Abstract
Biofilms pose significant challenges in various fields, including food, healthcare, and environmental industries, where they compromise safety, quality, and operational efficiency. Understanding their behavior, evaluating antimicrobial efficacy, developing control strategies, and implementing monitoring systems are crucial steps in mitigating biofilm-related risks. This review explores the integration of rheology and atomic force microscopy techniques as powerful tools for addressing these challenges. Rheological models provide insights into biofilm viscoelastic properties, aiding in monitoring and predicting their behavior under diverse environmental conditions. From bulk rheological characterizations to micro-scale measurements, studies elucidate the complex interplay between environmental factors and biofilm development, informing strategies for disinfection and product optimization. AFM enables visualization of biofilm morphology, quantification of surface roughness, and probing of mechanical interactions at the nanoscale. Integration with other analytical techniques offers comprehensive insights into biofilm structure–function relationships, guiding innovative biofilm management strategies. Current applications span antimicrobial effectiveness assessments, biofilm control strategy design, and monitoring of biofilm contamination across industries. Leveraging interdisciplinary approaches holds promising potential to deepen our understanding of biofilms and develop more effective interventions, safeguarding product quality and human health. This review underscores the pivotal role of rheology and AFM in characterizing biofilms and addressing biofilm-related challenges in these fields, where continued research and innovation are essential for advancing our understanding and enhancing control strategies.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biofilms are complex communities composed of bacteria, fungi, and other microorganisms, with a strong ability to adhere to surfaces and form protective matrices composed of extracellular polymeric substances (EPS) [1]. These matrices not only shield the microorganisms from environmental stresses but also facilitate their survival and proliferation, making biofilms a persistent threat in various natural and man-made settings. In the context of food processing, spoilage organisms and pathogenic bacteria including Escherichia, Listeria and Salmonellae are capable of forming biofilms, thereby increasing the risk of contamination throughout the production process [2, 3]. The ability of these microorganisms to colonize surfaces and resist conventional cleaning and disinfection measures poses a significant challenge to food safety protocols. Biofilm embedded actively contributes to food deterioration, leading to off-flavors, odors, and other undesirable changes, ultimately compromising the quality of food products [4]. This deterioration not only affects consumer perceptions but also has economic implications for food producers and distributors. Moreover, traditional cleaning agents and disinfectants often prove ineffective against biofilms, allowing microbial populations to persist and potentially proliferate. Another concerning aspect of biofilms is the resilience conferred by biofilm formation against antimicrobial interventions. This resilience not only compromises food safety but also increases the likelihood of developing antibiotic-resistant strains [5, 6], further exacerbating the challenge of controlling microbial contamination within food processing environments.
Biofilms represent a challenge within food processing environments due to their intricate nature and significant implications for food safety and quality [7]. Multiple factors influence their behaviors in practical settings, including bacterial strains, manufacturing conditions, surface properties, etc. [8, 9]. The ability to comprehend the composition, structure, and dynamics of biofilms is crucial for understanding their behaviors, especially within the context of food processing and handling environments [2]. Therefore, leveraging analytical techniques is indispensable for developing effective biofilm control strategies and advancing innovations in food processing and hygiene. Various techniques and strategies have been developed to study biofilms, ranging from conventional microbiological methods to sophisticated imaging and molecular techniques [10,11,12]. Microscopic techniques such as confocal laser scanning microscopy (CLSM) and scanning electron microscopy (SEM) offer high-resolution images that elucidate biofilm architecture and spatial distribution [13]. Molecular techniques such as polymerase chain reaction (PCR) and fluorescence in situ hybridization (FISH) facilitate the identification and quantification of specific microbial species within the biofilms [14]. Additionally, various biochemical assays enable the measurement of biofilm biomass, metabolic activity, and the composition of extracellular substances [15].
This review focuses on rheology and atomic force microscopy (AFM) as two contemporary tools with distinct advantages in characterizing biofilms. Rheology, the study of material flow and deformation, provides valuable insights into the mechanical properties of biofilms. By quantifying the viscosity, elasticity, and viscoelastic properties of biofilms, rheology helps in understanding their structural integrity and response to external forces [16]. AFM, a high-resolution imaging technique, allows the visualization and measurement of surface properties at the nanoscale, including surface roughness and topography of biofilms [17]. Additionally, AFM facilitates probing of mechanical properties such as Young's modulus and adhesive forces by using specialized cantilevers [18, 19]. Rheology provides a macroscopic understanding of biofilm behavior, offering insights into their overall mechanical properties and interactions with external environments [20, 21]. Meanwhile, AFM offers a detailed view of the structural organization of biofilm surfaces, allowing researchers to examine surface and mechanical characteristics with precision. Integrating these techniques allows researchers to gain comprehensive insights into biofilms, facilitating the development of effective strategies for biofilm prevention and control across various industries, including food processing. These interdisciplinary approaches enhance our understanding of biofilm dynamics and support the advancement of innovations in food processing and related fields.
2 Biofilm
2.1 Biofilm development and environmental factors
Biofilms are an accumulation or aggregation of growing bacteria and their secretions on surfaces [22]. The formation of biofilms serves as an important survival strategy for microorganisms because bacterial cells in biofilms are more resistant to disinfectants or antimicrobial agents than planktonic bacteria [23]. The formation and development of biofilm are highly dependent on the bacterial strain, material surface properties, and environmental parameters [24]. Generally, the development of biofilm can be divided into 5 stages: initial attachment (reversible attachment), irreversible attachment, microcolony formation, maturation, and dispersion, as illustrated in Fig. 1(A).
A Scheme of biofilm formation and development; B Bulk rheological strategies using a rheometer to measure the rheology of biofilms; C Representative stress–strain curve of an S. aureus biofilm microcolony exhibiting a characteristic “J” shape [25] (Adapted and reproduced with copyright permission from American Society for Microbiology); D Creep curve of an S. aureus biofilm microcolony [25] (Adapted and reproduced with copyright permission from Royal Society of Chemistry)
Initial attachment
The initial attachment of bacteria can be active or passive, depending largely on the physicochemical properties of the bacterial cell surface [4]. Bacteria produce small amounts of EPS and move freely by pilus-mediated twitching [26]. Attachment is reversible at this stage, with bacterial cells retaining their morphology and the ability to detach and return to the planktonic stage [27]. Surface properties such as texture, roughness, charge, hydrophobicity, pH, and temperature also significantly affect the attachment [24, 28, 29]. For instance, Salmonellae and Listeria were shown to be more likely to adhere and form biofilms on hydrophobic surfaces than hydrophilic ones. Additionally, studies have shown that Escherichia coli (E. coli) biofilms developed faster when cultured in low-nutrient media by increasing cell adhesion on surfaces [30]
Irreversible attachment
The transition from reversible to irreversible attachment marks a subtle but crucial shift from weak interactions to permanent binding mediated by EPS [27]. At this stage, biofilm removal becomes challenging and requires strong shear forces or the introduction of chemical agents (such as enzymes, detergents, surfactants, and sanitizers) or heat treatments [31,32,33]. The EPS matrix was shown to facilitate the formation of microcolonies and enhance biofilm maturation by promoting cell-to-cell adhesion and providing structural stability [34, 35].
Microcolony formation
This stage, also known as the early development of biofilm structure, involves the mass production of EPS, which strengthens the bond between bacterial cells and stabilizes the colony against environmental stress [36]. Quorum sensing facilitates intercellular communication, leading to the recruitment of planktonic cells from the surrounding media [37]. Type IV pili play a crucial role in biofilm formation, particularly for Gram-negative bacteria. The swimming motility enabled by these pili is essential for overcoming the repulsive forces at the interface. This motility allows microorganisms to clump together and form microcolonies, which in turn facilitate substrate exchange between species and the mutual removal of by-products from bacterial cells [38, 39].
Maturation
During the maturation stage, bacterial cells aggregate together and undergo a differentiation process, resulting in a morphological transformation of the microcolony into an organized structure, which can be flat or mushroom-shaped [36]. In addition to morphological changes, gene expression in bacteria within mature biofilms is altered significantly. In a study conducted by Whiteley, the genes of planktonic Pseudomonas aeruginosa (P. aeruginosa) and cells in its mature biofilm were compared by DNA microarray technology. The results showed that more than 70 genes encoding proteins involved in the translation, metabolism, and gene regulation were changed [40].
Dispersion
Dispersion represents the final stage in the biofilm formation cycle, which allows the bacterial cells to re-enter the media and transform into the planktonic phase [41]. External interferences such as increased shear force or reduced nutrient ingredients, as well as internal processes like endogenous enzymatic degradation or the releasing of surface binding proteins, can trigger detachment of biofilm. This enables bacterial cells to colonize new niches in search of a nutrient-rich environment or to contaminate other surfaces [42,43,44,45].
Biofilm formation is a dynamic process influenced by a variety of environmental factors, with temperature, pH, and nutrient levels crucially affecting their survival and persistence in various environments. Each microorganism has an optimal temperature range, and many pathogenic bacteria form biofilms most effectively at body temperature (37 °C). Nguyen et al. [46] demonstrated that Salmonella Typhimurium formed biofilms more rapidly at higher temperatures ranging from 28 °C to 42 °C, indicating the impact of temperature on metabolic rates and biofilm development. In marine settings, Rao found that a 5 °C rise in water temperature significantly increased biofilm thickness and biomass, suggesting that temperature also affects the structure and long-term stability of the biofilm [47]. The pH can affect the stability of the EPS matrix, which is crucial for biofilm integrity. Most bacteria prefer neutral to slightly alkaline conditions (pH 6.5–7.5), while some fungi and acidophilic bacteria thrive in more acidic environments. A study by Speranza et al. [48] has shown that pH 6.0 was optimal for biofilm formation by Salmonella sp., with reduced formation at more acidic or alkaline pH levels. Similarly, Iliadis et al. [49] found that biofilm formation was favored under low salinity conditions as pH increased, with an optimal pH around 7.0. Nutrient availability is another critical determinant of biofilm formation dynamics. Microorganisms tend to form more biofilms under nutrient-deficient conditions as a survival strategy [50]. Speranza et al. found that nutrient-poor media (1.0 to 1.5 g/L of peptone) led to increased biofilm formation by Salmonella sp [48]. Another study by Roy et al. further showed that glucose supplementation influenced biofilm formation by Salmonella enterica serotype Kentucky, with 0.025% glucose inducing biofilm formation at 37 °C and pH 7.0, while 0.4% glucose inhibited it [51]. Understanding and manipulating these factors are critical steps toward developing control strategies to prevent biofilm development in food processing and other industrial settings.
2.2 Biofilm-related food safety concerns and control strategies
Biofilms pose significant food safety concerns, as they enable the attachment and growth of pathogenic bacteria on food contact surfaces, thereby increasing the risk of contamination. Since the middle of last century, when Salmonella adhesion was first identified as a foodborne bacterial biofilm [52], numerous studies have reported biofilms in various food industries. Examples include Bacillus cereus and Shigella sp. in dairy processing facilities, Pseudomonas and Listeria in shrimp factories, and Enterobacteriaceae in fish factories [53,54,55]. Among all food products, fresh produce presents a particularly high risk of contamination and disease transmission since they are often consumed raw. While washing is an essential step to prolong the shelf life of fresh produce, disinfection typically results in a reduction of bacteria by only 2–3 logs during the process [56, 57]. The inefficacy of this process is due to the potential presence of bacteria hidden in plant tissue or self-formed biofilms [58].
During produce manufacturing, various processing steps such as trimming, cutting, rinsing, dewatering, and packaging can act as primary sources of cross-contamination [59]. Packing, the last step of processing, is critical and requires careful regulation to prevent re-contamination and guarantee the quality of fresh produce. For instance, a cantaloupe-related outbreak in 2011 caused by Listeria monocytogenes (L. monocytogenes) was traced back to unsanitary conditions in the packing house. The existence of biofilms in production lines contributes to disease transmission and foodborne illness [60]. The presence of the same bacteria strains on surfaces like conveyor belts and drying areas indicated firm bacterial attachment in inaccessible places [61]. Therefore, it is essential to develop cleaning and disinfection methods and control systems for food processing to prevent biofilm cross-contamination throughout the processing chain.
To reduce the risk of contamination, preventing biofilm formation by avoiding firm attachment to contact surfaces is crucial [62]. Disinfectants and antimicrobials are often used to kill bacteria to further reduce the density of microorganisms on the surface since a simple cleaning process only removes 90% of the bacteria and does not prevent cell reattachment [63]. However, conventional disinfectants such as ozone, chlorine, hydrogen peroxide, peroxyacetic acid, and iodine have been reported to be ineffective against bacterial biofilms [64]. For instance, E. coli could survive in a 20,000-ppm chlorine solution and grow to a level of 6 log colony forming units (CFU) [65]. In another study, cantaloupes pre-inoculated with E. coli 766, were soaked into 1% hydrogen peroxide solution for 2 h, but this resulted in no increase in efficacy of this disinfectant and only 1 log CFU decrease [66]. A good cleaning process should not only remove the residues or compounds that may promote proliferation or biofilm formation but also break up the EPS matrix and avoid biofilm reattachment [67]. Another effective approach is to remove bacteria before biofilm development or inhibit microbial adhesion by customizing the physicochemical properties of surface materials. For instance, modification of the contact surface could increase the disinfection efficacy, such as the pre-coating of silver or the use of de-adhesion surfactants [68, 69]. Furthermore, efficient methods for removing or controlling biofilms are developed by combining various disciplines after analyzing the properties of biofilms comprehensively. Therefore, a holistic study and understanding of the physicochemical properties and behaviors of biofilms are essential.
