprosky IIIB defect, which is higher than in BA group.
The hemi-pelvic von Mises stress in eight reconstruction models is shown in Fig. 5. The stress increased at the edge of the interface between the bone tissue and implant. The peak stress was located near the posterior wall of the deficient acetabulum along the direction of the body alignment, from acetabular to sacroiliac joint; these were 26.18, 31.55, 27.75, 37.40, 32.44, 27.65, 39.10, and 29.14 MPa as shown in Table 3, and BA had the smallest peak stress among the three groups.
Discussion
We have demonstrated a modular strategy with bispherical metal augment in severe acetabular deficiency reconstruction, and this new shape augment could achieve favourable outcomes in clinical application [18]. It is widely known that a new orthopaedic implant with good biocompatibility must meet certain biomechanical requirements after implantation. To evaluate the mechanical stability of modular reconstruction with a bispherical augment and uncemented hemispherical cup, we therefore carried out this FEA study.
In current study, the implants were selected according to the pelvic morphology of three specific patents, therefore the cup size was differentiated in BA and TA group in each defect simulate reconstruction model. Owing to the modular diameter and thickness parameters of bispherical augment, the cup size in BA group was smaller than TA group, which was conducive to avoiding hip centre elevation and iatrogenic host bone loss. Literature reported the morphological parameters of proximal femur and acetabulum was different between populations [25], therefore this bispherical augment allowed for wider application by intraoperative assemble of reconstruction construct depending on the acetabular bone loss pattern.
The FEA model was verified by comparing the von Mises stress of the pelvic bone with the results of other studies [24, 26]. The peak von Mises stress in current eight models were between 26 and 37 MPa, consistent with the results reported in the literature, which ranged from 15 to 30 MPa around the acetabulum [24]. Li et al. developed a series of acetabular reconstruction models to analyse the reconstructive stability for Paprosky III acetabular defects using three different reconstruction strategies with trabecular-metal augments [26]. The peak implant displacements in current eight models were between 0.6343 and 1.0004 mm, which was consistent with the published results. Since their acetabular defect models were produced by Boolean operations through Solidworks on the residual bone mass, instead of originating in patients, our assembling schemes and results were more in keeping with real clinical circumstances.
Although the difference between the maximum and minimum displacement values was only 0.3661 mm, but it was a true reflection of construct stability was more stable in the BA group rather than TA group. It can be concluded, both bispherical augment and wedge-shaped augment could provide support with stable fixation, which maintained the acetabular cup in a good anatomical position and meanwhile restored the typical biomechanical environment. Our results suggested that these modular reconstruction constructs achieved smaller displacement than the trabecular-metal augments under loading conditions in all models, as shown in Fig. 3. These results also could be predicted from the solid assembled models seen in Fig. 2, because the spatial regions of the acetabular defects were filled more fully in BA group. Furthermore, the contact area between the cup and augment was also larger, contributing to its bispherical shape. Moreover, the wedge-shaped disadvantages of the trabecular-metal augment also could be seen from the displacement results in the Paprosky IIIA and IIIB defect models. The installation orientation and number of augments used strongly influenced the mechanical stability of the final construct, which was also challenging for surgical skills and clinical experience. Maximise the contact area at the interface of implant and host bone and minimise residual defects cavity were important to improve prothesis stability.
Interfacial fretting is important for bone integration between host bone and metal implants, and contributes to favourable long-term stability. The maximum micromotion value between the bone-cup or bone-augment interface in the BA group was 167.8 μm, which was consistent with the bone ingrowth requirement [27]. This result also demonstrated that the bispherical metal augment could provide favourable support by stable fixation, which finally contributed to maintaining the good biomechanical environment of the cup. The bispherical augment owned similar shape of acetabular cup, after spherical treatment of defects surface with reamer, the bispherical augment could achieve press-fit with host bone in most cases without screws ancillary. The integrity of the acetabular reconstruction structure played an important role in the initial stability, and the initial stability in turn facilitated osteointegration between the prosthesis and host bone [26].
