Abstract
In this study, eighty isolates of Fusarium were obtained from the uncultivated soils and roots of chickpea plants showing typical black root rot symptoms from different areas of Kermanshah province, west Iran during 2015 to 2017. Based on colony morphology, growth pattern, and micromorphological characteristics, the most prevalent Fusarium species recovered from uncultivated soil were F. redolens, 38 isolates (64%), followed by F. oxysporum, 12 isolates (20%), F. solani, seven isolates (11%) and Neocosmospora vasinfecta, three isolates (5%). All isolates recovered from chickpea plants with black root rot symptom were identified as F. redolens according to macro-micromorphological and molecular characteristics. After grouping, 27 isolates were selected for molecular confirmation by phylogeny of DNA sequence data for the internal transcribed spacer (ITS) rDNA and translation EF1α intergenic regions. The results of the pathogenicity test under greenhouse condition revealed that all isolates of F. redolens obtained from chickpea plants with black root rot symptoms in this study and N. vasinfecta from uncultivated soil are pathogenic to chickpea cultivar Bivanij. Two weeks after inoculation with F. redolens and N. vasinfecta, symptoms developed as black cankers that extended upward and downward of roots of all emerged seedlings. Re-isolation from all inoculated plants after observation of symptoms were performed, and isolates were compared to original cultures thus fulfilling Koch’s postulates. According to the results of canonical correspondence analysis, soil texture, altitude, CaCO3, EC, carbon, organic matter and pH in descending order were recognized as the most important environmental variables for the distribution of Fusarium species in soil. Fusarium redolens reflected a soil with very low sand, carbon, organic matter and intermediate pH, EC and CaCO3. Results of this study suggest that previously reported F. oxysporum in western Iran on chickpea might have been mistaken. This is the first report of pathogenicity of F. redolens and N. vasinfecta on chickpea from Iran.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fusarium is one of the most important genera of soil-inhabiting fungi, commonly associated with plants as pathogens, saprobes, and endophytes (Summerell et al. 2011). The fungi have been isolated from various soil types of many parts of the world. This genus belongs to the division Ascomycota, sub-division Pezizomycotina, class Sordariomycetes, order Hypocreales, and family Nectriaceae (Kirk et al. 2008). Fusarium species play differing roles in the soil ecosystem. Some species of this genus have important roles through their saprobic ability and subsequent effects on decomposition and mineralization of organic residues in soil, releasing plant nutrients into the ecosystem for other soil organisms and nutrient cycling (Stoner 1981; Paul and Clark 1989; Ruiter et al. 1994). A number of Fusarium species are plant pathogens that cause various types of diseases on plants such as root rot, fruit and seed decay, bulb rot, stem rot, vascular wilt, canker, dieback, gall, and foliar disease (Dean et al. 2012; Chehri et al. 2017; Trabelsi et al. 2017; Sharma and Marques 2018). Non-pathogenic Fusarium spp. have been shown to grow endophytically in the endorhiza (Dababat and Sikora 2007). These non-pathogenic Fusarium have been used effectively for the control of soilborne plant pathogens (Steinberg et al. 2007; Zhang et al. 2015; Šišić et al. 2017; Shadmani et al. 2018).
Kermanshah province is a mountainous area and situated at an elevation of 1200 m. The mean annual precipitation is 400–500 mm and the mean annual temperature is about 13 °C. There are seven different climatic zones in the province (Karam et al. 2014). With the exception of cold and highland climates, chickpea (Bivanij cultivar) is planted in the rest of the region. Bivanij cultivar is mostly common and popular than other chickpea cultivars (cultivars Azad, Hashem and ILC482) due to its shorter phenological stages time as complete ripening and a highest biomass and grain yield. In recent years, chickpea cultivation has increased and great damage is caused to the crop every year as a result of Fusarium disease (Jalali and Chand 1992). Chickpea cultivation has also increased in newly cultivated soils of foothill areas. Considering the importance of this crop, it is important to investigate the presence of Fusarium species infecting chickpea in these soils for proper management strategy.
The distribution of Fusarium species in soil is influenced by climatic factors and differs by adaptation to specific sets of climatic and environmental conditions in different areas (Saremi and Burgess 2000). Factors such as temperature, vegetation, rainfall, and soil organic matter have a major influence on distribution of some Fusarium species (Burgess and Summerell 1992). In Iran, a number of studies have been done on the distribution and presence of Fusarium species in cultivated soils compared to uncultivated soils (Hasanzade et al. 2008; Haji-Allahverdipoor et al. 2011; Zokaee et al. 2012; Nourollahi et al. 2017). In other parts of the world, due to the widespread distribution of Fusarium in different geographical areas, many scientists and researchers around the world have identified Fusarium species in cultivated and uncultivated soils (Jeschke et al. 1990; Stahl et al. 1999; Latiffah et al. 2007; Tafinta et al. 2018), but little has been published on the effect of environmental variables on Fusarium distribution especially in uncultivated soils. The aims of this study were (1) to isolate Fusarium species from uncultivated soils and from chickpea plants with disease symptoms, (2) to identify the isolates through morphological and molecular characterization, (3), to study the effect of environmental parameters on species distribution in uncultivated soils, and (4) to determine their pathogenicity on seedlings in a greenhouse to fulfill Koch’s postulates.
Materials and methods
Soil sampling
During 2015–2017, a total of fifteen samples of uncultivated soils (rangelands and foothill) were collected from different areas of Kermanshah province (west Iran) including Bistoon, Harsin, Sarab-e Niloofar, Paveh, Sahneh, Kangavar, Eslamabad-e Ghrab, Ravansar, Javanrud, Dalahu, Sarpol-e Zhab, Sumar, Qasr-e Shirin, Gilan-e Gharb and Sonqor during 2015 to 2017 (Fig. 1). The sampling sites covered all localities of the study area. Twenty soil subsamples from each area (50 × 50 m) to a depth of 20 cm after the litter removal were randomly collected and pooled to get one composite sample (approximately 2 kg). These soil samples were placed in sterile labeled plastic bags and transferred to the laboratory and stored at 4 °C until processing. The geographic location information (longitude, latitude, altitude) were recorded using GPSMAP device model 76CSx.
Map of study site location in the Kermanshah province, western Iranian state of Iran. Color circles refer to the locations in this study from which Fusarium isolates were obtained from chickpea fields and uncultivated soils. [S1 = Paveh; S2 = Javanrud; S3 = Ravansar; S4 = Qasr-e Shirin; S5 = Dalahu; S6 = Sarab-e Niloofar; S7 = Sahneh; S8 = Harsin; S9 = Eslamabad-e Gharb; S10 = Bistoon; S11 = Sarpol-e Zahab; S12 = Kangavar; S13 = Gilan-e Gharb; S14 = Sonqor; S15 = Sumar]
Isolation from soil
The standard soil dilution plate method (Nash and Snyder 1965) was used to isolate species of Fusarium from uncultivated soil. For this purpose, each soil sample was first passed through two mm, 40 and 60 mesh sieves. Ten grams of each subsample (four subsamples from each composite sample) were ground using mortar and pestle and suspended in 90 ml of 0.1% water-agar containing 100 ppm NPX (nonyl phenyl polyethyleneglycol ether containing a concentration of 10.5 mol of ethylene oxide) and shaken for 30 min. Three replicates (0.5 ml) of the serially diluted suspension (10−2, 10−3) were plated on sterile Petri dishes containing peptone-PCNB-agar (Raper and Fennell 1965) amended with 250 ppm ampicillin to prevent bacterial growth. Petri dishes were incubated at 25 °C for 3 to 5 days under a photoperiod of 12 h light/12 h dark and under fluorescent illumination. All Fusarium-like colonies were transferred to water-agar medium and purified using single spore method.
Isolation from chickpea plants
During 2016–2018, twenty isolates of Fusarium were obtained from roots of chickpea plants showing typical black root rot symptoms (Fig. 2a, b) from 20 different fields which they distributed in a similar pattern to the uncultivated soils sampled in Kermanshah province, west Iran (Fig. 1). Root samples were collected and carried separately to the laboratory for isolation. In order to isolate the pathogen, after washing roots to remove soil and debris with running tap water for 30 min, small root pieces, approximately 5 mm in size were taken from the interface of healthy and diseased tissues. Then, the pieces were surface disinfected with 1.5% solution sodium hypochlorite (2% available chlorine v/v) for 30 s, rinsed three times in sterile distilled water to remove surface sterilization agents, and plated on potato dextrose agar amended with chloramphenicol (25 μg/ml) (Jamali and Nasimi 2014). Plates were incubated at 25 °C for 3 to 5 days to allow the fungi to grow. All Fusarium-like colonies were transferred to water-agar medium and purified using single spore method. For fungal identification, during the incubation period, plates were observed daily for the appearance of fungal colonies.
