Abstract
The periodontal disease (PD) etiology is mainly associated with some bacterial strains, such as Porphyromonas gingivalis (P. gingivalis). Nonsurgical root scaling (e.g., antibiotics) may achieve a temporary decrease in the P. gingivalis level, yet it cannot eradicate the microorganism. Moreover, antibiotics can lead to bacterial resistance and undesirable side effects. This systematic review was performed to identify animal data defining antimicrobial photodynamic therapy (PACT) role on experimental PD models in the treatment of P. gingivalis. Embase, MEDLINE, and PubMed were examined for studies published from January 1980 to August 2018. MeSH terms and Scopus data were used to find more related keywords. Four studies were selected and reviewed by two independent researches with a structured tool for rating the research quality. The beneficial effect of PACT included reductions in P. gingivalis counts, bleeding on probing, redness, and inflammation on multiple sites (i.e., first molar, dental implants; subgingival; and mandibular premolars). Although our results suggest that PACT displays antimicrobial action on P. gingivalis, thus improving the PD, a nonuniformity in the PACT protocol and the limited number of studies included lead to consider that the bactericidal efficacy of PACT against periodontal pathogens in PD remains unclear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental biofilm is a main etiological factor for periodontal disease (PD) [1], and it develops over a period of several weeks, initially developing supragingival, with a mature subgingival biofilm that establishes up to 12 weeks [2]. As the biofilm accumulates, there is colonization of several periodontal bacteria (e.g., Aggregatibacter actinomycetemcomitans; Fusobacterium sp.; Porphyromonas gingivalis (P. gingivalis); Prevotella sp.; Treponema denticola; Streptococcus beta-hemolytic) [3,4,5]. The bacterial biofilm leads to a wide range of inflammatory settings including the activation of leucocytes, neutrophils, and T lymphocytes and the release of antibodies, lipopolysaccharides, and chemical inflammatory mediators that include cytokines and chemokines [6,7,8,9]. Chronic periodontitis produces tissue signs such as periodontal pockets, periodontal attachment apparatus loss, bleeding, bone loss, resulting ultimately in tooth loss [9,10,11,12,13].
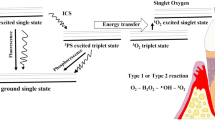
Nonsurgical root scaling may achieve a temporary decrease in the subgingival bacteria levels, yet it cannot eradicate the microorganism. The location of these bacteria in unreachable areas, such as furcations or the base of periodontal pockets, probably accounts for the failure of mechanical therapy [4]. Therefore, combination of treatments such as non-resective periodontal surgery, antibiotics, and good oral hygiene are means to control the bacteria [14]. Conversely, it has been reported that antibiotics can lead to bacterial resistance and undesirable side effects [14, 15]. These limitations have led to the search for new approaches that are effective and easily applied in the bacteremia of PD. In this regard, photodynamic antimicrobial chemotherapy (PACT), which uses a low-level light (led or laser), was introduced as a promising strategy antimicrobial platform to adjuvant treat PD. PACT has been a scientific demonstrated effect against microorganism since Raab has published it in 1900; Paramecium caudatum would die following irradiation in combination with acridine dye [16]. The PACT reduces microbes with little effect on keratinocytes, thereby constituting a safe alternative for antimicrobial treatment [17]. The surface of both Gram-positive and Gram-negative bacteria is negatively charged, which makes anionic photosensitizers ineffective [1]. PACT is based on the use of light at a specific wavelength in combination with a photosensitizer (PS); it leads to phototoxic reactions to induce bacterial destruction in a reaction called photodynamic effect. The PACT requires two components, a light source and a photosensitizer (photo reactive drug) capable of binding to the targeted cell. The photosensitizer becomes activated by light at a certain wavelength, thereby producing singlet oxygen as well as other reactive agents, which are toxic to bacteria [18, 19]. PACT begins when a PS absorbs a resonant photon (visible light or near-infrared) and it may impact the electron orbital by a given energy to PS molecule, which goes to singlet excited form. At this point, PS tends to decay, and it has two ways (i) emitting light by fluorescence or (ii) making an intercrossing system to a triplet state. The triplet state of PS has a long-term life and it has the opportunity to transfer energy to oxygen on substrate. The O2 receives the PS’s energy and it becomes toxic to every cell, especially to those that have less enzymatic content against reactive oxygen species (ROS). One single molecule of PS may go to this route about 10,000 times until it is destroyed [17]. Mechanism of PACT is showed in Fig. 1.