In this study, the peak von Mises stress of the hemi-pelvis under the maximum hip joint force was significantly lower than the yield strength of cortical bone, the maximum peak von Mises stress of pelvic bone was 39.1 MPa, which was significantly less than 93.4 MPa of the yield strength of cortical bone. Current results indicated that both BA and TA techniques were sufficient to support walking after implantation [28]. In the BA group, the stress distribution around the acetabulum rim was uniform in Paprosky IIC and IIIB defects models, which indicates that this bispherical metal augment is potentially advantageous in reconstructing medial and superior defects. The stress in the Paprosky IIIA defect model was concentrated at the supero-posterior and infero-anterior quadrant of the acetabulum rim in both groups, and the maximum value of stress in the two groups showed the same magnitude, consistent with clinical practice. Changes in pelvic stress distribution significantly intervened in bone metabolism, osteogenesis was active at higher stress levels while steoclastogenesis was inhibition. Suitable concentrated stress at supero-posterior and infero-anterior quadrant of the acetabulum rim was conducive to bone ingrowth which further enhanced the long-term stability of prothesis.
This study had some limitations. First, the analysis in current study did not select the loading of the entire gait cycle, but only selected the moment of the maximum load in the gait cycle for comparative analysis, a typical gait cycle with 3D loading and motion profiles was reasonable to further clarify the scientific validity of the results. Second, the current study focused on the reconstruction techniques, and therefore only the immediate post-operative biomechanical stability of the constructs was evaluated. Lastly, an in vitro cadaveric experiment is also needed for the wide application of this bispherical metal augment. All these should be investigated in future studies.
Conclusions
Based on the results of this study, the bispherical metal augment could acquire smaller augment-cup system displacement, less interface micromotion, and lower pelvic von Mises stress in Paprosky IIC, IIIA, and IIIB defect reconstruction rather than wedge-shaped augment. This technique was a reliable alternative method in severe acetabular deficiency reconstruction.
Data availability
The data used to support the findings of this study are included within the article.
References
Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100(17):1455–60.
Schwartz AM, Farley KX, Guild GN, Bradbury TL Jr. Projections and epidemiology of revision hip and knee arthroplasty in the United States to 2030. J Arthroplasty. 2020;35(6S):S79–85.
Ahmad AQ, Schwarzkopf R. Clinical evaluation and surgical options in acetabular reconstruction: a literature review. J Orthop. 2015;12(Suppl 2):S238–43.
Garcia-Cimbrelo E, Garcia-Rey E. Bone defect determines acetabular revision surgery. Hip Int. 2014;24(Suppl 10):S33–6.
Waddell BS, Valle AGD. Reconstruction of non-contained acetabular defects with impaction grafting, a reinforcement mesh and a cemented polyethylene acetabular component. Bone Joint J. 2017;99–B:25–30.
Makita H, Kerboull M, Inaba Y, Tezuka T, Saito T, Kerboull L. Revision total hip arthroplasty using the Kerboull Acetabular Reinforcement Device and structural allograft for severe defects of the Acetabulum. J Arthroplasty. 2017;32(11):3502–9.
Nwankwo CD, Ries MD. Do jumbo cups cause hip center elevation in revision THA? A radiographic evaluation. Clin Orthop Relat Res. 2014;472(9):2793–8.
Webb JE, McGill RJ, Palumbo BT, Moschetti WE, Estok DM. The double-cup construct: a novel treatment strategy for the management of paprosky IIIA and IIIB acetabular defects. J Arthroplasty. 2017;32(9S):S225–31.
Wang C, Huang Z, Wu B, Li W, Fang X, Zhang W. Cup-cage solution for massive acetabular defects: a systematic review and meta-analysis. Orthop Surg. 2020;12(3):701–7.
Hao Y, Wang L, Jiang W, Wu W, Ai S, Shen L, et al. 3D printing hip prostheses offer accurate reconstruction, stable fixation, and functional recovery for revision total hip arthroplasty with complex acetabular bone defect. Engineering. 2020;6(11):1285–90.
Loppini M, Schiavi P, Rocca AD, Traverso F, Rocca FD, Mazziotta G, et al. Double-trabecular metal cup technique for the management of paprosky type III defects without pelvic discontinuity. Hip Int. 2018;28(2suppl):66–72.
Nehme A, Lewallen DG, Hanssen AD. Modular porous metal augments for treatment of severe acetabular bone loss during revision hip arthroplasty. Clin Orthop Relat Res. 2004;429:201–8.
Stiehl JB. Revascularization of a total bulk acetabular allograft at 14 years. J Arthroplasty. 2004;19(4):508–12.
Abolghasemian M, Tangsataporn S, Sternheim A, Backstein DJ, Safir OA, G AE. Porous metal augments: big hopes for big holes. Bone Joint J. 2013;95–B(11 Suppl A):103–8.