3 Rheology
The microorganisms that form the biofilm are bound together by a protective matrix consisting of EPS, including polysaccharides, proteins, nucleic acids, and lipids. EPS is composed of 50–90% of the total organic matter in the biofilm and forms a highly hydrated viscoelastic gel that provides additional flexibility to cope with environmental stresses [1]. As biofilm has been widely recognized as a living structure, its structural and mechanical properties change over time and are highly dependent on growth conditions such as temperature, pH, nutrition status, hydrophobicity of contact surface, flow rate, and oxygen content of the fluid medium, which have been summarized and discussed elsewhere before [16, 70,71,72,73]. Biofilm is a multiscale assembled viscoelastic material that conventionally can be treated as a homogeneous substance and exhibits greater mechanical stability against shear flow fragmentation [20]. Bulk rheological devices, such as rheometers, are commonly used to characterize the macroscopic mechanical properties of biofilms because they are relatively easy to operate and widely available [74]. However, biofilm, formed by cells embedded in an EPS matrix, can also be considered a heterogeneous composite due to the presence of EPS-formed pores and channels, which allow the circulation of nutrient-rich fluids and the removal of waste products. This heterogeneity is highly correlated with local microstructure and can be influenced by the nutrient and oxygen gradients, leading to distinct differences in mechanical and viscoelastic properties [75]. The complexity of biofilms endows them with unique properties and structures but also poses challenges for the study of their mechanical and viscoelastic properties. The choice of the test and measurement device highly depends on the type and location of biofilm and the scale of parameters involved, so it is important to choose appropriate techniques to assess their mechanical and viscoelastic properties.
3.1 Bulk rheological strategies
The macro-mechanical and viscoelastic properties of biofilm have been studied over the past few decades using conventional bulk rheological techniques [76]. As illustrated in Fig. 1(B), the analysis methods can be roughly divided into two different categories based on the direction of the applied forces: indentation (axial force) or shear (transverse force) [77, 78]. For the mechanical indentation method, where a normal force that can be either compression (pushing) or tension (pulling) is applied to the surface of the biofilm, then the force and displacement of the biofilm matrix are recorded by a rheometer. By calculating the slope of the resulting force–displacement cure, Young’s modulus (E), which is the indicator of stiffness (elasticity), is determined [79]. The shear method that can measure the deformation of biofilm is further divided into two categories: spinning plate system or flowing liquid system (will be discussed in Sect. 3.2) based on the format of the forces and applications. In a spinning plate system, a parallel plate probe is brought into contact with the biofilm that is either transferred/grown on the base of the rheometer. The applied forces can be either rotational or oscillatory and the applied strains or stresses can be either constant or varying. In the stress–strain test, one of the most commonly used rheological tests, the biofilm samples are subjected to shear stress loading for a relatively short period, followed by an unloading process. The resulting curve usually exhibits a J-shaped hysteresis loop (Fig. 1(C)), indicating that viscous flow and mechanical energy dissipation have occurred, as the sample under test will never return to its original state [25]. To investigate the recovery behavior of biofilms, creep tests are performed. Biofilms located inside the flow cell are exposed to shear stress for a long period of time and then the stress is removed to observe their recovery behavior. As shown in Fig. 1(D), due to their inherent viscoelastic, the creep curves for biofilm typically display five characteristic regions: a) an immediate elastic deformation; b) a transition to a nonlinear viscous response; c) a steady-state viscous flow with constant viscosity; d) the initiation of elastic recoil caused by the removal of stress; e) a residual deformation due to the fluid-behavior [80]. The creep test also allows the calculation of the viscosity (η) and the shear modulus (G), which describes the degree to which the material can resist deformation and represents the stiffness of the material (similar to Young's modulus). In addition to the aforementioned tests, the spinning plate system can be used for oscillation analyses, where the stress or strain is cyclically oscillated in a sinusoidal manner at a steady or changing frequency (characterize the dynamic behavior of biofilms in response to periodic loading). Using the corresponding phase shift, the storage modulus (G’, energy that can be stored) and loss modulus (G”, energy that is lost) that describe the rigidity and fluidity of a material can be quantified [81].
3.2 Dynamic rheological strategies (in situ)
The utilization of conventional bulk techniques to characterize the rheological properties of biofilms processes certain drawbacks, such as disruption of biofilm integrity during measurements, undesirable disturbances during transport from the medium to the measurement equipment, and discrepancies in mechanical characteristics related to differences between natural and experimental conditions [82]. To address this situation, various in situ techniques have been introduced. As shown in Fig. 2(A), the flowing liquid system in which biofilms are grown within a channel with a constant flow of nutrients is often used in conjunction with time-lapse microscopy to study the growth of biofilms. In this measurement, the liquid flow that provides a continuous supply of nutrients to the bacteria can be regulated so that the force applied to the biofilm can be controlled, and therefore the response of the biofilm to the shear rate can be determined by image analysis of the biofilm deformation, and ultimately the shear modulus can be calculated [83]. The most significant advantage of these measurements is that all data are performed and collected in situ and biofilms are formed under more realistic conditions, indicating that biofilms maintain their heterogeneity and complexity during the growth process. However, the imperfection of the techniques is that the strain of biofilm is measured by the deformation of the streamers growing off the biofilm. With the onset of continuous hydrodynamic flow, the biofilm generates an elongated and flow-mediated structure called a “streamer” that is typically tethered to the surface of the biofilm at one end, while the rest of the slender soft structure floats in the flowing fluid. Although streamers are derived from and share a similar composition with biofilm, researchers have found that streamers possess unique mechanical properties, viscoelastic behavior, growth kinetics, and flowing/clogging dynamics [84]. It has been revealed that the formation of streamers is faster than the development of flat biofilm, leading to the rapid formation of problematic clogging in the channel and pipes [85]. Due to the continuous breakage of the streamer, a significant residual material transport was observed downstream of the biofilm, which could lead to the transmission of pathogens and cross-contamination of drinking water [86]. Thus, the flowing liquid system may not be suitable for all conditions. In order to mimic the growth process, biofilms are incubated within a rheometer, thus allowing real-time measurement of structural specifications during substrate development. By combining the rheometer with a bioreactor, the mechanical behavior of bacterial biofilms in response to various types of physical stimuli can be assessed instantaneously. Pavlovsky et al. developed a method to characterize in situ the rheological properties of Staphylococcus epidermidis biofilms by loading a continuously fed bioreactor into a parallel plate rheometer. In this case, the small-amplitude oscillatory rheology and creep rheology of the biofilm can be determined due to the elimination of the deformation produced by transferring the sample [87].
In recent years, interfacial rheology has been introduced as a new technique to complement the methods used to study bacterial adhesion and network formation. Unlike traditional rheology, which is considered to study the flow of substances, interfacial rheology addresses the flow behavior between two immiscible phases, such as water–oil or water–air [88]. The double wall-ring flow cell is one of the most common devices for determining the real-time interfacial rheology of biofilms, especially those located at the air–water interface. As shown in Fig. 2(A), the Teflon flow cell is filled with media, and the du Noüy ring is located at the air–liquid interface. The growth medium is added or removed from the outer chamber to compensate for evaporative losses during the experiment or to keep the liquid level of the inner and outer chambers constant throughout the experiment without disturbing the biofilm. The shear stress and strain applied to the interface are calculated by the required torque of the oscillating du Noüy ring and its angular displacement, respectively [89]. In general, the modulus of elasticity gradually increases after inoculation, which is attributed to bacterial colonization and early formation of EPS. The subsequent explosive increase in the elastic modulus represents an accelerated production and accumulation of EPS. After a slight increase in viscosity, the elastic modulus returns to a steady increase and begins to decrease after the nutrients in the medium are depleted [89,90,91]. However, this trend is not invariant and is correlated with the matrix components in biofilm formation. In a recent study, two bacteria with different rates of film formation were inoculated together into a double wall-ring flow cell. The results showed that Vibrio cholerae, which formed biofilms about 15–18 h faster than E. coli, inhibited the formation of wrinkled structures exclusive to E. coli species [92].
As shown in Fig. 2(A), the rotating biconical rheometer is another commonly used device in recent years to determine the interfacial rheology of biofilms located at the air–liquid or liquid–liquid interface [93,94,95,96]. The basic setup of this equipment is similar to a double-wall ring flow cell. Information about the viscoelastic energy is obtained by sinusoidal oscillations of a double cone located at the interface of two immiscible phases. A glass measuring cell is used as a bioreactor, with conditions can be precisely controlled and modified. For example, Bertsch et al. demonstrated that kombucha biofilms at the air/tea interface have two growth phases: an initial bacterial uptake determined by bacterial content and species, and a final EPS secretion influenced by the nutrient content of the medium [97]. In a study conducted by Rühs et al. [98], the attachment of bacteria at the water–oil interface and the growth of biofilms over time were monitored and recorded to investigate the application of biofilms to wastewater treatment, because previous studies have shown that some bacteria could aggregate at the oil–water interface of emulsions and use crude oil as a carbon source to degrade alkanes and polycyclic aromatic compounds into harmless by-products while enhancing carbon uptake by forming a biofilm [99,100,101]. In a more recent study, Subbiahdoss et al. investigated the mechanism of bacterial absorption and biofilm formation at the water/oil interface of three bacteria (P.aeruginosa, Staphylococcus aureus (S. aureus), and Staphylococcus epidermidis (S. epidermidis)) [102]. The results showed that bacteria with higher hydrophobicity (P. aeruginosa) tended to accumulate rapidly at the water/oil interface, S. epidermidis, which had negligible hydrophobicity, formed the most resilient biofilm because of its ability to secrete biosurfactants [91, 102, 103]. To sum up, biofilm formation is not solely determined by cell hydrophobicity, but the secretion of biosurfactants and the metabolism of the interface also play an important role.
3.3 Micro-rheology
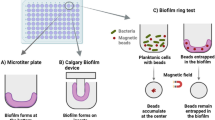
The mechanical properties of biofilms are spatially heterogeneous and highly correlated with microstructural organization. Most of traditional mechanical studies consider biofilm as a homogeneous material and analyze its mechanical responses averaged over the whole sample, which smooths out its heterogeneous as the micro properties may be significantly different from macro properties [74]. Thus, micro or nano-scale measurements are required to fix this situation. Micro-rheology is a powerful tool, which provides detailed, local mechanical information about biofilms, capturing spatial heterogeneity in mechanical properties that traditional bulk measurements might miss [104]. In this method, beads of glass, steel, or any other material (which can be easily seen under the microscope within the biofilm matrix) are added to the biofilm and tracked throughout the growth process (biofilm heterogeneity). By tracking individual beads or particles within the biofilm matrix, micro-rheology can detect subtle changes in viscoelastic properties due to factors like genotype variations, EPS composition, and environmental influences. Importantly, it also allows for non-destructive measurements of biofilm mechanical responses [105], preserving the biofilm structure and providing an accurate simulation of its behavior under natural conditions. Such capabilities are essential for studying real-time mechanical adaptations and responses to external stimuli such as stress and nutrient fluctuations.
As illustrated in Fig. 2(B), passive micro-rheology uses thermally driven bead motion (Brownian motion) to probe its local microenvironment. In this approach, the shear compliance of the biofilm can be calculated using the mean square displacements of individual beads, which are tracked and analyzed by the microscope and software. Up to now, this method has been used to characterize biofilms of various bacteria and investigate the interactions between bacteria and their biofilm [83, 106]. For example, Chew et al. employed particle tracking micro-rheology to measure the temporal and spatial variation of stiffness of the biofilms formed with different P.aeruginosa genotypes [107]. The results showed that the viscoelastic properties of biofilms were closely related to the type of EPS produced and could affect the survival of the species. EPS with effective cross-linking and enhanced elasticity promoted the formation of microcolonies, while a more viscous matrix facilitated biofilm spreading [107]. In addition to using external beads, the bacteria themselves could be used as passive trackers to measure the viscoelasticity of the biofilm when the environment was precisely controlled [108]. Although passive micro-rheology provides valuable information on the viscoelastic properties of heterogeneous biofilms as it is perceived by individual cell motility, its application to motile cells is not as applicable as to non-motile cells because it cannot distinguish between thermally induced movement and active bacterial motion.