Chickpea field affected with Fusarium redolens (a), Black root rot caused by Fusarium redolens on chickpea in fields (b), Pathogenicity tests of Fusarium redolens from chickpea plants with black root rot symptoms under greenhouse conditions: chickpea plants non-inoculated with Fusarium redolens (c), inoculated plants with Fusarium redolens (d), black root rot symptom on inoculated plants with Fusarium redolens (e, f), and control (g) in greenhouse trial
Pathogenicity tests
Inoculation tests were performed with 20 purified isolates of F. redolens isolates obtained from chickpea plants and three isolates of Neocosmospora. For this, 200 g of wheat grain and 120 ml of distilled water were autoclaved for 1 h at 121 °C three times in 24 h interval in 1 L Erlenmeyer flasks. The wheat was inoculated with 5–6 blocks of 7-day-old potato dextrose agar plate containing the fungus and incubated at 25 °C for 2 weeks in the dark (Westerlund et al. 1974). Inocula were mixed thoroughly with a sterilized vermiculite: perlite: soil (2:1:1 v:v:v) mix at a rate of 1:12 (wt/wt) to reach an inoculum density of approximately 105 CFU/g of soil for each of the isolates (Jimenez-Fernández et al. 2011). Noninfested wheat grain with the autoclaved soil mixture at the same rate as above served as the control. Chickpea seeds of the cultivar Bivanij were disinfected with sodium hypochlorite 10% w/v, and then washed three times with sterile distilled water, germinated in transparent plastic germination boxes (Gerbox) (11 × 11 × 3.5 cm), selected for uniformity (length of radicle = 1 to 2 cm), and sown into pots with a 0.5 kg capacity. Four seeds were used in each pot and inoculated plants placed in a greenhouse at 24 °C for 35–42 days in a completely randomized design with four replications. Starting 5 days after inoculation, the seedlings were examined for symptomatic leaves and dead plants at 3 days intervals until the end of the experiment, 6 weeks after inoculation. Disease severity on each plant was rated using the following scale: 0 = no symptoms; 1 = 1 to 33% of leaves with symptoms; 2 = 34 to 66% of leaves with symptoms; 3 = 67 to 100% of leaves with symptoms; and 4 = plant dead. The disease severity index was calculated for each isolate according to the parameters in the disease scale (Landa et al. 2006; Navas-Cortés et al. 2007) DSI = Σ (A × n)/Σ (B) × 100. In this formula; A: disease scale, n: number of plants in specific scale, and B: total number of plants.
Morphological identification
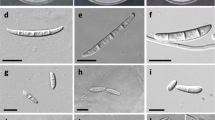
Morphological and micromorphological characteristics of isolates in the pure culture were studied using a light microscope (Olympus model BH2). Fusarium isolates were grown on carnation leaf agar (CLA), Synthetischer Nährstoffarmer agar (SNA) and potato dextrose agar in Petri plates at 25 °C with 12 h light/12 h darkness for 10 days. Species of the Fusarium isolates were identified based on their colony texture, growth pattern, growth rate, pigmentation on potato dextrose agar, and micromorphology of phialides, microconidia, macroconidia, and chlamydospores on SNA (Leslie and Summerell 2006). Images were captured with a camera (Canon Powershot model SX10). Measurements of the observed fungal structures were made using the BioloMICS Measure software (Robert et al. 2011). The identification was carried out according to the authentic mycology taxonomic keys (Nelson et al. 1983; Leslie and Summerell 2006).
Genomic DNA isolation
For molecular studies, isolates were grown on potato dextrose agar for 5 days at 25 °C in the dark. Approximately 100 mg of fungal mycelium was scraped from the pure cultures and mechanically disrupted by grinding the mycelium with a mortar and pestle to a fine powder under liquid nitrogen (Gardes and Bruns 1993). Fungal genomic DNA was extracted using a genomic DNA purification Kit (50 t-PR881613-EX6011, Sinagen co., Iran) according to the manufacturer’s instructions. The nuclear ribosomal DNA internal transcribed spacer (ITS) of the fungal isolates were amplified using the forward primer, ITS1 (5´-TCC GTA GGT GAA CCT GCG G-3′) and the reverse primer, ITS4 (5´-TCCTCC GCT TAT TGA TAT G-3′) (White et al. 1990). All PCR reactions were conducted in 25 μl containing 2.5 μl of a diluted genomic DNA (1:10 or 1:100 dilutions of the original extract), 20 pmol of each primer, 1.25 nmol of each deoxynucleotide, 0.5 U Taq DNA polymerase (CinnaGen, Iran), 1.5 mM of MgCl2, 2.5 μl of 10 × PCR buffer (CinnaGen, Iran), and 14.5 μl H2O. The PCR reactions were performed on a Biometra model T-Personal thermocycler. Thermocycling parameters for ITS were as follows: an initial denaturation step at 95 °C for 2 min, then 35 cycles at 95 °C for 60 s, 55 °C for 80 s, and 72 °C for 90, with a final extension step of 72 °C for 10 min before cooling or removing the tubes. A portion of the translation elongation factor 1-alpha (TEF-1α) gene was amplified using primers EF1F (5-ATGGGTAAGGAGGACAAGAC-3) and EF2R (5-GGAAGTACCAGTGATCATGTT-3) (Geiser et al. 2004) under these parameters: initial denaturation step at 95 °C for 5 min, then 35 cycles at 95 °C for 60 s, 57 °C for 50 s, and 72 °C for 90, with a final extension step of 72 °C for 7 min. The amplification products were visualized under UV light after electrophoresis on 1% TBE-agarose gel stained with ethidium bromide and run in 1× TBE buffer. The controls, with no DNA, were included in every set of amplification to check the DNA contamination in reagents and reaction buffers.
Sequencing of the amplified ITS and TEF-1α regions
The amplification products were purified using the GeneJET PCR purification Kit (Fermentas, UK) to remove excess primers and nucleotides. Sequencing reaction was performed on purified PCR products in forward orientation using the same primers as in PCR. The sequence was determined with an Applied Biosystems (ABI prism 377) DNA sequencer according to the manufacturer’s instruction (Macrogene, South Korea). All DNA sequences were deposited in the National Center for Biotechnology Information GenBank (NCBI, http://www.ncbi.nlm.nih.gov/Entrez) (Bethesda, MD, USA) and are listed in Table 1.
Phylogenetic analysis
After sequencing, the nucleotide sequences were edited using BioEdit Sequence Alignment Editor version 7.2.5 software (Hall 1999), and sequence similarity searching in the GenBank sequence database was performed using BLAST service in NCBI (http://blast.ncbi.nlm.nih.gov). The multiple alignment program ClustalW (http://www.clustal. org/download) (Thompson et al. 1994) was implemented using MEGA5 software (http://megasoftware.net/) to align the ITS and TEF-1α sequences generated in this study (Table 1) with sequences available in GenBank (Table 2), mainly those from previously published studies. Alignments were manually optimized with BioEdit version 7.2.5. Poorly aligned positions and gaps of the sequences were excluded from the final alignment of each dataset using Gblocks software version 0.91b (Castresana 2000) under the less stringent parameters selected. The evolutionary history was inferred using the Neighbor-Joining (Saitou and Nei 1987) and Maximum Likelihood (Kimura 1980) methods based on the p-distance and Kimura 2-parameter model respectively. The analysis involved 72 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 334 positions in the final dataset. Evolutionary analyses were conducted in MEGA5 software (http://megasoftware.net/). Evaluation of the reliability of tree topologies were performed by calculating 1000 bootstrap re-samplings (Felsenstein 1985).
Soil physicochemical parameters
The collected soil from uncultivated soils and chickpea fields (4 subsamples per pooled soil sample) was analyzed for physicochemical parameters included organic carbon, pH, electrical conductivity (EC), texture, and CaCO3 content. Analysis of soil particle size distribution was performed by a Bouyoucos hydrometer method (Gee and Bauder 1986). The soil pH in the saturation extract was measured according to Thomas (1996) and electrical conductivity (EC) measured using an electrical conductivity meter in a soil and water suspension (Consort C833 pH/EC Meter). Total carbonates (CaCO3) were measured with a Scheibler calcimeter (Loeppert and Suarez 1996). Organic carbon was determined by the Walkley and Black (1934). These characteristics are listed in Table 3. Soil parameters were tested for significant differences between the sites using Tukey’s HSD test (Tukey 1953) in the statistical analysis system (SAS) software for Windows version 8.2 (SAS, Institute Inc., Cary, NC, USA).