Although several researchers have found a PACT effect alone or in combination with alternative therapies to reduce bacterial infection [20, 21], there are studies showing null results [22, 23] or even a higher bacterial load [24]. Therefore, the aim of the present study was to systematically review the bactericidal efficacy of PACT in experimental PD models. In this primer, we focus on the PACT action in P. gingivalis because it is commonly found in PD [4, 13, 14] and accounts for the majority of periodontal tissue damage [25]. Moreover, to our knowledge, there are not many systematic reviews aimed at the antimicrobial PACT against P. gingivalis, in which there is data illustrating a positive [26] or negative [27] efficacy.
Materials and methods
Search strategy
The search scheme was carried out in accordance with the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) guidelines for systematic review. Original research articles published in English on Embase, MEDLINE, and PubMed, from January 1980 to August 2018, were retrieved and evaluated by two independent authors.
The keywords from related articles were selected, and MeSH terms and Scopus international data lines were used to find more related keywords with close meanings. The entire search strategy used was (Porphyromonas gingivalis OR P. gingivalis) AND (photodynamic therapy OR photodynamic OR phototherapy OR photochemotherapy OR photo-activated disinfection) AND (antimicrobial photodynamic therapy OR antimicrobial photodynamic OR antimicrobial phototherapy OR antimicrobial photodynamic OR antimicrobial photochemotherapy). MeSH terms were used individually or combined to increase the findings. The search was repeated following a review of the eligible papers on experimental methodology, outcomes, and irradiation parameters. The retrieved articles were also reviewed to identify possible additional studies.
Study selection
Title and abstract screening of citations were examined for potentially eligible studies, and two independent reviewers applied predetermined inclusion criteria to full studies. Conflicts were solved by a third independent researcher.
Inclusion criteria were as follows:
-
1.
live animal subjects;
-
2.
random allocation of treatment;
-
3.
type of irradiation was provided as an intervention to at least one of the treatment groups;
-
4.
a quantitative or semi-quantitative measure;
-
5.
English language.
The exclusion criteria were as follows:
-
1.
in vitro, clinical and systematic review articles with or without meta-analysis;
-
2.
papers not published in English language.
Quality of studies
Potentially eligible articles were printed, reviewed, and critically judged for quality rating by two independent reviewers. Systematic reviews are commonly performed in clinical trials but rarely in animal studies. Quality rating scales commonly used consider issues as the appropriateness of the animal model being evaluated; therefore, the quality of study was analyzed using a scale targeted for animal/tissue researches (QATRS) [28]. QATRS is a 20-point scale evaluation chart designed to assess randomization, blinding, standardization, and reliability of measurements, management of study withdrawals, appropriateness of statistical methods, and similarity of the animal/tissue model with clinical studies.
Results
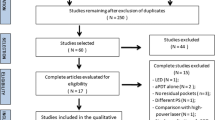
Overall, 76 articles were found in a first screening on the databases, and abstracts were used to identify studies who were repeatedly found in more than one database (i.e., repeated study; n = 27). Thereby, 49 studies were prescreened for overall review. Among these, 45 studies were excluded because they did not meet the inclusion criteria of this systematic review: in vitro studies (n = 18); clinical trials (n = 20); systematic reviews (n = 5); no experimental model of PD (n = 1); lipopolysaccharide inoculation (n = 1). Therefore, four studies were included for critical evaluation of the bacterial infection protocol and PACT (Fig. 2).
Table 1 shows data extracted from the papers, in which the studies evaluated two animal species (i.e., rat and dog), and several bacterial strains (Actinomyces, Fusobacterium nucleatum, Fusobacterium sp, P. gingivalis, Prevotella sp, Streptococcus beta-hemolytic, Treponema denticola) [3, 29,30,31]. The induction of PD in multiple sides (first molar, dental implants, subgingival regions, and mandibular premolars) was mainly caused by using a ligature model (three studies), and only one study was conducted with the direct P. gingivalis inoculation. According to the QATRS, the studies showed a range of methodological quality between 14 and 18 points on a scale until 20.