In recent studies, active micro-rheology, which measures the viscoelasticity of biofilms by artificially introducing tunable external forces to passive micro-rheology, has gained widespread attention. As shown in Fig. 2(B), using magnetic or optical tweezers to control and move (stress) the beads that are embedded in EPS, the strain response to these stresses can be recorded and analyzed to reveal the mechanical properties of biofilms and the spatial distribution of all the small changes that occur in this structure [83]. In the study conducted by Zrelli, magnetic beads with a diameter of approximately 2.8 μm were incorporated into E. coli biofilms to study the mechanical integrity of their EPS structure after treatment. The results indicated that antibiotics that disrupted DNA replication and membrane assembly killed the majority of bacteria, but did not alter the mechanical properties of the biofilm. This technique has also been used to study the spatial heterogeneity of biofilms at the micro-scale, as it allows researchers to fine-tune the applied force at any location within the biofilm structure and derive the associated creep profile by measuring the deflection of the inserted particles. Relevant experimental results show that shear compliance appears to vary dramatically from location to location, and viscoelastic heterogeneity is influenced by environmental conditions, such as nutrient levels, oxygen concentration, salt, and flow rate [109,110,111].
3.4 Microfluidic rheometry
Traditional methods for investigating biofilm rheology are low throughput and difficult to achieve temporal and spatial control of biofilm community formation [112]. In the last decade, microfluidics, as a promising tool, has become an integral part of biofilm rheology techniques because it can be used to study the effects of various types of materials on bacterial movement as well as adhesion through in vitro mimicry [80]. This approximation is achieved with three-dimensional cultures made by embedding microorganisms in hydrogels used to mimic the extracellular matrix while mimicking the interstitial flow that transports nutrients and other microorganisms by introducing flow fields [113]. The use of artificial microdevices involves the use of small volumes, saving reagents, biological materials, residues, and space. In addition, the ability to better control environmental conditions during experiments, such as precise modification of physical (stiffness, pressure, fluid flow, etc.) and chemical (pH, ion strength, etc.) conditions, makes it easier to study the adaptation of biofilms to environmental changes in a high throughput manner [114]. In a study conducted by Hohne, approximately 200 pL of the test solution was injected into the bottom of the microfluidic chamber (Fig. 2(C) [115]. A soft membrane was used to separate the fluid chamber from the upper air chamber, and the flow rate of air could be adjusted to apply different forces to the membrane and subsequently to the test sample. The corresponding mechanical strain was then determined by measuring the deflection of the soft membrane using a confocal laser scanning microscope (CLSM). Measurements of the linear elastic moduli and viscoelastic relaxation times of the gellan gum solutions using this microdevice agree well with the results obtained by conventional mechanical rheometers. This microdevice also allowed for the first time to estimate Young's moduli and relaxation times of biofilms formed by S. epidermidis and Klebsiella pneumoniae. In addition, a hardening behavior (increased rigidity) was observed when biofilms were subjected to higher airflow rates [115]. As mentioned earlier, biofilms are influenced by fluids (for nutrient supply), but they can in turn influence flow. For example, when biofilms almost block the porous medium, a strong intermittent inflow occurs, manifested by a rapid opening and slow closing of individual preferential flow paths (PFPs) [116, 117]. In a more recent study, microfluidic chips were recruited to investigate the mechanistic origin of intermittency [118]. The results show that the closure of PFPs is driven by microbial growth and controlled by nutrient mass flow. In contrast, the rapid opening of PFPs is driven by flow-induced shear stress that increases with microbial growth, leading to compression and rupture of the biofilm [118]. Although microfluidic devices have been widely used to probe the mechanical properties of various materials, few of them have been specifically used to measure the rheological properties of bacterial biofilms. Therefore, more investigations are needed to explore the full potential of microfluidic devices for rheological applications.
4 AFM
AFM has been one of the most important tools in the study and analysis of nanotechnology since its inception in 1986, evolving from the scanning tunneling microscope (STM) [119]. Particularly noteworthy is its role at the intersection of microbiology and nanoscience, where AFM has garnered considerable attention in the realm of nanoscale biofilm analysis [11, 120, 121]. Serving as a vital surface visualization technique, AFM not only captures the three-dimensional (3D) microscopic morphology of biofilms and reveals the surface characteristics but also enables quantitative measurement of mechanical properties and interaction forces through nanoindentation. Table 1 summarizes specific examples of systematic biofilm studies, showcasing the functionalities that enable such research, which were also demonstrated in previous comprehensive reviews [122,123,124]. Recently, AFM methodologies and modalities have undergone continuous innovation and have been synergized with modern techniques, such as confocal fluorescence microscopy and various spectroscopy methods [125]. Thus, AFM has emerged as an invaluable tool for broader microbial investigations at the biofilm level, encompassing dynamic characterization of bacterial cell surface components and EPS.
The fundamental principle of AFM is its intricate interactions between their components, as illustrated in Fig. 3(A), leading to the precise probing of sample surfaces at the atomic level [137]. The core of AFM comprises a cantilever, a slender beam, and a sharp tiny tip. The tip is usually made of materials such as silicon or silicon nitride and has a nanoscale size that allows it to interact closely with the sample under test. During operation, the cantilever is positioned in close proximity to the sample surface, and forces between the tip and the atoms of samples cause the cantilever to deflect. These deflections are meticulously tracked using a laser beam reflected from the cantilever onto a position-sensitive photodetector, allowing precise measurement of the forces. As the tip traverses the surface, its interactions with the sample induce variations in the deflection of the cantilever. These variations are meticulously recorded, generating a detailed topographic map of the surface features with remarkable spatial resolution. Moreover, by analyzing the forces required to maintain the cantilever at a constant distance from the sample surface, AFM can provide insights into the mechanical properties of the sample, such as its stiffness or adhesive characteristics [138].
A Scheme of AFM working principle; B AFM morphology and topography of biofilm layer formed on 304 SS substrates after 3, 14, and 21 days of exposure in Pseudomonas-containing medium, respectively [139] (Reproduced with copyright permission from De Gruyter)
One of the distinguishing features of AFM is its versatility in operating environments. Unlike many other microscopy techniques that require vacuum conditions, AFM can function robustly in ambient air or even in liquid environments [140]. This adaptability makes AFM particularly suited for studying, including biofilms, which are often best to be examined in their native habitats. This also facilitates the sample preparation process for AFM, avoiding the need for extensive dehydration and metal coating. These attractive advantages have led to the rapid development of biofilm investigations with AFM in recent years. However, along with the growing interest in biofilm research, several limitations and challenges have come to the fore. These include the need to ensure the integrity and authenticity of the biofilms under study, as well as the use of AFM to obtain comprehensive chemical information [17, 141]. Given these considerations, it’s imperative to develop and implement the appropriate strategies tailored to the specific requirements of biofilm exploration using AFM.
4.1 Morphology and topography
AFM has widespread application in biofilm investigations, particularly in visualizing the surface morphology of biofilms to obtain high-resolution images and elucidate their characteristics. This technique has been extensively utilized across various species of biofilms and related processes. For instance, AFM has been instrumental in studying the morphological changes induced by sanitizers and other environmental conditions [131, 142]. Additionally, it has facilitated investigations into the diverse physiological states exhibited during different stages of biofilm formation by comparing the surfaces of bare substrates with those hosting biofilms [143]. Figure 3(B) illustrates the surface morphology and three-dimensional images of biofilm layers provided by AFM. Typically, AFM operates with the tip in contact with the surface, as this mode offers direct imaging capabilities. However, when studying biofilms, this approach can pose challenges. Contact with the surface may potentially damage the biofilm, leading to alterations in its structure and the risk of bacterial deposition on the tip [144]. To mitigate these risks, dynamic modes, including non-contact mode and tapping mode, are often employed. Among these dynamic modes, tapping mode (also known as semi-contact mode) is favored for its advantages. It offers faster scanning speeds and eliminates issues arising from moisture interference and lateral force effects from the tip. Moreover, the tapping mode facilitates the detection of bacteria and changes in surface topography, enhancing its suitability for biofilm analysis [145].
As with other microscopes, sample preparation is a critical aspect of AFM that directly influences the authenticity of the obtained results. While air drying serves as a straightforward and commonly employed method in numerous studies [146, 147], imaging hydrated diffuse biofilms presents significant challenges without proper immobilization. The methods for immobilizing biofilms in AFM experiments vary, broadly falling into two categories: chemical fixation and mechanical entrapment. Chemical fixation methods entail the use of substances such as poly-lysine [148, 149], glutaraldehyde [150], cross-linking carboxyl groups [151], and gelatin [152]. For the mechanical entrapment, early work employed membranes with pore diameters akin to the bacteria cell dimensions [153], which have since evolved to encompass more complex or functionalized surfaces, such as lithographically patterned substrates [154]. These methods allow imaging of ultrastructural features of microorganisms, such as fimbriae or flagella [155], and are frequently utilized to observe the dynamics of bacterial growth [156] or biofilm formation [157]. However, immobilization often emerges as a challenging step in imaging, as the method must strike a balance between withstanding lateral forces and avoiding inducing physiological and nanomechanical changes in biofilms [158]. Consequently, despite advancements, challenges still persist in real-time and further microscopic biofilm studies due to the varied morphologies encountered. Further exploration is needed to overcome these limitations.
Imaging cellular microscopic features using AFM encompasses both air and liquid environments. While AFM studies of biofilms typically prioritize obtaining morphological information about surface features [159, 160], imaging in air is more commonly employed due to its convenience and typically higher resolution compared to imaging in liquid [122]. However, a drawback of air imaging is the limitation to acquiring static images, coupled with the inevitable changes in biofilms resulting from the water loss-induced hardening process during air drying. For AFM studies with specific requirements, biofilms have been studied in the aqueous environment [161] in an attempt to understand their more realistic properties. For instance, in the study on drinking water-associated biofilms [162], AFM was utilized to successfully observe the three-dimensional topography and microstructure of mixed culture drinking water biofilms both in liquid and air. Notably, the results showed that the resolution in liquid is comparable to that in air and that continuous imaging in liquid is possible without fixing the sample, losing spatial resolution, or damaging the sample [162].
4.2 Surface roughness
In addition to the visual morphological features, AFM images can provide a variety of mechanical properties of biofilm. As previously discussed regarding the operation of AFM, the image produced by AFM is not merely a visual representation but rather a dataset obtained through scanning and analysis. From the dataset of 3D surface topography, various quantitative height (amplitude) parameters can be derived. Among these height parameters, the commonly utilized metrics for characterizing biofilms include surface roughness, denoted as Sa, and Sq, which can be calculated using the following Eq. (1) and (2).
Sa represents the average roughness, while Sq represents the root mean square roughness (RMS), both serving as comprehensive measures of the texture comprising the biofilm surface [163]. Sa is particularly effective in directly evaluating bacterial attachments to the surface [164]. However, it's noteworthy that studies have indicated a potential lack of correlation between surface roughness and bacterial attachments [165]. Additionally, it's important to acknowledge that Sa or Sq may be misleading due to their insensitivity in distinguishing between peaks, valleys, and the spacing of various texture features [166]. Nevertheless, despite their limitations, these parameters remain useful and widely employed for evaluating biofilm surface roughness. In the research work by Ammar et al., the authors modeled and investigated how the surface roughness of the substrate and biofilm affects bacterial adhesion, utilizing average roughness and RMS obtained from AFM statistical parameters analysis [167]. Furthermore, in the AFM study by Mahto & Das, RMS was employed as a crucial roughness parameter to assess the biofilm formation ability of P.aeruginosa on various substrates such as glass, polystyrene, steel, ceramics, and rubber [126].
4.3 Surface charge
In biofilm-related studies, surface potential emerges as a critical property associated with biofilm adhesion to substrate surfaces [168]. Advancements in AFM modes, particularly electrostatic force microscopy (EFM) and Kelvin probe force microscopy (KPFM), have proven invaluable for measuring surface contact potential differences at the nanoscale, even in liquid environments [169, 170]. These techniques offer new insights into the electrical characteristics of biofilms, enhancing our understanding of their behavior in real-world conditions. Simultaneous acquisition of topography and surface potential maps enables visualization of differences in surface potential among biofilms on different material substrates. In the study by Birkenhauer & Neethirajan, KPFM mode was utilized to investigate changes in the bacterial surface potential of Pseudomonas aeruginosa and methicillin-resistant S.aureus (MRSA) on positively charged poly-L-lysine and negatively charged gold surfaces [134]. Figure 4 illustrates the topography and surface potential maps of P. aeruginosa in their study. Insights gleaned from the electrical dynamics of the surface and its impact on bacterial adhesion through KPFM mode can inform strategies to prevent microbial adhesion and the formation of recalcitrant biofilms [134]. Moreover, potential changes on the biofilm surface obtained via EFM or KPFM mode can contribute to understanding the interaction of nanoparticles with biofilms. In research conducted by Ikuma et al. concerning the deposition of nanoparticles (NP) onto coated surfaces with polysaccharides, the major component of EPS, results indicated that NP deposition is primarily governed by electrostatic forces, with surface charge distribution contributing to different NP deposition behaviors [171]. These findings underscore the potential application of the electrical properties of biofilms obtained from AFM modeling in the study of biofilm adhesion and formation.