Effect of pH on Fusarium redolens radial growth
To evaluate the influence of pH on F. redolens mycelial growth (19 isolates that were molecularly identified), a 5 mm diameter mycelial block was cut from the margin of 3-day-old F. redolens colonies and placed on potato dextrose agar plates with pH adjusted to 5.8, 7.3, 8.4, 9.19, 9.72, 10.44 with 0.1 N HCl and NaOH before autoclaving, and incubated at 25 °C. The colony diameters of the F. redolens colonies were measured in 2 directions for each isolate replicated on 4 separate potato dextrose agar plates at each pH level 1 day after inoculation. The means were analyzed by analysis of variance (ANOVA) at 5% significant level with SAS software for Windows version 8.2 (SAS, Institute Inc., Cary, NC, USA).
Statistical analysis
Normality and homogeneity of the variances of the disease severity data were assessed using the Shapiro–Wilk and Levene tests, respectively. The Levene statistics for testing the homogeneity of variances indicated unequal variances. Therefore, Welch’s F test was used and post hoc comparisons conducted with the Games-Howell procedure (SPSS software version 18.0).
To examine the relationships between environmental variables and Fusarium species, at first detrended correspondence analysis (DCA) was used to determine the length of the gradient (length > 2 SD: indicates unimodal variation and a length < 2 SD indicates a linear variation). Gradient analysis showed a length of 7 for DCA, therefore the canonical correspondence analysis (CCA) (unimodal method) was used (Gauch and Wentworth 1976). Analyses were performed in CANOCO for Windows v4.5 (Ter Braak 2003).
Results
Sampling and morphological identification
A total of 60 Fusarium isolates were obtained from uncultivated soil in different areas of Kermanshah province (west Iran). Based on their colony texture, growth pattern, and micromorphological characteristics, four different species were identified, namely F. redolens, F. oxysporum, F. solani and Neocosmospora vasinfecta. The most prevalent Fusarium species recovered were F. redolens, 38 isolates (64%), followed by F. oxysporum, 12 isolates (20%), F. solani, seven isolates (11%) and N. vasinfecta, three isolates (5%).
Isolation from chickpea plants
The disease was present on chickpea plants in all of the chickpea growing regions. In this study, many samples with black root rot symptoms were collected from each field and cultured in media, but because the Fusarium isolates obtained from each plant were the same in terms of morphological characteristics, so only one plant was randomly selected from each field and each isolate was from a different field. A total of 20 fungal isolates were obtained from 20 fields in Kermanshah province, west Iran. All the 20 isolates were identified as F. redolens on the basis of colony texture, growth pattern, growth rate, pigmentation on potato dextrose agar and micromorphological characteristics. Morphological characteristics were consistent with descriptions of F. redolens in Leslie and Summerell (2006). Fungal colonies were white to pink color with abundant aerial mycelium. Colony diameter on potato dextrose agar varied from 25 mm to 30 mm after 3 days of incubation at 25 °C in the dark. Sporodochia produced on CLA were cream to orange. Abundant spherical chlamydospores, 8–12 μm in size, were formed either singly or grouped in chains on CLA medium after 4 weeks. Macroconidia with 3 to 5 septa and 3–5.4 × 35–44 μm size were also present. Microconidia were abundant, oval or cylindrical shape and 3–5.1 × 6–16 μm in size.
Molecular identification
In order to confirm the morphological identification, after grouping the isolates based on morphological and cultural characteristics, the molecular techniques were used for 17 isolates from uncultivated soils and 10 isolates from chickpea plants. The TEF-1α partial gene was successfully amplified using EF1/EF2 primers from these 27 isolates, and yielded a single band ranging in size from about 600 to 700 base pair. Results of the sequencing and blast search for similar TEF-1α sequences in the Genbank DNA database using Blast program (http://blast.ncbi.nlm.nih.gov/blast.cgi) showed that all ten isolates recovered from chickpea plants showed 100% identity with valid sequences of F. redolens deposited in GenBank. From 17 isolates obtained from uncultivated soil, nine isolates showed 100% similarity with F. redolens isolates deposited at GenBank, four isolates showed 100% homology with F. oxysporum at GenBank, one isolate showed 100% homology with N. vasinfecta at GenBank and three isolates showed 100% homology with those previously identified as F. solani at GenBank. Analysis of the ITS sequence of 17 isolates from uncultivated soil revealed that nine isolates had 98–99% identity with the reference sequences of F. redolens, four isolates had 98% identity with F. oxysporum at GenBank and three isolates 100% identity with F. solani at GenBank. The sequenced isolates with accession numbers are listed in Table 1.
Phylogenetic analysis
Phylogenetic reconstruction of the combined ITS1 + 5.8S + ITS2 regions of the genomic ribosomal RNA tandem gene repeat which was applied for preliminary identification of species, resulting in poor resolution of species discrimination between the investigated Fusarium taxa. The results indicated that ITS sequences did not have significant nucleotide variations (data not shown). The TEF-1α gene had a higher resolution than ITS for discrimination between different Fusarium species (Fig. 3). Both methods used for phylogenetic analysis showed the same topology, in spite of slight differences in bootstraps value among equivalent branches. In neighbor-joining method, the optimal tree was with the sum of branch length = 0.636 (data not shown). In maximum likelihood method, the tree with the highest log likelihood (−1357.57) is shown (Fig. 3). In this method the Kimura 2-parameter model was determined as the best fit nucleotide substitution model of evolution for each dataset based on the Bayesian information criterion (BIC) by MEGA 5. Phylogenetic analysis of isolates based on TEF-1α gene put them into four monophyletic lineages. Two main clades supported by high bootstrap values were distinguished. Clade I was divided into five subclades. Subclade I comprised isolates from uncultivated soil (9 isolates) and chickpea plants (10 isolates), which together with authentic isolates of F. redolens constitute a monophyletic group with high bootstrap value (97% NJ, 87% ML). The reference isolates were from Spain, Italy, Germany, USA and Finland (Riccioni et al. 2008; Balmas et al. 2010; Jimenez-Fernández et al. 2011; Christ et al. 2011; Haapalainen et al. 2016). Subclade II comprised two isolates described as F. hostae. Fusarium hostae with high bootstrap value (99% NJ, 88% ML) is a sister taxon of F. redolens. Subclade III comprised two isolates described as F. nisikadoi. Subclade IV comprised Gibberella fujikuroi complex, three described as F. verticillioides, three as F. subglutinans and three as F. proliferatum. Subclade V comprised four isolates from uncultivated soil in this study and F. oxysporum from other authors within F. oxysporum complex (O'Donnell et al. 2009; Short et al. 2011; Koyyappurath et al. 2016). The distances among TEF-1α gene of Fusarium species were calculated using Mega 5 software both between and within the five subclades. The sequence divergence between the five subclades was generally much higher than the distances within the subclades. The two highest within-clade distances characterized subclades 4 and 5, respectively, which included Gibberella fujikuroi complex and F. oxysporum complex isolates. The lowest sequence divergence was between subclade F. redolens (subclade I) and F. hostae (subclade II). In clade II, three of the Fusarium isolates in our study grouped with F. solani and one isolate grouped with N. vasinfecta isolates within the F. solani complex (99% NJ, 99% ML).
Soil analysis
The results of physico-chemical analyses on uncultivated soil samples from different parts of Kermanshah province are shown in Table 3 (physico-chemical data for chickpea fields not shown). Statistical analysis using Tukey’s HSD test showed differences in soil structure parameters among sites. Soils collected from different parts of Kermanshah province (field and uncultivated soils) were mostly loamy and silty loamy, alkaline pH and low in organic matter. These soils are considered as non saline and basic soils.
Relationships between Fusarium species and environmental factors
The relative importance of the determined soil parameters and altitude to the distribution of Fusarium species is shown in canonical correspondence analysis (CCA) biplot (Fig. 4) where altitude, soil parameters and Fusarium species are arranged on the basis of their scores on two axes. The eigenvalue for the first axis was 0.98 and 0.78 for the second axis. The correlation between species and environmental parameters was highly significant (99.3% for the first axis and 89.4% for the second axis). Where electrical conductivity (EC), carbon, organic matter, silt, clay and altitude are negatively correlated with the first CCA axis, pH, sand and CaCO3 correlated positively (Fig. 4). Also, the species on the second axis have a negative correlation with CaCO3, pH, conductivity, silt and altitude and have a positive correlation with clay, sand, organic matter and soil carbon. The species at the margins of the axes are usually uncorrelated with environmental variables, and those at the center of the biplot can be highly correlated or uncorrelated to environmental variables. In canonical correspondence analysis arrow length indicates the importance of variables on species. According to the arrow lengths, soil texture (sand, silt, clay), altitude, CaCO3, EC, carbon and organic matter and pH in descending order were recognized as the most important environmental variables on the distribution of Fusarium species in soil. Fusarium redolens was most numerous in soil with very low sand, while F. oxysporum and F. solani responded to soil with intermediate sand (Fig. 4). Fusarium redolens were most numerous in uncultivated soil with intermediate pH, EC and CaCO3. The two species F. solani and F. oxysporum are much more abundant in soil with very low pH, EC and CaCO3. F. solani and F. oxysporum were most common at higher carbon and organic matter; F. redolens at very low carbon and organic matter. For the altitude, F. solani and F. oxysporum were found at lower altitude than F. redolens. Neocosmospora vasinfecta is located on the edge of the axis and was isolated only from the soil of Sumar region with its CaCO3 content higher than the other locations.
