Abstract
Dental lasers have numerous applications for periodontal and peri-implant disease therapy which include non-surgical treatments, such as pathogen reduction, removal of surface accretions, and photobiomodulation; along with surgical procedures of soft tissue and osseous structures. A non-surgical protocol is the first approach, but surgery may follow to alleviate unresolved problems. This manuscript will describe how lasers can be utilized in this treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Quick reference/description
Non-surgical therapy represents a protocol for removal of maximum amount of calculus for disruption or elimination of biofilm and associated microorganisms. In addition, the root or implant surface should be restored to biocompatibility to reestablish the periodontal attachment or the peri-implant integration without inflammation. Lasers can be used adjunctively for non-surgical periodontal therapy to enhance its effectiveness. It is essential to monitor the treatment outcome for non-surgical laser therapy to determine the need for surgery. It is a well-accepted therapy that can also improve a patient’s postoperative home care regime.
Indications
Initial non-surgical treatment of periodontal and peri-implant disease.
Materials/instruments
-
Scaling instruments
-
Carbon fiber or plastic curettes
-
Diode laser system
-
Erbium laser systems (Er,Cr:YSGG, Er:YAG)
-
Nd:YAG laser system
-
CO2 laser system
Procedure
Periodontal and peri-implant disease therapies must be carefully planned. A thorough oral examination must include observations, radiographs, and pocket probing. A patient’s risk factors, such as medical health, heredity, stress, and prescribed medications, must be integrated into those findings, so that a diagnosis can be made. The recent (2017) periodontal and peri-implant disease classification scheme describes both the staging of the severity and complexity of the disease, and grading to help assess the outcome and progression of the disease. Once the diagnosis is reached, a treatment plan can be developed.
Initial periodontal therapy can then be commenced, and is termed, ‘non-surgical therapy.’ The term represents removal of maximum amount of calculus for disruption or elimination of the biofilm and associated microorganisms. This aids in decreasing inflammation and is considered as the initial treatment for periodontal and peri-implant disease. The patient’s periodontal status is assessed following this therapeutic phase, which can lead to two possible outcomes; one, the patient will continue to undergo periodontal maintenance, or two, the patient will undergo surgical therapy.
During initial non-surgical therapy, thorough removal of calculus from root or implant surfaces is crucial. Conventional periodontal therapy starts with removal of biofilm and calculus using scaling instruments on tooth surfaces and carbon fiber or plastic curettes on implant fixtures. This procedure is simple in patients with gingivitis or initial peri-implant mucositis with good ease of access. Debridement of root or implant surfaces becomes difficult with increase in disease severity. It has been shown that in spite of meticulous root planning or implant debridement, some calculus deposits remain and can impair treatment outcomes in deeper pockets. Hence, surgical access to these sites followed by placement of regenerative materials becomes essential. Sub-gingival debridement with conventional ultrasonic and sonic scalers may be ineffective for a bactericidal effect.
Usually, the initial therapy is performed in a general dentist’s office, when an immediate referral to a periodontist is not indicated. In this appointment, a dental hygienist can provide all or partial treatment within scope of his/her practice. During the initial treatment session, instructions for maintaining good oral hygiene should be provided to the patient. Multiple sessions may be required to complete the initial non-surgical therapy. Appropriate durations for evaluation can determine the success of the clinician’s treatment and the patient’s compliance.
Commonly used laser wavelengths
In general, dental lasers are used in adjunct to the initial non-surgical therapy. The commonly used laser instrument has a minimum output of about 0.5 W of average power. Any of the commercially available dental lasers can be used in initial non-surgical periodontal or peri-implant disease therapy for debriding the soft tissue aspect of periodontal pockets. Currently, the types and emission wavelengths of these lasers include diode (445, 810, 940, 980, and 1064 nm), Er,Cr:YSGG (2780 nm), Er:YAG (2940 nm), Nd:YAG (1064 nm) and CO2 (9300 and 10,600 nm) (Table 1). Both erbium wavelengths are currently indicated for removal of calculus from tooth structure.
Adjunctive laser use
The principle of using laser adjunctively to periodontal and peri-implant disease therapy is to aid in conventional instrumentation for disrupting or eliminating the biofilm and calculus deposits. All available wavelengths can be used, although only the erbium family is currently indicated for calculus removal. Conventional non-surgical instrumental of periodontal pockets does not facilitate complete elimination of bacterial toxins and deposits. Adjunctive use of lasers can improve therapeutic outcomes.