A Topography and surface potential maps of P. aeruginosa on poly-L-lysine coated stainless steel surfaces; B Topography and surface potential maps of P. aeruginosa on gold surfaces. [134] (Reproduced with copyright permission from Royal Society of Chemistry)
4.4 Force-distance curve
The AFM is capable of assessing the interaction between its tip and the surface of biofilms through the force-distance curve obtained via force spectroscopy. This curve is generated by measuring the change in cantilever deflection, which reflects the force applied to a specific point on the specimen. This unique capability of AFM has introduced a novel approach to investigating the mechanical properties, such as adhesion and elasticity, as well as the interactions of biofilms [172]. To analyze forces and interactions, researchers commonly utilized three experimental designs for biofilms. The first method involves adhering bacterial cells to the substrate and directly probing them using the tip. The second method entails attaching specific molecules to the cantilever. The third method involves affixing bacterial cells to the cantilever and then probing the substrate or biofilm. By analyzing the slope of the force-distance curve, Young’s modulus (E) can be determined, providing insight into biofilm stiffness and elasticity [173]. Several studies have utilized the force-distance curve by AFM to investigate adhesion and elasticity parameters, as well as to assess cell-substrate/cell–cell interaction forces during biofilm formation. For instance, in the study by Q. Huang et al., AFM was employed to examine the forces between bacteria and goethite in water, shedding light on the mechanism of bacterial interaction with mineral surfaces during biofilm formation in environmental settings [146]. Figure 5 presents the representative force-distance curves between E. coli and goethite, along with a summary of the maximum adsorption force demonstrated by the results. Similarly, Wang et al. utilized the force-distance curve to explore the role of capsular polysaccharides during biofilm formation of Klebsiella pneumoniae [174]. They developed a mechanical model to fit the four-stage force curves to the cell-tip interaction theory, providing insights into the biofilm layer. In addition, another study demonstrated that AFM force-distance curves were employed to show the adhesion force at the cell-cell interface was significantly higher compared to the bacterial cell surface in both 1-week-old and 3-week-old biofilms, which underscores the increased complexity and evolving interactions within biofilms over time [175].
A Representative force-distance curves between E. coli and goethite as a function of the surface contact time in water; B Summary of the corresponding maximum adhesion forces and fracture lengths. [146] (Reproduced with permission from Springer Nature under the terms of the Creative Commons public use license)
4.5 Complementary study with other analytical techniques
Complementary studies with other analytical techniques are essential to overcome the inherent limitations of AFM as a stand-alone approach in biofilm research. While AFM provides valuable information on biofilm morphologies, properties, and formation processes, it may lack molecular details and changes in chemical signals crucial for mechanistic studies [176]. Integrating AFM with other methods allows for a more comprehensive understanding of microbial systems. The current trend involves combining AFM with techniques such as confocal laser scanning microscopy (CLSM) and various spectroscopy methods. This multidisciplinary approach holds significant promise for enhancing the application of AFM in biofilm research, enabling researchers to obtain a more holistic view of biofilm structure, composition, and behavior.
4.5.1 AFM with confocal laser scanning microscopy
Integration of AFM with confocal laser scanning microscopy (CLSM) enhances the capabilities of biofilm analysis by providing complementary insights into morphology and composition. While AFM offers detailed observations of morphological and property changes following antimicrobial treatments, CLSM allows for a more intuitive visualization of these results. For instance, in a study by López-Jiménez et al., changes in morphological damage and surface roughness of Enterococcus faecalis biofilms treated with photodynamic therapy were observed using AFM, while CLSM was employed to analyze bacterial cell survival [177]. Similarly, in research conducted by Yang et al., CLSM was utilized to reveal the presence of a high abundance of extracellular DNA (eDNA) and low protein-to-polysaccharide ratios in the EPS of sulfide-based biofilms, while the effect of eDNA on biofilm cohesion and adhesion was determined through analysis with AFM [178]. By combining AFM with CLSM, researchers can leverage the strengths of each technique to gain a comprehensive understanding of biofilm structure, composition, and response to various treatments. This integrated approach facilitates more robust and insightful investigations into biofilm dynamics and behavior.
In recent years, there has been a growing trend towards utilizing combined CLSM/AFM and fluorescence microscopy (FM)/AFM equipment for microbial analysis [179,180,181]. This combined approach offers significant advantages, particularly in simultaneously observing biofilm morphology and identifying chemical components. By utilizing fluorescent labels for key biochemicals such as DNA, RNA, proteins, and lipids, CLSM/AFM equipment enables researchers to distinguish between living and dead cells and provides valuable insights into biofilm composition [182,183,184,185]. For example, Kuyukina et al. utilized the combined CLSM/AFM to generate high-resolution integrated CLSM/AFM images of live, dead, and damaged bacterial cells under varying solvent pressures [186]. Moreover, the combination of these techniques allows for the association of biochemical molecules within biofilms with specific external conditions, contributing to a deeper understanding of biofilm formation and growth dynamics. The CLSM/AFM system is particularly useful for studying living biofilms, as it provides valuable insights into the spatial organization of bacteria and the rheological properties of biofilms [78]. This integrated approach enables researchers to comprehensively analyze biofilm structure, composition, and behavior, thereby advancing our understanding of microbial systems.
4.5.2 AFM with infrared spectroscopy
Infrared spectroscopy (IR) is an important method for biofilm analysis and widely used for biofilm characterization [187, 188] as well as the study of EPS [189]. Atomic force microscope-infrared spectroscopy (AFM-IR) has emerged as a powerful technique for biofilm analysis, combining the chemical analysis capabilities of infrared spectroscopy with the high spatial resolution of AFM. This integration allows for the measurement of infrared light absorption by a sample with nanoscale resolution, enabling precise characterization of biofilm composition and structure [190, 191]. In biofilm studies, AFM-IR offers the capability to probe the chemical composition of molecules within bacterial cells, facilitating their characterization and identification during biofilm formation [17]. For instance, a study by Otzen et al. utilized AFM-IR to explore the functional amyloid proteins from E. coli, Pseudomonas, and Archaea Methanosaeta in situ. AFM-IR was able to identify amyloid proteins based on their characteristic cross β-sheet secondary structure at a single-cell level [141]. The results of the average properties of E. coli without curli in this study are shown in Fig. 6, including the 3D morphology, IR absorption maps, and IR spectra. Moreover, by employing AFM-IR, Barlow et al., observed the accumulation of particles near broader biofilms and identified them as secondary microplastics comprising urethane-rich aggregates [192]. Overall, AFM-IR provides valuable insights into biofilm composition, structure, and dynamics at the molecular level, offering a deeper understanding of biofilm behavior and facilitating innovative approaches for biofilm analysis and characterization.
A 3D morphology of E. coli without curli production; B IR absorption maps of E. coli without curli production; C IR spectra of E. coli without curli production. [141] (Reproduced with permission from John Wiley & Sons under the terms of the Creative Commons public use license)
4.5.3 AFM with Raman spectroscopy
Raman spectroscopy, a label-free technique offering nanoscale chemical characterization technique [193], is widely employed in biofilm studies to obtain information about the chemical molecular and surface structure of living bacterial cells [194]. While the fundamental theory of Raman spectroscopy is akin to that of IR spectroscopy, with Raman providing a scattering spectrum and IR an absorption spectrum, they often complement each other. Despite the weaker Raman signal compared to IR, Raman spectroscopy is advantageous for its ability to measure aqueous environments, as the water bands in Raman spectra are less intense [176]. Therefore, the combination of AFM with Raman spectroscopy, known as AFM-Raman, offers more than just a functional overlay of chemical analysis and high-resolution observation. The advent of tip-enhanced Raman spectroscopy (TERS) enhances the Raman signal in the nanoscale region between the AFM tip and the surface using conductive metal-coated AFM tips, such as gold or silver nanoparticles [193]. AFM-TERS has demonstrated promise in microbial research at the single bacterial cell level, enabling surface structure analysis, surface heterogeneity analysis, and investigation of surface chemical distribution [125, 195,196,197]. While AFM-TERS holds potential for biofilm applications, studies at the whole biofilm scale are limited due to weak Raman signals. To address this challenge, surface-enhanced Raman scattering (SERS) can be employed. Common approaches include modifying bacterial surfaces with metal nanoparticles or utilizing bacteria with nanostructures on their surfaces for study [176]. For instance, Ravindranath et al. utilized SERS to detect multiple metal ions in biofilms, characterizing biofilms using gold nano island-based SERS mapping substrates [198]. Additionally, Zheng et al. proposed a two-dimensional scanning SERS probe to overcome SERS limitations and accurately delineate biofilm boundaries within a short incubation period [199]. These studies highlight the potential of combining AFM with SERS to overcome challenges encountered in AFM-Raman spectroscopy at the whole biofilm scale, offering novel insights into biofilm composition, structure, and dynamics.
5 Current applications of rheological and AFM strategies
5.1 Effect of environmental factors on biofilm behavior
Understanding how environmental factors, including temperature, pH, and nutrient availability, influence the behavior of biofilms is crucial for mitigating their impact on food safety [200]. These factors can profoundly impact the formation, structure, and resilience of biofilms, ultimately affecting their potential to contaminate food products. Developing rheological models based on biofilm viscoelastic properties is essential for predicting their behavior under different processing conditions in realistic food environments. Recent studies have employed rheology devices to investigate how external conditions affect the viscoelastic properties of biofilms formed by foodborne bacteria. For instance, Rühs et al. explored the impact of nutrient concentration, temperature, pH, surfactants, and genetic changes on the biofilm formation of three common foodborne bacteria, E. coli, Pseudomonas fluorescens, and Bacillus subtilis [70]. Their research aimed to elucidate the complex interplay between these factors and biofilm development, providing insights into novel disinfection strategies to improve the safety of ready-to-eat foods. Additionally, AFM is instrumental in exploring the effects of extrinsic factors on the structural and mechanical properties of biofilms. In a study by Allen et al., the growth of Pseudomonas fluorescens biofilms was examined under various nutrient levels, and shear conditions employing liquid-phase AFM. Under semi-static conditions, AFM analysis revealed that biofilms grown in high-nutrient environments were less stiff compared to those grown in low-nutrient conditions, as indicated by lower Young’s modulus values [201]. Conversely, under dynamic conditions, biofilms exposed to low-nutrient conditions and high shear rates exhibited significantly higher adhesion compared to biofilms grown under other dynamic conditions [201]. Furthermore, Costello et al. investigated the influence of structural composition, growth location, and natural antimicrobial nisin on the microbial dynamics of Listeria Innocua (L. Innocua) in viscoelastic food model systems by rheological analysis. Their study revealed selective growth of L. innocua on the protein phase of biphasic systems and significant variations in colony size and distribution in monophasic systems based on growth type and Xanthan gum concentration [202]. These findings underscored the role of environmental composition and complexity in shaping biofilm behavior and highlighted the importance of considering these factors in the development of food processing methods aimed at mitigating biofilm-related risks.
5.2 Antimicrobial assessment against foodborne pathogen biofilms
Biofilms are complex microbial communities with important biological functions including enhanced resistance to external challenges. The penetration of antimicrobials and nutrients into biofilms relies on factors such as biofilm channelization and medium availability for molecular transport [20]. Although it is now clear that the protection provided by biofilms against physical treatments or antimicrobial agents consists of a combination of mechanisms, the exact mechanisms are still not fully understood [203]. Assessing the effectiveness and mechanism of antimicrobial agents in disrupting biofilms formed by foodborne pathogens is crucial. Using rheological analysis to quantify changes in biofilm mechanical properties provides insights into the effectiveness of antimicrobial agents, including their elasticity, viscosity, and adhesion strength. Alterations in biofilm viscoelasticity following antimicrobial exposure indicate shifts in biofilm matrix integrity and microbial viability. In the study by Jones et al., rheometry was employed to measure changes in the viscoelastic properties of bacterial biofilms following chemical and antimicrobial treatments [204]. Exposure to various chloride salts, urea, industrial biocides, and antibiotics, the biofilm mechanical properties altered significantly, and there was no consistent response between the two biofilm types to a specific treatment [204]. Concurrently, AFM aids in visualizing the structural damage caused by antimicrobial agents and elucidating their mode of action against biofilms. For instance, the antimicrobial efficacy of soy isoflavones against biofilms of food-borne pathogens (L. monocytogenes, E. coli, P. aeruginosa, and Methicillin-Resistant S. aureus) was evaluated, which may involve potential induction of cell dispersion and cell wall disruption [205]. Similarly, the morphological and antibacterial efficacy of quercetin-loaded nanoparticles against food-borne bacteria (Bacillus subtilis, E. coli, S. aureus, and Salmonella Typhimurium) were also explored by AFM [206]. The topography of the stainless steel coupons coated with silver zeolite for food contact surfaces was investigated by AFM to support the antimicrobial efficacy of silver zeolite coatings [207]. Further, Young's modulus obtained from AFM serves as a probe of the hierarchical structure–property relationship, which is very sensitive to the internal structural details of heterogeneous materials [208]. In the study by Zou et al., the effects of proanthocyanidins on the structural, adhesive, and mechanical properties of the cell envelope were investigated. The morphology, Young's modulus, and adhesion force obtained from the AFM curve were employed to clarify the antibiofilm mode of proanthocyanidins against S. epidermidis [208]. These multifaceted approaches offer invaluable insights into antimicrobial mechanisms and effectiveness, crucial for developing effective control strategies.