Correspondence analysis (CA) of the Fusarium species communities found in different parts of Kermanshah province. The eigenvalues of the first and second axes in the two dimensional ordination diagrams are as: CA1 = 0.98 and CA2 = 0.78. [S1 = Paveh; S2 = Javanrud; S3 = Ravansar; S4 = Qasr-e Shirin; S5 = Dalahu; S6 = Sarab-e Niloofar; S7 = Sahneh; S8 = Harsin; S9 = Eslamabad-e Gharb; S10 = Bistoon; S11 = Sarpol-e Zahab; S12 = Kangavar; S13 = Gilan-e Gharb; S14 = Sonqor; S15 = Sumar]
Greenhouse pathogenicity tests
The results of the pathogenicity test under greenhouse condition revealed that all 20 isolates of F. redolens obtained from chickpea plants with black root rot symptoms in this study are pathogenic to the chickpea of the cultivar Bivanij (Fig. 2d). Two weeks after inoculation with F. redolens, symptoms developed as foliar yellowing and black cankers that extended upward and downward of roots of all emerged seedlings (Fig. 2e, f). Discoloration in the vascular tissues of inoculated chickpea plants were not observed. Disease severity index (DSI) ranged between 46 and 89 among the F. redolens isolates on chickpea cultivar Bivanij. Fusarium redolens isolate FuRe12 was most virulent with DSI 89 (Fig. 5). Three isolates of N. vasinfecta obtained from uncultivated soil could infect chickpea plants. Disease symptoms were observed as yellowing and black root rot. Except for three isolates of Neocosmospora, the Fusarium isolates from uncultivated soil samples were not tested for pathogenicity on chickpea. Re-isolation from all inoculated plants after observation of symptoms were performed, and isolates were compared to original cultures all fulfilling Koch’s postulates.
Effect of pH on Fusarium redolens mycelial growth
The results of pH test on F. redolens growth showed the mycelial growth of this species was highest at pH 9.72. The mycelial growth was lowest at pH 5.8 (data not shown).
Discussion
In this study, a total of 60 Fusarium isolates were obtained from uncultivated soil in different areas of Kermanshah province (west of Iran) with F. redolens most prevalent. In most studies F. oxysporum and F. solani are considered cosmopolitan species and have been reported to be among the most frequently isolated fungi from different soils such as sandy soils (Mandeel 2006), grassland soils (Burgess and Summerell 1992), desert soils (El Gindy and Saad 1990), forest soils (Latiffah and Azaman 2011), cultivated soils (Saremi and Saremi 2013) and different climates such as tropical, temperate, arctic, arid and mediterranean regions (Sangalang et al. 1995; Mandeel 2006; Manshor et al. 2012; Stefańczyk et al. 2016).
In all of these studies, the identification was based on morphological characteristics. However, due to the overlapping in several characters among morphologically similar Fusarium spp., such as F. oxysporum and F. redolens some misidentifications may have been made when using these characteristics (Jimenez-Fernández et al. 2011; Jamali 2017). Recent molecular studies have shown that the causal agent of Fusarium yellows of some plants is F. redolens that was misidentified as F. oxysporum(Baayen et al. 1997; Baayen et al. 2000a, 2000b). Due to similar morphological characteristics, F. redolens has been considered to be conspecific with F. oxysporum or a variety of F. oxysporum (Baayen et al. 2001; Jimenez-Fernández et al. 2011) or even F. solani (Šišić et al. 2018). Our results showed that the abundance of F. redolens in uncultivated soils was higher than other species. Shadmani et al. (2018), showed that the most isolated Fusarium isolate from barley roots was F. redolens (Shadmani et al. 2018). In their study, the identification was performed by molecular characters.
In this study, the pathogenicity of all isolates of F. redolens obtained from symptomatic chickpea plants that showed yellowing (aerial parts) and black root rot were confirmed. In Iran, most studies are based on morphological characteristics, and F. oxysporum was reported as the most pathogenic agent of chickpea with black root rot and yellowing symptoms in most parts of the country (Afshari-Azad 1998; Mohammadi and Banihashemi 2005, 2006; Zamani et al. 2001, 2004; Hasanzade et al. 2008; Haji-Allahverdipoor et al. 2011; Zokaee et al. 2012; Nourollahi et al. 2017). In this study, all isolates obtained from chickpea plants were identified as F. redolens based on morphological and molecular characteristics.
Based on the morphological characters, F. oxysporum f. sp. ciceri has been reported as the most important causal agent of chickpea disease in many parts of the world, including India, Ethiopia, Egypt, Turkey, Spain, Syria, Pakistan, Peru, Australia, United States, Tunisia, Canada, and other countries (Chattopadhyay and Sen Gupta 1967; Echandi 1970; Westerlund et al. 1974; Trapero-Casas and Jimnez-Diaz 1985; Nene et al. 1996; Esmaeili Taheri et al. 2011). Then, in a number of these countries including Tunisia, Netherlands, Morocco, Pakistan, Canada, Spain and Lebanon, using molecular methods, F. redolens was identified as the causal agent of chickpea root rot (Baayen et al. 2000a, 2000b; Esmaeili Taheri et al. 2011; Jimenez-Fernández et al. 2011; Leisso et al. 2011; Bouhadida et al. 2017; Rafique et al. 2020).
Among various species of Fusarium being reported as the most important causal agent of chickpea disease in Iran, F. solani and F. oxysporum are the most common isolated species. To our knowledge, this is the first report of F. redolens causing root rot of chickpea in Iran. There was no previous report of this species causing diseases on chickpea and other crops in Iran. Different crop management procedures including sanitation, crop rotation, resistant chickpea cultivars and use of fungal or bacterial antagonists have been proposed to control the disease. Thus, accurate identification and clarifying the ecology and biology of this fungus are crucial for proper management strategy, especially if resistant cultivars are the most effective control measures. Differentiation between F. oxysporum and F. redolens, based on morphology, is difficult due to the presence of isolates with intermediate forms and it is possible that F. redolens has been found previously and misidentified as F. oxysporum. Use of molecular methods is needed in order to identify and separate Fusarium species correctly. We used the combined ITS1 + 5.8S + ITS2 regions of the genomic ribosomal RNA tandem gene repeat and partial TEF-1α gene for identification of species. Our results showed poor resolution of species discrimination between the investigated Fusarium taxa with the ITS regions, but the TEF-1α gene had a higher resolution than ITS for discrimination between different Fusarium species (Fig. 3). These results agree with the results of other authors (Zhao et al. 2011; Raja et al. 2011; Šišić et al. 2018; Alhawatema et al. 2019). Based on previous research, DNA-based studies showed that F. redolens is distinct from F. oxysporum (O’Donnell et al. 1998; Baayen et al. 2000a, 2000b; Baayen et al. 2001; Bogale et al. 2007). These studies revealed that they even lack a sister taxon relationship. Baayen et al. (2001) showed that the F. nisikadoi-F. miscanthi clade is more closely related to the F. oxysporum clade than it is to the F. redolens-F. hostae clade. In this study, the estimated transition/transversion bias (R) was 2.2, that could be suitable for phylogenetic analysis and clearly resolved species boundaries in the constructed phylogram (Fig. 3). The maximum Log likelihood for this computation was −1357.573. In our phylogenetic trees, F. hostae with high bootstrap value (99% NJ, 88% ML) was a sister taxon of F. redolens. Our results agree with results of Baayen et al. (2001) and other authors (Jimenez-Fernández et al. 2011). To clarify the ecology and biology of this fungus in Iran, more investigation is needed.
Little is known about the influence of environmental factors on the distribution of F. redolens under agricultural and natural soil conditions. Environmental factors and climate that contribute to the distribution of Fusarium species could predict the potential presence of different species of Fusarium in specific locations. The study of the effective factors on the distribution of Fusarium fungi and modeling species distribution using new and advanced software is useful and valuable, but has received far less attention. Saremi and Burgess (2000), showed that the distribution of Fusarium species is restricted due to adaptation to specific sets of soil environmental conditions. Their studies have demonstrated that some species of Fusarium are cosmopolitan while others are restricted to a particular climatic region. It has been concluded that the distribution of Fusarium species is closely related to a variety of climatic factors (Burgess et al. 1993; Saremi et al. 1997). It has been reported that non-pathogenic F. oxysporum, F. solani and F. equiseti typically were cosmopolitan and occur in most parts of the world, in contrast F. acuminatum and F. sambucinum that were restricted to the cool temperate areas (Abbas et al. 1987; Backhouse and Burgess 1995; Burgess et al. 1988; Backhouse et al. 2001). Summerell et al. (2010) have reported that Fusarium species distribution is influenced by environmental factors, such as temperature, soil texture, rainfall, drought tolerance, and local vegetation.