Whitehouse MR, Masri BA, Duncan CP, Garbuz DS. Continued good results with modular trabecular metal augments for acetabular defects in hip arthroplasty at 7 to 11 years. Clin Orthop Relat Res. 2015;473(2):521–7.
Jenkins DR, Odland AN, Sierra RJ, Hanssen AD, Lewallen DG. Minimum five-year outcomes with porous tantalum acetabular cup and augment construct in complex revision total hip arthroplasty. J Bone Joint Surg Am. 2017;99(10):e49.
Schierjott RA, Hettich G, Graichen H, Jansson V, Rudert M, Traina F, et al. Quantitative assessment of acetabular bone defects: a study of 50 computed tomography data sets. PLoS ONE. 2019;14(10):e0222511.
Li G, Zhang X, Chen M, Luo Z, Ji X, Shang X. Modular revision strategy with bispherical augments in severe acetabular deficiency reconstruction. Int Orthop. 2022;46(2):215–22.
Amirouche F, Solitro GF, Walia A, Gonzalez M, Bobko A. Segmental acetabular rim defects, bone loss, oversizing, and press fit cup in total hip arthroplasty evaluated with a probabilistic finite element analysis. Int Orthop. 2017;41(8):1527–33.
WangY, Wang M, Li C, Nakamura Y, Deng L, Yamako G, Chosa E, Pan C. Biomechanical effect of metal augment and bone graft on cup stability for acetabular reconstruction of total hip arthroplasty in hip dysplasia: a finite element analysis. BMC Musculoskelet Disord. 2022;23(1):277.
Lewinnek GE, Lewis JL, Tarr R, Compere CL JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217–20.
Dong E, Wang L, Iqbal T, Li DC, Liu YX, He JK, Zhao BH, Li Y. Finite element analysis of the pelvis after customized prosthesis reconstruction. J Bionic Eng. 2018;15(3):443–51.
Levine DL, Dharia MA, Siggelkow E, Crowninshield RD, Degroff DA, Wentz DH. Repair of periprosthetic pelvis defects with porous metal implants: a finite element study. J Biomech Eng. 2010;132(2):021006.
Ghosh R, Pal B, Ghosh D, Gupta S. Finite element analysis of a hemi-pelvis: the effect of inclusion of cartilage layer on acetabular stresses and strain. Comput Methods Biomech Biomed Engin. 2015;18(7):697–710.
Chantarapanich N, Rojanasthien S, Chernchujit B, Mahaisavariya B, Karunratanakul K, Chalermkarnnon P, Glunrawd C, Sitthiseripratip K. 3D CAD/reverse engineering technique for assessment of Thai morphology: proximal femur and acetabulum. J Orthop Sci. 2017;22(2):703–9.
Li P, Tang H, Liu X, Chen Z, Zhang X, Zhou Y, Jin Z. Reconstruction of severe acetabular bone defects with porous metal augment in total hip arthroplasty: a finite element analysis study. Proc Inst Mech Eng H. 2022;236(2):179–87.
Perona PG, Lawrence J, Paprosky WG, Patwardhan AG, Sartori M. Acetabular micromotion as a measure of initial implant stability in primary hip arthroplasty. An in vitro comparison of different methods of initial acetabular component fixation. J Arthroplasty. 1992;7(4):537–47.
Nyman JS, Gorochow LE, Adam Horch R, Uppuganti S, Zein-Sabatto A, Manhard MK, Does MD. Partial removal of pore and loosely bound water by low-energy drying decreases cortical bone toughness in young and old donors. J Mech Behav Biomed Mater. 2013;22:136–45.
Funding
This work was supported by the Joint Fund for Medical Artificial Intelligence (MAI2023Q027), the Natural Science Foundation of Anhui Province (2308085MH251) and the National Natural Science Foundation of China (81902201).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Guoyuan Li], [Xiaoqi Zhang], [Min Chen]and [Zhengliang Luo]. Simulated operation schemes were performed by [Xiaofeng Ji] and [Xifu Shang]. FEA were performed by [Chunang Pan] and [Hui Li]. The first draft of the manuscript was written by [Guoyuan Li] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The first two authors contributed equally to this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval was obtained from the ethics committee of The First Affiliated Hospital of University of Science and Technology of China (No. 2023KY-445). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, G., Zhang, X., Chen, M. et al. Bispherical metal augment improved biomechanical stability in severe acetabular deficiency reconstruction: a comparative finite element analysis. BMC Musculoskelet Disord 25, 691 (2024). https://doi.org/10.1186/s12891-024-07816-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-024-07816-0