5.3 Biofilm control strategies for food processing
Ensuring the safety and quality of food processing requires the implementation of stringent protocols such as Good Manufacturing Practices (GMP) and Hazard Analysis and Critical Control Points (HACCP) [55]. To effectively prevent and control biofilms, the first step is to find critical points where biofilms may proliferate [209]. Investigating the effectiveness of different biofilm control strategies is crucial, including chemical sanitation protocols and surface coatings, aimed at inhibiting biofilm formation on food processing equipment. Rheological analysis provides a way to assess the impact of control strategies on biofilm mechanical properties and AFM imaging enables the visualization of the coating surfaces and their effects on biofilm structure and organization. In the food industry, a variety of disinfectants are commonly used, including quaternary ammonium compounds (Quats), chlorine compounds such as bleach, iodine compounds, peroxyacetic acid (PAA), hydrogen peroxide, acetic acid found in vinegar, and ethanol/isopropanol. However, conventional biofilm strategies have been reported to be insufficient in removing biofilms [210, 211]. Zrelli et al. proposed that strategies relying solely on cell-targeted disinfectants may be off-target, as they preserve the structural integrity of the biofilm, promoting its resilience. In their study, micro-rheology analysis revealed that the biofilms lost their original viscoelastic properties after treatment with a proteolytic enzyme that cleaves EPS into short peptides, while the viability of the bacteria inside did not show detectable alterations [212]. Thus, designing disinfection strategies that target not only the bacteria themselves but also the EPS through the viscoelasticity analysis could provide a promising direction in the fight against biofilms. Biofilm formation heavily relies on substrate properties, including hydrophobicity/hydrophilicity, structure, surface charge, and roughness [213]. Another approach to controlling biofilms is preventing cell adhesion by modifying surfaces through chemical strategies, physical strategies, and multifunctional strategies [214, 215]. Multipotent antibacterial platforms incorporate both bacteria-killing and self-cleaning capabilities. To investigate the impact of surface modifications, such as nanostructured coatings and surface functionalization, on the adhesion and growth of biofilms using AFM helps understand the nanoscale interactions between biofilms and modified surfaces [17]. In a study conducted by Lorite et al., AFM was employed to investigate the physicochemical changes induced by Periwinkle wilt (PW) culture medium conditioning film formation on different surfaces (glass and silicon) and their influence on Xylella. fastidiosa biofilm development [216]. AFM imaging revealed a reduction in surface roughness on glass surfaces with conditioning film formation, contrary to the commonly observed increase in cell attachment on hydrophobic and rough surfaces. The study underscores the importance of surface functional groups resulting from PW conditioning film formation, particularly phosphate groups, in facilitating X. fastidiosa adhesion and biofilm development, emphasizing the significance of chemical surface changes over surface roughness or hydrophobicity in biofilm formation.
5.4 Monitoring biofilm in food-related fields
The monitoring system that integrates rheological and AFM measurements could revolutionize the detection and management of biofilm contamination on food contact surfaces during processing operations. Investigating the feasibility of using this system for early detection of biofilm contamination and implementing timely intervention strategies is crucial in food-related fields [217]. Rheological data offers valuable insights into the mechanical properties and stability of biofilms throughout their formation and growth dynamics. Tarifa et al. investigated the factors influencing the mechanical properties of biofilms formed by yeast species isolated from juice processing industries. Their findings revealed that the flow type (turbulent flow and static conditions) during juice processing had a significant effect on the biofilm's mechanical properties, with velocity and nutrient status also influencing biofilm thickness and biomass [218]. Monitoring these parameters can aid in the development of effective biofilm control strategies. In parallel, high-resolution AFM imaging allows for detailed visualization of biofilm structure. Examination of biofilms in an aqueous environment using AFM is required for in situ observation to avoid the potential important structural changes during transfer or dehydration. For instance, due to the complexity of mixed culture drinking water biofilm, direct visual observation under in situ conditions has been challenging. In the study by Daniels et al., AFM revealed the three-dimensional morphology and arrangement of drinking water-relevant biofilm in air and aqueous solution. Biofilm and the structural topography of individual bacterial cells were resolved and continuously imaged in liquid without fixation of the sample, loss of spatial resolution, or sample damage, allowing future in situ investigations to temporally monitor structural changes in mixed culture drinking water biofilm [162]. By integrating rheological and AFM measurements in a monitoring system, researchers in the food-related industry can gain comprehensive insights into biofilm dynamics, enabling proactive measures to control contamination and ensure food safety.
6 Conclusions and future directions
As a significant challenge in food and related industries, it is crucial to monitor and mitigate biofilm not only to prevent microbial contamination but also to ensure product safety and quality. However, the complexity and dynamism of biofilms, which can vary significantly in composition, structure, and mechanical properties across different environments, adds to the difficulty of developing universal characterization methods. The characterization of biofilms using rheology and AFM has witnessed remarkable advancements, offering invaluable insights into their structural and mechanical properties. By understanding biofilm behavior at various scales, from macroscopic rheological analyses to nanoscale AFM imaging, researchers are better equipped to tackle the challenges. The diverse array of rheological techniques discussed, ranging from macroscopic bulk methods to microscale analyses, enables the assessment of biofilm viscoelastic properties and response to environmental stimuli with high precision. Similarly, AFM provides detailed information on biofilm morphology, topography, and mechanical characteristics, facilitating a comprehensive understanding of biofilm formation and dynamics. The current applications of these techniques in assessing antimicrobial effectiveness, developing control strategies, and monitoring biofilms underscore their significance in ensuring food safety and quality.
Integrating rheological and AFM approaches has greatly expanded our understanding of biofilm characterization, enabling researchers to unravel the complex interplay between biofilm structure, mechanics, and environmental factors. Despite these advances, several challenges remain, including the need to replicate real-world conditions more accurately in laboratory settings and to improve the consistency and reliability of biofilm characterization methods. Rheological techniques are well-suited for bulk analyses of biofilms but may struggle to capture the fine details of biofilm microstructure. AFM provides high-resolution imaging, but its small field of view, high costs, and operational complexity can make it less accessible for routine analysis, particularly in industrial settings. Moving forward, addressing these issues will require the development of advanced environmental control systems and standardized protocols to improve accuracy and reduce variability, bridging the gap between laboratory research and industrial application. Secondly, developing high-throughput screening methods that incorporate both rheological and AFM measurements could accelerate the study of biofilms, particularly in industrial or environmental contexts where large numbers of samples need to be analyzed quickly. Furthermore, cross-disciplinary collaboration will be key to overcoming current limitations and advancing biofilm research. Except the chemical analysis methods (CLSM/FM/IR/Raman) discussed, leveraging machine learning algorithms could help assist in identifying patterns in biofilm behavior and predicting how biofilms will respond to different treatments. Specifically, applying machine learning to AFM object recognition and image processing could automate the identification and quantification of biofilm features, making routine analysis more feasible for industrial use. In conclusion, significant progress has been made in biofilm characterization using rheology and AFM, and future research will continue to advance these techniques and develop new approaches that enhance our ability to understand and control biofilms in food and related fields.
Data Availability
No datasets were generated or analysed during the current study.
References
Flemming H-C, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623–633
Carrascosa C, Raheem D, Ramos F, Saraiva A, Raposo A (2021) Microbial biofilms in the food industry—A comprehensive review. Int J Environ Res Public Health 18(04):2014
Galie S, García-Gutiérrez C, Miguélez EM, Villar CJ, Lombó F (2018) Biofilms in the food industry: health aspects and control methods. Front Microbiol 9:898
Kumar CG, Anand SK (1998) Significance of microbial biofilms in food industry: a review. Int J Food Microbiol 42(1–2):9–27
Shemesh M, Ostrov I (2020) Role of Bacillus species in biofilm persistence and emerging antibiofilm strategies in the dairy industry. J Sci Food Agric 100(6):2327–2336
Bridier A, Sanchez-Vizuete P, Guilbaud M, Piard J-C, Naitali M, Briandet R (2015) Biofilm-associated persistence of food-borne pathogens. Food Microbiol 45:167–178
Wimpenny J, Manz W, Szewzyk U (2000) Heterogeneity in biofilms. FEMS Microbiol Rev 24(5):661–671
Shineh G, Mobaraki M, Perves Bappy MJ, Mills DK (2023) Biofilm formation, and related impacts on healthcare, food processing and packaging, industrial manufacturing, marine industries, and sanitation–a review. Applied Microbiol. 3(3):629–665
Giaouris E, Heir E, Hébraud M, Chorianopoulos N, Langsrud S, Møretrø T, Habimana O, Desvaux M, Renier S, Nychas G-J (2014) Attachment and biofilm formation by foodborne bacteria in meat processing environments: causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Sci 97(3):298–309
M.J. Franklin, C. Chang, T. Akiyama, B. Bothner (2015) New technologies for studying biofilms. Microbial Biofilms 1–32. https://doi.org/10.1128/9781555817466.ch1
Azeredo J, Azevedo NF, Briandet R, Cerca N, Coenye T, Costa AR, Desvaux M, Di Bonaventura G, Hébraud M, Jaglic Z (2017) Critical review on biofilm methods. Crit Rev Microbiol 43(3):313–351
Neu TR, Manz B, Volke F, Dynes JJ, Hitchcock AP, Lawrence JR (2010) Advanced imaging techniques for assessment of structure, composition and function in biofilm systems. FEMS Microbiol Ecol 72(1):1–21
Neu TR, Lawrence JR (2014) Investigation of microbial biofilm structure by laser scanning microscopy. Productive Biofilms 146:1–51
Frickmann H, Zautner AE, Moter A, Kikhney J, Hagen RM, Stender H, Poppert S (2017) Fluorescence in situ hybridization (FISH) in the microbiological diagnostic routine laboratory: a review. Crit Rev Microbiol 43(3):263–293
White DC, Findlay RH (1988) Biochemical markers for measurement of predation effects on the biomass, community structure, nutritional status, and metabolic activity of microbial biofilms. Hydrobiologia 159:119–132
Tallawi M, Opitz M, Lieleg O (2017) Modulation of the mechanical properties of bacterial biofilms in response to environmental challenges. Biomaterials science 5(5):887–900
Huang Y, Chakraborty S, Liang H (2020) Methods to probe the formation of biofilms: applications in foods and related surfaces. Anal Methods 12(4):416–432
James SA, Hilal N, Wright CJ (2017) Atomic force microscopy studies of bioprocess engineering surfaces–imaging, interactions and mechanical properties mediating bacterial adhesion. Biotechnol J 12(7):1600698
Abu-Lail NI, Beyenal H (2013) Characterization of Bacteria-Biomaterial Interactions, from a Single Cell to Biofilms. Elsevier, Characterization of Biomaterials 207–253. https://doi.org/10.1016/B978-0-12-415800-9.00006-1
Peterson BW, He Y, Ren Y, Zerdoum A, Libera MR, Sharma PK, Van Winkelhoff A-J, Neut D, Stoodley P, Van Der Mei HC (2015) Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol Rev 39(2):234–245
Charlton SG, White MA, Jana S, Eland LE, Jayathilake PG, Burgess JG, Chen J, Wipat A, TPCurtis, (2019) Regulating, measuring, and modeling the viscoelasticity of bacterial biofilms. J Bacteriol 201:18. https://doi.org/10.1128/jb.00101-19