Dental lasers cause a rise in the temperature of target tissues that can affect the pathogens and the resultant inflammation. Most non-sporulating bacteria (periodontopathic anaerobes) are easily deactivated at a temperature of 50 °C. At temperatures of 60 °C, coagulation of the inflamed soft tissue wall of periodontal pockets and hemostasis can be achieved. Surgical excision of soft tissue can occur at 100 °C. Hence, use of lasers at such low temperatures represents non-surgical therapy. In case of calculus removal using erbium lasers, the primary interaction occurs when the photonic energy vaporizes the interstitial water of the mineralized matrix at a minimum temperature of 100 °C. However, the rapid pulsing of the lasers along with a water spray reduces a marked rise in temperature in the adjacent tissues.
Considering the elimination of the microbial component, laser irradiation has marked potential as an adjunct to conventional mechanical therapy. The lasers mentioned above use the photothermal effect for strong detoxification and bactericidal effects. In addition, debridement of the infected soft tissue wall of the pocket and coagulation of the blood and lymphatic vessels is possible to enhance healing.
General protocol
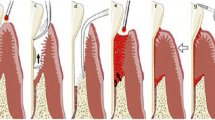
After examination and diagnosis, the clinician should check the periodontal charting and perform the initial non-surgical therapy (Figs.1a, 2a, 3a, 4a). The protocol is as follows:
-
Before any instrumentation, low power laser irradiation is utilized to decrease the microbial population in the sulcus. This reduces the risk of bacteremia and minimizes aerosol contamination during conventional instrumentation. While using the Nd:YAG, CO2 and diode wavelengths, prolonged laser contact with sub-gingival calculus and root surfaces should be avoided. When the Nd:YAG laser is used on implant surfaces, laser beam placement should be carefully done. Occasionally, calculus and biofilm removal is done prior to laser irradiation (Figs.1b, 2b, 4c).
-
Calculus removal from root or implant surfaces should be done with proper conventional instrumentation. As mentioned, both erbium wavelengths can be used primarily or adjunctively to remove this accretion.
-
Laser irradiation is performed for decontamination of pocket epithelium. The biofilm and microbial parts are disrupted when the photonic energy interacts with different components of the inflamed soft tissue. The average power employed for this irradiation is lower than that utilized for excisional surgery. Hence, the clinician should read the instrument’s operating manual to confirm the power settings. The treatment’s aim is to direct the laser beam towards the soft tissue with overlapping strokes to ensure irradiation of the complete pocket. The pocket anatomy (shape, depth, and width) governs the time requirement for this task. Occasionally, debris can collect on the contact tip of certain lasers or they can be flushed out of the pocket. When fresh bleeding ensues from the pocket, the decontamination is considered complete (Figs. 1b, 2d, e, 4c).
-
Laser energy is implemented to achieve coagulation and sealing of the blood and lymphatic vessels. Usually, this takes place in a short period with the last beam placement at the pocket entrance. In shallow pockets, the decontamination itself can bring about hemostasis without supplemental irradiation. After switching off the laser, digital pressure helps in re-adapting the tissue to the tooth, particularly in deeper pockets. In shallow pockets, the decontamination aids the initial healing.
-
Postoperative and oral hygiene instructions are provided to the patient. The tissue at the treated site should be minimally manipulated to prevent disruption of the fibrin clot. For a couple of days, brushing and flossing should be done very gently. Crunchy and spicy food items should be avoided for at least a day. Warm salt water rinses should be gently performed three times a day to soothe the tissues in a short duration. The patient is informed to expect only mild discomfort. It is essential to avoid sub-gingival irrigation (Figs. 1c, 2f, 3c, 4d).
a Preoperative view of a 5 mm pocket. b Preoperative view of a 6 mm pocket. c Preoperative periodontal probe chart. d, e After hand and ultrasonic scaling, an Nd:YAG laser is used with a 400 μm fiber and an average power of 1.8 W (30 mJ/pulse and 60 Hz) and directed toward the soft tissue side of the pocket. f, g 6-month postoperative view showing pocket depth reduction and lack of inflammation. h 6 month post operative periodontal probe chart
a Preoperative view of an 8 mm pocket with bleeding on probing. b Preoperative radiograph of the pockets. After ultrasonic removal of the calculus, an Er,Cr:YSGG laser was used with a 500µ diameter radial firing tip at an average power of 1.5 W (50 mJ, 30 Hz) with a pulse duration of 60microseconds for debridement. c 7-month postoperative probing shows significant pocket depth reduction without bleeding on probing. d 7-month postoperative radiograph depicts a more stable periodontium.
a Preoperative view of a 7 mm pocket with bleeding on probing around an implant. b Preoperative radiograph. c After careful conventional debridement of any calculus with conventional instrumentation, an 810 nm diode laser with a 400µ tip was used with an average power of 0.4 CW emission directed toward the soft tissue and away from the implant fixture. d 6-month postoperative view showing pocket depth reduction and lack of inflammation. e 6-month post operative radiograph
Treatment planning
Initial non-surgical therapy should be performed for all patients with periodontal or peri-implant disease. The disease extent is determined during periodontal examination and diagnosis. There are several points of consideration for treatment planning.