The three isolates of Neocosmospora obtained from uncultivated soil in Qasr-e Shirin, Kermanshah province, west Iran with an arid and hot climate, were identified as N. vasinfecta and their pathogenicity on chickpea cultivar Bivanij was confirmed. The locality was situated at an elevation of 682 m. In the CCA analysis N. vasinfecta is located on the edge of the axis. The species at the margins of the axes are usually uncorrelated with environmental variables (Ter Braak 1988). Qasr-e Shirin is a hot area of Iran and therefore the correlation with temperature is likely. In most studies, disease severity is positively correlated with soil temperature, so planting this cultivar in this area with warm weather can be a threat for this crop. Cannon and Hawksworth (1982), showed that N. vasinfecta is found mostly in the soil of tropical or subtropical areas. This species has been reported as phytopathogenic fungus from chickpea in Pakistan (Ali et al. 2011), Hungary, Ethiopia and India (Nene et al. 1996), peanuts in South Africa, Australia, Vietnam and Taiwan (Fuhlbohm et al. 2007), Arachis hypogaea plant in Guinea (Lombard et al. 2015), and other plants (Cannon and Hawksworth 1982; Manikandan et al. 2007). This species has also been reported from clinical materials in Senegal and France (Ben Hamida et al. 1993; Kac et al. 1999; Gabriel et al. 2013), soil in India and South Africa (Lombard et al. 2015), and animal dung (Doveri 2011). This is the first report of pathogenicity of N. vasinfecta on chickpea from Iran. More studies are needed to clarify the ecology, biology and host range of this fungus in Iran.
The results of CCA analysis showed that the correlation between species and environmental parameters was highly significant (99.3% for the first axis and 89.4% for the second axis). In this study, all the soils sampled were mostly alkaline in the range of 7.2 to 9. Jones and Woltz (1981), showed that the most Fusarium wilt (F. oxysporum)- suppressive soils have a pH value greater than 7. Such soils are inhibitory to F. oxysporum species and increasing soil pH reduces Fusarium wilt (Borrero et al. 2004; Fang et al. 2012; Deltour et al. 2017). In contrast, F. redolens responded to a soil with intermediate pH, while F. oxysporum and F. solani responded to soil with very low pH (Fig. 4) and all isolates obtained from chickpea plants with black root rot symptom were identified as F. redolens. The results of acidity test on F. redolens growth showed the mycelial growth of this species was highest at pH 9.72. The mycelial growth was lowest at pH 5.8. The soil in most parts of Iran and Kermanshah province (in this study) is alkaline, and has a high pH of 7.4 and 8.2 (Qadir et al. 2008; Heidari et al. 2008) so it can be expected that root rot of chickpea is caused by F. redolens.
Soil pH modulates the bioavailability of macro- and micronutrients such as manganese, iron, copper and zinc (Collins and Buol 1970). To obtain micronutrients many organisms produce siderophores with stability constants differing in magnitude and pH dependence. Therefore, the relative ability of different species to obtain essential micronutrients differs with pH, due to the effect of pH on solubility of the metals and stability of the chelated forms (Boukhalfa and Crumbliss 2002; Dhungana and Crumbliss 2005). According to the arrow lengths in the CCA analysis, soil texture (sand, silt, clay), altitude, CaCO3, EC, carbon and organic matter and pH in descending order were recognized as the most important environmental variables on the distribution of Fusarium species in soil. Fusarium redolens was most numerous in soil with low clay, while F. oxysporum and F. solani responded to soil with very low clay (Fig. 4). Deltour et al. (2017) identified a negative correlation between clay content and Fusarium wilt severity. Clay may influence suppression by pH buffering, availability of nutrients, and altering oxygen diffusion (Lavie and Stotzky 1986; Dominguez et al. 2001).
The abundance of F. redolens was significantly highest at very low carbon and organic matter. Organic matter and microbial biomass carbon contents in soil with loam and sandy loam texture are low (Vujanovic et al. 2006). Several studies have found a positive correlation between organic matter and suppression of Fusarium disease of melon, chrysanthemum, and flax (van Rijn et al. 2007; Saadi et al. 2010). Soil organic matter impacts the structure of soil, pH, pH buffering capacity, and nutrient availability (Brady and Weil 2000; Baum et al. 2015). Gehlker and Scholl reported that low soil pH, high soil organic matter, high clay content, or inadequate drainage favor Fusarium disease of asparagus (Gehlker and Scholl 1974). Our results showed that F. redolens was most abundant in uncultivated soil with intermediate EC and CaCO3. Increased EC of the nutrient solution in hydroponic culture can also influence plant diseases positively or negatively. Nam et al. (2018) showed that the increase of EC of the nutrient solution had no significant effects on the Fusarium wilt of lettuce. Research on the effect of CaCO3 on the survival of Fusarium is rather limited. Calcium carbonate (CaCO3) could not only serve as a soil amendment to change soil pH but also increase soil Ca2+ content (He et al. 2014). Ca2+ has been reported to affect many soil-borne diseases (Benson et al. 2009).
Despite the overwhelming impact of F. redolens, there is limited information available on the pathogenicity of this species in relation to soil and climate and further research is required to investigate the effect of soil texture, pH, EC, CaCO3, temperature, nutrients, and organic matter on disease severity of F. redolens. According to phylogenetic studies, the alkalinity of the soils, the frequency of F. redolens in the studied soils, optimal mycelial growth of F. redolens at pH 9.72, suppressiveness of alkaline soils against Fusarium wilt caused by F. oxysporum, and its pathogenicity on chickpea, it is possible that F. redolens also is the causal of agent of chickpea black root rot in other parts of Iran, and likely has been mistakenly called F. oxysporum based on morphological characteristics. Further studies in other regions of Iran should be performed to confirm whether F. redolens is the major causal agent of chickpea root rot.