Table 2 illustrates PACT effectiveness data. The studies applied diode to the target tissue with a wavelength ranging from 660 to 662 nm, a fluence varying between 12.7 and 212.3 J/cm2, and irradiance between 0.06 and 1.06 W/cm2. Unfortunately, only one study had a complete description of the irradiation parameters [3]. Several photosensitizers were used on the bacteria, including toluidine blue, 25% azulene, chlorin e6, and BLC 1010 and phenothiazine chloride. The irradiation time per point was reported between 10 and 180 s per site. Table 2 also shows the main benefits of PACT as a noninvasive approach to control oral bacteremia. Notwithstanding, PACT is distinctly advantageous in reducing the periodontal signs of redness, bleeding on probing, and inflammation.
Discussion
The PD is mainly associated with the presence of distinct bacterial strains, in which P. gingivalis is a Gram-negative bacterium leading to colonization of subgingival plaque with consequent tissue invasion and destruction in several forms of PD [4, 13]. Thus, although the included studies had the periodontal biofilm as a target in which it has multiple bacteria, this systematic review of experimental studies reviewed the in vivo antimicrobial efficacy of PACT against a major periodontal pathogen in PD—P. gingivalis. The application of eligibility criteria resulted in the inclusion of only four studies derived from an initial screening of 49 papers. Although the studies that fulfilled our eligibility criteria applied different PACT protocols, the reduction of periopathogenic microorganism counts was a common finding. On the other hand, an issue should be clarified for the study of de Oliveira et al. [31] in which P. gingivalis counts were significantly reduced 1 week after a single application of PACT. However, after 4 weeks, the authors reported a regrowth of microorganism. A reasonable explanation for this finding is the frequency of the PACT application, in which there are indications that PACT treatment should be performed weekly [31].
Multiple factors for the bactericidal effect of PACT are proposed. These include DNA breakdown, efflux of potassium ion, abnormalities of sarcolemmal proteins, and interruptions in the cell wall synthesis [26]. Typically, the light absorption by the photosensitizer results in excited singlet state and triplet excited state to cause type I and type photo-oxidative reactions. There is a burst of radicals and reactive oxygen species, and if sufficient oxidative damage ensues, this will result in target-bacterial death [32,33,34,35].
Photosensitizers and light protocol
It is noteworthy that the included studies had substantial heterogeneity in the parameters related to PACT. For example, each study has applied different photosensitizers on different laser irradiation fluences to cause decontamination. Azulene, toluidine blue, chorin E6, BLC 1010, and phenothiazine chloride of methylene blue or toluidine blue as photosensitizers were included. This criterion was selected because these dyes are the most used for oral PACT [36]. Moreover, methylene blue and toluidine blue present high effectiveness in both Gram-positive and Gram-negative bacteria [37].
All studies included demonstrated that the combination of dyes and a light source PACT led to lower bacterial proliferation compared with samples of control animals (not treated with PACT). It has been reported that the effectiveness of PACT is greater to control or eliminate oral bacteria in planktonic phase than in biofilms [38]. The probable reasons for the lower effectiveness of PACT in biotopes may be the distinct and protected phenotypes, such as those of dental plaque microorganisms, which are able to adhere to the teeth [39].
The therapeutic light corresponds to a small share of the total electromagnetic radiation with wavelengths from visible to infrared from 300 to 1100 nm [40]. All included studies have applied irradiations with a wavelength of 660 to 662 nm combined with the dye. This wavelength range has been well-reported to be within a suitable “phototherapeutic window” to excite the photosensitizer to produce radicals and/or reactive oxygen species [41]. Further, although a relationship between laser irradiance and the bactericidal efficacy of PACT remains to be established, we have reported very low irradiance values for the four included studies, in which it can limit the clinical translation of the findings. In fact, clinical studies that showed a significant reduction in P. gingivalis had a higher range of irradiance [26].
Limitations
There are some limitations that can be raised from our review. First, it is evident from the previous discussion that the differences in laser fluence and irradiance and types of photosensitizer would have resulted in a nonstandardized overall dose of PACT in the included studies. Then, studies with standardized inclusion criteria and treatment regimens are recommended in this regard. Moreover, the variance of PACT patterns and the absence of all irradiation data make the comparison between studies complex. Second, the small number of studies included in this systematic review is insufficient to perform a meta-analysis, which could better illustrate the PACT efficacy relevant to clinical observations. Third, there are heterogeneity between the four included studies such as the use of the two animal species (i.e., rat and dog) and three distinct procedures to induce PD (i.e., ligature, peri-implant, or bacterial injection). These observations have important implications to evaluate PACT role. For example, the rate of PD in dogs is high, increases with aging, and hence, the etiopathology is closely associated with humans [42]. On the other hand, the occurrence of PD in rats is less frequent than in human and there is continuous growth and migration of the teeth [43], which might not be subtle for studying the repercussion of the PACT over long periods. In addition, variability in host responses to bacterial infection among dog and rat can contribute significantly to the severity of PD and thus, the effect of the PACT. Finally, ligatures or seeding with exogenous pathogens (bacterial inoculation) to induce PD can elicit different disease evolution [44] and therefore a non-singular response to PACT treatment.