-
Patient’s physical limitations, such as temporomandibular joint disease or posture
-
Patient’s pain sensitivity during procedures and tolerance towards medications required to control it (local anesthetics, sedation)
-
Patient’s systemic health and risk factors that would impact the treatment outcome
-
Number of periodontal pockets requiring treatment and their anatomy
-
Amount and tenacity of debris and biofilm to be eliminated or disrupted
-
Any occlusal problems or restorations that could compromise the access for therapy and its success
-
Patient’s ability to continue proper oral hygiene techniques
The disease severity governs the appointment schedule for therapy and the patient’s tolerance for treatment. It is crucial that the treatment plan is customized for every patient. Occasionally, treatment for gingivitis may include only full-mouth disinfection and debridement, which can be achieved in two appointments along with polishing. In advanced conditions with excess calculus and biofilm, it becomes essential to treat only a few teeth per visit.
Each appointment length can also change. Usually, moderate generalized disease is treated with appointments of 1 h for each diseased site. Certain clinicians prefer to divide the oral cavity into quadrants for treatment, while others prefer to treat the deep pockets first. The second approach is advantageous as the pockets with severe disease can be retreated in subsequent visits, particularly if inflammation persists following the first appointment. To achieve maximum debridement of pockets, the use of laser can be repeated in the subsequent visits.
Development of the biofilm can be suppressed by placing locally delivered chemotherapeutic agents, such as minocycline hydrochloride, chlorhexidine gluconate and doxycycline hyclate in the pockets. These agents are more effective when placed after disrupting the biofilm during debridement. In general, these agents should be applied only following the last laser session.
During initial non-surgical therapy, the patient’s oral hygiene skills should be regularly monitored and reinforced, because the intended healing does not occur if the biofilm persists. It is essential to schedule a follow-up appointment every 4 weeks after completing the non-surgical therapy.
Three months after the initial non-surgical therapy, a follow-up appointment should evaluate the patient’s periodontal status and the effectiveness of his/her home care regimen. The clinician expects to observe absence or reduction of inflammation, decreased pocket depths without bleeding and a healthier tissue tone. In this appointment, a minimum probing force should be applied for evaluation to prevent disruption of the attachment apparatus. Comprehensive probing can be done at the 6-month post-treatment visit. Re-evaluation can be continued at intervals of 3 months for careful assessment of the manner of disease resolution. For preserving the improved clinical attachment and to maintain minimum inflammation, a supportive therapy involving additional debridement and laser decontamination can be continued with the patient’s daily oral care regime.
Considerations about laser use in initial non-surgical therapy
The absorption of photonic energy is based on several factors in laser-tissue interactions. In periodontal and peri-implant laser therapy, the same factors work in a very restricted area viz., the periodontal pocket and adjacent structures. Hence, it is important to consider the following points:
-
Each wavelength interacts differently with different tissue parts. For example, the near-infrared wavelengths are easily scattered and only absorbed by inflammation. They have significant penetration depth in sulcular fluid, and the energy can easily travel beyond the target tissue. Erbium lasers can be implemented efficiently for removal of sub-gingival calculus, but it is not very selective. These lasers can also remove the root cementum.
-
Lasers, when used carefully, are generally considered safe for the treatment of peri-implant mucositis. The usual emission mode of Nd:YAG lasers generates pulses of very short durations with a very high peak power per pulse. These high power pulses can damage titanium surfaces. Erbium lasers produce the same duration of pulses without any surface alterations and with low energy density. A clinician should know these differences to be able to select the proper wavelength for treatment.
-
To minimize healthy tissue ablation, the laser device should generate low average power. Every laser instrument has particular operating instructions with recommended settings, which should be followed for the therapy.
-
Every laser has an emission device (a small tube or an optical fiber tip) or a specific handpiece. It is essential to ensure that the laser beam is precisely directed towards the target tissue. For example, dark calculus readily absorbs diode photonic energy causing a marked temperature rise. Therefore, the laser tip should be angled towards the soft tissue. Similarly, when an erbium laser is used for calculus debridement, the laser tip should be parallel to the tooth axis to prevent excess removal of cementum.
-
During removal of granulation tissue, it can accumulate around the laser tube or tip. The tip or tube should be observed and frequently cleaned to prevent energy concentration in the debris.
-
Proper case selection and continuous monitoring is crucial. If the diseased areas do not respond to non-surgical therapy, surgical therapy is indicated.