References
Abbas HK, Mirocha CJ, Berdal BP, Sundheim L, Gunther R, Johnsen B (1987) Isolation and toxicity of Fusarium species from various areas of Norway. Acta Agric Scand 37(4):427–435
Afshari-Azad H (1998) Identification of Iranian fungal isolates causing yellow disease in ckickpea. 13th Iranian Plant Protection Congress, Karaj, 22–26 August
Alhawatema M, Ali Alqudah A, Al Tawaha AR (2019) Separation of different Trichoderma species based on partial TEF-1α and RPB2 protein coding genes sequences against ITS regions. Biosci Res 16(1):161–170
Alidadi A, Javan-Nikkhah M, Kowsari M, Karami S, Ebrahimi Rastaghi M (2018) Some species of fungi associated with declined Persian oak trees in Ilam province with emphasis on new records to mycobiota of Iran. Rostaniha 19(2):75–91
Ali H, Akhtar KP, Shah TM, Khan AA (2011) First report of Neocosmospora vasinfecta causing root of chickpea in Pakistan. J Plant Pathol 93(4):63–89
Baayen RP, Van Dreven F, Krijger MC, Waalwijk C (1997) Genetic diversity in Fusarium oxysporum f. sp. dianthi and Fusarium redolens f. sp. dianthi. Eur J Plant Pathol 103:395–408
Baayen RP, O'Donnell K, Bonants PJ, Cigelnik E, Kroon LP, Roebroeck EJ, Waalwijk C (2000a) Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing wilt and rot disease. Phytopathology 90:891–900
Baayen RP, Van den Boogert PH, Bonants PJ, Poll JT, Blok W, Waalwijk C (2000b) Fusarium redolens f. sp asparagi, causal agent of asparagus root rot, crown rot and spear rot. Eur J Plant Pathol 106:907–912
Baayen RP, O'Donnell K, Breeuwsma S, Geiser DM, Waalwijk C (2001) Molecular relationships of fungi within the Fusarium redolens-F. hostae clade. Phytopathology 91(11):1037–1044
Backhouse D, Burgess LW (1995) Mycogeography of Fusarium: climatic analysis of the distribution within Australia of Fusarium species in section Gibbosum. Mycol Res 99:1218–1224
Backhouse D, Abubakar AA, Backhouse D, Burgess LW, Summerell BA (2001) Chapter 9-biogeography of Fusarium. In: Summerell BE, Leslie JF, Backhouse D, Bryden WL, Burgess LW (eds) Fusarium–Paul E. Nelson Memorial Symposium. APS Press, St. Paul, pp 122–137
Balmas V, Migheli Q, Scherm B, Garau P, O’Donnell K, Ceccherelli G, Kang S, Geiser DM (2010) Multilocus phylogenetics show high levels of endemic fusaria inhabiting Sardinian soils (Tyrrhenian Islands). Mycologia 102:803–812
Baum C, Eichler-Löbermann B, Hrynkiewicz K (2015) Impact of organic amendments on the suppression of Fusarium wilt. In: Meghvansi MK, Varma A (eds) Organic amendments and soil suppressiveness in plant disease management. Springer International Publishing, Cham, pp 353–362
Ben Hamida F, Achard JM, Westeel PF et al (1993) Leg granuloma due to Neocosmospora 6asinfecta in a renal graft recipient. Transplant Proc 25:2292
Benson JH, Geary B, Miller JS, Jolley VD, Hopkins BG, Stevens MR (2009) Phytophthora erythroseptica (pink rot) development in russet Norkotah potato grown in buffered hydroponic solutions I. calcium nutrition effects. Am J Potato Res 86(6):466–471
Bogale M, Wingfield BD, Wingfield MJ, Steenkamp ET (2007) Species-specific primers for Fusarium redolens and a PCR-RFLP technique to distinguish among three clades of Fusarium oxysporum. FEMS Microbiol Lett 271(1):27–32
Borrero C, Trillas MI, Ordovás J, Tello JC, Avilés M (2004) Predictive factors for the suppression of Fusarium wilt of tomato in plant growth media. Phytopathology 94(10):1094–1101
Bouhadida M, Jendoubi W, Gargouri S, Beji M, Kharrat M, Chen W (2017) First report of Fusarium redolens causing Fusarium yellowing and wilt of chickpea in Tunisia. Plant Dis 101(6):1038
Boukhalfa H, Crumbliss AL (2002) Chemical aspects of siderophore mediated iron transport. Biometals 15:325–339
Brady NC, Weil RR (2000) Elements of the nature and properties of soils. Prentice Hall, Upper Saddle River
Burgess LW, Summerell BA (1992) Mycogeography of Fusarium: survey of Fusarium species in sub-tropical and semi-arid grassland soils from Queensland, Australia. Mycol Res 96:780–784
Burgess LW, Liddell CM, Summerell BA (1988) Laboratory manual for Fusarium research, 2nd edn. The University of Sydney, Sydney
Burgess LW, Forbes C, Nelson PE, Marasas WFO, Gott KP (1993) Characterization and distribution of Fusarium acuminatum Subsp. Armeniacum Subsp NOv. Mycologia 85:119–124
Cannon PF, Hawksworth DL (1982) A revision of the genus Neocosmospora (Hypocreales). Trans Br Mycol Soc 82:673–688
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552
Chattopadhyay SB, Sen Gupta PK (1967) Studies on wilt diseases of pulses. I Variation and taxonomy of Fusarium species associated with wilt disease of pulses. Indian J Mycol Res 5:45–53
Chehri K, Hajeb S, Maassoumi SM (2017) Morphological and molecular identification and PCR amplification to determine the toxigenic potential of Fusarium graminearum species complex (FGSC) isolated from wild grasses in Iran. J Agric Sci Technol 19:1617–1629
Christ DS, Marlander B, Varrelmann M (2011) Characterization and mycotoxigenic potential of Fusarium species in freshly harvested and stored sugar beet in Europe. Phytopathology 101:1330–1337
Collins JF, Buol SW (1970) Effects of fluctuations in the eh-pH environment on iron and/or manganese equilibria. Soil Sci 110:111–118
Dababat AA, Sikora RA (2007) Importance of application time and inoculum density of Fusarium oxysporum 162 for biological control of Meloidogyne incognita on tomato. Nematropica 37:267–275
Dean R, Van Kan JAL, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD (2012) The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430
Deltour P, França SC, Liparini Pereira O, Cardoso I, De Neve S, Debode J, Höfte M (2017) Disease suppressiveness to Fusarium wilt of banana in an agroforestry system: influence of soil characteristics and plant community. Agric Ecosyst Environ 239:173–181
Dhungana S, Crumbliss AL (2005) Coordination chemistry and redox processes in siderophore-mediated iron transport. Geomicrobiol J 22:87–98
Dominguez J, Negrin MA, Rodriguez CM (2001) Aggregate water-stability, particle size and soil solution properties in conducive and suppressive soils to Fusarium wilt of banana from Canary Islands (Spain). Soil Biol Biochem 33:449–455
Doveri F (2011) Additions to “Fungi Fimicoli Italici”: an update on the occurrence of coprophilous basidiomycetes and ascomycetes in Italy with new records and descriptions. Mycosphere 2(4):331–427
Echandi E (1970) Wilt of chickpeas or garbanzo beans (Cicer arietinum) incited by Fusarium oxysporum. Phytopathology 60:1539
El Gindy AA, Saad RR (1990) Fungi in virgin and cultivated soil of Salhiah desert Egypt. Zbl Mikrobiol 145:547–551
Esmaeili Taheri A, Hamel C, Gan Y, Vujanovic V (2011) First report of Fusarium redolens from Saskatchewan and its comparative pathogenicity. Can J Plant Pathol 33(4):559–564
Fang X, You MP, Barbetti MJ (2012) Reduced severity and impact of Fusarium wilt on strawberry by manipulation of soil pH, soil organic amendments and crop rotation. Eur J Plant Pathol 134(3):619–629
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fuhlbohm MF, Tatnell JR, Ryley MJ (2007) Neocosmospora vasinfecta is pathogenic on peanut in Queensland. Australas Plant Dis Not 2:3–4
Gabriel FD, Almeida M, Albert O, Fitton-Ouhabi V, Noel T, Accoceberry I (2013) A disseminated infection with the antifungal-multiresistant teleomorphic fungus Neocosmospora vasinfecta in apatient with acute B-lymphoblastic leukemia. Med Mycol Case Rep 2:44–47
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes: application to identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gauch HG, Wentworth TR (1976) Canonical correlation analysis as an ordination technique. Vegetatio 33:17–22
Gee G, Bauder J (1986) Particle-size analysis. In Page AL (ed) Methods of soil analysis, part 1, physical and mineralogical methods, 2nd edn. Agronomy monograph 9, American Society of Agronomy, Madison, pp 383–411
Gehlker H, Scholl W (1974) Ecological factors and cultivation problems in connection with parasitic root rot of asparagus. Z Pflanzenk Pflanzen 81:394–406
Geiser DM, Jimenez-Gasco MDM, Kang S, Makalowska I, Veeraraghavan N, Ward TJ, Zhang N, Kuldau GA, O’Donnell K (2004) FUSARIUM-ID v. 1.0: a DNA sequence database for identifying Fusarium. Eur J Plant Pathol 110:473–479
Haapalainen M, Latvalab S, Kuivainena E, Qiua Y, Segerstedtb M, Hannukkalaa AO (2016) Fusarium oxysporum, F. proliferatum and F. redolens associated with basal rot of onion in Finland. Plant Pathol 65:1310–1320