Conclusion
Although PACT is a promising strategy to eradicate pathogenic microorganisms such as P. gingivalis, and this systematic review has shown some benefit concerning the effectiveness of therapy, some limitations show and should be considered so to assume a well-defined bactericidal efficacy of PACT against periodontal pathogens in PD. A valuable route could be to establish a well-standardized PACT to be applied homogeneously in future experimental studies. This could result in less heterogeneous data for antimicrobial effectiveness. Notwithstanding, Gram-negative bacteria as P. gingivalis are far more resistant to PACT [45]. Therefore, search for new approaches (e.g., polymyxin B nonapeptide or ethylene diamine tetraacetic acid) that can permeabilize the outer membrane to allow non-cationic photosensitizer [45] could have better antibacterial results compared with data reported in this review.
References
Oruba Z, Labuz P, Macyk W, Chomyszyn-Gajewska M (2015) Antimicrobial photodynamic therapy-a discovery originating from the pre-antibiotic era in a novel periodontal therapy. Photodiagn Photodyn Ther 12:612–618. https://doi.org/10.1016/j.pdpdt.2015.10.007
Lovegrove JM (2004) Dental plaque revisited: bacteria associated with periodontal disease. J N Z Soc Periodontol 87:7–21
Theodoro LH, Pires JR, Fernandes LA, Gualberto Júnior EC, Longo M, de Almeida JM, Garcia VG (2015) Effect of antimicrobial photodynamic therapy on periodontally infected tooth sockets in rats. Lasers Med Sci 30:677–683. https://doi.org/10.1007/s10103-013-1400-8
Carvalho C, Cabral CT (2007) Role of Porphyromonas gingivalis in periodontal disease. Rev Port Estomato Cir Maxilofac 48:167–171. https://doi.org/10.1016/S1646-2890(07)70136-X
Papapanou PN (2002) Population studies of microbial ecology in periodontal health and disease. Ann Periodontol 7:54–61. https://doi.org/10.1902/annals.2002.7.1.54
Graves DT, Cochran D (2003) The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol 74:391–401. https://doi.org/10.1902/jop.2003.74.3.391
Taubman MA, Valverde P, Han X, Kawai T (2005) Immune response: the key to bone resorption in periodontal disease. J Periodontol 76:2033–2041. https://doi.org/10.1902/jop.2005.76.11-S.2033
Kinney JS, Ramseier CA, Giannobile WV (2007) Oral fluid based biomarkers of alveolar bone loss in periodontitis. Ann N Y Acad Sci 1098:230–2351. https://doi.org/10.1196/annals.1384.028
Kinane DF, Stathopoulou PG, Papapanou PN (2017) Periodontal diseases. Nat Rev Dis Primers 3:17038. https://doi.org/10.1038/nrdp.2017.38
Mayer C, Moritz R, Kirschner C, Borchard W, Maibaum R, Wingender J, Flemming HC (1999) The role of intermolecular interactions: studies on model systems for bacterial biofilms. Int J Biol Macromol 26:3–16. https://doi.org/10.1016/S0141-8130(99)00057-4
Dentino A, Lee S, Mailhot J, Hefti AF (2013) Principles of periodontology. Periodontol 61:16–53. https://doi.org/10.1111/j.1600-0757.2011.00397.x
Meulman T, Peruzzo DC, Stipp RN, Gonçalves PF, Sallum EA, Casati MZ, Goncalves RB, Nociti FH Jr (2011) Impact of Porphyromonas gingivalis inoculation on ligature-induced alveolar bone loss. A pilot study in rats. J Periodontal Res 46:629–636. https://doi.org/10.1111/j.1600-0765.2011.01385.x
Tuite-McDonnell M, Griffen AL, Moeschberger ML, Dalton RE, Fuerst PA, Leys EJ (1997) Concordance of Porphyromonas gingivalis colonization in families. J Clin Microbiol 35:455–461
Slots J, Jorgensen MG (2000) Efficient antimicrobial treatment in periodontal maintenance care. J Am Dent Assoc 131:1293–1304. https://doi.org/10.14219/jada.archive.2000.0383
Baym M, Stone LK, Kishony R (2016) Multidrug evolutionary strategies to reverse antibiotic resistance. Science 351:aad3292. https://doi.org/10.1126/science.aad3292