Alotaibi GF, Bukhari MA (2021) Factors influencing bacterial biofilm formation and development. Am J Biomed Sci Res 12(6):617–626
Rather MA, Gupta K, Mandal M (2021) Microbial biofilm: formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol 54(4):1–18
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8(9):881
Rupp CJ, Fux CA, Stoodley P (2005) Viscoelasticity of Staphylococcus aureus biofilms in response to fluid shear allows resistance to detachment and facilitates rolling migration. Appl Environ Microbiol 71(4):2175–2178
O’Toole GA, Kolter R (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30(2):295–304
Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annual Reviews in Microbiology 56(1):187–209
Abdallah FB, Chaieb K, Zmantar T, Kallel H, Bakhrouf A (2009) Adherence assays and slime production of Vibrio alginolyticus and Vibrio parahaemolyticus. Braz J Microbiol 40:394–398
Nilsson RE, Ross T, Bowman JP (2011) Variability in biofilm production by Listeria monocytogenes correlated to strain origin and growth conditions. Int J Food Microbiol 150(1):14–24
Straub H, Eberl L, Zinn M, Rossi RM, Maniura-Weber K, Ren Q (2020) A microfluidic platform for in situ investigation of biofilm formation and its treatment under controlled conditions. Journal of nanobiotechnology 18:1–12
Augustin M, Ali-Vehmas T, Atroshi F (2004) Assessment of enzymatic cleaning agents and disinfectants against bacterial biofilms. J Pharm Pharm Sci 7(1):55–64
Maukonen J, Mättö J, Wirtanen G, Raaska L, Mattila-Sandholm T, Saarela M (2003) Methodologies for the characterization of microbes in industrial environments: a review. J Ind Microbiol Biotechnol 30(6):327–356
Sinde E, Carballo J (2000) Attachment of Salmonella spp. and Listeria monocytogenes to stainless steel, rubber and polytetrafluorethylene: the influence of free energy and the effect of commercial sanitizers. Food Microbiol 17(4):439–447
Luo A, Wang F, Sun D, Liu X, Xin B (2022) Formation, development, and cross-species interactions in biofilms. Front Microbiol 12:757327
Limoli DH, Jones CJ, Wozniak DJ (2015) Bacterial extracellular polysaccharides in biofilm formation and function. Microbial Biofilms 3(3):223–247
Chmielewski R, Frank J (2003) Biofilm formation and control in food processing facilities. Comprehensive reviews in food science and food safety 2(1):22–32
McLean RJ, Whiteley M, Stickler DJ, Fuqua WC (1997) Evidence of autoinducer activity in naturally occurring biofilms. FEMS Microbiol Lett 154(2):259–263
Costerton JW, Lewandowski Z, DeBeer D, Caldwell D, Korber D, James G (1994) Biofilms, the customized microniche. J Bacteriol 176(8):2137–2142
Skerker JM, Berg HC (2001) Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci 98(12):6901–6904
Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, Greenberg E (2001) Gene expression in Pseudomonas aeruginosa biofilms. Nature 413(6858):860–864
Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG (2002) Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. Am Soc Microbiol 184(4):1140–1154
Kaplan JB, Ragunath C, Velliyagounder K, Fine DH, Ramasubbu N (2004) Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 48(7):2633–2636
O’Toole G, Kaplan HB, Kolter R (2000) Biofilm formation as microbial development. Annual Reviews in Microbiology 54(1):49–79
Stoodley P, Cargo R, Rupp CJ, Wilson S, Klapper I (2002) Biofilm material properties as related to shear-induced deformation and detachment phenomena. J Ind Microbiol Biotechnol 29(6):361–367
Li P, Yin R, Cheng J, Lin J (2023) Bacterial biofilm formation on biomaterials and approaches to its treatment and prevention. Int J Mol Sci 24(14):11680
Nguyen H, Yang Y, Yuk H (2014) Biofilm formation of Salmonella Typhimurium on stainless steel and acrylic surfaces as affected by temperature and pH level. LWT-Food Science and Technology 55(1):383–388
Rao T (2010) Comparative effect of temperature on biofilm formation in natural and modified marine environment. Aquat Ecol 44(2):463–478
Speranza B, Corbo MR, Sinigaglia M (2011) Effects of nutritional and environmental conditions on Salmonella sp. Biofilm Formation. J. Food Sci 76(1):M12–M16
Iliadis I, Daskalopoulou A, Simões M, Giaouris E (2018) Integrated combined effects of temperature, pH and sodium chloride concentration on biofilm formation by Salmonella enterica ser. Enteritidis and Typhimurium under low nutrient food-related conditions, Food Research International 107:10–18
Passy SI, Larson CA (2011) Succession in stream biofilms is an environmentally driven gradient of stress tolerance. Microb Ecol 62:414–424
Roy PK, Ha AJ-W, Mizan MFR, Hossain MI, Ashrafudoulla M, Toushik SH, Nahar S, Kim YK, Ha S-D (2021) Effects of environmental conditions (temperature, pH, and glucose) on biofilm formation of Salmonella enterica serotype Kentucky and virulence gene expression. Poult Sci 100(7):101209
Duguid J, Anderson E, Campbell I (1966) Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol 92(1):107–137
Bagge-Ravn D, Ng Y, Hjelm M, Christiansen JN, Johansen C, Gram L (2003) The microbial ecology of processing equipment in different fish industries—analysis of the microflora during processing and following cleaning and disinfection. Int J Food Microbiol 87(3):239–250
Guobjoernsdottir B, Einarsson H, Thorkelsson G (2005) Microbial adhesion to processing lines for fish fillets and cooked shrimp: influence of stainless steel surface finish and presence of gram-negative bacteria on the attachment of Listeria monocytogenes. Food Technology and Biotechnology 43(1):55–61
Sharma M, Anand S (2002) Biofilms evaluation as an essential component of HACCP for food/dairy processing industry–a case. Food Control 13(6–7):469–477
Goodburn C, Wallace CA (2013) The microbiological efficacy of decontamination methodologies for fresh produce: a review. Food Control 32(2):418–427
Joshi K, Mahendran R, Alagusundaram K, Norton T, Tiwari B (2013) Novel disinfectants for fresh produce. Trends Food Sci Technol 34(1):54–61
Whipps JM, Hand P, Pink DA, Bending GD (2008) Human pathogens and the phyllosphere. Adv Appl Microbiol 64:183–221
T.V. Suslow (2001) Water disinfection: a practical approach to calculating dose values for preharvest and postharvest applications.
Shi X, Zhu X (2009) Biofilm formation and food safety in food industries. Trends Food Sci Technol 20(9):407–413
Srey S, Jahid IK, Ha S-D (2013) Biofilm formation in food industries: a food safety concern. Food Control 31(2):572–585
Simões M, Simões LC, Vieira MJ (2010) A review of current and emergent biofilm control strategies. LWT-Food Science and Technology 43(4):573–583
Gram L, Bagge-Ravn D, Ng YY, Gymoese P, Vogel BF (2007) Influence of food soiling matrix on cleaning and disinfection efficiency on surface attached Listeria monocytogenes. Food Control 18(10):1165–1171
Ölmez H, Temur S (2010) Effects of different sanitizing treatments on biofilms and attachment of Escherichia coli and Listeria monocytogenes on green leaf lettuce. LWT-Food Science and Technology 43(6):964–970
Fransisca L, Zhou B, Park H, Feng H (2011) The effect of calcinated calcium and chlorine treatments on Escherichia coli O157: H7 87–23 population reduction in radish sprouts. J Food Sci 76(6):M404–M412
Sapers G, Sites J (2003) Efficacy of 1% hydrogen peroxide wash in decontaminating apples and cantaloupe melons. J Food Sci 68(5):1793–1797
Simoes M, Pereira MO, Vieira MJ (2005) Effect of mechanical stress on biofilms challenged by different chemicals. Water Res 39(20):5142–5152
Choi N-C, Park S-J, Lee C-G, Park J-A, Kim S-B (2011) Influence of surfactants on bacterial adhesion to metal oxide-coated surfaces. Environmental Engineering Research 16(4):219–225
Knetsch ML, Koole LH (2011) New strategies in the development of antimicrobial coatings: the example of increasing usage of silver and silver nanoparticles. Polymers 3(1):340–366
Rühs PA, Böni L, Fuller GG, Inglis RF, Fischer P (2013) In-situ quantification of the interfacial rheological response of bacterial biofilms to environmental stimuli. PLoS ONE 8(11):e78524
Paramonova E, Krom BP, van der Mei HC, Busscher HJ, Sharma PK (2009) Hyphal content determines the compression strength of Candida albicans biofilms. Microbiology 155(6):1997–2003
Das T, Sharma PK, Krom BP, van der Mei HC, Busscher HJ (2011) Role of eDNA on the adhesion forces between Streptococcus mutans and substratum surfaces: influence of ionic strength and substratum hydrophobicity. Langmuir 27(16):10113–10118
Krsmanovic M, Biswas D, Ali H, Kumar A, Ghosh R, Dickerson AK (2021) Hydrodynamics and surface properties influence biofilm proliferation. Adv Coll Interface Sci 288:102336
Safari A, Tukovic Z, Walter M, Casey E, Ivankovic A (2015) Mechanical properties of a mature biofilm from a wastewater system: from microscale to macroscale level. Biofouling 31(8):651–664
Stewart PS, Franklin MJ (2008) Physiological heterogeneity in biofilms. Nat Rev Microbiol 6(3):199–210
Billings N, Birjiniuk A, Samad TS, Doyle PS, Ribbeck K (2015) Material properties of biofilms—a review of methods for understanding permeability and mechanics. Rep Prog Phys 78(3):036601
Gloag ES, Fabbri S, Wozniak DJ, Stoodley P (2020) Biofilm mechanics: Implications in infection and survival. Biofilm 2:100017
Boudarel H, Mathias J-D, Blaysat B, Grédiac M (2018) Towards standardized mechanical characterization of microbial biofilms: analysis and critical review. NPJ biofilms and microbiomes 4(1):1–15
S.P. Timoshenko, J.N. Goodier, Theory of elasticity, (1951).
Karimi A, Karig D, Kumar A, Ardekani A (2015) Interplay of physical mechanisms and biofilm processes: review of microfluidic methods. Lab Chip 15(1):23–42
Ferry JD (1980) Viscoelastic properties of polymers. John Wiley & Sons, New York
Branda SS, Vik Å, Friedman L, Kolter R (2005) Biofilms: the matrix revisited. Trends Microbiol 13(1):20–26
Gordon VD, Davis-Fields M, Kovach K, Rodesney CA (2017) Biofilms and mechanics: a review of experimental techniques and findings. J Phys D Appl Phys 50(22):223002
Ghosh UU, Ali H, Ghosh R, Kumar A (2021) Bacterial streamers as colloidal systems: Five grand challenges. J Colloid Interface Sci 594:265–278
Drescher K, Shen Y, Bassler BL, Stone HA (2013) Biofilm streamers cause catastrophic disruption of flow with consequences for environmental and medical systems. Proc Natl Acad Sci 110(11):4345–4350
Liu L-Y, Sun L, Zhong Z-T, Zhu J, Song H-Y (2016) Effects of titanium dioxide nanoparticles on intestinal commensal bacteria. Nucl Sci Tech 27(1):5
Pavlovsky L, Younger JG, Solomon MJ (2013) In situ rheology of Staphylococcus epidermidis bacterial biofilms. Soft Matter 9(1):122–131
de Wouters T, Jans C, Niederberger T, Fischer P, Rühs PA (2015) Adhesion potential of intestinal microbes predicted by physico-chemical characterization methods. PLoS ONE 10(8):e0136437
Hollenbeck EC, Fong JC, Lim JY, Yildiz FH, Fuller GG, Cegelski L (2014) Molecular determinants of mechanical properties of V. cholerae biofilms at the air-liquid interface. Biophys J 107(10):2245–2252
Wu C, Lim JY, Fuller GG, Cegelski L (2012) Quantitative analysis of amyloid-integrated biofilms formed by uropathogenic Escherichia coli at the air-liquid interface. Biophys J 103(3):464–471
Dahlbäck B, Hermansson M, Kjelleberg S, Norkrans B (1981) The hydrophobicity of bacteria—An important factor in their initial adhesion at the air-water inteface. Arch Microbiol 128(3):267–270
Abriat C, Enriquez K, Virgilio N, Cegelski L, Fuller GG, Daigle F, Heuzey M-C (2020) Mechanical and microstructural insights of Vibrio cholerae and Escherichia coli dual-species biofilm at the air-liquid interface. Colloids Surf, B 188:110786
Erni P, Fischer P, Windhab EJ, Kusnezov V, Stettin H, Läuger J (2003) Stress-and strain-controlled measurements of interfacial shear viscosity and viscoelasticity at liquid/liquid and gas/liquid interfaces. Rev Sci Instrum 74(11):4916–4924
Rühs PA, Scheuble N, Windhab EJ, Mezzenga R, Fischer P (2012) Simultaneous control of pH and ionic strength during interfacial rheology of β-lactoglobulin fibrils adsorbed at liquid/liquid interfaces. Langmuir 28(34):12536–12543
Pandit S, Fazilati M, Gaska K, Derouiche A, Nypelö T, Mijakovic I, Kádár R (2020) The exo-polysaccharide component of extracellular matrix is essential for the viscoelastic properties of Bacillus subtilis biofilms. Int J Mol Sci 21(18):6755
Abriat C, Virgilio N, Heuzey M-C, Daigle F (2019) Microbiological and real-time mechanical analysis of Bacillus licheniformis and Pseudomonas fluorescens dual-species biofilm. Microbiology 165(7):747–756
Bertsch P, Etter D, Fischer P (2021) Transient in situ measurement of kombucha biofilm growth and mechanical properties. Food Funct 12(9):4015–4020
Rühs PA, Böcker L, Inglis RF, Fischer P (2014) Studying bacterial hydrophobicity and biofilm formation at liquid–liquid interfaces through interfacial rheology and pendant drop tensiometry. Colloids Surf, B 117:174–184
Head IM, Jones DM, Röling WF (2006) Marine microorganisms make a meal of oil. Nat Rev Microbiol 4(3):173–182
D. Dasgupta, R. Ghosh, T.K. Sengupta, (2013) Biofilm-mediated enhanced crude oil degradation by newly isolated Pseudomonas species, Intl Scholarly Res Notices 2013.