Haas D, Lesch S, Buzina W, Galler H, Gutschi AM, Habib J, Pfeifer B, Luxner J, Reinthaler FF (2016) Culturable fungi in potting soils and compost. Sabouraudia 54(8):825–834
Haji-Allahverdipoor K, Bahramnejad B, Amini J (2011) Selection of molecular markers associated with resistance to Fusarium wilt disease in chickpea (Cicer arietinum L.) using multivariate statistical techniques. Aust J Crop Sci 5(13):1801–1809
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Han KS, Park JH, Back CG, Park MJ (2015) First report of Fusarium subglutinans causing leaf spot disease on Cymbidium orchids in Korea. Mycobiology 43(3):343–346
Hasanzade F, Falahati Rastegar M, Jafarpour B, Kermani M (2008) Identification of Fusarium solani f.sp. pisi the cause of root rot in chickpea and assessment its genetic diversity using AFLP in Northeast Iran. Res J Biol Sci 3(7):737–741
He L, He B, Zhao L (2014) Effect of particle size distribution of lime sludge on the hydrophobicity of paper. BioResources 9(1):1361–1372
Heidari A, Mahmoodi S, Roozitalab MH, Mermut AR (2008) Diversity of clay minerals in the vertisols of three different climatic regions in Western Iran. J Agric Sci Technol 10:269–284
Huang CH, Roberts PD, Datnoff LE (2011) Silicon suppresses Fusarium crown and root rot of tomato. J Phytopathol 159(7–8):546–54
Jalali BL, Chand H (1992) Chickpea wilt. In: Singh US, Mukhopadhyay AN, Kumar J, Chaube HS (eds) Plant diseases of international importance, Diseases of cereals and pulses, vol 1. Prentice Hall, Englewood Cliffs, pp 429–444
Jamali S (2017) First report of identification and molecular characterization of Tuber aestivum in Iran. Agrofor Syst 91(2):335–343
Jamali S, Nasimi Z (2014) First report of black-foot disease, caused by Cylindrocarpon destructans, on ornamental marigold (Tagetes minuta) in Iran. J Plant Protect Res 54(2):139–143
Jeschke N, Nelson PE, Marasas WFO (1990) Fusarium species isolated from soil samples collected at different altitudes in the Transkei, southern Africa. Mycologia 82(6):727–733
Jimenez-Fernández D, Navas-Cortés JA, Montes-Borrego M, Jiménez-Díaz RM, Landa BB (2011) Molecular and pathogenic characterization of Fusarium redolens, a new causal agent of Fusarium yellows in chickpea. Plant Dis 95:860–870
Jones JP, Woltz SS (1981) Fusarium-incited diseases of tomato and potato and their control. In: Nelson PE, Toussoun TA, Cook RJ (eds) Fusarium: Diseases, Biology and Taxonomy. Penn State University Press, University Park, pp 157–168
Kac G, Piriou P, Gueho E, Roux P, Tremoulet J, Denis M et al (1999) Osteoarthritis caused by Neocosmospora vasinfecta. Med Mycol 37:213–217
Karam A, Ranjbar M, Eftekhari M, Yaghoob Nejad Asl N (2014) Classification of morph climatic zones of Kermanshah province using cluster analysis method. Geography 11:235–256
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kirk P, Cannon P, Minter D, Stalpers J (2008) Dictionary of the Fungi. Wallingford: 771
Koyyappurath S, Atuahiva T, Le Guen R, Batina H, Le Squin S, Gautheron N et al (2016) Fusarium oxysporum f. sp. radicis-vanillae is the causal agent of root and stem rot of Vanilla. Plant Pathol 65(4):612–625
Kwon SI, Anderson AJ (2001) Laccase isozymes: production by an opportunistic pathogen, a Fusarium proliferatum isolate from wheat. Physiol Mol Plant Pathol 59(5):235–242
Landa BB, Navas-Cortés JA, Jiménez-Gasco MM, Katan J, Retig B, Jiménez-Díaz RM (2006) Temperature response of chickpea cultivars to races of Fusarium oxysporum f. sp. ciceris, causal agent of Fusarium wilt. Plant Dis 90:365–374
Latiffah Z, Azaman RS (2011) Fusarium species isolated from forest soil samples. Malays J Microbiol 7(3):171–174
Latiffah Z, Mohd Zariman M, Baharuddin S (2007) Diversity of Fusarium species in cultivated soils in Penang. Malay J Microbiol 3:27–30
Lavie S, Stotzky G (1986) Interactions between clay minerals and siderophores affect the respiration of Histoplasma capsulatum. Appl Environ Microbiol 51:74–79
Leisso R, Miller Z, Jacobsen B, Burrows M (2011) Pathogenicity of Fusarium spp. to chickpea seed and seedlings (Cicer arietinum L.). Can J Plant Pathol 33(3):400–409
Leslie JF, Summerell BA (2006) The Fusarium laboratory manual. Blackwell, Ames
Li BJ, Li PL, Li J, Chai AL, Shi YX, Xie XW (2017) First report of Fusarium root rot of solanum melongena caused by Fusariumsolani in China. Plant Dis 101(11):1956
Loeppert RH, Suarez DL (1996) Carbonate and gypsum. In: Sparks DL (ed) Methods of soil analysis, Chemical methods, Soil Science Society of America. American Society of Agronomy, Madison, pp 437–474
Lombard L, van der Merwe NA, Groenewald JZ, Crous PW (2015) Generic concepts in Nectriaceae. Stud Mycol 80:189–245
López-Bautista V, Mora-Aguilera G, Gutiérrez-Espinosa MA, Mendoza-Ramos C, Inés V, Martínez-Bustamante JJ, Acevedo-Sánchez G, Santana-Peñaloza B (2020) Morphological and molecular characterization of Fusarium spp. associated to the regional occurrence of wilt and dry bud rot in Agave tequilana. Rev Mex Fit 38(1):79–106
Mandeel QA (2006) Biodiversity of the genus Fusarium in saline soil habitats. J Basic Microbiol 46(6):480–494
Manikandan P, Vismer HF, Kredics L, Doczi I, Marasas WFO et al (2007) Corneal ulcer due to Neocosmospora vasinfecta in an immunocompetent paitent. Med Mycol 46:279–284
Manshor N, Rosli H, Ismail NA, Salleh B, Zakaria L (2012) Diversity of Fusarium species from highland areas in Malaysia. Trop life Sci Res 23(2):1
Masratul Hawa M, Salleh B, Latiffah Z (2013) Characterization and pathogenicity of Fusarium proliferatum causing stem rot of Hylocereus polyrhizus in Malaysia. Ann Appl Biol 163(2):269–280
Mohammadi H, Banihashemi Z (2005) Distribution, pathogenicity and survival of Fusarium spp. the causal agents of chickpea wilt and root rot in the Fars province of Iran. Iranian J Plant Pathol 41(4):687–708
Mohammadi H, Banihashemi Z (2006) Vegetative compatibility groups of Fusarium solani f. sp. pisi, the causal agent of chickpea black root rot in Fars Province of Iran. Iranian J Plant Pathol 42(1):179–194
Nam MH, Lee HC, Kim TI, Lee EM, Yoon HS (2018) Effect of nutrition solution pH and electrical conductivity on Fusarium wilt on strawberry plants in hydroponic culture. Res Plant Dis 24(1):26–32
Nash SM, Snyder WC (1965) Quantitative and qualitative comparisons of Fusarium populations in cultivated fields and noncultivated parent soil. Can J Bot 43:939–945
Navas-Cortés JA, Landa BB, Méndez-Rodríguez MA, Jiménez-Díaz RM (2007) Quantitative modeling of the effects of temperature and inoculum density of Fusarium oxysporum f. sp. ciceris races 0 and 5 on the development of Fusarium wilt in chickpea cultivars. Phytopathology 97:564–573
Nelson PE, Toussoun TA, Marasas WFO (1983) Fusarium species: an illustrated manual for identification. Pennsylvania State University Press, University Park
Nene YL, Sheila VK, Sharma SB (1996) A world list of chickpea and pigeonpea pathogens. ICRISAT, Hyderabad
Nourollahi KH, Aliaran A, Yonessi H (2017) Genetic diversity of F. oxysporium f. sp. ciceri isolates causal agent of wilt chickpea in Kermanshah province using microsatellite markers. Mod Genet J 11(4):605–615
O’Donnell K, Cigelnik E, Nirenberg HI (1998) Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90:465–493
O'Donnell K, Gueidan C, Sink S, Johnston PR, Crous PW, Glenn A et al (2009) A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet Biol 46:936–948
O’Donnell K, Sink S, Libeskind-Hadas R, Hulcr J, Kasson MT, Ploetz RC, Konkol JL, Ploetz JN, Carrillo D, Campbell A, Duncan RE (2015) Discordant phylogenies suggest repeated host shifts in the Fusarium–Euwallacea ambrosia beetle mutualism. Fungal Genet Biol 82:277–290
Paul EA, Clark FE (1989) Soil microbiology and biochemistry. Academic Press, San Diego
Qadir M, Qureshi AS, Cheraghi SAM (2008) Extent and characterisation of salt-affected soils in Iran and strategies for their amelioration and management. Land Degrad Dev 19(2):214–227
Rafique K, Kang S, Mahmood T, Ullah I, Mahmood H (2020) First report of vascular wilt on lentil (Lens culinaris Medikus) caused by Fusarium redolens in Pakistan. Plant Dis 104(9):2524–2524
Raja HA, Schoch CL, Hustad VP, Shearer CA, Miller AN (2011) Testing the phylogenetic utility of MCM7 in the Ascomycota. MycoKeys 1:63–94