Raab O (1900) Uber die wirkung fluoreszierender stoffe auf infusorien. Z Biol 39:524–546
Zeina B, Greenman J, Corry D, Purcell WM (2002) Cytotoxic effects of antimicrobial photodynamic therapy on keratinocytes in vitro. Br J Dermatol 146:568–573. https://doi.org/10.1046/j.1365-2133.2002.04623.x
Kömerik N, Nakanishi H, MacRobert AJ, Henderson B, Speight P, Wilson M (2003) In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother 47:932–940. https://doi.org/10.1128/AAC.47.3.932-940.2003
Wainwright M, Maisch T, Nonell S, Plaetzer K, Almeida A, Tegos GP, Hamblin MR (2017) Photoantimicrobials-are we afraid of the light? Lancet Infect Dis 17:e49–e55. https://doi.org/10.1016/S1473-3099(16)30268-7
Corrêa MG, Oliveira DH, Saraceni CH, Ribeiro FV, Pimentel SP, Cirano FR, Casarin RC (2016) Short-term microbiological effects of photodynamic therapy in non-surgical periodontal treatment of residual pockets: a split-mouth RCT. Lasers Surg Med 48:944–950. https://doi.org/10.1002/lsm.22449
Birang E, Talebi Ardekani MR, Rajabzadeh M, Sarmadi G, Birang R, Gutknecht N (2017) Evaluation of effectiveness of photodynamic therapy with low-level diode laser in nonsurgical treatment of peri-implantitis. J Lasers Med Sci 8:136–142. https://doi.org/10.15171/jlms.2017.25
Borekci T, Meseli SE, Noyan U, Kuru BE, Kuru L (2018) Efficacy of adjunctive photodynamic therapy in the treatment of generalized aggressive periodontitis: a randomized controlled clinical trial. Lasers Surg Med. https://doi.org/10.1002/lsm.23010
Grzech-Leśniak K, Matys J, Dominiak M (2018) Comparison of the clinical and microbiological effects of antibiotic therapy in periodontal pockets following laser treatment: an in vivo study. Adv Clin Exp Med 27:1263–1270. https://doi.org/10.17219/acem/70413
Talebi M, Taliee R, Mojahedi M, Meymandi M, Torshabi M (2016) Microbiological efficacy of photodynamic therapy as an adjunct to non-surgical periodontal treatment: a clinical trial. J Lasers Med Sci 7:126–130. https://doi.org/10.15171/jlms.2016.21
Sochalska M, Potempa J (2017) Manipulation of neutrophils by Porphyromonas gingivalis in the development of periodontitis. Front Cell Infect Microbiol 7:197. https://doi.org/10.3389/fcimb.2017.00197
Akram Z, Al-Shareef SA, Daood U, Asiri FY, Shah AH, AlQahtani MA, Vohra F, Javed F (2016) Bactericidal efficacy of photodynamic therapy against periodontal pathogens in periodontal disease: a systematic review. Photomed Laser Surg 34:137–149. https://doi.org/10.1089/pho.2015.4076
Fraga RS, Antunes LAA, Fontes KBFDC, Küchler EC, Iorio NLPP, Antunes LS (2018) Is antimicrobial photodynamic therapy effective for microbial load reduction in peri-implantitis treatment? A systematic review and meta-analysis. Photochem Photobiol 94:752–759. https://doi.org/10.1111/php.12901
Dos Santos SA, Serra AJ, Stancker TG, Simões MCB, Dos Santos Vieira MA, Leal-Junior EC, Prokic M, Vasconsuelo A, Santos SS, de Carvalho PTC (2017) Effects of photobiomodulation therapy on oxidative stress in muscle injury animal models: a systematic review. Oxidative Med Cell Longev 2017:5273403. https://doi.org/10.1155/2017/5273403
Hayek RR, Araújo NS, Gioso MA, Ferreira J, Baptista-Sobrinho CA, Yamada AM, Ribeiro MS (2005) Comparative study between the effects of photodynamic therapy and conventionaltherapy on microbial reduction in ligature-induced peri-implantitis in dogs. J Periodontol 76:1275–1281. https://doi.org/10.1902/jop.2005.76.8.1275
Sigusch BW, Pfitzner A, Albrecht V, Glockmann E (2005) Efficacy of photodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog model. J Periodontol 76:1100–1105. https://doi.org/10.1902/jop.2005.76.7.1100