Timmis KN, McGenity T, Van Der Meer JR, de Lorenzo V (2010) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin
Subbiahdoss G, Reimhult E (2020) Biofilm formation at oil-water interfaces is not a simple function of bacterial hydrophobicity. Colloids Surf, B 194:111163
Kang Z, Yeung A, Foght JM, Gray MR (2008) Mechanical properties of hexadecane–water interfaces with adsorbed hydrophobic bacteria. Colloids Surf, B 62(2):273–279
Xia Q, Xiao H, Pan Y, Wang L (2018) Microrheology, advances in methods and insights. Adv Coll Interface Sci 257:71–85
Mao Y, Nielsen P, Ali J (2022) Passive and active microrheology for biomedical systems. Frontiers in bioengineering and biotechnology 10:916354
Rivas DP, Hedgecock ND, Stebe KJ, Leheny RL (2021) Dynamic and mechanical evolution of an oil–water interface during bacterial biofilm formation. Soft Matter 17(35):8195–8210
Chew SC, Kundukad B, Seviour T, Van der Maarel JR, Yang L, Rice SA, Doyle P, Kjelleberg S (2014) Dynamic remodeling of microbial biofilms by functionally distinct exopolysaccharides. MBio 5(4):e01536-e1614
Rogers S, Van Der Walle C, Waigh T (2008) Microrheology of bacterial biofilms in vitro: Staphylococcus aureus and Pseudomonas aeruginosa. Langmuir 24(23):13549–13555
Pavissich JP, Li M, Nerenberg R (2021) Spatial distribution of mechanical properties in Pseudomonas aeruginosa biofilms, and their potential impacts on biofilm deformation. Biotechnol Bioeng 118(4):1545–1556
Galy O, Zrelli K, Latour-Lambert P, Kirwan L, Henry N (2014) Remote magnetic actuation of micrometric probes for in situ 3D mapping of bacterial biofilm physical properties. JoVE (Journal of Visualized Experiments) 2014(87):e50857
Galy O, Latour-Lambert P, Zrelli K, Ghigo J-M, Beloin C, Henry N (2012) Mapping of bacterial biofilm local mechanics by magnetic microparticle actuation. Biophys J 103(6):1400–1408
Kim J, Hegde M, Kim SH, Wood TK, Jayaraman A (2012) A microfluidic device for high throughput bacterial biofilm studies. Lab Chip 12(6):1157–1163
Huang M, Fan S, Xing W, Liu C (2010) Microfluidic cell culture system studies and computational fluid dynamics. Math Comput Model 52(11–12):2036–2042
Pérez-Rodríguez S, García-Aznar JM, Gonzalo-Asensio J (2022) Microfluidic devices for studying bacterial taxis, drug testing and biofilm formation. Microb Biotechnol 15(2):395–414
Hohne DN, Younger JG, Solomon MJ (2009) Flexible microfluidic device for mechanical property characterization of soft viscoelastic solids such as bacterial biofilms. Langmuir 25(13):7743–7751
Bottero S, Storck T, Heimovaara TJ, van Loosdrecht MC, Enzien MV, Picioreanu C (2013) Biofilm development and the dynamics of preferential flow paths in porous media. Biofouling 29(9):1069–1086
Sharp RR, Stoodley P, Adgie M, Gerlach R, Cunningham A (2005) Visualization and characterization of dynamic patterns of flow, growth and activity of biofilms growing in porous media. Water Sci Technol 52(7):85–90
Kurz DL, Secchi E, Carrillo FJ, Bourg IC, Stocker R, Jimenez-Martinez J (2022) Competition between growth and shear stress drives intermittency in preferential flow paths in porous medium biofilms. Proc Natl Acad Sci 119(30):e2122202119
Hansma PK, Elings V, Marti O, Bracker C (1988) Scanning tunneling microscopy and atomic force microscopy: application to biology and technology. Science 242(4876):209–216
Dufrêne YF (2008) Towards nanomicrobiology using atomic force microscopy. Nat Rev Microbiol 6(9):674–680
Xiao J, Dufrêne YF (2016) Optical and force nanoscopy in microbiology. Nat Microbiol 1(11):1–13
Liu S, Wang Y (2010) Application of AFM in microbiology: a review. Scanning 32(2):61–73
Wright CJ, Shah MK, Powell LC, Armstrong I (2010) Application of AFM from microbial cell to biofilm. Scanning 32(3):134–149
Dorobantu LS, Gray MR (2010) Application of atomic force microscopy in bacterial research. Scanning 32(2):74–96
Touhami A (2020) Atomic Force Microscopy: A New Look at Microbes. Synthesis Lectures on Materials and Optics 1(3):1–111
Mahto KU, Das S (2021) Microscopic techniques to evaluate the biofilm formation ability of a marine bacterium Pseudomonas aeruginosa PFL-P1 on different substrata. Microsc Res Tech 84(10):2451–2461
Shen H, López-Guerra EA, Zhu R, Diba T, Zheng Q, Solares SD, Zara JM, Shuai D, Shen Y (2018) Visible-light-responsive photocatalyst of graphitic carbon nitride for pathogenic biofilm control. ACS Appl Mater Interfaces 11(1):373–384
Ripa R, Shen AQ, Funari R (2020) Detecting Escherichia coli biofilm development stages on gold and titanium by quartz crystal microbalance. ACS Omega 5(5):2295–2302
Das B, Kar A, Bhowmik R, Karmakar S, Tripathy S, Matsabisa MG, Mukherjee PK (2022) Quality Related Safety Evaluation of a South African Traditional Formulation (PHELA®) as Novel Anti-Biofilm Candidate. Molecules 27(4):1219
López-Guerra EA, Shen H, Solares SD, Shuai D (2019) Acquisition of time–frequency localized mechanical properties of biofilms and single cells with high spatial resolution. Nanoscale 11(18):8918–8929
Betancourt P, Merlos A, Sierra J, Camps-Font O, Arnabat-Dominguez J, Viñas M (2019) Effectiveness of low concentration of sodium hypochlorite activated by Er, Cr: YSGG laser against Enterococcus faecalis biofilm. Lasers Med Sci 34(2):247–254
Chauhan D, Agrawal G, Deshmukh S, Roy SS, Priyadarshini R (2018) Biofilm formation by Exiguobacterium sp DR11 and DR14 alter polystyrene surface properties and initiate biodegradation. RSC Adv 8(66):37590–37599
Tarafdar A, Lee J-U, Jeong J-E, Lee H, Jung Y, Oh HB, Woo HY, Kwon J-H (2021) Biofilm development of Bacillus siamensis ATKU1 on pristine short chain low-density polyethylene: A case study on microbe-microplastics interaction. J Hazard Mater 409
Birkenhauer E, Neethirajan S (2014) Characterization of electrical surface properties of mono-and co-cultures of Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus using Kelvin probe force microscopy. RSC Adv 4(80):42432–42440
Das NM, Singh AK, Ghosh D, Bandyopadhyay D (2019) Graphene oxide nanohybrids for electron transfer-mediated antimicrobial activity. Nanoscale Advances 1(9):3727–3740
Schickle K, Gołda-Cępa M, Vuslat-Parlak Z, Grigorev N, Desante G, Chlanda A, Mazuryk O, Neuhaus K, Schmidt C, Amousa N (2024) Revealing bactericidal events on graphene oxide nano films deposited on metal implant surfaces. J Mater Chem B12(10):2494–504
Binnig G, Quate CF, Gerber C (1986) Atomic force microscope. Phys Rev Lett 56(9):930
Alsteens D, Gaub HE, Newton R, Pfreundschuh M, Gerber C, Müller DJ (2017) Atomic force microscopy-based characterization and design of biointerfaces. Nat Rev Mater 2(5):1–16
Abdolahi A, Hamzah E, Ibrahim Z, Hashim S (2014) Microbially influenced corrosion of steels by Pseudomonas aeruginosa. Corros Rev 32(3–4):129–141
Modena MM, Rühle B, Burg TP, Wuttke S (2019) Nanoparticle characterization: what to measure? Adv Mater 31(32):1901556
Otzen DE, Dueholm MS, Najarzadeh Z, Knowles TP, Ruggeri FS (2021) In Situ Sub-Cellular Identification of Functional Amyloids in Bacteria and Archaea by Infrared Nanospectroscopy. Small Methods 5(6):2001002
Chen X, Tyagi A, Vijayalakshmi S, Chelliah R, Shabbir U, Oh D-H (2022) Anti-adhesion and anti-biofilm activity of slightly acidic electrolyzed water combined with sodium benzoate against Streptococcus mutans: A novel ecofriendly oral sanitizer to prevent cariogenesis. Microb Pathog 166:105535
Díaz C, Schilardi P, Salvarezza R, de Mele MFL (2011) Have flagella a preferred orientation during early stages of biofilm formation?: AFM study using patterned substrates. Colloids Surf, B 82(2):536–542
Cao Y, Su B, Chinnaraj S, Jana S, Bowen L, Charlton S, Duan P, Jakubovics NS, Chen J (2018) Nanostructured titanium surfaces exhibit recalcitrance towards Staphylococcus epidermidis biofilm formation. Sci Rep 8(1):1–13
R. Bava, The early stages of biofilm formation by Staphylococcus epidermidis studied by XPS and AFM, (2019).
Huang Q, Wu H, Cai P, Fein JB, Chen W (2015) Atomic force microscopy measurements of bacterial adhesion and biofilm formation onto clay-sized particles. Sci Rep 5(1):1–12
Dong H, Zhang W, Xuan Q, Zhou Y, Zhou S, Huang J, Wang P (2021) Binding peptide-guided immobilization of lipases with significantly improved catalytic performance using Escherichia coli BL21 (DE3) biofilms as a platform. ACS Appl Mater Interfaces 13(5):6168–6179
Gonçalves S, Silva PM, Felício MR, de Medeiros LN, Kurtenbach E, Santos NC (2017) Ps d1 Effects on Candida albicans Planktonic Cells and Biofilms. Front Cell Infect Microbiol 7:249
Yao M, Lu Y, Zhang T, Xie J, Han S, Zhang S, Fei Y, Ling Z, Wu J, Hu Y (2021) Improved functionality of Ligilactobacillus salivarius Li01 in alleviating colonic inflammation by layer-by-layer microencapsulation. NPJ Biofilms Microbiomes 7(1):1–10
Xi Y, Wang Y, Gao J, Xiao Y, Du J (2019) Dual corona vesicles with intrinsic antibacterial and enhanced antibiotic delivery capabilities for effective treatment of biofilm-induced periodontitis. ACS Nano 13(12):13645–13657
Camesano TA, Liu Y, Datta M (2007) Measuring bacterial adhesion at environmental interfaces with single-cell and single-molecule techniques. Adv Water Resour 30(6–7):1470–1491
Dufrêne YF (2015) Sticky microbes: forces in microbial cell adhesion. Trends Microbiol 23(6):376–382
Ahimou F, Touhami A, Dufrêne YF (2003) Real-time imaging of the surface topography of living yeast cells by atomic force microscopy. Yeast 20(1):25–30
Kailas L, Ratcliffe E, Hayhurst E, Walker M, Foster S, Hobbs J (2009) Immobilizing live bacteria for AFM imaging of cellular processes. Ultramicroscopy 109(7):775–780
Formosa-Dague C, Duval RE, Dague E (2018) Cell biology of microbes and pharmacology of antimicrobial drugs explored by atomic force microscopy, seminars in cell & developmental biology. Elsevier 73:165–176
Dufrêne YF, Viljoen A, Mignolet J, Mathelié-Guinlet M (2021) AFM in cellular and molecular microbiology. Cell Microbiol 23(7):e13324
Dash S, Lahiri D, Nag M, Das D, Ray RR (2021) Probing the surface-attached in vitro microbial biofilms with atomic force (AFM) and scanning probe microscopy (SPM). Springer, Analytical Methodologies for Biofilm Research, pp 223–241
S.A. James, L.C. Powell, C.J. Wright, (2016) Atomic force microscopy of biofilms—Imaging, interactions, and mechanics, Microb Biofilms-importance Appl.