Raper KB, Fennell DI (1965) The genus Aspergillus. Williams and Wilkins Company, Baltimore, p 686
Riccioni L, Haegi L, Valvassori M (2008) First report of vascular wilt caused by Fusarium redolens on lentil in Italy. Plant Dis 92(7):10
Rivera-Jiménez MN, Zavaleta-Mancera HA, Rebollar-Alviter A, Aguilar-Rincón VH, García-de-los-Santos G, Vaquera-Huerta H, Silva-Rojas HV (2018) Phylogenetics and histology provide insight into damping-off infections of ‘Poblano’pepper seedlings caused by Fusarium wilt in greenhouses. Mycol Prog 17(11):1237–1249
Robert V, Szoke S, Jabas B, Vu D, Chouchen O, Blom E, Cardinali G (2011) BioloMICS software: biological data management, identification, classification and statistics. Open Appl Info J 5:87–98
Ruiter PC, de Bloem J, Bouwman LA, Didden WAM, Hoenderboom GHJ, Lebbink G, Marinissen JCY, Vos JA, Vreeken-Buijs MJ, Zwart KB, Brussard L (1994) Simulation of dynamics in nitrogen mineralisation in the belowground food webs of two arable farming systems. Agric Ecosyst Environ 51:199–208
Saadi I, Laor Y, Medina S, Krassnovsky A, Raviv M (2010) Compost suppressiveness against Fusarium oxysporum was not reduced after one-year storage under various moisture and temperature conditions. Soil Biol Biochem 42:626–634
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sangalang AE, Burgess LW, Backhouse D, Duff J, Wurst H (1995) Mycogeography of Fusarium species in soils from tropical, arid and Mediterranean regions of Australia. Mycol Res 99(5):523–528
Saremi H, Burgess LW (2000) Effect of soil temperature on distribution and population dynamics of Fusarium species. J Agric Sci Technol 2:119–125
Saremi H, Saremi H (2013) Isolation of the most common Fusarium species and the effect of soil solarisation on main pathogenic species in different climatic zones of Iran. Eur J Plant Pathol 137(3):585–596
Saremi H, Backhouse D, Burgess LW (1997) Mycogeographic survey of Fusarium species in southeastern New South Wales, Australia. Cereal Res Commun 25(3):611–612
Scauflaire J, Gourgue M, Munaut F (2011) Fusarium temperatum sp. nov. from maize, an emergent species closely related to Fusarium subglutinans. Mycologia 103(3):586–597
Shadmani L, Jamali S, Fatemi A (2018) Effect of barley endophytic fungi against of two pathogenic fungi, Gaeumannomyces graminis and Pythium aphanidermatum. Biol Control Pests Plant Dis 7(2):153–158
Sharma L, Marques G (2018) Fusarium, an entomopathogen—a myth or reality? Pathogens 7(4):2–15
Short DPG, O’Donnell K, Zhang N, Juba JH, Geiser DM (2011) Widespread occurrence of diverse human pathogenic types of the fungus Fusarium detected in plumbing drains. J Clin Microbiol 49:4264–4272
Šišić A, Baćanović J, Finckh MR (2017) Endophytic Fusarium equiseti stimulates plant growth and reduces root rot disease of pea (Pisum sativum L.) caused by Fusarium avenaceum and Peyronellaea pinodella. Eur J Plant Pathol 148:271–282
Šišić A, Baćanović-Šišić J, AMS A-H, Karlovsky P, Ahmed SA, Maier W, de Hoog GS, Finckh M (2018) The ‘forma specialis’ issue in Fusarium: a case study in Fusarium solani f. sp. pisi. Sci Rep 8:1252–1269
Stahl PD, Parkin TB, Christensen M (1999) Fungal presence in paired cultivated and uncultivated soils in Central Iowa, USA. Biol Fertil Soils 29:92–97
Stefańczyk E, Sobkowiak S, Brylińska M, Śliwka J (2016) Diversity of Fusarium spp. associated with dry rot of potato tubers in Poland. Eur J Plant Pathol 145(4):871–884
Steinberg C, Edel-Hermann V, Alabouvette C, Lemanceau P (2007) Soil suppressiveness to plant diseases. In: van Elsas JD, Jansson JK, Trevors JT (eds) Modern soil microbiology, 2nd edn. CRC Press, Boca Raton, pp 455–477
Stoner MF (1981) Ecology of Fusarium in non-cultivated soil. In: Nelson PE, Toussoun TA, Cook RJ (eds) Fusarium: diseases, biology, and taxonomy. Pennsylvania State University Press, University Park, pp 276–286
Summerell BA, Laurence MH, Liew EC, Leslie JF (2010) Biogeography and phylogeography of Fusarium: a review. Fungal Divers 44(1):3–13
Summerell BA, Leslie JF, Liew EC, Laurence MH, Bullock S, Petrovic T, Bentley AR, Howard CG, Peterson SA, Walsh JL, Burgess LW (2011) Fusarium species associated with plants in Australia. Fungal Divers 46(1):1–27
Tafinta IY, Shehu K, Maishanu HM, Noma SS, Yusif SA, Umar M, Abubakar N (2018) Isolation and identification of soil Mycoflora in the upland and lowland soils of Usmanu Danfodiyo University, Sokoto, Sokoto state. S Asian J Res Microbiol 1(2):1–7
Ter Braak CJF (1988) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179
Ter Braak CJF (2003) Program CANOCO version 4.5A (1988-2003) Biometris– quantitative methods in the life and earth sciences. The Netherlands: plant research international, Wageningen University and Research Centre Wageningen
Thomas GW (1996) Soil pH and soil acidity. In: Sparks DL (ed) Methods of soil analysis: chemical methods, part 3. Soil Science Society of America, Madison
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Trabelsi R, Sellami H, Gharbi Y, Krid S, Cheffi M, Kammoun S, Dammak M, Mseddi A, Gdoura R, Triki MA (2017) Morphological and molecular characterization of Fusarium spp. associated with olive trees dieback in Tunisia. 3 Biotech 7:28
Trapero-Casas A, Jimnez-Diaz RM (1985) Fungal wilt and root rot diseases of chickpea in southern Spain. Phytopathology 75:1146–1151
Tukey JW (1953) “The problem of multiple comparisons.” In Multiple Comparisons, 1948–1983, edited by Braun HI, vol. 8 of The Collected Works of John W. Tukey (published 1994), 1–300. London: Chapman and Hall. Unpublished Manuscript
van Rijn E, Termorshuizen AJ, van Bruggen AHC (2007) Storage method affects disease suppression of flax wilt induced by composts. Soil Biol Biochem 39:2743–2749
Velarde Félix S, Valdez Rubio N, Zamora Galván F, López Molina R, Melgoza Villagómez CM, Garzón Tiznado JA (2018) Identificación molecular de Fusarium spp. aislados de maíz en Sinaloa, México. Rev Mex Cienc Agríc 9(8):1675–1689
Vujanovic V, Hamel C, Yergeau E, St-Arnaud M (2006) Biodiversity and biogeography of Fusarium species from northeastern north American asparagus fields based on microbiological and molecular approaches. Microb Ecol 51:242–255
Walkley A, Black IA (1934) An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38
Wang A, Haapalainen M, Latvala S, Edelenbos M, Johansen A (2018) Discriminant analysis of volatile organic compounds of Fusarium oxysporum f. sp. cepae and Fusarium proliferatum isolates from onions as indicators of fungal growth. Fungal Biol 122(10):1013–1022
Westerlund FV, Jr Campbell RN, Kimble KA (1974) Fungal root rots and wilt of chickpea in California. Phytopathology 64:432–436
White TJ, Bruns T, Lee SJ, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Xiao F, Zhang JZ, Tu YL (2012) First report of Fusarium oxysporum causing wilt of Dendrobium candidum in Zhejiang province, China. Plant Dis 96(9):1377
Yang JW, Nam SS, Lee HU, Choi KH, Hwang SG, Paul NC (2018) Fusarium root rot caused by Fusarium solani on sweet potato (Ipomoea batatas) in South Korea. Can J Plant Pathol 40(1):90–95
Zamani MR, Motallebi M, Harigh MJ (2001) Pectic enzyme patterns of Fusarium oxysporum virulent isolates from chickpea in Iran. J Dermatol Sci 12(1):17–21
Zamani MR, Motallebi M, Rostamian A (2004) Characterization of Iranian isolates of Fusarium oxysporum on the basis of RAPD analysis, virulence and vegetative compatibility. J Phytopathol 152:449–453
Zhang Q, Yang L, Zhang J, Wu M, Chen W, Jiang D, Li G (2015) Production of anti-fungal volatiles by non-pathogenic Fusarium oxysporum and its efficacy in suppression of verticillium wilt of cotton. Plant Soil 392:101–114
Zhao P, Luo J, Zhuang WY (2011) Practice towards DNA barcoding of the nectriaceous fungi. Fungal Divers 46:183–191
Zokaee S, Falahati Rastegar M, Jafar Poor B, Bagheri A, Jahanbakhsh Mashhadi V (2012) Genetic diversity determination of Fusarium oxysporum f. sp. ciceri the causal agent of wilting and chlorosis in chickpea by using RAPD and PCR-RFLP techniques in Razavi and northern Khorasan provinces. Iran J Pulses Res 2:7–16
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saeedi, S., Jamali, S. Molecular characterization and distribution of Fusarium isolates from uncultivated soils and chickpea plants in Iran with special reference to Fusarium redolens. J Plant Pathol 103, 167–183 (2021). https://doi.org/10.1007/s42161-020-00698-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-020-00698-w