de Oliveira RR Jr, Novaes AB, Garlet GP, de Souza RF Jr, Taba M, Sato S, de Souza SL, Palioto DB, Grisi MF, Feres M (2011) The effect of a single episode of antimicrobial photodynamic therapy in the treatment of experimental periodontitis. Microbiological profile and cytokine pattern in the dog mandible. Lasers Med Sci 26:359–367. https://doi.org/10.1007/s10103-010-0864-z
Takasaki AA, Aoki A, Mizutani K, Schwarz F, Sculean A, Wang CY, Koshy G, Romanos G, Ishikawa I, Izumi Y (2009) Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontol 51:109–140. https://doi.org/10.1111/j.1600-0757.2009.00302.x
Luksiene Z (2003) Photodynamic therapy: mechanism of action and ways to improve the efficiency of treatment. Medicina (Kaunas) 39:1137–1150
Demidova TN, Hamblin MR (2007) Photodynamic therapy targeted to pathogens. Int J Immunopathol Pharmacol 17:245–254. https://doi.org/10.1177/039463200401700304
Maisch T (2007) Anti-microbial photodynamic therapy: useful in the future? Lasers Med Sci 22:83–91. https://doi.org/10.1007/s10103-006-0409-7
Soukos NS, Goodson JM (2011) Photodynamic therapy in the control of oral biofilms. Periodontol 55:143–166. https://doi.org/10.1111/j.1600-0757.2010.00346.x
Usacheva MN, Teichert MC, Biel MA (2001) Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg Med 29:165–173. https://doi.org/10.1002/lsm.1105
Goulard C, Langrand S, Carniel E, Chauvaux S (2010) The Yersinia pestis chromosome encodes active addiction toxins. J Bacteriol 192:3669–3677. https://doi.org/10.1128/JB.00336-10
Fontana CR, Abernethy AD, Som S, Ruggiero K, Doucette S, Marcantonio RC, Boussios CI, Kent R, Goodson JM, Tanner AC, Soukos NS (2009) The antibacterial effect of photodynamic therapy in dental plaque derived biofilms. J Periodontal Res 44:751–759. https://doi.org/10.1111/j.1600-0765.2008.01187.x
Anders JJ, Borke RC, Woolery SK, Van de Merwe WP (1993) Low power laser irradiation alters the rate of regeneration of the rat facial nerve. Lasers Surg Med 13:72–82. https://doi.org/10.1002/lsm.1900130113
Star WM, Marijnissen JPA, van Gemert MJC (1987) Light dosimetry: status and prospects. J Photochem Photobiol B 1:149–167. https://doi.org/10.1016/1011-1344(87)80023-4
Attstrom R, Graf-de Beer M, Schroeder HE (1975) Clinical and histologic characteristics of normal gingiva in dogs. J Periodontal Res 10:115–127
Struillou X, Boutigny H, Soueidan A, Layrolle P (2010) Experimental animal models in periodontology: a review. Open Dent J 4:37–47. https://doi.org/10.2174/1874210601004010037
Oz HS, Puleo DA (2011) Animal models for periodontal disease. J Biomed Biotechnol 2011:754857. https://doi.org/10.1155/2011/754857
Sperandio FF, Huang YY, Hamblin MR (2013) Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat Antiinfect Drug Discov 2:108–120. https://doi.org/10.2174/1574891X113089990012
Acknowledgments
We are grateful to the National Council for Scientific and Technological Development – CNPq (grant no. 305527/2017-7). The study was supported in part by São Paulo Research Foundation – FAPESP (grant no. 2018/06865-7). The funding had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors are grateful to Gisele Dalben for professionally editing this paper.
Funding
This work was funded in part by the Brazilian National Council for Scientific and Technological Development – CNPq (grant 305,527/2017-7) and Coordination for the Improvement of Higher Education Personnel – CAPES (grant 178,283).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peron, D., Bergamo, A., Prates, R. et al. Photodynamic antimicrobial chemotherapy has an overt killing effect on periodontal pathogens? A systematic review of experimental studies. Lasers Med Sci 34, 1527–1534 (2019). https://doi.org/10.1007/s10103-019-02806-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02806-4