Yadav N, Dubey A, Shukla S, Saini CP, Gupta G, Priyadarshini R, Lochab B (2017) Graphene oxide-coated surface: inhibition of bacterial biofilm formation due to specific surface–interface interactions. ACS Omega 2(7):3070–3082
Zeng G, Vad BS, Dueholm MS, Christiansen G, Nilsson M, Tolker-Nielsen T, Nielsen PH, Meyer RL, Otzen DE (2015) Functional bacterial amyloid increases Pseudomonas biofilm hydrophobicity and stiffness. Front Microbiol 6:1099
Kwon T-H, Kim S (2017) Measuring elastic modulus of bacterial biofilms in a liquid phase using atomic force microscopy. Geomechanics Eng 12:863–870
Daniels SL, Pressman JG, Wahman DG (2016) AFM structural characterization of drinking water biofilm under physiological conditions. RSC Adv 6(7):5812–5816
Kogbara RB, Masad EA, Woodward D, Millar P (2018) Relating surface texture parameters from close range photogrammetry to Grip-Tester pavement friction measurements. Constr Build Mater 166:227–240
Whitehead KA, Verran J (2015) Formation, architecture and functionality of microbial biofilms in the food industry, Current Opinion in Food. Science 2:84–91
Hilbert LR, Bagge-Ravn D, Kold J, Gram L (2003) Influence of surface roughness of stainless steel on microbial adhesion and corrosion resistance. Int Biodeterior Biodegradation 52(3):175–185
Cressie N (2015) Statistics for spatial data. John Wiley & Sons
Ammar Y, Swailes D, Bridgens B, Chen J (2015) Influence of surface roughness on the initial formation of biofilm. Surf Coat Technol 284:410–416
E. Birkenhauer, S. Neethirajan 2014 Surface potential measurement of bacteria using Kelvin probe force microscopy, JoVE: J Vis Exp 93:e52327
J Xu, D Chen, W Li, J Xu (2018) Surface potential extraction from electrostatic and Kelvin-probe force microscopy images, J Appl Phys 123(18)
L. Collins, S.A. Weber, B.J. Rodriguez, Applications of KPFM-based approaches for surface potential and electrochemical measurements in liquid, Kelvin Probe Force Microscopy: From Single Charge Detection to Device Characterization (2018) 391–433.
Ikuma K, Madden AS, Decho AW, Lau BL (2014) Deposition of nanoparticles onto polysaccharide-coated surfaces: implications for nanoparticle–biofilm interactions. Environ Sci Nano 1(2):117–122
Vu B, Chen M, Crawford RJ, Ivanova EP (2009) Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 14(7):2535–2554
Oliver WC, Pharr GM (1992) An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res 7(6):1564–1583
Wang H, Wilksch JJ, Strugnell RA, Gee ML (2015) Role of Capsular Polysaccharides in Biofilm Formation: An AFM Nanomechanics Study. ACS Appl Mater Interfaces 7(23):13007–13013
Li H, Liu H, Zhang L, Hieawy A, Shen Y (2023) Evaluation of extracellular polymeric substances matrix volume, surface roughness and bacterial adhesion property of oral biofilm. Journal of Dental Sciences 18(4):1723–1730
Sportelli MC, Kranz C, Mizaikoff B, Cioffi N (2022) Recent advances on the spectroscopic characterization of microbial biofilms: a critical review. Analytica Chimica Acta 1195:339433
López-Jiménez L, Fusté E, Martínez-Garriga B, Arnabat-Domínguez J, Vinuesa T, Viñas M (2015) Effects of photodynamic therapy on Enterococcus faecalis biofilms. Lasers Med Sci 30(5):1519–1526
Yang Y, Li M, Zheng X, Ma H, Nerenberg R, Chai H (2022) Extracellular DNA plays a key role in the structural stability of sulfide-based denitrifying biofilms. Sci Total Environ 838
Micic M, Hu D, Suh YD, Newton G, Romine M, Lu HP (2004) Correlated atomic force microscopy and fluorescence lifetime imaging of live bacterial cells. Colloids Surf, B 34(4):205–212
Kassies R, Van der Werf K, Lenferink A, Hunter C, Olsen J, Subramaniam V, Otto C (2005) Combined AFM and confocal fluorescence microscope for applications in bio-nanotechnology. J Microsc 217(1):109–116
Doak SH, Rogers D, Jones B, Francis L, Conlan RS, Wright C (2008) High-resolution imaging using a novel atomic force microscope and confocal laser scanning microscope hybrid instrument: essential sample preparation aspects. Histochem Cell Biol 130(5):909–916
Burns AR (2003) Domain structure in model membrane bilayers investigated by simultaneous atomic force microscopy and fluorescence imaging. Langmuir 19(20):8358–8363
Deng Z, Zink T, Chen H-Y, Walters D, Liu F-T, Liu G-Y (2009) Impact of actin rearrangement and degranulation on the membrane structure of primary mast cells: a combined atomic force and laser scanning confocal microscopy investigation. Biophys J 96(4):1629–1639
Baclayon M, Roos WH, Wuite GJ (2010) Sampling protein form and function with the atomic force microscope. Mol Cell Proteomics 9(8):1678–1688
Wu S, Liu Y, Zhang H, Lei L (2019) A new transformation method with nanographene oxides of antisense carrying yycG RNA improved antibacterial properties on methicillin-resistant Staphylococcus aureus biofilm. J Vet Med Sci 81(10):1540–6
Kuyukina MS, Ivshina IB, Korshunova IO, Rubtsova EV (2014) Assessment of bacterial resistance to organic solvents using a combined confocal laser scanning and atomic force microscopy (CLSM/AFM). J Microbiol Methods 107:23–29
Stenclova P, Freisinger S, Barth H, Kromka A, Mizaikoff B (2019) Cyclic changes in the amide bands within Escherichia coli biofilms monitored using real-time infrared attenuated total reflection spectroscopy (IR-ATR). Appl Spectrosc 73(4):424–432
Lorite GS, de Souza AA, Neubauer D, Mizaikoff B, Kranz C, Cotta MA (2013) On the role of extracellular polymeric substances during early stages of Xylella fastidiosa biofilm formation. Colloids Surf, B 102:519–525
Zhang P, Feng B, Chen Y-P, Dai Y-Z, Guo J-S (2019) In situ characterizations for EPS-involved microprocesses in biological wastewater treatment systems. Crit Rev Environ Sci Technol 49(11):917–946
Ruggeri F, Longo G, Faggiano S, Lipiec E, Pastore A, Dietler G (2015) Infrared nanospectroscopy characterization of oligomeric and fibrillar aggregates during amyloid formation. Nat Commun 6(1):1–9
Ruggeri FS, Habchi J, Chia S, Horne RI, Vendruscolo M, Knowles TP (2021) Infrared nanospectroscopy reveals the molecular interaction fingerprint of an aggregation inhibitor with single Aβ42 oligomers. Nat Commun 12(1):1–9
Barlow DE, Biffinger JC, Estrella L, Lu Q, Hung C-S, Nadeau LJ, Crouch AL, Russell JN Jr, Crookes-Goodson WJ (2020) Edge-localized biodeterioration and secondary microplastic formation by Papiliotrema laurentii unsaturated biofilm cells on polyurethane films. Langmuir 36(6):1596–1607
Kurouski D (2017) Advances of tip-enhanced Raman spectroscopy (TERS) in electrochemistry, biochemistry, and surface science. Vib Spectrosc 91:3–15
Neugebauer U, Schmid U, Baumann K, Ziebuhr W, Kozitskaya S, Deckert V, Schmitt M, Popp J (2007) Towards a detailed understanding of bacterial metabolism—spectroscopic characterization of Staphylococcus epidermidis. ChemPhysChem 8(1):124–137
Schmid T, Sebesta A, Stadler J, Opilik L, Balabin RM, Zenobi R (2010) Tip-enhanced Raman spectroscopy and related techniques in studies of biological materials. SPIE, Synthesis and photonics of nanoscale materials VII, pp 15–27
Kumar N, Mignuzzi S, Su W, Roy D (2015) Tip-enhanced Raman spectroscopy: principles and applications. EPJ Techniques and Instrumentation 2(1):9
Liu Q, Yang H (2019) Application of atomic force microscopy in food microorganisms. Trends Food Sci Technol 87:73–83
Ravindranath SP, Kadam US, Thompson DK, Irudayaraj J (2012) Intracellularly grown gold nanoislands as SERS substrates for monitoring chromate, sulfate and nitrate localization sites in remediating bacteria biofilms by Raman chemical imaging. Anal Chim Acta 745:1–9
Zheng Z, Xing J, Shi H, Wu M, Yang R, Yao P, Xu RX (2022) 2D scanning SERS probe for early biofilm boundary determination. Sens Actuators, B Chem 363:131822
Cappitelli F, Polo A, Villa F (2014) Biofilm formation in food processing environments is still poorly understood and controlled. Food Engineering Reviews 6:29–42
Allen A, Habimana O, Casey E (2018) The effects of extrinsic factors on the structural and mechanical properties of Pseudomonas fluorescens biofilms: A combined study of nutrient concentrations and shear conditions. Colloids Surf, B 165:127–134
Costello KM, Gutierrez-Merino J, Bussemaker M, Ramaioli M, Baka M, Van Impe JF, Velliou EG (2018) Modelling the microbial dynamics and antimicrobial resistance development of Listeria in viscoelastic food model systems of various structural complexities. Int J Food Microbiol 286:15–30
Ito A, Taniuchi A, May T, Kawata K, Okabe S (2009) Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl Environ Microbiol 75(12):4093–4100
Jones WL, Sutton MP, McKittrick L, Stewart PS (2011) Chemical and antimicrobial treatments change the viscoelastic properties of bacterial biofilms. Biofouling 27(2):207–215
Dhayakaran RPA, Neethirajan S, Xue J, Shi J (2015) Characterization of antimicrobial efficacy of soy isoflavones against pathogenic biofilms. LWT-Food science and technology 63(2):859–865
Kumar VD, Verma PRP, Singh SK (2016) Morphological and in vitro antibacterial efficacy of quercetin loaded nanoparticles against food-borne microorganisms, LWT-Food. Science and Technology 66:638–650
Griffith A, Neethirajan S, Warriner K (2015) Development and evaluation of silver zeolite antifouling coatings on stainless steel for food contact surfaces. J Food Saf 35(3):345–354
Zou M, Tao W, Ye X, Liu D (2020) Evaluation of antimicrobial and antibiofilm properties of proanthocyanidins from Chinese bayberry (Myrica rubra Sieb et Zucc) leaves against Staphylococcus epidermidis. Food Sci & Nutr 8(1):139–149
Cortés ME, Bonilla JC, Sinisterra RD (2011) Biofilm formation, control and novel strategies for eradication. Sci Against Microbial Pathog Commun Curr Res Technol Adv 2:896–905
Sadekuzzaman M, Yang S, Mizan M, Ha S (2015) Current and recent advanced strategies for combating biofilms. Comprehensive Reviews in Food Science and Food Safety 14(4):491–509
Wu H, Moser C, Wang H-Z, Høiby N, Song Z-J (2015) Strategies for combating bacterial biofilm infections. Int J Oral Sci 7(1):1–7
Zrelli K, Galy O, Latour-Lambert P, Kirwan L, Ghigo J, Beloin C, Henry N (2013) Bacterial biofilm mechanical properties persist upon antibiotic treatment and survive cell death. New J Phys 15(12):125026
Al-Amshawee S, Yunus MYBM, Lynam JG, Lee WH, Dai F, Dakhil IH (2021) Roughness and wettability of biofilm carriers: A systematic review. Environ Technol Innov 21:101233
Uneputty A, Dávila-Lezama A, Garibo D, Oknianska A, Bogdanchikova N, Hernández-Sánchez J, Susarrey-Arce A (2022) Strategies applied to modify structured and smooth surfaces: A step closer to reduce bacterial adhesion and biofilm formation. Colloid and Interface Science Communications 46:100560
Bazaka K, Jacob MV, Crawford RJ, Ivanova EP (2012) Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl Microbiol Biotechnol 95:299–311
Lorite GS, Rodrigues CM, De Souza AA, Kranz C, Mizaikoff B, Cotta MA (2011) The role of conditioning film formation and surface chemical changes on Xylella fastidiosa adhesion and biofilm evolution. J Colloid Interface Sci 359(1):289–295
Sahoo M, Panigrahi C, Aradwad P (2022) Management strategies emphasizing advanced food processing approaches to mitigate food borne zoonotic pathogens in food system. Food Frontiers 3(4):641–665
Tarifa MC, Genovese D, Lozano JE, Brugnoni LI (2018) In situ microstructure and rheological behavior of yeast biofilms from the juice processing industries. Biofouling 34(1):74–85
Funding
The authors have not received any specific funds to complete this work.
Author information
Authors and Affiliations
Contributions
Xinhao Wang and Jingyi Xue wrote the main manuscript text and prepared all figures. Honglin Zhu, Sunni Chen, and Yi Wang revised and edited the manuscript and strengthened the discussion. Zhenlei Xiao proofread the manuscript and polished the language. Yangchao Luo conceptualized this review, supervised the writing process, edited and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These two authors contributed equally to this work and are designated as co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Xue, J., Zhu, H. et al. Advances in biofilm characterization: utilizing rheology and atomic force microscopy in foods and related fields. Adv Compos Hybrid Mater 7, 143 (2024). https://doi.org/10.1007/s42114-024-00950-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42114-024-00950-2