-
A newer adjunctive modality, antimicrobial photodynamic therapy, shows promise for pathogen reduction. This process uses a nontoxic photoactive dye, which when exposed to photonic energy, produces a cytotoxic product. Visible and near infrared lasers can initiate the reaction, and studies are ongoing to scrutinize the benefit.
-
Another novel adjunctive modality, photobiomodulation, utilizes non thermal laser energy, principally from diode lasers, to facilitate periodontal wound healing. There are numerous citations in the literature showing some effectiveness for this therapy when used in conjunction to non-surgical and surgical procedures.
-
In addition, locally delivered antimicrobials (Minocycline hydrochloride, Chlorhexidine gluconate, and Doxycycline hyclate) can be added to the therapy after laser use.
Acronyms for non-surgical initial periodontal and peri-implant therapy
There are several acronyms in scientific literature or in the operating manuals of different laser devices. These terms mean to provide the first treatment phase. Terms such as LAPT (laser-assisted periodontal therapy), LAD/LABR (laser-assisted decontamination/laser-assisted bacterial reduction) and LCPT (laser-assisted comprehensive treatment) provide additional details about the procedure. Few companies have legally protected their acronyms:
-
REPaiR (regenerative Er,Cr:YSGG periodontitis regimen) uses the company’s Er,Cr:YSGG laser for root surface cleaning and sulcular debridement.
-
WPT™ (wavelength-optimized periodontal therapy) uses the company’s Nd:YAG laser for removal of diseased epithelial lining and the Er:YAG laser for debridement of root surface calculus.
Irrespective of the acronyms or terminology used, several laser wavelengths can be advantageous for periodontal and peri-implant disease therapy.
Pitfalls and complications
-
The clinician should be mindful of the periodontal anatomy of each site so that both conventional and laser therapy can proceed with as much precision and effectiveness as possible, while minimizing trauma to the surrounding tissue.
-
As described above, the interaction of the periodontal tissues and implant fixtures can be quite different with each wavelength. Careful attention to laser parameters, placement of the beam, and time of exposure are essential for beneficial therapy.
Further reading
Coluzzi DJ, Aoki A, Chiniforush N (2017) Laser treatment of periodontal and peri-implant disease in lasers in dentistry—current concepts. In: Coluzzi DJ, Parker SPA (Eds) Springer Nature, Cham, pp 295–309, ISBN 978-3-319-51943-2
Papapanou P et al (2018) Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol 89(Suppl 1):S173-182
Yu S et al (2022) Clinical effectiveness of adjunctive diode laser on scaling and root planing in the treatment of periodontitis: is there an optimal combination of usage mode and application regimen? A systematic review and meta-analysis. Lasers Med Sci 37(2):759–769
Nammour S et al (2021) Clinical evaluation of diode (980 nm) laser-assisted nonsurgical periodontal pocket therapy: a randomized comparative clinical trial and bacteriological study. Phorobio Photomed Laser Surg 39(1):10–22
Qadri T et al (2015) Role of diode lasers (800–980 nm) as adjuncts to scaling and root planing in the treatment of chronic periodontitis: a systematic review. Photomed Laser Surg 33(11):1–8
Ciurescu CE et al (2019) Adjunctive use of InGaAsP and Er, Cr:YSGG lasers in nonsurgical periodontal therapy: a randomized controlled clinical study. Quintessence Int 50:436–447
Zhou X, Lin M, Zhang D, Song Y, Wang Z (2019) Efficacy of Er: YAG laser on periodontitis as an adjunctive non-surgical treatment: a split-mouth randomized controlled study. J Clin Periodontol 46:539–547
Martelli FS et al (2016) Long-term efficacy of microbiology-driven periodontal laser-assisted therapy. Eur J Clin Microbiol Infect Dis 35(3):433–441
Peron D et al (2019) Photodynamic antimicrobial chemotherapy has an overt killing effect on periodontal pathogens? A systematic review of experimental studies. Lasers Med Sci 34(8):1527–1534
Mizutani K, et al. Lasers in minimally invasive periodontal and peri-implant therapy. Periodontol 2000.2016 71(1):185–212.
Aoki A, Mizutani K, Schwarz F et al (2015) Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000 68(1):217–269
Coluzzi DJ et al (2020) Do lasers have an adjunctive role in initial non-surgical periodontal therapy? A systematic review. Dent J 8(3):93. https://doi.org/10.3390/dj8030093
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Coluzzi, D.J., Aoki, A. & Chiniforush, N. Non-surgical laser therapy for periodontal and peri-implant disease. Clin Dent Rev 6, 6 (2022). https://doi.org/10.1007/s41894-022-00120-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41894-022-00120-x